Abstract
The article describes a green and efficient synthesis method for pyrano[2,3-c]-pyrazoles, employing DL-alpha-tocopherol methoxypolyethylene glycol succinate solution (TPGS-750-M) as a green and biodegradable surfactant in water. The utilization of water as a reaction medium and TPGS-750-M as a surfactant obviates the necessity for organic solvents, thereby enhancing the environmental sustainability of the synthesis. The compounds synthesized using this novel method was characterized using various spectroscopic techniques, including 1H-NMR, 13C-NMR, and mass spectrometry.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vitamin E is a group of fat-soluble compounds with antioxidant properties that are commonly found in foods such as nuts, seeds, and vegetable oils [1]. TPGS-750-M is a water-soluble derivative of Vitamin E that has been developed as an emulsifying agent and surfactant [2]. TPGS-750-M derivatives have been studied extensively for their potential as green alternatives in various applications, including pharmaceuticals, cosmetics, and food products [3, 4]. In recent years, natural compounds such as Vitamin E and TPGS-750-M have gained significant attention as green alternatives to traditional synthetic compounds in various applications. Vitamin E has been shown to have potential as a natural antioxidant and stabilizer in drug formulations in the pharmaceutical industry, and studies have demonstrated that vitamin E can protect drugs from oxidation and enhance their stability, leading to improved shelf life and reduced waste [5]. In addition, TPGS has been used as a solubilizing agent and enhancer of drug absorption, making it a promising candidate for improving the bioavailability of poorly soluble drugs [6]. TPGS-750-M has also been used as a natural alternative to synthetic emulsifiers and surfactants, improving the stability and texture of cosmetic formulations. TPGS-750-M has been used as an emulsifying agent and stabilizer in food formulations, leading to improved texture and shelf life [7]. The use of TPGS-750-M in green chemistry has several advantages over traditional synthetic compounds. These natural compounds are biodegradable and non-toxic, reducing the environmental impact of chemical products. They are also renewable resources, reducing dependence on fossil fuels and other non-renewable resources. Furthermore, their use in various applications has been shown to improve the quality and stability of products, reduce waste, and increase efficiency. The use of TPGS-750-M in green chemistry has significant potential as a sustainable and environmentally friendly alternative to traditional synthetic compounds [8, 9]. Further research and development are needed to fully realize the benefits of these natural compounds in various applications.
Green chemistry is an approach to chemical research and engineering that seeks to reduce or eliminate the use and generation of hazardous substances in the design, manufacture, and application of chemical products [10]. However, green synthesis is a rapidly growing area of research that focuses on the development of sustainable and environmentally friendly methods for synthesizing a wide range of materials [11]. This field has gained significant attention in recent years due to its potential to address some of the key environmental and health issues associated with traditional chemical synthesis methods. Green synthesis involves the use of non-toxic and renewable starting materials, as well as energy-efficient and environmentally benign reaction conditions. It offers a promising alternative to conventional synthetic methods that rely on toxic solvents, high temperatures, and pressures and produce harmful waste products [12, 13]. The use of green synthesis methods not only reduces the environmental impact of chemical synthesis but also offers a range of benefits, such as improved product purity, increased reaction efficiency, and reduced manufacturing costs.
Pyrano[2,3-c]pyrazoles are characterized by a pyrazole ring fused to a pyran ring, rendering them structurally unique and intriguing from a synthetic standpoint. This class of heterocyclic compounds has garnered considerable attention in recent years due to its diverse biological activities and potential applications in medicinal chemistry, as depicted in Fig. 1 [14,15,16,17,18,19]. These compounds have been shown to exhibit anticancer, antimicrobial, anti-inflammatory, and antiviral activities [20,21,22,23,24]. For example, several studies have reported the antitumor activity of pyrano[2,3-c]pyrazoles against a variety of cancer cell lines, thus making them promising candidates for the development of novel anticancer agents [25]. Furthermore, pyrano[2,3-c]pyrazoles have also shown potential as enzyme inhibitors. For instance, several studies have demonstrated their ability to inhibit key enzymes such as acetylcholinesterase, tyrosinase, and α-glucosidase [26, 27]. The inhibition of these enzymes is also important for the treatment of diseases such as Alzheimer's, Parkinson's, and diabetes, respectively. In addition to their biological activities, pyrano[2,3-c]pyrazoles are also useful intermediates for the synthesis of other important compounds. They can be used as starting materials for the synthesis of pyrazolo[1,5-a]pyrimidines, which have been shown to exhibit potent anticancer and antiviral activities [28, 29].
The use of TPGS-750-M as a catalyst in the synthesis of pyrano[2,3-c]pyrazole can represent an environmentally friendly and economical approach in line with the principles of green chemistry. The aim of this developed method is to provide a more sustainable and environmentally friendly alternative to traditionally used chemicals with high toxic and environmental impacts. It will contribute to make pyrano[2,3-c]pyrazole synthesis processes more efficient, environmentally friendly, and economical. Furthermore, the use of TPGS-750-M can be considered to make a significant contribution to the efforts to achieve environmental sustainability goals in the field of industrial chemistry, as it has led to the reduction of waste products and toxic substances, as well as energy savings. Therefore, the use of TPGS-750-M as a catalyst in the synthesis of pyrano[2,3-c]pyrazole will be an important and beneficial development, both scientifically and environmentally.
Experimental
General
The chemicals utilized in this study were purchased from two different companies, Merck and Sigma-Aldrich. No further purification was performed on the reagents and solvents used. The progress of the reactions was observed by analyzing them with thin-layer chromatography (TLC) on aluminum sheets pre-coated with Merck silica gel 60 F254, visualized under UV light. The obtained samples underwent measurement of their melting points employing an Electrothermal Gallenkamp apparatus, and the resultant values were reported without any adjustments. Nuclear Magnetic Resonance (NMR) spectra, specifically 1H (400 MHz) and 13C (100 MHz), were recorded in deuterated dimethyl sulfoxide (DMSO-d6) solutions. Thermo Scientific hybrid quadrupole-Orbitrap mass spectrometer, equipped with a refractive index detector, was employed for the determination of the molecular weights of the acquired samples.
General procedure for synthesis of pyrano [2,3-c] pyrazoles
A mixture of ethyl acetoacetate 1 (1 mmol), hydrazine hydrate 2 (1 mmol), and 2 mL TPGS-750-M solution (2 wt% in H2O) was stirred at room temperature for 3 min, then molanonitrile 3 (1 mmol), and aldehydes 4a-y (each 1 mmol) were added to the mixture. It was stirred at 50 °C. The reaction progress was monitored by TLC under UV light. After completion of the reaction, the resulting precipitate was subjected to washing with cold ethanol and subsequent filtration. Crude products were recrystallized from ethanol (96%) to afford the title pure compounds. The confirmation of the identity of established products was achieved through a meticulous comparison of their spectroscopic data and inherent physical characteristics with the comprehensive information provided in contemporary literature. Conversely, the novel products were discerned through rigorous analysis of their spectral data, encompassing 1H-NMR, 13C-NMR, and Mass data.
Selected data
6-amino-5-cyano-3-methyl-4-phenyl-1,4-dihydropyrano[2,3-c]pyrazole (5a)
White solid, Yield 99%; m.p: 243–245 °C. 1H NMR (400 MHz, DMSO-d6, δ ppm): 12.21 (s, 1H, NH), 7.60–7.08 (m, 5H), 6.90 (s, 2H, NH2), 4.51 (s, 1H), 1.95 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6): 159.3, 153.8, 145.3, 136. 3, 129.5, 129.3, 128.6, 128.2, 120.3, 97.4, 57.7, 37.9, 10.1; HRMS (ESI): m/z Calcd for C14H13N4O [M + H]+: 253.10859; Found, 253.10873.
6-amino-4-(4-fluorophenyl)-3-methyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (5e)
White solid, Yield 96%; m.p: 238–240 °C. 1H NMR (400 MHz, DMSO-d6, δ ppm): 11.99 (s, 1H, NH), 7.33–7.12 (m, 4H), 6.96 (s, 2H, NH2), 4.59 (s, 1H), 1.56 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6): 159.5, 153.9, 144.9, 130.6, 129.4, 127.6, 123.4, 122.2, 119.1, 115.3, 99.4, 58.8, 36.4, 9.5; HRMS (ESI): m/z Calcd for C14H12FN4O [M + H]+: 271.09917; Found, 271.09924.
6-amino-3-methyl-4-(3-nitrophenyl)-2,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (5j)
Yellow solid, Yield 83%; m.p: 191–193 °C. 1H NMR (400 MHz, DMSO-d6, δ ppm): 12.01 (s, 1H, NH), 7.63–7.04 (m, 4H), 6.85 (s, 2H, NH2), 4.65 (s, 1H), 1.97 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6): 160.0, 154.9, 149.3, 133.6, 132.5, 129.8, 127.8, 122.3, 121.0, 116.0, 99.9, 58.3, 35.2, 10.0; HRMS (ESI): m/z Calcd for C14H12N5O3 [M + H]+: 298.09347; Found, 298.09363.
6-amino-4-(3,4-dimethoxyphenyl)-3-methyl-2,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (5o)
White solid, Yield 62%; m.p: 190–192 °C. 1H NMR (400 MHz, DMSO-d6, δ ppm): 12.06 (s, 1H, NH), 7.53–7.18 (m, 3H), 6.91 (s, 2H, NH2), 4.51 (s, 1H), 3.45 (s, 3H, CH3), 3.26 (s, 3H, CH3), 1.98 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6): 160.9, 159.3, 155.1, 152.8, 134.8, 130.8, 127.5, 124.8, 122.6, 116.4, 99.8, 61.1, 55.5, 51.0, 36.3, 9.9; HRMS (ESI): m/z Calcd for C16H16N4O3Na [M + Na]+: 311.11256; Found, 311.11288.
6-amino-3-methyl-4-(1λ.2-pyrrol-2yl)-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (5u)
Yellow solid, m.p: 201–203 °C. 1H NMR (400 MHz, DMSO-d6, δ ppm): 11.85 (s, 1H, NH), 10.08 (s, 1H, NH), 7.64–6.53 (m, 3H), 6.81 (s, 2H, NH2), 4.70 (s, 1H), 1.88 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6): 160.6, 158.1, 144.9, 129.4, 122.2, 119.8, 118.9, 115.5, 100.1, 59.8, 34.8, 10.2; HRMS (ESI): m/z Calcd for C12H11N5OK [M + K]+: 279.05139; Found, 279.05106.
Results and discussion
Overall, the scientific significance of pyrano[2,3-c]pyrazoles lies in their diverse biological activities and potential applications in medicinal chemistry [30, 31]. Further research is necessary to develop environmentally friendly synthetic strategies for producing these compounds as significant agents and to thoroughly evaluate their potential. The aim of our study was to investigate the catalytic efficiency of the TPGS-750-M catalyst in the eco-friendly synthesis of pyrano[2,3-c]pyrazole derivatives. We optimized the reaction conditions, such as employing a green solvent system, utilizing low catalyst loading, maintaining mild reaction temperatures, and achieving high reaction yields, to establish a sustainable and environmentally benign process. Table 1 depicts the outcomes of the effect of the TPGS-750-M catalyst on the synthesis of compound 5a. Initially, the model reaction was conducted without a TPGS-750-M catalyst in a solvent-free condition.

The reaction was monitored using TLC, and after 2 h, it was observed that pyrano[2,3-c]pyrazole 5a was not formed (Table 1, entry 1). When only 2 mL of water without catalyst was used, a 15% product yield was obtained within 2 h.
Then, we attempted to ascertain the optimal effect of catalyst quantity on the reaction by altering the concentration of TPGS-750 M catalyst in the synthesis of pyrano[2,3-c]pyrazole. The model reaction yielded 40% yield of pyrano[2,3-c]pyrazole in 60 min at room temperature when a 0.5% weight in water catalyst was utilized (Table 1, entry 2). When the temperature was doubled, the reaction time decreased, and the yield increased. Upon using 1% TPGS-750-M catalyst, it was observed that the product yield increased further under the same reaction conditions (Table 1, entries 3–5). This indicates that both the amount of catalyst and temperature should be adjusted. The model reaction was conducted using 2% weight and 4% weight of TPGS-750-M catalyst, resulting in yields of 76% and 81% yields in 15 min and 17 min at room temperature, respectively (Table 1, entries 7 and 9). In order to achieve high yields and reduce the reaction time, the temperature was increased from 25 to 50 °C, resulting in a 99% yield in 10 min using TPGS-750-M catalyst at 2% weight in water catalyst (Table 2, entry 8). Similarly, 96% product efficiency was attained with a 4% weight TPGS-750-M catalyst (Table 2, entry 10). The study aimed to determine the optimal effect of TPGS-750-M catalyst on the synthesis of compound 5a. Furthermore, an investigation was conducted to evaluate the influence of aldehydes on the synthesis of pyrano[2,3-c]pyrazole using the TPGS-750-M catalyst in the model reaction. After optimizing the catalyst amount and temperature, various aldehydes 4a-y were employed under the same reaction conditions for the synthesis of pyrano[2,3-c]pyrazole, using hydrazine hydrate 1, ethyl acetoacetate 2, and malononitrile 3 (1 mmol each). The green synthesis was conducted as depicted in Scheme 1. The TPGS-750-M-catalyzed multi-component reaction proved to be effective for a range of aldehydes, including those featuring both electron-donating and electron-withdrawing substituents on the aromatic rings. The reaction model yielded pyrano[2,3-c]pyrazole products with high yields, which were conveniently purified through recrystallization in ethanol. Moreover, the straightforward purification process via recrystallization from ethanol highlights the practicality of this methodology for synthetic chemists and researchers in the field. A short reaction time of 6 min was observed for 5 s, while a longer reaction time of 22 min was determined for 5 l. The reaction efficiencies were found to range between 55 and 99%. When the reaction was carried out using model reactants (1 mmol each) with 2% weight of TPGS-750-M catalyst in 2 mL of water at 50 °C, the pyrano[2,3-c]pyrazole products were obtained in abundant yields without column chromatography purification. The successful application of the TPGS-750-M catalyst in this multi-component reaction underscores its versatility in accommodating a wide range of aldehyde substrates, regardless their electronic properties.
The structure of the synthesized pyrano[2,3-c]pyrazoles was identified by Mass, 1H, and 13C NMR spectroscopy (see Supplementary Material). When the NMR results of all pyrano[2,3-c]pyrazole compounds are examined in general, in the structures of pyrano[2,3-c]pyrazoles 5a-y, the amine proton signals of the pyrazole were observed around δ 12.00 ppm, and the amine protons attached to the pyran ring were observed around δ 6.80 ppm. Methyl protons in the structure of pyrano[2,3-c]pyrazole were also observed around δ 1.90 ppm. Structure elucidations of pyrano[2,3-c]pyrazole 5a-y are mainly based upon NMR spectroscopy. Signals of carbon atoms of the pyrano[2,3-c]pyrazole rings are assigned at about ∼160.0 (C-2 pyran), ∼155.0 (C-5 pyrazole), ∼136.0 (C-3 pyrazole), ∼122.0 (C-4 pyrazole), ∼120.0 (CN), ∼98.0 (C-3 pyran), ∼115.0 (C-3a) and∼57.0 (C-4 pyran) ppm. The characterization results of these obtained compounds were consistent with the structures of current pyrano[2,3-c]pyrazoles [32,33,34]. The efficient synthesis of pyrano[2,3-c]pyrazole compounds with substantial yields offers a promising approach for the preparation of these biologically relevant molecules. Currently, the mechanism of pyrano[2,3-c]pyrazole synthesis with the TPGS-750-M catalyst is not well understood. Although the reaction mechanism was not investigated in this study, a possible explanation for the reaction progress for the synthesis of pyrano[2,3-c]pyrazoles with the TPGS-750-M catalyst system is depicted in Scheme 2 [32, 35]. According to this reaction mechanism, the first step involves the formation of intermediate pyrazolone 8 through the reaction between hydrazine hydrate 1 and ethyl acetoacetate 2. The Knoevenagel condensation reaction between malonitrile 3 and aldehydes 4a-y on the active sites of the TPGS-750-M surface synthesizes arylidenemalonitriles 9. Subsequently, intermediates 8a-y and 9a-y undergo Michael addition to give 10a-y, which upon intramolecular cyclization yield the desired pyrano[2,3-c]pyrazoles 5a-y.
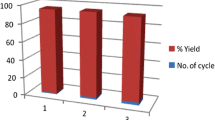
The reusability of the TPGS-750-M catalyst was assessed to determine its practical and industrial applications. After the synthesis reaction of pyrano[2,3-c]pyrazole 5a was completed, the product was filtered from the reaction mixture with water mixture (see experimental). Subsequently, the used catalyst was present in the aqueous phase, which could be isolated. The remaining aqueous suspension was regenerated by concentrating it with excess water, enabling the catalyst to be reused. Achieving 100% reusability of the TPGS-750-M catalyst is beneficial for scaling up the synthesis process. The regenerated TPGS-750-M catalyst was tested for further pyrano[2,3-c]pyrazole 5a synthesis reactions as shown in Fig. 2, demonstrating cost-effective potential. In the first, second, and third reaction runs, a slight decrease in product yield was observed, with yields of 99%, 96%, and 94%, respectively. However, in the fourth reaction, a more significant decrease in yield was recorded, with a yield of 84%. These experiments confirmed that the catalyst could be recycled for up to four cycles with minimal loss of activity.
Various catalysts previously employed in the synthesis of pyrano[2,3-c]pyrazole, as reported in the literature, have been investigated within the context of green synthesis, and the results are presented in Table 2. As indicated in Table 2, many of the listed methodologies exhibit certain limitations. These include extended reaction durations, elevated temperatures, reliance on non-aqueous solvents, and the use of non-natural catalysts. In contrast, the proposed catalyst offers distinct advantages, including high yields, shorter reaction times, minimal catalyst usage, compatibility with water, and, most notably, its environmentally friendly nature. These characteristics position it as superior to other catalysts. The comparative analysis presented in Table 2 underscores the significant advancements achieved with the proposed catalyst. Its ability to deliver high yields while simultaneously minimizing reaction times and catalyst quantities is particularly noteworthy. Furthermore, its compatibility with water and its environmentally friendly nature align with the principles of green chemistry, enhancing its overall adherence to sustainable and eco-friendly practices. This not only enhances the sustainability of the synthesis process but also aligns with the broader goals of environmentally conscious chemistry. The catalyst's compatibility with water is a significant advantage as it simplifies the reaction conditions, reduces the need for hazardous organic solvents, and contributes to a more environmentally friendly synthesis process. This aligns perfectly with the core principles of green chemistry, emphasizing the importance of sustainability, safety, and reduced environmental impact in chemical processes [59,60,61]. It also opens up opportunities for more eco-conscious approaches to chemical synthesis, a crucial aspect in modern research and industry [62, 63]. Overall, the proposed catalyst represents a promising development in the field, addressing several drawbacks associated with alternative methodologies. This reaction model showcases its flexibility in modulating molecular complexity and diversity. Notably, the reactions occurred almost instantaneously, yielding pure products without the need for chromatographic techniques, through a simple recrystallization process using ethanol. The rapid completion of reactions and the ease of product purification make this method practical and efficient for synthetic endeavors.
Conclusion
In conclusion, we present a sustainable and environmentally friendly method for synthesizing pyrano[2,3-c]pyrazole compounds utilizing TPGS-750-M as a catalyst in an aqueous medium at room temperature. The application of TPGS-750-M in pyrano[2,3-c]pyrazole synthesis represents a novel approach. The notable advantages of this novel synthesis method, including environmentally benign reaction conditions, facile product isolation, impressive yields, reduced reaction times, and catalyst recyclability, underscore its alignment with the fundamental principles of green chemistry. The study's findings endorse TPGS-750-M as a more practical and efficient catalyst for green chemistry. Additionally, it is conceivable that TPGS-750-M may have potential applicability as a catalyst in various multicomponent reactions or other organic synthesis reactions.
References
A.R.Y.B. Lee, A. Tariq, G. Lau, N.W.K. Tok, W.W.S. Tam, C.S.H. Ho, Nutrients 14, 656 (2022)
B.H. Lipshutz, S. Ghorai, A.R. Abela, T. Nishikata, C. Duplais, R.C. Gadwood, J. Org. Chem. 76, 4379 (2011)
P. Mohan, J. Rajeswari, K. Kesavan, Mater 28, 101711 (2023)
Y. Xiong, T. Wang, L. Liu, Y. Kou, Z. Zhao, M. Yuan, S. Song, J. Chem. Eng. 451, 138889 (2023)
Q. Sheng, M. Yuan, D. Wang, L. Liu, Y. Chen, S. Song, Langmuir 39, 11839 (2023)
L. Tang, K. Huang, W. Jiang, L. Fu, R. Zhang, L. Shen, Z. Zhang, Drug Deliv. 30, 2183830 (2023)
A.K. Mehata, A. Setia, A.K. Malik, R. Hassani, H.G. Dailah, H.A. Alhazmi, M.S. Muthu, Pharmaceutics. 15, 722 (2023)
C.J. Hastings, M.S. DiNola, E. Petratos, E.J. Veltri, Tetrahedron 133, 133271 (2023)
V. Singhania, M. Cortes-Clerget, J. Dussart-Gautheret, B. Akkachairin, J. Yu, N. Akporji, B.H. Lipshutz, Chem. Sci. 13, 1440 (2022)
M. Shi, N. Ye, W. Chen, H. Wang, C. Cheung, M. Parmentier, B. Wu, Org. Process Res. Dev. 24, 1543 (2020)
R.A. Sheldon, D. Brady, Chemsuschem 15, e202102628 (2022)
K. Fabitha, C.G. Arya, M. Chandrakanth, J. Banothu, Res. Chem. Intermed. 49, 997 (2023)
I. Ijaz, E. Gilani, A. Nazir, A. Bukhari, Green Chem. Lett. Rev. 13, 223 (2020)
H.T. Nguyen, M.N.H. Truong, T.V. Le, N.T. Vo, H.D. Nguyen, P.H. Tran, ACS Omega 7, 17432 (2022)
K.D. Dwivedi, B. Borah, L.R. Chowhan, Front. Chem. 7, 944 (2020)
S.R. Mandha, S. Siliveri, M. Alla, V.R. Bommena, M.R. Bommineni, S. Balasubramanian, Bioorgan. Med. Chem. Lett. 22, 5272 (2012)
M. Khoobi, F. Ghanoni, H. Nadri, A. Moradi, M.P. Hamedani, F.H. Moghadam, A. Shafiee, Eur. J. Med. Chem. 89, 296 (2015)
R.M. Vala, V. Tandon, L.G. Nicely, L. Guo, Y. Gu, S. Banerjee, H.M. Patel, Ann. Med. 54, 2548 (2022)
M.G. Abouelenein, A.E.H.A. Ismail, A. Aboelnaga, M.A. Tantawy, N.M. El-Ebiary, S.A. El-Assaly, J. Mol. Struct. 1275, 134587 (2023)
A.K. Allayeh, A.H. El-Boghdady, M.A. Said, M.G. Saleh, M.T. Abdel-Aal, M.G. Abouelenein, Pharmaceuticals. 17, 198 (2024)
J.A. Mokariya, D.P. Rajani, M.P. Patel, ChemistrySelect 7, e202201341 (2022)
A.M. Fouda, R.A. El-Eisawy, M.A. El-Nassag, H.M. Mohamed, A.H. Fekry, H.K. El-Mawgoud, A.M. El-Agrody, J. Mol. Struct. 1295, 136518 (2024)
N. Nagasundaram, K. Padmasree, S. Santhosh, N. Vinoth, N. Sedhu, A. Lalitha, J. Mol. Struct. 1263, 133091 (2022)
S. Patra, P. Patra, Polycycl. Aromat. Compd. 44, 818 (2023)
S.K. Biswas, D. Das, Mini-Rev. Org. Chem. 19, 552 (2022)
P.H. Parikh, J.B. Timaniya, M.J. Patel, K.P. Patel, J. Mol. Struct. 1249, 131605 (2022)
M. Mamaghani, R. Hossein Nia, F. Shirini, K. Tabatabaeian, M. Rassa, Med. Chem. Res. 24, 1916 (2015)
L. Pourabdi, M. Khoobi, H. Nadri, A. Moradi, F.H. Moghadam, S. Emami, A. Shafiee, J. Med. Chem. 123, 298 (2016)
T.E. Ali, M.A. Assiri, H.M. El-Shaaer, S.M. Abdel-Kariem, W.R. Abdel-Monem, S.M. El-Edfawy, S.E.I. Elbehairi, Synth. Commun. 51, 2478 (2021)
S.K. Salama, M.F. Mohamed, A.F. Darweesh, A.H. Elwahy, I.A. Abdelhamid, Bioorg. Chem. 71, 19 (2017)
D. Das, R. Banerjee, A. Mitra, J. Chem. Pharm. Res. 6, 108 (2014)
S. Karami, M.G. Dekamin, E. Valiey, P. Shakib, New J. Chem. 44, 13952 (2020)
S. Sikandar, A.F. Zahoor, J. Heterocycl. Chem. 58, 685 (2021)
B. Myrboh, M.R. Rohman, M. Rajbangshi, I. Kharkongor, B.M. Laloo, B. Kshiar, Org. Prep. Proced. Int. 45, 253 (2013)
S. Talaiefar, S.M. Habibi-Khorassani, M. Shaharaki, Polycycl. Arom. Compd. 42, 791 (2022)
G. Vasuki, K. Kumaravel, Tetrahedron Lett. 49, 5636 (2008)
M.M. Reddy, V.P. Jayashankar, M.A. Pasha, Synth. Commun. 40, 2930 (2010)
H. Mecadon, M.R. Rohman, I. Kharbangar, B.M. Laloo, I. Kharkongor, M. Rajbangshi, B. Myrboh, Tetrahedron Lett. 52, 3228 (2011)
M. Kangani, N. Hazeri, M.T. Mghsoodlou, S.M. Habibi-khorasani, S. Salahi, Res. Chem. Intermed. 41, 2513 (2015)
K. Kanagaraj, K. Pitchumani, Tetrahedron Lett. 51, 3312 (2010)
K.S. Dalal, Y.A. Tayade, Y.B. Wagh, D.R. Trivedi, D.S. Dalal, B.L. Chaudhari, RSC Adv. 6, 14868 (2016)
G.M. Reddy, J. Raul-Garcia, J. Heterocycl. Chem. 54, 89 (2017)
M. Zakeri, M.M. Nasef, T. Kargaran, A. Ahmad, E. Abouzari-Lotf, J. Asadi, Res. Chem. Intermed. 43, 717 (2017)
R.H. Vekariya, K.D. Patel, H.D. Patel, Res. Chem. Intermed. 42, 7559 (2016)
A.P. Katariya, M.V. Katariya, J. Sangshetti, S.U. Deshmukh, Polycycl. Arom. Compd. 43, 3761 (2023)
S.K. Shinde, M.U. Patil, S.A. Damate, S.S. Patil, Res. Chem. Intermed. 44, 1775 (2018)
D. Azarifar, S.M. Khatami, R. Nejat-Yami, J. Chem, Science 126, 95 (2014)
S.W. Kshirsagar, N.R. Patil, S.D. Samant, Synth. Commun. 41, 1320 (2011)
M. Babaie, H. Sheibani, Arab. J. Chem. 4, 159 (2011)
M.M. Amer, M.H. Abdellattif, S.M. Mouneir, W.A. Zordok, W.S. Shehab, Bioorg. Chem. 114, 105136 (2021)
P. Beigiazaraghbelagh, A. Poursattar Marjani, Res. Chem. Intermed. (2024).
H.R. Shaterian, K. Azizi, Res. Chem. Intermed. 40, 661 (2014)
E. Ahmadi, A. Khazaei, T. Akbarpour, Res. Chem. Intermed. 49, 2099 (2023)
I. Yellapurkar, S. Bhabal, M.M.V. Ramana, K. Jangam, V. Salve, S. Patange, P. More, Res. Chem. Intermed. 47, 2669 (2021)
K.G. Patel, N.M. Misra, R.H. Vekariya, R.R. Shettigar, Res. Chem. Intermed. 44, 289 (2018)
H. Mecadon, M.R. Rohman, M. Rajbangshi, B. Myrboh, Tetrahedron Lett. 52, 2523 (2011)
M. Bakherad, A. Keivanloo, M. Gholizadeh, R. Doosti, M. Javanmardi, Res. Chem. Intermed. 43, 1013 (2017)
N. Aslam, J.M. White, A.M. Zafar, M. Jabeen, A. Ghafoor, N. Sajid, M.A. Khan, ARKIVOC 6, 139 (2018)
F. Tamaddon, M. Alizadeh, Tetrahedron Lett. 55, 3588 (2014)
V.H. Kumar, R. Tamminana, J. Heterocycl. Chem. 60, 18 (2023)
F. Mohamadpour, J. Chem. Sci. 135, 74 (2023)
S.A. Shaikh, V.S. Kamble, R.K. Zemase, S.K. Patil, B.D. Aghav, Res. Chem. Intermed. 49, 5255 (2023)
R. Moradi, G. Mohammadi Ziarani, A. Badiei, Res. Chem. Intermed. 49, 1427 (2023)
Acknowledgment
This work was supported by the Van Yuzuncu Yil University, Scientific Research Projects Chairmanship (BAP) (Project Number: FYL-2023-10529).
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Conceptualization (AC), investigation (AC, MYB), validation (AC, MYB), visualization (AC, MYB), writing of original draft (AC), reviewing and editing (AC, MYB).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cetin, A., Bayden, M.Y. Aqueous TPGS-750-M-mediated synthesis of pyrano[2,3-c]-pyrazoles: a sustainable and efficient approach. Res Chem Intermed 50, 2827–2840 (2024). https://doi.org/10.1007/s11164-024-05280-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-024-05280-y