Abstract
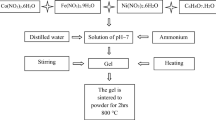
Magnetically separable magnesium ferrichromate nanoparticles (MgFeCrO4 NPs) were synthesized by aqueous combustion synthesis (ACS) using glycine as the fuel. The as-synthesized powder was characterized using XRD, FTIR, SEM–EDX and SQUID techniques. Rietveld refinement studies inferred the single-phase cubic structure of the nanoferrite. Using Williamson–Hall plot, the lattice strain and the crystallite size were evaluated. Small value of lattice strain is indicative of absence of strain in the crystal lattice. The M–O bonding at the tetrahedral as well as the octahedral position was confirmed by FTIR Spectroscopy. Morphological investigations demonstrated sphere-like nanostructures with 50–100 nm particle size using SEM. Absence of any impurity in the EDX spectrum suggests formation of extremely pure MgFeCrO4 NPs. The room temperature magnetization was studied using vibrating sample magnetometer, and the nanoferrite exhibits superparamagnetic behaviour. The pyrano[2,3-c]pyrazole derivatives were synthesized in water/ethanol solvent system using MgFeCrO4 NPs heterogenous catalyst. A very short reaction time, maximum yield, easy method of separation of product and environmentally safer and milder reaction conditions are some of the advantages of our work along with the retention of catalytic activity up to five cycles.
Graphic abstract
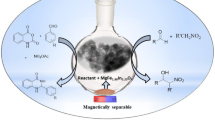
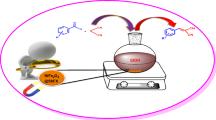
Synthesis of chromene derivatives using magnetically separable magnesium ferrichromate (MgFeCrO4).








Similar content being viewed by others
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
F. Al-Assar, K.N. Zelenin, E.E. Lesiovskaya, I.P. Bezhan, B.A. Chakchir, Pharm. Chem. J. 36, 598 (2002)
R.P. Jain, J.C. Vederas, Bio-org. Med. Chem. Lett. 14, 3655 (2004)
T. Raj, R.K. Bhatia, A. Kapur, M. Sharma, A.K. Saxena, M.P. Ishar, Eur. J. Med. Chem. 2(45), 790 (2010)
S.J. Mohr, M.A. Chirigos, F.S. Fuhrman, J.W. Pryor, Cancer Res. 35, 3750 (1975)
M.T. Flavin, J.D. Rizzo, A. Khilevich, A. Kucherenko, A.K. Sheinkman, V. Vilaychack, L. Lin, W. Chen, E.M. Greenwood, T. Pengsuparp, J.M. Pezzuto, S.H. Hughes, T.M. Flavin, M. Cibulski, W.A. Boulanger, R.L. Shone, Xu ZQ. J. Med. Chem. 39, 1303 (1996)
A.G. Martinez, L. Marco, J. Bio org. Med. Chem. Lett. 7, 3165 (1997)
L. Bonsignore, G. Loy, D. Secci, A. Calignano, Eur. J. Med. Chem. 28, 517 (1993)
I.H. ElAzab, M. Youssef, M. Amin, Molecules 19, 19648 (2014)
A. Shaabani, R. Ghadari, A. Sarvary, A.H. Rezayan, J. Org. Chem. 74, 4372 (2009)
M. Costa, T. Dias, A. Brito, F. Proenca, Eur. J. Med. Chem 123, 487 (2016)
M. Syamala, Org. Prep. Proceed. Int. 41(1), 1 (2009)
R.W. Armstrong, A.P. Combs, P.A. Tempest, S.D. Brown, T.A. Keating, Acc. Chem. Res. 29, 123 (1996)
S. Kamijo, Y. Yamamoto, J. Am. Chem. Soc. 124, 11940 (2002)
H. Mehrabi, H. Abusaidi, J. Iran. Chem. Soc. 7(4), 890 (2010)
S. Gowravaram, K. Arundhathi, K.B.S. Sudhakar, J.S. Yadav, Synth. Commun. 39, 433 (2009)
M. Safaiee, M.A. Zolfigol, F. Afsharnadery, S. Baghery, RSC Adv. 5, 102340 (2015)
M.G. Dekamin, M. Eslami, A. Maleki, Tetrahedron 69, 1074 (2013)
S. Banerjee, A. Horn, H. Khatri, G.A. Sereda, Tetrahedron Lett. 52, 1878 (2011)
R. Fareghi-Alamdari, N. Zekri, F. Mansouri, Res Chem Intermed. 43, 6537 (2017)
H. Ahankar, A. Ramazani, K. Slepokura, T. Lis, J. Chem. 42, 719 (2018)
Y. Pourshojaei, F. Zolalal, K. Eskandari, M. Talebi, L. Morsali, M. Amiri, A. Khodadadi, R. Shamsimeymandi, E. Faghih-Mirzaei, A. Asadipour, J. Nanosci. 20, 3206 (2020)
J. Rajput, P. Arora, G. Kaur, M. Kaur, Ultrason Sonochem 26, 229 (2015)
Z. Zarnegar, J. Safari, J. Mol. Struc. 1072, 53 (2014)
S. Rostamizadeh, N. Zekri, Res. Chem. Intermed. 42, 2329 (2016)
K. Jangam, K. Patil, S. Balgude, S. Patange, P. More, J. Phys. Chem. Solids. 148, 109700 (2021)
K. Jangam, K. Patil, S. Balgude, S. Patange, P. More, RSC Adv. 10, 42766 (2020)
L. Weil, F. Bertaut, L. Bochirol, J. Phys. Radium. 11, 208 (1950)
S.H. Lee, S.J. Yoon, G.J. Lee, H.S. Kim, C.H. Yo, K. Ahn, D.H. Lee, K.H. Kim, Mat. Chem. Phys. 61, 147 (1999)
M.K. Fayek, S.S. Ata Allah, Phys. Stat. Sol. 198, 457 (2003).
A. Gismelseed, A. Yousif, Phys. B 370, 215 (2005)
R. Waldron, Phys. Rev. 99, 1727 (1955)
B. Pourgolmohammad, S.M. Masoudpanah, M.R. Aboutalebi, Ceram. Int. 43(4), 3797 (2017)
T. Slatineanu, A.R. Iordan, M.N. Palamaru, O.F. Caltun, V. Gafton, L. Leontie, Mater. Res. Bull. 46, 1455 (2011)
W.B. Carpenter, J. A. Fournier, R. Biswas, G. A. Voth, and A. Tokmakoff , The Journal of Chemical Physics 147, 084503 (2017).
S.K. Banerjee, W. O’Reilly, IEEE Trans. Magnets. 3, 463 (1966)
W. Zheng, F. Gao, H. Gu, J. Magn. Mater. 288, 403 (2005)
W.S. Mohamed, M. Alzaid, M.S.M. Abdelbaky, Z. Amghouz, Nanomaterials 9, 1602 (2019)
J.K. Oh, J.M. Park, Prog. Polym. Sci. 36, 168 (2011)
R. Vekariya, K. Patel, H. Patel, Indian J. Chem. 57B, 576 (2018)
J. Zheng, Li. Yiqun. Mendeleev Commun. 280, 21 (2011)
S. Tekale, S. Kauthale, K. Jadhav, R. Pawar, J. Chem. (2013).
H. Mecadon, M. Rohman, M. Rajbangshi, B. Myrboh, Tetrahedron Lett. 52, 2523 (2011)
A. Atar, J. Kim, K. Lim, Y. Jeong, Synth. Commun. 44, 2679 (2014)
M. Nasseri, S. Sadeghzadeh, Monatsh Chem. 144, 1551 (2013)
M. Bakherad, A. Keivanloo, M. Gholizadeh, R. Doosti, M. Javanmardi, Res Chem Intermed 43, 1013 (2017)
R. Guo, Z. An, L. Mo, S. Yang, H. Liu, S. Wang, Z. Zhang, Tetrahedron 9331, 69 (2013)
Q. Zhange, B. Liu, W. Chen, Q. Lin, X. Lin, Green Chem. 10, 972 (2008)
K. Pradhan, S. Paul, A.R. Das, Catal. Sci. Technol. 4, 822 (2014)
F. Moeinpour, A. Khojastehnezhad, Chinese Chem. Lett. 26(5), 575 (2015)
F. Moeinpour, A. Khojastehnezhad, Arab. J. Chem. 10, 3468 (2017)
H. Mecadon, R. Md Rumum, M. Rajbangshi, B. Myrboh, Tetrahedron Lett. 52, 2523 (2011)
A. Siddekha, A. Nizam, M.A. Pasha, Spectrochim. Acta A. 81, 431 (2011)
J.B. Gujar, M.A. Chaudhari, D.S. Kawade, M.S. Shingare, Tetrahedron Lett. 55, 6030 (2014)
Y.A. Tayade, S.A. Padvi, Y.B. Wagh, D.S. Dalal, Tetrahedron Lett. 56(19), 2441 (2015)
M. Bihani, P.P. Bora, G. Bez, H. Askari, A.C.S. Sustain, Chem. Eng. 1, 440 (2013)
H. Mecadon, H.; R. Rohman,Md.; I. Kharbangar, B. Laloo, I. Kharkongor, M. Rajbangshi B. Myrboh, Tetrahedron Lett. 52, 3228 (2011)
K.S. Dalal, Y.A. Tayade, Y.B. Wagh, D.R. Trivedi, D.S. Dalal, B.L. Chaudhari, RSC Adv 6, 14868 (2016)
P.P. Bora, M. Bihani, G. Bez, J. Mol Catal. B Enzym. 92, 24 (2013)
M. R. Bhosle, L.D. Khillare, S.T. Dhumal, R.A. Mane Chinese Chem. Lett. 27, 370 (2016)
Acknowledgements
The researchers are grateful to TIFR (Mumbai), SAIF (IIT Bombay) and Microanalytical Laboratory (University of Mumbai) for characterization assistance. PM is thankful to Dr. B. B. Sharma, Principal V. G. Vaze College for the infrastructure and laboratory facilities.
Funding
It is a self-funded project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yellapurkar, I., Bhabal, S., Ramana, M.M.V. et al. Magnesium ferrichromate nanoparticles: an efficient and recyclable catalyst in the synthesis of pyrano[2,3-c]pyrazole derivatives. Res Chem Intermed 47, 2669–2687 (2021). https://doi.org/10.1007/s11164-021-04435-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-021-04435-5