Abstract
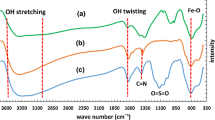
In the present study, a novel, facile and environmentally benign protocol for the synthesis of pharmaceutically active 6-amino-1,4-dihydropyrano[2,3-c]-pyrazole-5-carbonitrile derivatives using CoCeO2 (nanoparticles) NPs as an efficient, reusable and heterogeneous catalyst in aqueous medium is described. The CoCeO2 NPs were successfully synthesised using a simple co-precipitation method and confirmed using XRD, FESEM and HRTEM techniques. The high yields of the product, short reaction time, use of non-toxic materials, simple workup and reusability of the catalyst without loss of activity are the superior advantages of the present protocol.









Similar content being viewed by others
References
Z. Hu, Y. Men, Z. Xu, T. Wu, X. Xu, B. Tang, Org. Chem. Front. 7(22), 3720 (2020)
S. Chitra, N. Paul, S. Muthusubramanian, P. Manisankar, Green Chem. 13(10), 2777 (2011)
M.C. Pirrung, Chem. Eur. J. 12(5), 1312 (2006)
B. Maleki, N. Nasiri, R. Tayebee, A. Khojastehnezhad, H.A. Akhlaghi, RSC adv. 6(82), 79128 (2016)
A. Maleki, S. Azadegan, J. Inorg. Organomet. Polym. Mater. 27, 714 (2017)
A. Maleki, V. Eskandarpour, J. Iran. Chem. Soc. 16, 1459 (2019)
Z. Hajizadeh, A. Maleki, Mol. Catal. 460, 87 (2018)
B. Maleki, Org. Prep. Proced. Int. 47(2), 173 (2015)
B. Maleki, S.S. Ashrafi, R. Tayebee, RSC Adv. 4(78), 41521 (2014)
M. Fallah-Mehrjardi, M. Shirzadi, S.H. Banitaba, Polycycl. Aromat. Compd. 42(5), 2198 (2022)
A. Maleki, Z. Hajizadeh, SILICON 11(6), 2789 (2019)
B. Maleki, H. Eshghi, M. Barghamadi, N. Nasiri, A. Khojastehnezhad, S.S. Ashrafi, O. Pourshiani, Res. Chem. Intermed. 42, 3071 (2016)
A. Kamal, M. Nazari, M. Yaseen, M.A. Iqbal, M.B.K. Ahamed, A.S.A. Majid, H.N. Bhatti, Bioorg. Chem. 90, 103042 (2019)
N.N. El-Sayed, M.A. Abdelaziz, W.W. Wardakhan, R.M. Mohareb, Steroids 107, 98 (2016)
R.M. Mohareb, M.Y. Zaki, N.S. Abbas, Steroids 98, 80 (2015)
S.A. Shaikh, V.S. Kamble, S.T. Salunkhe, S.K. Patil, B.D. Aghav, Org. Prep. Proced. Int. 55(5), 393 (2023)
D. Iwan, K. Kaminska, M. Denel-Bobrowska, A.B. Olejniczak, E. Wojaczynska, Biomed. Pharmacother. 153, 113473 (2022)
L. Fabian, M.T. Porro, N. Gomez, M. Salvatori, G. Turk, D. Estrin, A. Moglioni, Eur. J. Med. Chem. 188, 111987 (2020)
M. Sayed, A.M.K. El-Dean, M. Ahmed, R. Hassanien, Synth. Commun. 48(4), 413 (2018)
S. Desai, V. Desai, S. Shingade, Bioorg. Chem. 94, 103382 (2020)
A.J. Marcus, I. Iezhitsa, R. Agarwal, P. Vassiliev, A. Spasov, O. Zhukovskaya, V. Anisimova, B.B. Johari, N.M. Ismail, Eur. J. Pharm. Sci. 114, 245 (2018)
N.O. Al-Harbi, S.A. Bahashwan, A.A. Fayed, M.S. Aboonq, A.E.G.E. Amr, Int. J. Biol. Macromol. 57, 165 (2013)
S.C. Yavuz, S. Akkoc, B. Turkmenoglu, E. Saripinar, J. Heterocycl. Chem. 57(6), 2615 (2020)
J.Y.C. Wu, W.F. Fong, J.X. Zhang, C.H. Leung, H.L. Kwong, M.S. Yang, D. Li, H.Y. Cheung, Eur. J. Pharmacol. 473(1), 9 (2003)
K. Saravana Mani, S.P. Rajendran, Synth. Commun. 47(22), 2036 (2017)
M. Koohshari, M. Dabiri, P. Salehi, RSC Adv. 4(21), 10669 (2014)
P.H. Parikh, J.B. Timaniya, M.J. Patel, K.P. Patel, J. Mol. Struct. 1249, 131605 (2022)
F.M. Abdelrazek, P. Metz, N.H. Metwally, S.F. El-Mahrouky, Int. J. Pharm. Chem. 339(8), 456 (2006)
G.M. Reddy, J.R. Garcia, V.H. Reddy, A.K. Kumari, G.V. Zyryanov, G. Yuvaraja, J. Saudi Chem. Soc. 23(3), 263 (2019)
A. Venkatesham, R.S. Rao, K. Nagaiah, J.S. Yadav, G. RoopaJones, S.J. Basha, B. Sridhar, A. Addlagatta, MedChemComm 3(6), 652 (2012)
G. Mali, B.A. Shaikh, S. Garg, A. Kumar, S. Bhattacharyya, R.D. Erande, A.V. Chate, ACS Omega 6(45), 30734 (2021)
S.U. Tekale, S.S. Kauthale, K.M. Jadhav, R.P. Pawar, J. Chem. 2013, 8 (2013)
A.S. Waghmare, S.S. Pandit, J. Saudi Chem. Soc. 21(3), 286 (2017)
F. Hassanzadeh-Afruzi, S. Asgharnasl, S. Mehraeen, Z. Amiri-Khamakani, A. Maleki, Sci. Rep. 11(1), 19852 (2021)
A. Saha, S. Payra, S. Banerjee, Green Chem. 17(5), 2859 (2015)
N. Salehi, B.B.F. Mirjalili, Org. Prep. Proced. Int. 50(6), 578 (2018)
R.H. Vekariya, K.D. Patel, H.D. Patel, Res. Chem. Intermed. 42, 4683 (2016)
R.H. Vekariya, K.D. Patel, H.D. Patel, Res. Chem. Intermed. 42, 7559 (2016)
R. Kumari, A. Varghese, L. George, K.B. Akshaya, J. Mol. Liq. 222, 828 (2016)
M. Mehravar, B.B.F. Mirjalili, E. Babaei, A. Bamoniri, Polycycl. Aromat. Compd. 42(6), 3191 (2022)
M. Fatahpour, F.N. Sadeh, N. Hazeri, M.T. Maghsoodlou, M.S. Hadavi, S. Mahnaei, J. Saudi Chem. Soc. 21(8), 998 (2017)
F. Heydari, M. Bakhtiarian, M.M. Khodaei, J. mater. sci. eng., B 296, 116686 (2023)
B. Maleki, A.V. Mofrad, R. Tayebee, A. Khojastehnezhad, H. Alinezhad, E.R. Seresht, Russ. J. Gen. Chem. 87, 2922 (2017)
A.S. Fudala, W.M. Salih, F.F. Alkazaz, Mater. Today Proc. 49, 2786 (2022)
D. Parimi, V. Sundararajan, O. Sadak, S. Gunasekaran, S.S. Mohideen, A. Sundaramurthy, ACS Omega 4(1), 104 (2019)
T.M. Nimbalkar, Y.M. Jadhav, R.N. Dhanawade, N.S. Pawar, A.C. Molane, S.S. Gavande, G.T. Chavan, C.W. Jeon, S.D. Sartale, V.B. Patil, J. Alloys Compd. 966, 171461 (2023)
G. Jayakumar, A.A. Irudayaraj, A.D. Raj, S.J. Sundaram, K. Kaviyarasu, J. Phys. Chem. Solids 160, 110369 (2022)
V. Pandey, I.A. Badruddin, T.T. Terfasa, B.B. Tesfamariam, G.M.S. Ahmed, C.A. Saleel, H. Alrobei, Fuel 317, 123393 (2022)
M. Hatami, B.F. Azarkar, M. Qandalee, H. Hasanabadi, Polym. Eng. Sci. 55(10), 2339 (2015)
A. Miri, H. Beiki, A. Najafidoust, M. Khatami, M. Sarani, Bioprocess Biosyst. Eng. 44(9), 1891 (2021)
S.I. Ahmad, Int J Nano Rech 1(1), 11 (2018)
M.P. Woods, P. Gawade, B. Tan, U.S. Ozkan, Appl. Catal. B Environ. 97(1–2), 28 (2010)
Y.S. Khadar, A. Balamurugan, V.P. Devarajan, R. Subramanian, S.D. Kumar, J. Mater. Res. Technol. 8, 267 (2019)
K.S. Ranjith, P. Saravanan, S. Chen, C. Dong, C. Chen, S. Chen, K. Asokan, R.T.R. Kumar, J. Phys. Chem. C 46(118), 27039 (2014)
V.S. Kamble, R.K. Zemase, R.H. Gupta, B.D. Aghav, S.A. Shaikh, J.M. Pawara, S.K. Patil, S.T. Salunkhe, Opt. Mater. 131, 112706 (2022)
Q. Maqbool, M. Nazar, S. Naz, T. Hussain, N. Jabeen, R. Kausar, S. Anwaar, F. Abbas, T. Jan, Int. J. Nanomed. 4(11), 5015 (2016)
R.Y. Guo, Z.M. An, L.P. Mo, S.T. Yang, H.X. Liu, S.X. Wang, Z.H. Zhang, Tetrahedron 69(47), 9931 (2013)
F. Tamaddon, M. Alizadeh, Tetrahedron Lett. 55(26), 3588 (2014)
M.A. Chaudhari, J.B. Gujar, D.S. Kawade, N.R. Jogdand, M.S. Shingare, Cogent Chem. 1(1), 1063830 (2015)
J.M. Khurana, A. Chaudhary, Green Chem. Lett. Rev. 5(4), 633 (2012)
A.B. Atar, J.T. Kim, K.T. Lim, Y.T. Jeong, Synth. Commun. 44(18), 2679 (2014)
H. Mecadon, M.R. Rohman, I. Kharbangar, B.M. Laloo, I. Kharkongor, M. Rajbangshi, B. Myrboh, Tetrahedron Lett. 52(25), 3228 (2011)
S.H.S. Azzam, M.A. Pasha, Tetrahedron Lett. 53(50), 6834 (2012)
Author information
Authors and Affiliations
Contributions
SAS: Conceptualization, Methodology, Software, Writing—Original Draft and Investigation. VSK: Visualization, Software, Resources and Data Curation. RKZ: Methodology and Resources. SKP: Project administration, Methodology and Writing—Review. BDA: Conceptualization, Supervision, Formal analysis, Writing—Review and Editing, Resources and Software
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shaikh, S.A., Kamble, V.S., Zemase, R.K. et al. A green and one-pot synthesis of 6-amino-1,4-dihydropyrano[2,3-c]-pyrazole-5-carbonitrile derivatives using CoCeO2 nanoparticles as an efficient, reusable and heterogeneous catalyst. Res Chem Intermed 49, 5255–5272 (2023). https://doi.org/10.1007/s11164-023-05156-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-023-05156-7