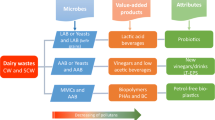
Abstract
The second cheese whey (SCW) is the liquid fraction that remains after the production of whey-cheeses. SCW appears as a white to yellow/green opalescent liquid with suspended solids and contains up to 6% lactose and variable amounts of proteins, fats, and mineral salts. Due to its organic load, SCW is characterized by levels of Biochemical Oxygen Demand and Chemical Oxygen Demand that are significantly higher than urban wastewater. Therefore, it poses an environmental challenge and represents a significant cost and a problem for cheese production facilities when it comes to disposal. On the flip side, SCW contains valuable nutrients that make it a cost-effective substrate for bio-based productions including lactose extraction, and the production of lactic acid, bioethanol, eco-friendly bioplastics, biofuels, beverages, bioactive peptides, and microbial starters. A search in Scopus database indicates that despite the numerous potential applications, interest in SCW exploitation is surprisingly limited and, accordingly, sustainable management of SCW disposal remains an unresolved issue. In this review, which marks the first exclusive focus on SCW, with the aim of contributing to increase the interest of both the scientific community and the stakeholders in the exploitation of this by-product, the processes aimed at SCW valorisation will be described, with particular attention to its use in the production of beverages, food and feed, single cell proteins and as a source of biodegradable bioplastics, organic acids and renewable energy. Moreover, to provide valuable insights into its applications and innovations, an overview on patents regarding the exploitation of SCW will be presented.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The second cheese whey (SCW), also known as ricotta cheese, exhausted whey, deproteinized whey, scotta, sorelho, and others, depending on the country, is the liquid fraction that remains after the production of whey-cheeses. These last are produced worldwide under various names and have historically represented a sustainable option to utilize whey, which is the primary by-product of the cheese-making process.
As outlined in the Codex Alimentarius (2010), these cheeses are obtained either through whey concentration or the coagulation of whey by heat, with or without the addition of organic (citric, lactic) acids, and may be produced with ovine, caprine, bovine or buffalo cheese whey (CW). In the case of heat/acid coagulation, the residual SCW accounts for about 90% of the original whey and its composition varies depending the origin (animal species) and the quality of whey, which in turns depends on seasonality, animal feeding, and on the technology applied by the dairy industry in the whey-cheese production process. Regardless of the technology utilized, SCW maintains a significant organic load that results in Biochemical Oxygen Demand (BOD) and Chemical Oxygen Demand (COD) levels of approximately 50 and 80 g L−1, respectively (Maragkoudakis et al. 2016), notably higher than those of urban wastewater (Rabaioli Rama et al. 2019). Therefore, SCW can be considered either a waste or a by-product. In particular, according to the Waste Framework Directive (WFD; Directive 2008/98/EC), SCW qualifies as waste unless repurposed as a raw material or ingredient in specific production processes. In this scenario, its handling and disposal are subject to stringent environmental and health regulations, such as Regulation (EC) No. 1069/2009 and Regulation (EC) No. 1774/2002. To prevent environmental pollution and disease transmission, direct discharge of SCW into the environment or surface waters is prohibited, and transportation must adhere to waste or organic material transport regulations. Given the substantial costs associated with current waste treatment methods, the disposal of SCW represents a problem for the majority of cheese production facilities. Consequently, many of them opt to provide it to farmers as animal feed, following guidelines established by local health authorities or, at times, without any oversight.
On the flip side, SCW contains valuable nutrients and qualifies as a by-product when employed as a natural source of high-value compounds or as a cost-effective substrate for microbial growth and bio-based productions. So far, SCW has been explored for various purposes, including lactose extraction and the production of lactic acid, bioethanol, eco-friendly bioplastics (e.g., polylactic acid, polyhydroxyalkanoates), biofuels, fermented sports beverages, synbiotic drinks, bioactive peptides and cheese starters (Pereira et al. 2002; Sansonetti et al. 2009; Secchi et al. 2012; Maragkoudakis et al. 2016; Cabizza et al. 2021; Colombo et al. 2019).
Despite the numerous potential applications, interest in SCW exploitation and valorisation remains surprisingly limited. A search in the Scopus database (accessed 28 December 2023), using query statements like "Scotta," "Second cheese whey," "Deproteinized cheese whey," and "Ricotta exhausted cheese whey" to identify relevant literature, resulted in only 147 peer-reviewed scientific articles published from 1980 to 2023. While during this time there has been a gradual increase in interest, as evidenced by the average number of publications per year (rising from 1.86 between 1980 and 2010 to 8.15 from 2011 to 2023), the analysis of documents by country or territory indicates that researchers from Italy and Greece are significantly more active in exploring and understanding the characteristics and potential applications of this by-product while, despite the widespread production of whey-cheeses, other countries show lower levels of interest (Fig. 1). This might be due, at least in part, to the seasonality of production, variability in composition, and perishability. In fact, SCW management necessitates strict adherence to proper hygienic practices to avoid microbial spoilage during storage and transportation to processing plants. Therefore, is not surprising that sustainable management of SCW disposal remains an unresolved issue, while more attention ought to be paid to the utilization of this by-product as an alternative to costly disposal solution.
This review, which marks the first exclusive focus on SCW, aims to contribute to arousing the interest of the scientific community and the stakeholders in the exploitation of this by-product. Thus, the processes aimed at SCW valorisation will be described, with particular attention to its utilisation in the production of beverages, food and feed as well as a source of renewable energy, biodegradable bioplastics and organic acids. Additionally, to provide valuable insights into its applications and innovations, an overview of patents related to the exploitation of SCW will also be presented.
2 Second cheese whey composition
SCW appears as a white to yellow/green opalescent liquid with suspended solids. Its pH falls within the acidic range of 5–6 and contains up to about 3–6% lactose, variable amounts of proteins and fats depending on the origin of whey and the whey cheese manufacturing process (Table 1). Moreover, the addition of salts during whey-cheese manufacturing may influence its mineral content (Table 1).
SCW proteins include β-lactoglobulin, α-lactalbumin, immunoglobulins and serum albumin whose amino acid profile differs from that of caseins due to a greater amount of sulphur-containing amino groups. These, besides serving as precursors of the antioxidant glutathione, are involved in metabolic health in animals and their greater content in SCW proteins in respect to casein may be of interest from a nutritional point of view. Despite their limited concentration in SCW, proteins may be a source of bioactive peptides upon enzymatic treatment or fermentation (Sommella et al. 2016; Cabizza et al. 2021; Monari et al. 2019; Pontonio et al. 2021). SCW contains also glycomacropeptide, a biologically active acidic peptide released in whey from κ-casein during cheese making (Monti et al. 2018). Bergamaschi et al. (2017) highlighted that the fatty acids profile of SCW differs from that of ricotta cheese. SCW tends to contain more saturated fatty acids and Ѡ-6 fatty acids, and less monounsaturated acids, Ѡ-3 fatty acids and conjugated linoleic acid compared to ricotta cheese. Furthermore, compared to CW, SCW contains higher levels of butyric and palmitic acids (C14:0 and C16:0, respectively) both exerting positive effects on human health, and lower levels of monounsaturated fatty acids. SCW fats, besides being an important source of nutrients, may also affect SCW flavour. Interestingly, the concentration of volatile organic compounds (VOCs) in SCW is more than double (+136%) that in CW, especially concerning alcohols, aldehydes, and ketones. This difference arises from physicochemical and microbial modifications during processing of whey-cheeses. The main VOC identified in terms of relative abundance is 3-methyl-butan-1-ol, while ethyl lactate and butyl butanoate appear in very low quantities in SCW (Bergamaschi et al. 2018). Generally, SCW exhibits a higher mineral content compared to milk and conventional whey (CW), akin to acid-type whey. Specifically, findings by Pasta et al. (2024) indicate that consuming 355 mL of SCW provides over 20% of the daily value (DV) of calcium (Ca) and phosphorus (P), more than 10% of the DV for potassium (K) and magnesium (Mg), and approximately 6% of the DV for sodium (Na) as prescribed by Food and Drug Administration. Calcium deficiency is a global concern, especially concerning bone health. Given the abundant calcium content in SCW, it can serve as a valuable nutritional supplement to address this issue. Additionally, the sodium content in SCW, crucial for hydration, parallels that of sports beverages (Shirreffs 2009; Veniamakis et al. 2022). Overall, SCW emerges as a noteworthy source of essential minerals such as calcium, phosphorus, potassium, sodium, and magnesium, making it a potentially valuable addition to one's diet to address mineral deficiencies and support health and hydration.
3 Bio-based processes for second cheese whey valorisation
3.1 Second cheese whey in the production of food and beverages
Given the increasing consumer awareness regarding the importance of maintaining a healthy and sustainable diet, both the scientific community and the agri-food industry are actively engaged in developing innovative, high-quality foods. These are designed to incorporate elements such as prebiotics, probiotics, microbial metabolites, and bioactive compounds (Terpou et al. 2019; Cunningham et al. 2021) while upholding the sustainability of the production process. In this context, the potential of SCW as a possible ingredient for the production of food and beverages has been explored by several authors (Table 2).
Pontonio et al. (2021) demonstrated the potential of fermenting SCW to produce bioactive peptides with angiotensin-I-converting enzyme (ACE) inhibitory properties to enhance the health promoting effect of foods like ricotta cheese. For this purpose, pasteurized SCW was ultrafiltered and the resulting protein-rich retentate was inoculated with a selected strain of Lactobacillus helveticus, known for proteolytic activity. The fermented retentate contained 3230 ± 25 mg L−1 peptides and, after spray-drying, it was utilized in different proportions (1–5%) to fortify ricotta cheese. Samples obtained with 5% of the spray-dried fermented retentate contained 30 mg of bioactive peptides per 100 g serving and exhibited a nine-fold increase in ACE-inhibitory activity compared to the control produced without fortification. Minor modifications in terms of hardness, chewiness, acidity, odour, and taste were observed while an improvement in flavour persistence and savouriness was noted. Moreover, according to these authors, the fortification process for ricotta cheese is easily scalable, thus enabling its application for the valorisation of SCW.
Similarly, Garcia et al. (2022), based on the evidence that whey cheeses is suitable to deliver probiotic strains (Madureira et al. 2005), proposed the utilization of SCW in probiotic goat whey cheese making, thus highlighting its potential for enhancing both the nutritional and health-promoting aspects of dairy products. To achieve this, goat whey was added with 5% freeze dried diafiltered SCW and 5% of commercial probiotic cultures including Bifidobacterium animalis subsp. lactis and Lactobacillus rhamnosus, along with thyme essential oil and sodium citrate. Probiotics viability at 21 days of storage of the resulting cheese reached an average of 1.7×108 CFU g−1 and was therefore largely above the minimum required for a probiotic product (107 CFU g−1).
Additionally, Borges et al. (2020) explored the use of SCW as a fat replacer in reduced-fat washed curd cheese. For this purpose, 5% of ultrafiltered concentrated SCW, buttermilk and CW were added to milk and their impact on cheese colour, texture and sensorial properties was evaluated. The experimental cheeses obtained with SCW and CW received lower scores for both texture and taste in comparison to those obtained with buttermilk thus suggesting that further works need to be done to evaluate the fat replacer properties of these by-products.
Furthermore, Pires et al. (2023) showcased the utilization of concentrated SCW in the production of probiotic ice creams. In this case SCW was ultrafiltered, pasteurized, homogenised, and added with sucrose, inulin, citric acid, and xanthan gum, before inoculation either with thermophilic yogurt starter culture, kefir culture or a mixture of probiotic cultures. Fermentation was carried out to pH 4.6 and the resulting ice creams were stored in a freezer at – 21 °C for 120 days. Although with significant differences among the products in terms of hardness, viscosity and meltdown rates, all of them were well-received by a consumer panel and retained their probiotic characteristics for up to 2 months.
For the development of a probiotic beverage, Maragkoudakis et al. (2016) inoculated pasteurized SCW with mixed cultures including Streptococcus thermophilus, Lactobacillus delbrueckii subsp. bulgaricus and Lb. acidophilus. These bacteria lowered SCW pH to 4.2 and reached viable cell counts of about 8–9×108 CFU mL−1 thus suggesting that pasteurized SCW can be employed of the production of a fermented beverage rich in potentially beneficial bacteria. Similarly, Tirloni et al. (2020) inoculated SCW with Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus. The fermented SCW (pH 4.0) was then supplemented with 13.75% (w/v) pasteurized fruit purees along with pectin, potassium sorbate, sodium citrate and stored into 125-mL bottles at 4 and 12 °C. Under these conditions, the fermented SCW remained microbiologically stable for up to 14 days thus supporting the potential of SCW in the production of fermented beverages.
Other authors combined uninoculated SCW and fruit juices to obtain unfermented SCW-based beverages (de Souza Silva et al. 2022). For the microbiological stabilization of SCW a thermal treatment at 100 °C for 15 minutes was carried out, and three formulations, containing different proportions of SCW, passion fruit pulp and sucrose, were produced, heated at 85 °C for 30 s and stored at 7 °C. Through these processes an isotonic beverage composed by 30% SCW, 12% passion fruit pulp, 5% sucrose was developed. This exhibited good sensory acceptance and showed higher phenolic content and antioxidant activity compared to commercially available isotonic beverages. While further in vivo studies are required to assess the impact of these beverages on hydro-electrolytic replenishment and sports performance, their findings suggest the potential usefulness of unfermented SCW as an ingredient in athlete supplements. Interestingly, according to Rizzolo and Cortellino (2017, 2018), by varying the type of fruit juice used in the formulation it is possible to modulate the levels of antioxidants, sugars, and organic acids present in SCW-based beverages. Such flexibility in formulation allows for the creation of a diverse range of products with varying nutritional and flavour profiles, catering to different consumer preferences and dietary needs.
More recently, Kurnick et al. (2024) explored the possibility of utilizing sheep and goat CW and SCW to produce unfermented (containing Lactobacillus casei as bioprotectant) and fermented beverages (with a commercial yogurt starter culture). Also in this case, CW and SCW were thermally treated (75 °C for 5 min) and added with sugars, flavouring agents and stabilizers. SCW fermented beverages suffered of lower consumers acceptance and physical instability during storage, due to the effect of acidic pH on proteins, their denaturation level and solubility.
Another possibility regards the utilization of SCW to partly replace water and sugar in the production of a sweet milk stout and a salty and acidified Gose style beer. Different amount of SCW (37% for sweet milk stout and 16% for Goose style beer) were mixed with water for grain mashing and the resulting worts (25 L) were inoculated with Saccharomyces cerevisiae for the sweet milk stout and with a mixed culture including S. cerevisiae and Lachancea thermotolerans for the Gose-style beer. Since these two yeasts do not ferment lactose, the Goose style wort was treated with lactase. After fermentation, the sweet milk stout contained 1.6 % (w/w) residual lactose and a calcium level of 14.3 mg 100 g−1, about the double of the content reported in other stouts. The Goose style beer showed no residual lactose and a calcium level of 7.06 mg 100 g−1, at the high end for many beers. Sensory attributes of the two beers were in line with that reported for other beers in the market. According to these results, the use of SCW in beer manufacturing serves to add sugars, replace up to 37% of the water used in the brewhouse, and increase beer mineral content with no marked detrimental effects on the sensory attribute of the final products. This opens new ways towards more sustainable productions in the dairy and brewing industries (Pasta et al. 2024)
Based on all these findings, SCW emerges as a promising ingredient for the production of food and beverages, and the approaches presented open avenues for reducing waste in the dairy industry while meeting the demands of consumers seeking healthier and more sustainable dietary options. Nevertheless, the processes described have ample room for technological improvement, and several issues still need to be addressed. The first is related to the perishability of SCW. So far, thermal treatments (i.e., pasteurization) have been employed to ensure the safety of SCW-based products, while the application of less invasive emerging technologies such as High Pressure Processing (HPP), cold plasma, and others could be advisable to ensure microbial inactivation and preserve the nutritional quality of the final product. Moreover, further work needs to be done to improve both the physical properties and sensory profile of SCW-based products. One of the main problems related to the production of SCW-based fermented beverages concerns their structural properties and the formation of precipitates during storage. Thus, the effects of protein content and integrity and/or their interaction with stabilizers under acidic pH should be thoroughly evaluated. Regarding the sensory properties, SCW is characterized by high concentrations of free fatty acids, esters, and ketones (Bergamaschi et al. 2018), which are associated with rancid odour descriptors thus requiring the utilization of flavouring agents in the manufacturing of SCW-containing products. Finally, while the production processes described have been implemented at the laboratory scale, their feasibility at the industrial scale needs to be assessed.
3.2 Second cheese whey for the production of microbial starters, single cell proteins, single cell oils, and bioactive molecules
The utilization of by-products and wastes of the agri-food industry for biobased productions represents a key strategy for their valorisation within the framework of circular economy and sustainable development. In line with this approach, several authors have explored the practicability of utilizing SCW or deproteinized whey as substrates for cultivating algae, yeast, and bacteria for the production of starters, biomass enriched in proteins (single cell proteins, SCP), oils (single-cell oils, SCO) or other valuable metabolites.
In the context of microbial starters production, Rabaioli Rama et al. (2020) investigated the possibility of using unsupplemented SCW and CW for the growth and spray drying encapsulation of Lactobacillus paracasei ATR6. After culturing the selected strain in pasteurized SCW and CW (65 °C for 30 min) in a laboratory-scale bioreactor, they found that L. paracasei ATR6 exhibited a significantly higher increase in cell density when grown in SCW compared to CW while showing comparable survival rates after spray drying on both media (>78%). The potential of SCW as a substrate for the propagation of LAB cultures was confirmed by Chessa et al. (2020) and Naziri et al. (2023). In particular, Chessa et al. (2020) proposed SCW for the propagation of mixed starter cultures for Pecorino Romano PDO cheese. At first, by utilizing commercial powder SCW rehydrated in water at different proportions they observed that halving the concentration of SCW did not affect the propagation of starter cultures, thus indicating that the nutrients in SCW are more than sufficient to support LAB growth. Then they proved that SCW is suitable for maintaining the biodiversity and technological characteristics of mixed starter cultures including termophilic lactobacilli and cocci, enterococci and mesophilic lactobacilli. Similarly, Naziri et al. (2023) after having observed that Lactobacillus delbrueckii subsp. lactis, Lactobacillus delbrueckii subsp. jakobsenii, Lactobacillus leichmannii, and Lactobacillus crispatus, constitute the dominant microflora in SCW derived from the manufacturing of Halloumi whey cheese, proved that LAB growth in unsupplemented SCW and SCW supplemented with skim milk was comparable to that on MRS medium, reaching approximately 108 CFUmL−1. Additionally, SCW supplemented with skim milk improved LAB freeze-drying tolerance (with survival rates ranging from 75 to 91% depending on the species) and viability (> 60%) over a three-month storage period at 4 °C in vacuum-packed powders.
Interestingly, although lactose utilization is not common in yeast (Kurtzman 2011), Gottardi et al. (2023) showed that Yarrowia lipolytica strains may be cultured on dairy by-products including SCW. In particular, they observed that 18 out of 20 Y. lypolytica isolates grew on unpasteurized SCW reaching cell densities ranging from 1×106 to 8 ×107 cell ml−1 within 72 h. Interestingly, due to their proteolytic and lipolytic activities, Y. lipolytica strains released a variety of volatile compounds that were compatible with cheese flavours. Therefore, SCW was indicated as suitable substrate to produce Y. lypolitica cultures to be used as co-starters and/or food adjuncts in cheese making (Gottardi et al. 2023).
Another possible application of SCW regards its utilization for SCP productions. SCP are unicellular proteins produced by microbial fermentation of waste substrates and utilized as supplements in human and animal nutrition for their high nutritional profile. In this context, Schultz et al. (2006) attempted the bioconversion of deproteinized sweet and sour whey concentrates into yeast SCP. For such application the yeast Kluyveromyces marxianus CBS 6566 was selected. Having been recognized the QPS and GRAS status K. marxianus is considered safe for human and animal consumption. Moreover, it metabolizes lactose with no ethanol production thus channelling carbon flow through the production of biomass. The selected strain was inoculated in 100 L stirred bioreactor filled with 13.5 or 55 L filter sterilized deproteinized whey containing 140 g L−1 lactose and supplemented with trace elements, vitamins and ammonium sulphate. Growth was carried out at 30 °C under controlled pH and aeration. In these conditions biomass production reached 50 and 65 g L−1 with yields of 0.52 and 0.48, and a COD reduction of 90 and 83% in sweet and sour deproteinized whey concentrates, respectively. The amino acid composition indicated that when produced in sweet and sour deproteinized whey, K. marxianus SCP contain amounts of valine (6.89–7.5 g 100 g protein−1), leucine (7.62–7.74 g 100 g protein−1), isoleucine (5.07–5.48 g 100 g protein−1) and threonine (7.45–6.94 g 100 g protein−1) that are higher than those reported in World Health Organization (WHO) guidelines for SCPs.
Lactose utilizing yeasts have been proposed also for the valorisation of dairy by-products through the fermentative production of SCO, a biodiesel source compatible with the majority of traditional diesel engines. Taskin et al. (2014) showed that when grown in SCW at laboratory scale, Y. lipolytica produced 7.4 g L−1 biomass containing 58% total lipids of which 80.54% monounsaturated fatty acids. Better results were obtained by Carota et al. (2017) with Cryptococcus curvatus NRRL Y-1511 and C. laurentii UCD 68-201. When grown in SCW these strains were capable of producing 6.83 g L−1 and 5.06 g L−1 biomass, while determining COD reductions of 86.7 and 77.9%, respectively. Notably, laboratory scale bioreactor cultivation of C. laurentii led to significant increases in biomass production (up to 14.37 g L−1) and lipids productivity (9.93 g L−1 at 60 h). Moreover, the fatty acid methyl ester (FAME) composition under bioreactor conditions was characterized by a predominance of palmitic, oleic, and linoleic fatty acids and closely resembled that of Jatropha oil, a well-established feedstock in biodiesel production (Carota et al. 2017). More recently, Vasilakis et al. (2022) showed that fed-batch cultivation of C. curvatus ATCC20509 with whey lactose pulse resulted in the production of 38.1 g L−1 cell biomass and 21.7 g L−1 total lipids. Notably, the lipids were found to be rich in unsaturated fatty acids, particularly oleic acid (up to 14.3 g 100 g−1 total FA) and, based on their composition, this yeast could be considered a possible candidate for the synthesis of second-generation biodiesel.
Since mixotrophic growth enhances microalgal biomass productivity, Tsolcha et al. (2016) assessed the feasibility of microalgal SCO production in SCW wastewater (SCWW) obtained by diluting SCW with tap water at different proportion. By doing so they obtained SCWW growth media differing in the initial concentrations of total sugars and mineral compounds, and utilized it for the cultivation of a mixed microbial population dominated by a Choricystis-like chlorophyte, in a 4 L aerobic photobioreactor. In the presence of the highest concentration of SCW, biomass productivity reached a maximum of 228.25 mg L−1d−1 and the growth rate recorded (0.45 d−1) was among the highest reported in the literature for similar microbial populations. Similarly, the maximum oil concentration, that ranged in between 60.75 ± 3 to 120.50 ± 8 mg L−1, was obtained in SCWW containing higher concentrations of SCW. Interestingly, the fatty acids profile of the lipids accumulated was in agreement with that reported for other chlorophytes in dairy effluents and appeared suitable for biodiesel production. Moreover, the microbial population utilized determined up to 92.3% reduction of COD. Other authors assessed SCW as a substrate for the production of algal, yeast and bacterial biomass enriched in carotenoids and other valuable metabolites. In particular Ribeiro et al. (2017a) focused on the microalga Chlorella protothecoides which, due to the production of appreciable amounts of proteins, chlorophyll, lipids, and valuable carotenoids, may be suitable for nutraceuticals and food/feed supplements (Campenni’ et al. 2013). When cultured in SCW containing medium within 1 L photobioreactor C. protothecoides shifted to mixotrophic growth. Under these conditions, the growth rate increased and biomass production more than doubled compared to that produced under autotrophic conditions, reaching up to 3.6 g L−1, possibly due to lactose and mineral nutrients content in SCW. Similarly, the volumetric productivities of chlorophyll (42.17 mg L−1) and carotenoids (11.98 mg L−1) were higher than under autotrophic growth and were achieved in a shorter time, thus suggesting that the mixotrophic cultivation of C. protothecoides in SCW represents a viable strategy to economize the costs associated with microalgal biomass production while contributing to SCW disposal. Similarly, Russo et al. (2021) utilized SCW for the cultivation of the heterotrophic microalga Galdieria sulphuraria, a promising biorefinery platform for the production of pigments, antioxidants and for the removal of nitrogen, sugars and phosphorus from wastewaters (Zimermann et al. 2020; Pan et al. 2021). Microfiltered heat treated SCW was diluted in water to obtain different concentrations of reducing sugars (1.0÷2.5%). At 2% reducing sugars G. sulphuraria produced up to about 5 g L−1 biomass enriched in glycogen and polyunsaturated fatty acids, and with a protein content that was comparable to that obtained under standard conditions of cultivation. Moreover, supplementation of SCW-based medium with glucose and nitrogen significantly increased biomass production (10.65 g L−1). Also, the red yeast Rhodotorula glutinis was explored for the production of biomass and carotenoids in SCW (Ribeiro et al. 2017b). Biomass production ranged in between 2.59 and 2.81 g L−1 within 21 days batch cultivation and was comparable to that obtained in Malt Extract broth employed as a reference medium. Similarly, carotenoids production, that reached a maximum of 0.5 µg gdw−1 after 21 days in SCW, showed not significant differences in the two substrates.
Coronas et al. (2023) assessed the possibility of utilizing SCW for the cultivation of Propionibacterium freudenreichii, to produce bacterial biomass and vitamin B12. For this purpose, four isolates of P. freudenreichii, selected for lactose utilization and in vitro probiotic features such as resistance to bile salts, acid stress, osmostress and lyophilization, were cultured in unpasteurized SCW. After 72 hours incubation at 30 °C, the four isolates reached cell densities ranging from 108 to 109 CFU mL−1. Moreover, they showed a total production of cobalamin derivatives (vitamin B12) in the range 0.49–1.31 mg L−1 thus suggesting that by choosing the appropriate starter, SCW can be converted into a probioactive preparation that combines probiotic properties and bioactive compounds.
Following an important breakthrough that showed the feasibility of lactobionic acid (LBA) production in CW-based medium (Alonso et al. 2011; Goderska et al. 2015), also SCW was utilized to produce LBA. This is a complex polyhydroxy acid with a wide range of significant applications. Due to its various beneficial effects, including prebiotic, bioactive, gelling, stabilizing, and antimicrobial properties LBA is utilized in functional foods (Nielsen et al. 2009; Sarenkova et al. 2018; Cardoso et al. 2019; Sáez-Orviz et al. 2022). SCW fermentation with Pseudomonas taetrolens resulted in 34.25±2.86 g L−1 LBA with an 85% yield (De Giorgi et al. 2018).
Overall, these studies underscore the versatility and potential of SCW as a cost-effective and sustainable substrate for microbial starter production and biobased applications. While careful selection of the cultivation system and microbial strains is crucial for all these production processes, using dairy effluents as culture media offers numerous advantages. SCW not only supports the proliferation of LAB while preserving the biodiversity and technological characteristics of microbial consortia but also serves as a suitable cell protectant for long-term preservation of starter cultures. Moreover, although the feasibility of large-scale production requires further evaluation, SCW, possibly due to its high C/N ratio, appears to meet the nutritional requirements for producing cell biomass enriched in microbial oils and proteins. Additionally, it serves as a growth medium for microalgae, yeast, and bacteria, resulting in biomass production enriched in lipids, pigments, and other bioactive metabolites while leading, at the same time, to wastewater treatment.
3.3 Second cheese whey in the formulation of animal feed
In Italy the Legislative Decree 22/97, known as the "Ronchi Decree," deems SCW a “waste” if directly used for animal feeding. Consequently, farmers intending to use it for this purpose must obtain specific authorization. On the other side SCW is categorized as by-product of animal origin with low risks when utilized in the formulation of animal feed, as per Regulation (EC) No. 1774/2002. The value of SCW in the formulation of animal feed lies in its nutritional properties (Minhalma et al. 2007; Pontonio et al. 2021). Also, LAB involved in spontaneous SCW acidification contribute notably to its dietary value. Therefore, SCW is a composite mixture with various constituents, some of which holding significant value for livestock nutrition. However, SCW is inherently very diluted, and its use is restricted by the gastric capacity of the animals to which it is fed. Consequently, SCW concentration is advised to achieve optimal results in animal nutrition.
Regarding the amount of SCW to be used for animal feed formulation no data were found in literature while more information is available on CW or CW permeate. CW permeate, obtained by membrane treatment to remove proteins and other solids (Menchik et al. 2019), is a deproteinized CW, assimilable to SCW, that contains higher amounts of lactose and milk oligosaccharides compared to other milk co-products (Barile et al. 2009; Dallas et al. 2014).
Lactose is the main nutrient for weanling pigs to achieve maximum performance during the initial period after weaning (Grinstead et al. 2000). Newborn piglets rely on milk from sows during lactation and, as a result, their digestive tracts are adapted to lactose digestion upon weaning. The supplementation of lactose in nursery feeds positively improves the growth performance of pigs, although the growth responses to lactose gradually disappears with age due to reduced lactase activity after weaning (Mahan et al. 2004; Cromwell et al. 2008; Gahan et al. 2009). When this happens, residual undigested lactose may result in excessive fermentation by gut microbiota thus aggravating enteric diseases and determining post-weaning diarrhoea (Pierce et al. 2005). Lactose also plays a role in stimulating the synthesis of B-vitamins by gut microbiota in both mammals and poultry (Atkinson et al. 1957). Furthermore, it demonstrates favourable effects on the absorption, retention, and utilization of essential minerals such as calcium, phosphorus, and magnesium (Atkinson et al. 1957). Thus, the inclusion of lactose in the diet has the potential to enhance nutrient digestibility. In cattle, this improvement may be linked to lactose's impact on ruminal fermentation and its role in increasing butyrate production which, in turn, could potentially enhance absorption capacity by promoting rumen papillae development and growth (Dirksen et al. 1985; Xu 1999; DeFrain et al. 2004, 2006). Moreover, the decrease in ruminal ammonia nitrogen (NH3–N) and milk urea nitrogen suggests the possibility of greater microbial protein synthesis in cows fed with lactose (Susmel et al. 1995; DeFrain et al. 2004). Ammonia can be utilized in ruminants by bacteria for the synthesis of amino acid required for growth. Considering that this process is energy-dependent, lactose could provide an adequate amount of energy for utilization of ammonia in the rumen. As the content of urea in milk is an indicator of the effective use of diet protein by the ruminant, a low urea milk content indicates a better utilization of diet protein, hence suggesting a positive role of lactose in ruminal protein metabolism.
Also, milk oligosaccharides have been well documented for their beneficial prebiotic effects (Bering 2018; Ramani et al. 2018) preventing intestinal dysfunction and aiding in the development of infant brain (Bode 2012; Moukarzel and Bode 2017). One possible mechanism for these positive effects could be related to the binding of milk oligosaccharides to pathogenic bacteria in the small intestine (Morrow et al 2005), thus inhibiting possible attachment of pathogenic bacteria and their toxins to enterocytes (El-Hawiet et al. 2015; Nguyen et al. 2016). Another possible mode of action could be an increase of Bifidobacterium and lactobacilli in the small intestine, resulting in the production of short-chain fatty acids used as energy sources by enterocytes (Garrido et al. 2013; Yu et al. 2013), and the reduction of the lumen pH (Fukuda et al. 2011; Kim 2018). Accordingly, dietary inclusion of CW permeates determined a significant increase in the growth of nursery pigs, partly attributed to the stimulation of the intestinal immune response and enterocyte proliferation with positive changes in jejunal mucosa-associated microbiota (Jang et al. 2021).
3.4 Second cheese whey in the production of infant formula ingredients
Infant formula (IF) is a highly processed food specifically formulated and marketed for the nourishment of babies and infants under 12 months of age. IF is based on bovine skim milk solids, whey protein, lactose, vegetable oils, and mineral/vitamin premixes, carefully balanced to replicate the nutritional composition of human milk and must meet or exceed the amino acid content of essential and semi-essential amino acids found in human milk (Koletzko et al. 2005; Thompkinson & Kharb 2007). The application of SCW in IF preparation can be aimed at including lactose (Atkinson et al. 1957) and galacto-oligosaccharides or harnessing the rich peptide fraction with distinctive biological properties, considering that the protein fraction of SCW comprises approximately 50% peptides, which may enhance digestibility (Monti et al. 2018). In addition, SCW includes other bioactive molecules, with glycomacropeptide standing out. Notably, glycomacropeptide has demonstrated therapeutic effects in various inflammatory disorders, as highlighted by Córdova-Dávalos et al. (2019). When included in the diet, it has been shown to protect weaning piglets against E. coli K88 infection (Rong et al. 2015).
Additionally, glycomacropeptide in SCW can neutralize enterotoxins (Oh et al. 2000), inhibit virus or bacterial adhesion to cells (Brück et al. 2006; Feeney et al. 2017), regulate gastrointestinal secretions (Brody 2000), promote beneficial bacteria proliferation (Janer et al. 2004), and exhibit immunoregulatory functions (Otani & Hata 1995). Concerns have been raised about adding glycomacropeptide to IF due to its high threonine content, leading to elevated plasma threonine concentrations (hyperthreoninemia) in infants compared to those fed breast milk (Rigo et al. 2001). However, the collective evidence suggests that a unifying feature and potential mechanism for the reported health-promoting properties of glycomacropeptide may be its role as a prebiotic, fostering a beneficial gut microbiota and modulating immune function. Furthermore, Sommella et al. (2016) and Cabizza et al. (2021) highlighted in buffalo and ovine SCW a plethora of bioactive peptides with a range of healthy properties comprising antihypertensive, antimicrobial, immunomodulating, opioid, antioxidant, and antithrombotic.
3.5 Second cheese whey in the production of polymers for biodegradable bioplastics
With estimates suggesting that the production of petroleum-based plastics will exceed 34 billion tons by 2050, and the majority of these plastics being discarded into the environment with less than 10% being recycled, it has become imperative to explore the use of renewable polymers for producing biobased plastics. Among them, the polyesters polylactic acid (PLA), obtained by condensation of lactic acid, and polyhydroxyalkanoates (PHAs) emerge as promising candidates (Sudesh et al. 2000; Oliveira et al. 2017; Akinmulewo and Nwinyi 2019; Ahmad et al. 2022). However, their large-scale production and utilization are still hampered by high production costs (Salehizadeh and van Loosdrecht 2004; Sabapathy et al. 2020). Nonetheless, recent reports have shown that by using low-cost carbon sources as feedstock for bioplastics production it is possible to significantly reduce their production costs.
In the study conducted by Secchi et al. (2012), SCW was evaluated as a substrate for lactic acid production. Fermentations were carried out in 3 L bioreactors and SCW, either unsupplemented or supplemented with yeast extract and magnesium sulphite, was inoculated with LAB (Lactobacillus casei, Lactobacillus helveticus and Streptococcus thermophilus) in pure and mixed cultures. In unsupplemented SCW, lactic acid productivities of mixed cultures reached a maximum of 1.643 ± 0.13 g L−1 h−1 and were higher than that of pure cultures, (ranging from 0.715±0.02 to 1.080 ± 0.04 g L−1 h−1), possibly due to the synergistic effect of LAB in lactose and protein utilization. SCW supplementation positively affected LAB fermentative performances and under these conditions lactic acid productivity reached an average of 1.6 g L−1 h−1 and 2 g L−1 h−1 in pure and mixed cultures respectively. Moreover, by selecting the proper starter, it was possible to modulate the percentage of L-lactic and D-lactic acid obtained by fermentation. This is an important issue, given that polylactic acid (PLA) structural properties depend on its enantiomeric constitution (de Franca et al. 2022).
Regarding PHAs production, Colombo et al. (2019) reached very high yield of PHA in SCW and CW. Based on the assumptions that volatile organic acids are better substrates than sugars for the production of PHAs, and that these acids can be produced by wastes dark fermentation (Villegas Calvo et al. 2018), these authors articulated the production process into two steps. The first was aimed at the production of biohydrogen and organic acids via dark fermentation of SCW or CW permeate by a mixed microbial population coming from an activated sludge. Fermentation was carried out in 4 L bioreactors fed in continuous with SCW or CW permeate enzymatically treated for lactose hydrolysis. During the second step, the microbial cultures selected from activated sludge in sequencing batch reactors during 30 days, bio converted organic acids into PHA reaching 274±60 gPHAkg−1 CODin and 268±100 gPHAkg−1 CODin in SCW and CW, respectively. The fermentative performances of the microbial cultures in SCW and CW permeate were comparable thus highlighting a good reproducibility of PHA production process when different fermented dairy streams are utilized (Colombo et al. 2019). Furthermore, the net revenue of the process, although higher in CW (59.3 €m−3) than in SCW (33.6 €m−3), indicated that both dairy by-products are economically viable. Bosco et al. (2021) employed a microbial consortium coming from activated sludge and including Citrobacter freundii and Leuconostoc mesenteroides, to produce PHAs from SCW. For that a 4 L bioreactor operating in batch mode at 30 °C and pH 7 was utilized. Under these conditions PHA production reached 1.065 g L−1 after 29 hours with a yield of 52% (wv−1), higher than that obtained with H. mediterranei (7–9% wv−1). Therefore, SCW can support PHAs production without the need for additional nutrients, making it a cost-effective substrate for bioplastic production.
SCW was utilized also by Raho et al. (2020) to produce poly(3-hydroxybutyrate-co-3-hydroxyvalerate) a polyhydroxyalkanoate-type polymer, commonly known as PHBV, with the halophilic archeon Haloferax mediterranei. For this purpose, SCW was subjected to membrane multi-step fractionation and, since H. mediterranei is unable to ferment lactose, the retentate obtained after nanofiltration (R-NF) was enzymatically hydrolysed before being used as the sole carbon source. Process conditions were optimized in terms of temperature (37 °C), stirring rate (500 rpm) and NaCl concentration (10%) and after 72 hours of R-NF fermentation in a 3L bioreactor 1.27 g L−1 PHBV were produced.
These findings underscore the potential of repurposing SCW, for sustainable and cost-effective bioplastic production. Further investigations are needed both to optimize process conditions (fed-batch, continuous) and assess the thermal/mechanical properties of the PHBV synthetized, especially in view of implementing a production process at the industrial level. Nonetheless, SCW appears to be an interesting alternative to counteract the economic uncompetitiveness of bioplastic production, and its exploitation aligns with the global imperative to reduce plastic pollution and dependence on non-renewable resources for a more sustainable future.
3.6 Second cheese whey in the production of biofuels
The continuous increase in the world population is leading to a progressive rise in energy demand. This can partly be satisfied by biofuels that, being produced through the valorisation of agro-industrial wastes and by-products, align to the principles of circular economy (Zhang et al. 2016; Kaur et al. 2020). The biofuels that have attracted the interest of both the scientific community and the industry include ethanol, butanol, biogas/biomethane, and hydrogen. In the following paragraphs, the production of these biofuels will be discussed. Since information available for SCW is relatively limited, when required, reference will be made to processes based on the fermentation of CW.
3.6.1 Bioethanol
Ethanol (C2H6O) stands out as the most important biofuel, covering about 75% of the current biofuel market in developed countries, where it is mainly blended with petrol and utilized as vehicles fuel. Ninety-five percent of global ethanol production is obtained through fermentation.
The production of ethanol through the fermentation of the by-products of dairy industry, reported in scientific literature since the 1940s (Whittier 1944, Rogosa et al. 1947, Webb et al. 1948), involves yeasts such as K. marxianus and Kluyveromyces lactis, naturally capable of lactose fermentation (Sansonetti et al. 2009). Indeed, the choice of microbial strain is a critical factor in achieving high ethanol yields (>80%) from these substrates (Osorio-González et al. 2022) and the inhibition of microorganisms by ethanol concentrations in the range of 6–15% (wv−1) (Roohina et al. 2016) poses a challenge to conversion yields.
To overcome ethanol inhibition problems, yeasts immobilization within solid matrices (Tesfaw 2023) and the utilization of other yeast species (Osorio-González et al. 2022) have been realized. S. cerevisiae and Candida pseudotropicalis show an ethanol tolerance up to 4-fold than K. marxianus, but are not able to directly metabolize lactose that, therefore, must be enzymatically pre-hydrolyzed to glucose and galactose (Osorio-González et al. 2022).
For that, engineered microorganisms that use lactose to produce ethanol were developed (Roohina et al. 2016; Pasotti et al. 2017; Saini et al. 2017). Ethanol production can be carried out in batch or continuous mode (Bernstein et al. 1977, Moulin et al. 1978, Maiorella et al. 1984), and when CW is fermented, two-stage processes and co-cultures are commonly used.
An emerging bioprocess is the electrofermentation which controls fermentative microbial metabolism by means of specific electrodes. The electrodes can act as sinks and sources of electrons, modifying the redox balance of the culture medium and generating unbalanced fermentations (Moscoviz et al. 2016). As there are no studies on ethanol production from SCW that apply this technology, a new area of research could be opened to explore this bio electrochemical system (Yamada et al. 2022).
SCW holds promise as a potential source of bioethanol, although the scientific literature on its use for ethanol production is still limited. Table 3 shows the main studies carried out in recent years on the treatment of SCW for the production of bioethanol. Sansonetti et al. (2010a, b) analysed the effects of temperature, pH, stirring speed, initial lactose concentration for SCW batch fermentation by means of response surface methodology. The predicted optimal operating parameters of the reactor were: temperature of 32.35 °C, pH of 5.41, stirring speed of 195.56 rpm and initial concentration of lactose of 40g L−1. Saraceno et al. (2010) utilized a hybrid multiple neural model (HMNM) to predict biomass, lactose and ethanol concentration profiles of SCW in a simulated batch fermentation. Zoppellari et al. (2103) utilized the yeast K. marxianus var. marxianus, under aerobic and anaerobic batch conditions and semicontinuous conditions for the fermentation of SCW, CW and a mixture of the two at two different process temperatures (28 °C and 40 °C). As a result, SCW and CW resulted suitable substrates to produce bioethanol under anaerobic conditions. However, while CW proved useful under batch and semicontinuous anaerobic conditions, SCW led to good results solely in semi-continuous fermentations.
Lactose content in SCW and CW is insufficient for economically sustainable ethanol production at the industrial scale, as final ethanol concentration should reach a value of 20% w/w (Pisponen et al. 2013, Zou et al. 2022). Therefore, the concentration of the SCW and CW through distillation, evaporation under vacuum, or pre-concentration using membrane treatments followed by evaporation under vacuum, is mandatory to address this problem.
Another possible solution is to add other low-cost carbon sources to the substrates (Sampaio et al. 2020; Leonel et al. 2021; Pires et al. 2021; Zou et al. 2022). However, in mixtures with high sugar content the yeasts undergo catabolic repression and are not able to degrade lactose (Oda and Nakamura 2009).
Industrial facilities using dairy-by products for ethanol production are located in Ireland, New Zealand, the United States, Denmark and Germany. The first industrial plant to operate in the production of ethanol from CW was that of Carbery Milk Products Ltd. The Carbery process, adopted in the United States and New Zealand, involves batch fermentation followed by continuous distillation. CW permeate is adjusted to a pH of 5, heat-treated at 85 °C for 15 seconds, cooled to 34 °C, fed into the bioreactor, and inoculated with K. marxianus. The fermentation process lasts for 12 hours, followed by 6 hours of cooling before distillation. Recently, modifications to the process have introduced continuous fermentation and the use of concentrated whey as the raw material.
The three industrial plants of Anchor Ethanol limited of the Fonterra cooperative group in New Zealand produce approximately 19,000,000 L year−1 of ethanol. The two plants in the United States collectively produce approximately 30,000,000 L year−1 (Zou et al. 2022). Based on our knowledge there are not currently news of plants producing ethanol using SCW.
3.6.2 Bio-butanol
Butanol (C4H10O) has several advantages compared to ethanol. Besides being more suitable for distribution through petrol pipelines and mixable with gasoline, it shows higher energy, density and boiling point and lower volatility, hygroscopicity and corrosivity. Its production can occur by chemical synthesis or through acetone-butanol-ethanol (ABE) fermentation (Ndaba et al. 2015). In the latter case, Clostridium sp. is the biocatalyst of choice and fermentation substrates such as corn flour, cassava starch or flour, oil palm sap, or glucose, account for over 70% of the production cost (Gu et al. 2011; Zhang et al. 2016). Given the ability of Clostridium sp. to ferment lactose, also CW and SCW are being considered as promising alternatives for biobutanol production. In fact, their lactose content is close to the optimal value (40–50 g L−1) required by the ABE process to avoid butanol feedback inhibition (Becerra et al. 2015; Kaur et al. 2020). Since all efforts so far have been focused on CW, a brief description of CW utilization for bio-butanol production will be provided, with the understanding that most of the work done waits to be extended to SCW.
To favour the production of butanol (solventogenesis) instead of acids (acidogenesis) it is necessary to operate at low pH (4.7–5.5) or with high initial concentrations of lactose. Other parameters that can influence bio-butanol production include inoculum preparation, substrate composition, and temperature. For example, using ultrafiltered cheese whey and operating at 30 °C in batch conditions, Schoutens et al. (1984), achieved total lactose fermentation and a remarkable increase in butanol production. Fermentation can be carried out in batch, fed-batch and continuous mode. Continuous processes, in general, have several advantages such as the reduction of sterilization and accumulation times, high productivity, reduction of inhibition by butanol. On the other hand, continuous processes are characterized by high product recovery costs due to the low concentration (2.7 g L−1) of bio-butanol (Qureshi and Maddox 1987). Fed-batch reactors generally start with a low concentration of substrate and therefore require the addition of more substrate to keep the level constant. Batch reactors generally allow an easier management of anaerobic conditions, temperature and pH (Visioli et al. 2014). However, bio-butanol may reach concentrations of about 20 g L−1 that inhibit microbial growth thus affecting productivity (Knoshaugh and Zhang 2009). To overcome this problem gas stripping (Maddox 1989; Groot et al. 1992), pervaporation (Maddox 1989; Friedl et al. 1991; Qureshi et al. 1999), adsorption (Qureshi et al. 2005), vacuum fermentation (Mariano et al. 2011) but also the genetic manipulation of the biocatalyst (Visioli et al. 2014) have been proposed.
Clostridium acetobutylicum and C. beijeinckii are the biocatalysts most frequently employed in the ABE process, and the utilization of immobilized C. acetobutylicum P262 for CW permeate fermentation has been studied since the 1980s. C. acetobutylicum P262 was utilized for the development of a mathematical model that describes a continuously operating packed bed reactor in the fermentation of CW permeate (Qureshi et al. 1988). Raganati et al. (2013) used C. acetobutylicum DSM 792 and CW powder with 69% lactose in a continuous laboratory scale production of bio-butanol from reaching butanol concentration of 4.93 g L−1.
C. acetobutylicum DSM 792 and AS1.224 were employed for batch production of bio-butanol from CW with 4.5–5.0% lactose (Foda et al. 2010). CW permeate containing 211 g L−1 of lactose and C. acetobutylicum P262 were utilized by Qureshi et al. (2014) and pervaporation membrane was applied to remove bio-butanol. By doing so, they obtained a butanol volumetric productivity of 0.43 g L−1 h−1, much higher than those obtained by per-estraction (0.21 g L−1 h−1) and gas stripping (0.32 g L−1 h−1).
While several plants already exist for the production of bioethanol from CW, the same cannot be said for butanol. The industrial production of biobutanol from CW can now be considered theoretically possible since Clostridia can use lactose as a carbon source and produce biobutanol.
The same statement can be made theoretically for the SCW, but it is necessary to proceed with targeted experimental work both on a laboratory and pilot scale to develop suitable transformation processes based on the knowledge already acquired for the CW. Finally, CW and SCW proteins could also be considered a good substrate for biobutanol production. In fact, Huo et al. (2011), highlighted the possibility of using engineered Escherichia coli for growth on amino acid mixtures. In this process protein hydrolysates, which are constituted from amino acids and peptides was subjected to enzymatic reactions that involve deamination, transamination or dehydrogenation. In particular, some amino acids are deaminated to keto acids that can be converted to several alcohols such as ethanol, 1-propanol, n-butanol, iso-butanol, 2-methyl -1-butanol and 3-methyl-1-butanol.
3.6.3 Biogas/biomethane
Biogas is composed mainly of methane and CO2 with traces of hydrogen sulphide ammonia, water, nitrogen, oxygen, and hydrogen. It is obtained through the anaerobic fermentation of carbon sources carried out by a microbial consortium which acts through four stages: hydrolysis, acidogenesis, acetogenesis, and methanogenesis (Kangle et al. 2012).
Agricultural residues, agro-industrial by-products, animal manure, organic urban wastes, livestock residues and wastewater are suitable for anaerobic digestion with low capital expenditure. Among agro-industrial by-products, SCW and CW undoubtedly represent important and low-cost feedstocks for biogas production. For the economic sustainability of the process, besides the quality of the feedstocks also the configuration of the reactors assumes considerable importance (Osorio-González et al. 2022).
Thus, Continuous Stirred Tank Reactors (CSTR) are frequently proposed for the treatment of CW and SCW diluted or mixed with other substrates, but also Up-flow Anaerobic Sludge Blanket (UASB) reactors seem particularly suitable as they can process high volumes in a short time. An updated review on the state of the art and recent technological innovations for UASB reactors, which also considers the treatment of CW, was done by Mainardis et al. (2020). However, other types of reactors are also used such as upflow anaerobic filter (UAF), upflow anaerobic sludge fixed film (UASFF), anaerobic sequencing batch reactor (ASBR), anaerobic membrane bioreactors (AMB), expanded granule sludge bed (EGSB), plug flow reactor (PFR).
Methane yield from SCW and CW is limited due to the accumulation of volatile fatty acids originating from the fermentation of lactose which leads to a lowering of pH, growth of acetogenic bacteria and inhibition of methanogenic activity (Poništ et al. 2021). To maintain the optimal pH for methanogenic bacteria, co-digestion with appropriate substrates, or pH correction using alkaline substances are advised (Jasko et al. 2011). An example is given by mixing CW with cow manure which has the appropriate alkalinity, as well as providing other nutrients and microelements necessary for the development of microorganisms. Many works have been carried out to evaluate the conditions to produce biogas and consequently biomethane from the co-digestion of CW, SCW and animal manure, most of which dedicated to the treatment of CW, while a small number regard SCW. Table 4 shows the main studies carried out in recent years on the treatment of SCW for the production of biogas.
The first work dealing with the anaerobic digestion of SCW as the sole substrate was carried out by Lembo et al. (2016). In this work, using mesophilic anaerobic digestion in continuous mode with single-stage and two two-stage CSTRs on a laboratory scale, methane production and microbial community composition were explored. The results showed an increase in methane production in two-stage CSTRs ranging between 17.4 and 21.3% for the first reactor and between 4.0 and 19.0% for the second. The predominant microflora in the first two-stage CSTRs was made up mainly of Methanosarcina, while in the second Methanosaeta predominated. Overall, the results indicate the feasibility of the methane production process from SCW. Marchetti and Vasmara (2019) evaluated the effect of deproteinized CW (assimilated to SCW) as the sole substrate or in co-digestion with pig slurry, at different initial pH values in mesophilic (35 °C) batch condition on laboratory scale. The combination of deproteinized CW and 25% pig slurry produced the greatest amount of methane. The authors conclude that it is advisable to separate the fermentation phase of deproteinized CW from the subsequent co-digestion phase with fresh pig slurry. Santana et al. (2020), evaluated the performance of four laboratory-scale Plug Flow Reactors (PFRs) at an ambient temperature (18 °C÷25 °C), in a fed batch mode. Reactors were fed with SCW concentration ranging from 20 to 80%, in co-digestion with cattle manure, using 100% cattle manure as control, with a 30 days Hydraulic Retention Time (HRT). The experiment lasted for 106 days and in these conditions a biogas production between 17 and 27 L d−1 was obtained with a CH4 concentration between 57.0 and 60.0%. Lembo et al. (2021), evaluated the performance in thermophilic conditions (55 °C) of a Gas Stirred Tank Reactor (GSTR), powered with raw SCW, with the addition of hydrogen and with the partial immobilization of the microbial community. The results obtained showed that the addition of hydrogen leads to a 25% increase in CH4 productivity but determines a 13% decrease in CH4 concentration compared to the control. Gomes Barreto da Motta et al. (2022) analysed the population dynamics of methanogenic archaea, using tubular reactors with a volume of 60 L, fed with a SCW concentration between 20 and 80% in co-digestion with bovine manure. The results indicate that Methanosaeta and Methanosarcina were predominant in the co-digestion of SCW, while the highest concentration of CH4 (58%) was found in the mixture with 20% SCW. Lembo et al. (2023), studied different ways to increase mass transfer in a packed GSTR with a volume of 49L fed continuously with SCW in thermophilic conditions (55 °C) and with an HRT of 30 days, aimed at upgrading biogas in situ, using H2, with three different recirculation ratios. With a recirculation ratio of 235 Lreactor−1d−1 the methane concentration increased from 49.3 to 75% with a methane production of 0.37 LCH4 Lreactor−1d−1.
Despite the scarcity of works in the literature, there are some industrial plants in Italy that deal with SCW. For example, it is worth to mention the anaerobic digester of the Latteria e Caseificio Moro (TV) which treats 170 td−1 of SCW with the production of 3,200 Nm3CH4d−1; the anaerobic digester with SCW, acid whey and UF washes of Tobaldo Srl (PD) which treats 87 td−1 with a production of 1,369 Nm3 CH4d−1; the anaerobic digester of the Soligo Dairy (TV) which treats 147 td−1, with the production of 2,720 Nm3CH4d−1. Finally, the co-digestion of maize silage, sheep manure, ovine cheese whey and SCW, olive mill wastewater, pitted olive pome, in mixtures with each other, was carried out on pilot and industrial scale. Compared to the industrial plant of the Olmeo farm (Bancali, Sassari), the pilot plant shows much higher biogas production rate and specific production rates for both biogas (about 0.64 and 0.86 Nm3 kg VS−1 vs 0.35 Nm3 kg VS−1) and methane (about 0.30 and 0.43 Nm3 kg VS−1 vs 0.20 Nm3 kg VS−1) (Scano et al. 2021).
3.6.4 Hydrogen
Hydrogen is considered a promising source of clean energy and various technologies for its production have been studied for several decades. Although the economic feasibility of H2 production is still low, biological processes including fermentation (dark fermentation), bio-photolysis (direct and indirect) and bioelectrochemistry (microbial electro-cells) (Gopalakrishnan et al. 2019), together with the use of low-cost raw materials can contribute to the economic sustainability of the process. According to several authors SCW and CW can be economically viable substrates for hydrogen production by fermentation (Ferchichi et al. 2005; Yang et al. 2007; Davila-Vazquez et al. 2008; Azbar et al. 2009; Castello et al. 2009; Venetsaneas et al. 2009; Rosales-Colunga et al. 2010; Kargi et al. 2012; Karadag et al. 2014; De Gioannis et al. 2017; Asunis et al. 2019). However, as already seen for bio-butanol, while several studies have been carried out to evaluate the use of CW as a substrate for hydrogen production, very little has been done on SCW.
In the fermentation of CW, several strictly anaerobic microorganisms such as Clostridium butyricum, Clostridium pasteurianum, Clostridium beijirinkii and other facultative anaerobes such as Enterobacter sp., Citrobacter sp, and Escherichia coli are used to produce hydrogen. Mixed cultures are grown under mesophilic (30–38 °C) or thermophilic (55 °C) conditions. Thermophilic conditions seem more convenient due to the speeding up of the metabolic phases achieving higher methane yields and a better control of pathogenic microorganisms. Besides, temperature, several other variables including pH, alkalinity, substrate composition, predominant microorganisms, hydraulic retention time (HRT) and nutrient supplementation, impact on the production of hydrogen. The reactors that are mainly used are stirred tank (STR), CSTR and UASB reactors (Singh 2014). Anaerobic digestion of CW may be carried out using single and two-stage processes (Ghaly 1996; Saddoud et al. 2007). By using the two-stage process it is possible to optimize the operating conditions for hydrolysis, acidogenesis and methanogenesis, and to recover the hydrogen produced in the first phase of fermentation (Gómez et al. 2011). As a part of integrated treatment processes for the valorization of CW, Asunis et al. (2020), reviewed dark fermentation in terms of process parameters and type of reactors used. Dareioti et al. (2021), optimized the process parameters for dark fermentation in terms of pH, substrate pretreatment and organic load of a substrate consisting of CW, sorghum stalks and liquid cow manure under mesophilic conditions (37 °C) with a laboratory-scale batch reactor. The maximum H2 yield (0.52 mol mol eq.glucose-1) was observed at pH 5.5. The importance of pH on H2 production was confirmed in dark fermentation using permeate from SCW with varying lactose content by Vasmara and Marchetti (2017). Other authors combined hydrogen and polyhydroxyalkanoate production by means of mixed microbial culture using SCW, CW permeate (similar to SCW) and concentrated CW permeate (Colombo et al. 2019) or ovine CW Asunis et al. 2022.
A comprehensive work, also from an economic point of view, on the combined production of H2 and CH4 for the valorisation of SCW through anaerobic digestion was carried out by Lembo et al. (2022). The authors took into consideration three different plant situations: an anaerobic digester single stage located a short distance from the SCW production site, a single stage anaerobic digester located at the SCW production site and a two-stage anaerobic digester located on the same site, performing a detailed cost analysis also from the point of view of environmental impacts. The most economically sustainable solution is represented by the installation of a two-stage reactor at the SCW production site.
4 Patents regarding the exploitation of second cheese whey
To gather information on the current state of inventions regarding SCW exploitation, patents were searched in the Espacenet Patent database (https://worldwide.espacenet.com and Google Patents database (https://patents.google.com) using: deproteinized CW , “scotta”, SCW” and ricotta exhausted CW. Summary overview of SCW related patents is shown in Fig.2.
Between 1950 and 2023, only 67 patents were issued for the utilization of SCW. Most of these patents were granted during the periods of 1981 to 1985 and 2001 to 2015. United States had the highest number of patents granted (59.7%), followed by Poland (6.9%) and Japan (4.5%). European countries accounted for 25.37% of the patents. According to the Cooperative Patent Classification (CPC) system (2023.08), the most commonly referred definitions of SCW related patents were under the category A23, which pertains to foods or foodstuffs and their treatment (Fig. 3).
Several patents related to the utilization of SCW to produce bioactive molecules, probiotic foods, and single cell proteins (SCPs) are catalogued under the subclass A23C.
Patent number IT-202100024911-A1 (2023), for instance, describes the formulation of a functional food either in liquid or solid for that utilizes around 40–80% de-fatted and de-lactosated SCW derived from buffalo milk.
Patent number IT-MI980389-A1 (1999) outlines a procedure that employs microorganisms to dispose of lactose present in whey and/or SCW, with the production of bio-proteins for animal feed. K. marxianus is proposed as a starter yeast for its lactose fermentation ability.
Patent number AU2005237210B8 (2011) describes a process that uses dairy derivatives (including SCW) to enzymatically convert lactose to LBA. The required enzyme (carbohydrate oxidase) is obtained from a fungus belonging to the genus Microdochium.
In subclass A23J different SCW-related patents regard the purification of the κ-casein glycomacropeptide. Patent no. JP-2976349-B2 (1999), for instance, provides a method to inexpensively produce κ-casein glycomacropeptide using raw materials like whey protein concentrate, CW, or deproteinized CW. In the subclass A23L, patent no. CA2459284A1 (2004) describes a process for producing an aqueous antimicrobial solution containing nisin and pediocin by fermenting ultrafiltered SCW with Pediococcus acidilactici.
Subclass C13K includes patents focused on the production of saccharides through the hydrolysis of naturally occurring disaccharides, oligosaccharides, or polysaccharides. In this subclass patent no. US2681858A (1950) describes the enzymatic hydrolysis of lactose in milk products by beta-glucosidase-producing microorganisms such as Saccharomyces fragilis. SCW, which is filtered and thermally treated before being utilized, is one of the substrates suggested for this process. More recently, patent no. AU-2017342361-B2 (2020), describes a system for obtaining high purity lactose from a natural medium, which is clarified and then treated by using an adsorbent resin. Among natural sources of lactose, the patent suggests the use of deproteinized SCW.
Subclass C12P covers patents related to the production of compounds by biochemical transformation of matter performed by using enzymes or microorganisms. In this subclass, patent no. WO2005118775A1 (2005) provides a method for producing hydrogen by fermenting a sugar containing medium with Clostridium spp. (e.g. Clostridium bifermentans). The rate of hydrogen production and yield from SCW could be enhanced after SCW supplementation with a suitable nitrogen source.
5 Conclusion
SCW, is one of the main by-products of the dairy industry. It fulfils some of the requirements demanded for biotechnological exploitation, including low market price and high content of valuable nutrients and represents therefore a substrate of interest for sustainable and cost-effective biobased production. Accordingly, it may find application in the production of beverages or food ingredients, not only due to its nutritional value but also for meeting the demands of consumers seeking healthier and more sustainable dietary options. It serves as a low-cost substrate for microbial starters growth and long-term preservation. In animal nutrition, although careful consideration of optimal concentrations is essential for effective utilization in livestock feeding programs, SCW offers nutritional benefits, particularly through the positive impact of lactose on digestive health and growth performance in pigs and cattle. Moreover, SCW can be proposed for the production non-food related goods such as SCO, SCP, pigments and monomers for bioplastic production and may be utilized for the production of biofuels, especially bioethanol and biogas. Thus, the biotechnological valorisation of SCW achieves diverse objectives, including the production of goods applicable in the food, feed pharmaceutical/nutraceutical, chemicals and fuel industries and the bioremediation of dairy effluents.
In accordance with its multifaceted potential, most of the patents related to the utilization of SCW for the production of bioactive molecules, probiotic foods, and single cell proteins (SCPs) are catalogued under the subclass A23C, which covers dairy products such as milk, cheese, butter, and their substitutes, as well as the manufacturing of protein compositions for foodstuffs.
However, with the exception of biogas production that already occurs at the industrial level, all other applications of SCW have thus far been implemented solely at laboratory or pilot scale. This limitation arises from several challenges associated with the large-scale utilization of SCW. Firstly, the production of SCW exhibits seasonality, resulting in fluctuations in availability and quantity throughout the year. Secondly, SCW is characterized by variability in chemical composition, which can affect its suitability for different applications and necessitates careful quality control measures. Additionally, SCW has a high water content, which poses challenges for transportation, often rendering it uneconomical to transport over long distances. Furthermore, SCW is susceptible to microbial spoilage, especially during storage, which can compromise its quality and safety.
Therefore, continued efforts in research and innovation are crucial for addressing these constraints and unlocking the full potential of SCW. Research endeavours aimed at developing technologies to mitigate seasonality effects, improve compositional consistency, and enhance storage stability are essential. Additionally, innovations in transportation and storage methods are needed to overcome the challenges posed by SCW's high water content and perishability. Collaborative efforts between academia, industry, and government agencies are necessary to drive advancements in SCW utilization and facilitate its integration into mainstream industrial processes. By overcoming these challenges, SCW can emerge as a valuable and sustainable resource with diverse applications across various sectors, contributing to a more efficient and environmentally friendly dairy industry.
References
Ahmad A, Banat F, Alsafar H, Hasan SW (2022) An overview of biodegradable poly (lactic acid) production from fermentative lactic acid for biomedical and bioplastic applications. Biomass Conv Bioref 14:3057–3076. https://doi.org/10.1007/s13399-022-02581-3
Akinmulewo A, Nwinyi O (2019) Polyhydroxyalkanoate: a biodegradable polymer (a mini review). J Phys Conf Ser 1378:042007. https://doi.org/10.1088/1742-6596/1378/4/042007
Alfano A, D’ambrosio S, Cimini D, Falco L, D’Agostino M, Finamore R, Schiraldi C (2022) No waste from waste: membrane-based fractionation of second cheese whey for potential nutraceutical and cosmeceutical applications, and as renewable substrate for fermentation processes development. Fermentation 8:514. https://doi.org/10.3390/fermentation8100514
Alonso S, Rendueles M, Díaz M (2011) Efficient lactobionic acid production from whey by Pseudomonas taetrolens under pH-shift conditions. Bioresour Technol 102:9730–9736. https://doi.org/10.1016/j.biortech.2011.07.089
Asunis F, De Gioannis G, Isipato M, Muntoni A, Polettini A, Pomi R, Rossi A (2019) Control of fermentation duration and pH to orient biochemicals and biofuels production from cheese whey. Biores Technol 289:121722. https://doi.org/10.1016/j.biortech.2019.121722
Asunis F, De Gioannis G, Dessì P, Isipato M, Lens Piet NL, Muntoni A, Polettini A, Pomi R, Rossi A, Spiga D (2020) The dairy biorefinery: integrating treatment processes for cheese whey valorisation. J Environ Manag 276:111240. https://doi.org/10.1016/j.jenvman.2020.111240
Asunis F, Carucci A, De Gioannis G, Farru G, Muntoni A, Polettini A, Pomi R, Rossi A, Spiga D (2022) Combined biohydrogen and polyhydroxyalkanoates production from sheep cheese whey by a mixed microbial culture. J Environ Manag 322:116149. https://doi.org/10.1016/j.jenvman.2022.116149
Atkinson RL, Kratzer FH, Stewart GF (1957) Lactose in animal and human feeding: a review. J Dairy Sci 40(9):1114–1132
Azbar N, Cetinkaya Dokgoz FT, Keskin T, Korkmaz KS, Syed HM (2009) Continuous fermentative hydrogen production from cheese whey wastewater under thermophilic anaerobic conditions. Int J Hydrog Energy 34(17):7441–7447. https://doi.org/10.1016/j.ijhydene.2009.04.032
Barile D, Tao N, Lebrilla CB, Coisson J-D, Arlorio M, German JB (2009) Permeate from cheese whey ultrafiltration is a source of milk oligosaccharides. Int Dairy J 19(9):524–530
Becerra M, Cerdán ME, González-Siso MI (2015) Biobutanol from cheese whey. Microb Cell Fact 14:27
Bergamaschi M, Bittante G (2017) Detailed fatty acid profile of milk, cheese, ricotta and by products, from cows grazing summer highland pastures. J Dairy Res 84(3):329–338. https://doi.org/10.1017/s0022029917000450
Bergamaschi M, Bittante G (2018) From milk to cheese: Evolution of flavor fingerprint of milk, cream, curd, whey, ricotta, scotta, and ripened cheese obtained during summer Alpine pasture. J Dairy Sci 101(5):3918–3934. https://doi.org/10.3168/jds.2017-13573
Bering SBJN (2018) Human milk oligosaccharides to prevent gut dysfunction and necrotizing enterocolitis in preterm neonates. Nutrients 10(10):1461. https://doi.org/10.3390/nu10101461
Bernstein S, Tzeng CH, Sisson D (1977) Commercial fermentation of cheese whey for the production of protein and/or alcohol. Biotechnol Bioeng Symp 19:7
Borges AR, Pires AF, Marnotes NG, Gomes DG, Henriques MF, Pereira CD (2020) Dairy by-products concentrated by ultrafiltration used as ingredients in the production of reduced fat washed curd cheese. Foods 9:1020. https://doi.org/10.3390/foods9081020
Bosco F, Cirrincione S, Carletto R, Marmo L, Chiesa F, Mazzoli R, Pessione E (2021) PHA production from cheese whey and “Scotta”: comparison between a consortium and a pure culture of Leuconostoc mesenteroides. Microorganisms 9:2426. https://doi.org/10.3390/microorganisms9122426
Brody EP (2000) Biological activities of bovine glycomacropeptide. Br J Nutr 84(S1):39–46
Brück WM, Kelleher SL, Gibson GR, Graverholt G, Lönnerdal BL (2006) The effects of α-lactalbumin and glycomacropeptide on the association of CaCo-2 cells by enteropathogenic Escherichia coli. Salmonella Typhimurium and Shigella Flexneri 259(1):158–162. https://doi.org/10.1111/j.1574-6968.2006.00268.x
Cabizza R, Fancello F, Petretto GL, Addis R, Pisanu S, Pagnozzi D, Piga A, Urgeghe PP (2021) Exploring the DPP-IV inhibitory, antioxidant and antibacterial potential of ovine “Scotta” hydrolysates. Foods 10:3137. https://doi.org/10.3390/foods10123137
Campenni L, Nobre BP, Santos CA, Oliveira AC, Aires-Barros MR, Palavra AMF, Gouveia L (2013) Carotenoid and lipid production by the autotrophic microalga Chlorella protothecoides under nutritional, salinity, and luminosity stress conditions. Appl Microbiol Biotechnol 97:1383–1393
Cardoso T, Marques C, Andreotti Dagostin JL, Masson ML (2019) Lactobionic acid as a potential food ingredient: recent studies and applications. J Food Sci 84(7):1672–1681. https://doi.org/10.1111/1750-3841.14686
Carota E, Crognale S, D’Annibale A, Gallo AM, Stazi SR, Petruccioli M (2017) A sustainable use of Ricotta Cheese Whey for microbial biodiesel production. Sci Total Environ 584–585:554–560. https://doi.org/10.1016/j.scitotenv.2017.01.068
Castelló E, Santos CG, Iglesias T, Paulino G, Wenzel J, Borzacconi L, Etchebehere C (2009) Feasibility of biohydrogen production from cheese whey using a UASB reactor: Links between microbial community and reactor performance. Int J Hydrogen En 34:5674–5682
Chessa L, Paba A, Daga E, Caredda M, Comunian R (2020) Optimization of scotta as growth medium to preserve biodiversity and maximise bacterial cells concentration of natural starter cultures for Pecorino Romano PDO cheese. FEMS Microbiol Lett. https://doi.org/10.1093/femsle/fnaa110
Codex Alimentarius (2010) CODEX STAN 284–1971; FAO/WHO Standard for Whey cheeses. FAO/WHO Food Standards: Rome, Italy.
Colombo B, Villegas Calvo M, Pepè Sciarria T, Scaglia B, Savio Kizito S, D’Imporzano G, Adani F (2019) Biohydrogen and polyhydroxyalkanoates (PHA) as products of a two-steps bioprocess from deproteinized dairy wastes. Waste Manage 95:22–31
Córdova-Dávalos LE, Jiménez M, Salinas E (2019) Glycomacropeptide bioactivity and health: a review highlighting action mechanisms and signaling pathways. Nutrients 11(3):598. https://doi.org/10.3390/nu11030598
Coronas R, Zara G, Gallo A, Rocchetti G, Lapris M, Petretto GL, Zara S, Fancello F, Mannazzu I (2023) Propionibacteria as promising tools for the production of pro-bioactive scotta: a proof-of-concept study. Front Microbiol 14:1223741. https://doi.org/10.3389/fmicb.2023.1223741
Cromwell G, Allee G, Mahan DC (2008) Assessment of lactose level in the mid-to late-nursery phase on performance of weanling pigs. J Anim Sci 86(1):127–133. https://doi.org/10.2527/jas.2006-831
Cunningham M, Azcarate-Peril MA, Barnard A et al (2021) Shaping the future of probiotics and prebiotics. Trends Microbiol 29(8):667–685. https://doi.org/10.1016/j.tim.2021.01.003
Dallas DC, Weinborn W, de Moura BJMLN et al (2014) Comprehensive peptidomic and glycomic evaluation reveals that sweet whey permeate from colostrum is a source of milk protein-derived peptides and oligosaccharides. Food Res Internat 63:203–209
Dareioti MA, Vavouraki AI, Tsigkou K, Zafiri C, Kornaros M (2021) Dark fermentation of sweet sorghum stalks, cheese whey and cow manure mixture: effect of pH, pretreatment and organic load. Processes 9(6):1017. https://doi.org/10.3390/pr9061017
Davila-Vasquez G, Alatriste Mondragon F, León Rodríguez A, Razo-Flores A (2008) Fermentative hydrogen production in batch experiments using lactose, cheese whey and glucose. Influence of initial substrate concentration and pH. Int J Hydrog Energy 33:4989–4997
de França JOC, da Silva VD, Paiva MF, Dias SCL, Dias JA (2022) Polymers based on PLA from synthesis using D, L-Lactic acid (or racemic lactide) and some biomedical applications: a short review. Polymers 14:2317. https://doi.org/10.3390/polym14122317
De Gioannis G, Muntoni A, Polettini A, Pomi R, Spiga D (2017) Energy recovery from one and two-stage anaerobic digestion of food waste. Waste Manag 68:595–602. https://doi.org/10.1016/j.wasman.2017.06.013
De Giorgi S, Raddadi N, Fabbri A, Gallina Toschi T, Fava F (2018) Potential use of ricotta cheese whey for the production of lactobionic acid by Pseudomonas taetrolens strains. New Biotechnol 42:71–76. https://doi.org/10.1016/j.nbt.2018.02.010
DeFrain JM, Hippen AR, Kalscheur KF, Schingoethe DJ (2004) Feeding lactose increases ruminal butyrate and plasma β-hydroxybutyrate in lactating dairy cows. J Dairy Sci 87(8):2486–2494
DeFrain JM, Hippen AR, Kalscheur KF, Schingoethe DJ (2006) Feeding lactose to increase ruminal butyrate and the metabolic status of transition dairy cows. J Dairy Sci 89(1):267–276
de Souza Silva G, Pinho da Costa Castro CD, da Silva Campelo Borges G, Tonetto de Freitas S, de TarsoAidar S, Camarão Telles Biasoto A, Poloni Rybka AC, Cardarelli HR (2022) Physicochemical and functional properties of new sports drink with ricotta cheese whey and a brazilian passion fruit variety. J Food Sci Technol 60(2):538–548. https://doi.org/10.1007/s13197-022-05636-5
Dirksen G, Liebich H, Mayer E (1985) Adaptive changes of the ruminal mucosa and their functional and clinical significance. The Bovine Pratictioner 20:116–120. https://doi.org/10.21423/bovine-vol1985no20p116-120
El-Hawiet A, Kitova EN, Klassen JS (2015) Recognition of human milk oligosaccharides by bacterial exotoxins. Glycobiology 25(8):845–854. https://doi.org/10.1093/glycob/cwv025
Feeney S, Ryan T, Kilcoyne M, Joshi L, Hickey R (2017) Glycomacropeptide reduces intestinal epithelial cell barrier dysfunction and infection of enterohemorrhagic and enteropathogenic Escherichia Coli in-vitro. Foods 6(11):93. https://doi.org/10.3390/foods6110093
Ferchichi M, Crabbe E, Gil G-H, Hintz W, Almadidy A (2005) Influence of initial pH on hydrogen production from cheese whey. J Biotechnol 120:402–409. https://doi.org/10.1016/j.jbiotec.2005.05.017
Foda Mervat I, Hongjung D, Dong Li Y (2010) Study the suitability of cheese whey for bio-butanol production by clostridia. J Am Sci 6(8):39–46
Friedl A, Qureshi N, Maddox IS (1991) Continuous acetone-butanol-ethanol (ABE) fermentation using immobilized cells of Clostridium acetobutylicum in a packed bed reactor and integration with product removal by pervatioration. Biotech Bioeng 38:518–527. https://doi.org/10.1002/bit.260380510
Fukuda S, Toh H, Hase K et al (2011) Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469(7331):543–547
Gahan DA, Lynch MB, Callan JJ, O’Sullivan JT, O’Doherty JV (2009) Performance of weanling piglets offered low-, medium-or high-lactose diets supplemented with a seaweed extract from Laminaria spp. Animal 3(1):24–31. https://doi.org/10.1017/s1751731108003017
Garcia MME, Dias Pereira CJ, Freitas AC, Pereira Gomes AM, Estevez Pintado MM (2022) Development and characterization of a novel sustainable probiotic goat whey cheese containing second cheese whey powder and stabilized with thyme essential oil and sodium citrate. Foods 11(17):2698. https://doi.org/10.3390/foods11172698
Garrido D, Ruiz-Moyano S, Jimenez-Espinoza R, Eom H-J, Block DE, Mills DA (2013) Utilization of galactooligosaccharides by Bifidobacterium longum subsp. infantis isolates. Food Microbiol 33(2):262–270. https://doi.org/10.1016/j.fm.2012.10.003
Ghaly AE (1996) A comparative study of anaerobic digestion of acid cheese whey and dairy manure in a two-stage reactor. Biores Technol 58:61–72
Goderska K, Juzwa W, Szwengiel A, Czarnecki Z (2015) Lactobionic acid production by glucose–fructose oxidoreductase from Zymomonas mobilis expressed in Escherichia coli. Biotechnol Lett 37(10):2047–2053. https://doi.org/10.1007/s10529-015-1887-0
Barreto G, da Motta I, Rezende Santana LA, Passe Pereira H, Romário de Paula V, Fonseca Martins M, da CostaCarneiro J, Otenio MH (2022) Population dynamics of methanogenic archea in co-digestion systems operating different industrial residues for biogas production. Sustainability 14(18):11536. https://doi.org/10.3390/su141811536
Gómez X, Fernández C, Fierro J, Sánchez ME, Escapa A, Morán A (2011) Hydrogen production: two stage processes for waste degradation. Biores Technol 102(18):8621–8627. https://doi.org/10.1016/j.biortech.2011.03.055
Gopalakrishnan B, Khanna N, Das D (2019) Dark-Fermentative Biohydrogen Production. In: Pandey A, Venkata Mohan S, Chang J-S, Hallenbeck PC, Larroche C (Eds) Biomass, Biofules, Biochemicals. 2nd edition pp 79–122.
Gottardi D, Siroli L, Braschi G, Rossi S, Bains N, Vannini L, Patrignani F, Lanciotti R (2023) Selection of Yarrowia lipolytica strains as possible solution to valorize untreated cheese whey. Fermentation 9:51. https://doi.org/10.3390/fermentation9010051
Grinstead GS, Goodband RD, Dritz SS et al (2000) Effects of a whey protein product and spray-dried animal plasma on growth performance of weanling pigs. J Anim Sci 78(3):647–657
Groot WJ, van der Lans RG, Luyben KCAM (1992) Tecnologies for butanol recovery integrated with fermentations. Proc Biochem 27:61–75
Gu Y, Jiang YY, Wu H et al (2011) Economical challenges to microbial producers of butanol: feedstock, butanol ratio and titer. Biotechnol J 6:1348–1357
Huo YK, Cho KM, Rivera JGL et al (2011) Conversion of proteins into biofuels by engineering nitrogen flux. Nat Biotechnol 29:346–351. https://doi.org/10.1038/nbt.1839
Jang KB, Purvis JM, Kim SW (2021) Dose-response and functional role of whey permeate as a source of lactose and milk oligosaccharides on intestinal health and growth of nursery pigs. J Anim Sci. https://doi.org/10.1093/jas/skab008
Jasko J, Skipsts E, Dubrovskis V, Zabarovskis E, Kotelenecs V (2011) Biogas production from cheese whey in two phases anaerobic digestion. Eng Rural Dev:373–376
Janer C, Peláez C, Requena T (2004) Caseinomacropeptide and whey protein concentrate enhance Bifidobacterium lactis growth in milk. Food Chem 86(2):263–267
Kangle KM, Kore SV, Kore VS, Kulkarni GS (2012) Recent trends in anaerobic codigestion: a review. Anaerobic digestion process. Univers J Environ Res Technol 2:210–219
Karadag D, Köroglu OE, Ozkaya B, Cakmakci M, Heaven S, Banks C (2014) A review on fermentative hydrogen production from dairy industry wastewater. J Chem Technol Biotechnol 89:1627–1636
Kargi F, Eren NS, Ozmihci S (2012) Bio-hydrogen production from cheese whey powder (CWP) solution: comparison of thermophilic and mesophilic dark fermentations. Int J Hydrog Energy 37(10):8338–8342
Kaur R, Panwar D, Panesar PS (2020) Biotechnological approach for valorisation of whey for value-added products. In: Kosseva MR, Webb C (eds) Food industry waste-assessment and recuperation of commodities. Elsevier
Kim CH (2018) Immune regulation by microbiome metabolites. Immunology 154(2):220–229
Knoshaug EP, Zhang M (2009) Butanol tolerance in a selection of microorganisms. Appl Biochem Biotechnol 153:13–20
Koletzko B, Baker S, Cleghorn G et al (2005) Global standard for the composition of infant formula: recommendations of an ESPGHAN Coordinated International Expert Group. J Pediatr Gastroenterol Nutr 41(5):584–599
Kurnick JV, Guarnieri Michellim MG, Yada RY, de Castro Leite Jr BR, Artigiani Lima Tribst A, (2024) Development of value-added beverages using sheep and goat cheese whey and secondary whey. Int Dairy J. https://doi.org/10.1016/j.idairyj.2024.105886
Kurtzman C (2011) The yeasts: a taxonomic study. Elsevier, Amsterdam, The Netherlands
Lembo G, Massini G, Mazzurco MV, Fenice M, Felici C, Liberatore R, Signorini A (2016) Anaerobic digestion of ricotta cheese whey effect of phase separation on methane production and microbial community structure. Proceedings European Biomass Conference and Exhibition, Amsterdam. https://doi.org/10.5071/24thEUBCE2016-2DO.1.4
Lembo G, Rosa S, Mazzurco MV, Marone A, Massini G, Fenice M, Signorini A (2021) Thermophilic anaerobic digestion of second cheese whey: microbial community response to H2 addition a partially immobilized anaerobic hybrid reactor. Processes 9:43. https://doi.org/10.3390/pr9010043
Lembo G, Signorini A, Marone A, Carbone C, Agostini A (2022) Hydrogen and methane production by single- and two-stage anaerobic digestion of second cheese whey: economic performances and GHG emissions evaluation. Energies 15:7869. https://doi.org/10.3390/en15217869
Lembo G, Rosa S, Marone A, Signorini A (2023) In situ biogas upgrading in a randomly packed gas-stirred tank reactor (GSTR). Energies 16:3296. https://doi.org/10.3390/en16073296
Leonel LV, Arruda PV, Chandel AK, Felipe MGA, Sen L (2021) Kluyveromyces marxianus: a potential biocatalyst of renewable chemicals and lignocellulosic ethanol production. Crit Rev Biotechnol 41:1131–1152
Maddox IS (1989) The acetone-butanol-ethanol fermentation: recent progress in technology. Biotechnol Genet Eng Rev 7:199–220
Madureira AR, Gião MS, Pintado ME, Gomes AMP, Freitas AC, Malcata FX (2005) Incorporation and survival of probiotic bacteria in whey cheese matrices. J Food Sci 70(3):M160–M165
Mainardis M, Buttazzoni M, Goi D (2020) Up-flow anaerobic sludge blanket (UASB) technology for energy recovery: a review on state-of-the-art and recent technological advances. Bioeng 7:43
Maiorella BL, Blanch HW, Wilke CR (1984) Feed component inhibition in ethanolic fermentation by Saccharomyces cerevisiae. Biotechnol Bioeng 26(10):1155–1166
Mahan DC, Fastinger ND, Peters JC (2004) Effects of diet complexity and dietary lactose levels during three starter phases on postweaning pig performance. J Anim Sci 82(9):2790–2797
Maragkoudakis P, Vendramin V, Bovo B, Treu L, Corich V, Giacomini A (2016) Potential use of scotta, the by-product of the ricotta cheese manufacturing process, for the production of fermented drinks. J Dairy Res 83:104. https://doi.org/10.1017/S002202991500059X
Marchetti R, Vasmara C (2019) Co-digestion of deproteinized dairy waste with pig slurry: effect of recipe and initial ph on biogas and volatile fatty acid production. BioEnergy Res 13:643–658
Mariano AP, Qureshi N, Filho RM, Ezeij TC (2011) Bioproduction of butanol in bioreactors: new insights from simultaneous in situ butanol recovery to eliminate product toxicity. Biotech Bioeng 108:1757–1765
Menchik P, Zuber T, Zuber A, Moraru CI (2019) Composition of coproduct streams from dairy processing: acid whey and milk permeate. J Dairy Sci 102(5):3978–3984. https://doi.org/10.3168/jds.2018-15951
Minhalma M, Magueijo V, Queiroz DP, de Pinho MN (2007) Optimization of “Serpa” cheese whey nanofiltration for effluent minimization and by-products recovery. J Environ Manag 82(2):200–206. https://doi.org/10.1016/j.jenvman.2005.12.011
Monari S, Ferri M, Russo C et al (2019) Enzymatic production of bioactive peptides from scotta, an exhausted by-product of ricotta cheese processing. PLoS ONE 14(12):e0226834. https://doi.org/10.1371/journal.pone.0226834
Monti L, Donati E, Zambrini AV, Contarini G (2018) Application of membrane technologies to bovine Ricotta cheese exhausted whey (scotta). Int Dairy J 85:121–128. https://doi.org/10.1016/j.idairyj.2018.05.007
Moscoviz R, Toledo-Alarcón J, Trably E, Bernet N (2016) Electro-fermentation: how to drive fermentation using electrochemical systems. Trends Biotechnol 34:856–865. https://doi.org/10.1016/j.tibtech.2016.04.009
Morrow AL, Ruiz-Palacios GM, Jiang X, Newburg DS (2005) Human-milk glycans that inhibit pathogen binding protect breast-feeding infants against infectious diarrhea. J Nutr 135(5):1304–1307
Moulin G, Galzy P (1978) Remarks on the metabolism of Kluyveromyces lactis van der walt. Mycopathologia 66:73–76
Moukarzel S, Bode L (2017) Human milk oligosaccharides and the preterm infant: a journey in sickness and in health. Clin Perinatol 44(1):193–207. https://doi.org/10.1016/j.clp.2016.11.014
Naziri E, Papadaki E, Savvidis I, Botsaris G, Gkatzionis K, Mugampoza E, Mantzouridou FT (2023) Exploring the potential of halloumi second cheese whey for the production of lactic acid cultures for the dairy industry. Sustainability 15:9082. https://doi.org/10.3390/su15119082
Ndaba B, Chiyanzu I, Marx S (2015) n-Butanol derived from biochemical and chemical routes: a review. Biotechnol Rep 8:1–9
Nguyen TTH, Kim JW, Park J-S, Hwang KH, Jang T-S, Kim C-H, Kim D (2016) Identification of oligosaccharides in human milk bound onto the toxin A carbohydrate binding site of Clostridium difficile. J Microbiol Biotechnol 26(4):659–665. https://doi.org/10.4014/jmb.1509.09034
Nielsen PM, Hoeier E (2009) Food products containing aldobionic acid. International Patent Application Pub. No.: WO 2009/007398 A1
Oda Y, Nakamura K (2009) Production of ethanol from the mixture of beet molasses and cheese whey by a 2-deoxyglucose-resistant mutant of Kluyveromyces marxianus. FEMS Yeast Res 9(5):742–748
Oh S, Worobo RW, Kim B-C, Rheem S, Kim SJB (2000) Detection of the cholera toxin-binding activity of κ-casein macropeptide and optimization of its production by the response surface methodology. Biosci Biotechnol Biochem 64(3):516–522. https://doi.org/10.1271/bbb.64.516
Oliveira CSS, Silva CE, Carvalho G, Reis MA (2017) Strategies for efficiently selecting PHA producing mixed microbial cultures using complex feedstocks: feast and famine regime and uncoupled carbon and nitrogen availabilities. Nat Biotechnol 37:69–79. https://doi.org/10.1016/j.nbt.2016.10.008
Osorio-González CS, Gómez-Falcon N, Brar SK, Avalos Ramírez A (2022) Cheese whey as a potential feedstock for producing renewable biofuels: a review. Energies 15:6828. https://doi.org/10.3390/en15186828
Otani H, Hata I (1995) Inhibition of proliferative responses of mouse spleen lymphocytes and rabbit Peyer’s patch cells by bovine milk caseins and their digests. J Dairy Res 62(2):339–348
Pan S, Dixon KL, Nawaz T, Rahman A, Selvaratnam T (2021) Evaluation of Galdieria sulphuraria for nitrogen removal and biomass production from raw landfill leachate. Algal Res 54:102183. https://doi.org/10.1016/j.algal.2021.102183
Pasotti L, Zucca S, Casanova M, Micoli G, Cusella De Angelis MG, Magni P (2017) Fermentation of in cheese of lactose to ethanol whey permeate and concentrated permeate by engineered Escherichia coli. BMC Biotechnol 17:48
Pasta C, Caccamo M, Petriglieri R et al (2024) Sicilian Whey: Utilization of Ricotta Whey in the Production of Value-Added Artisanal Beers. Fermentation 10:19. https://doi.org/10.3390/fermentation10010019
Pereira CD, Diaz O, Cobos A (2002) Valorization of by-products from ovine cheese manufacture: clarification by thermocalcic precipitation/microfiltration before ultrafiltration. Int Dairy J 12:773–783. https://doi.org/10.1016/S0958-6946(02)00070-5
Pierce KM, Sweeney T, Brophy PO, Callan JJ, Mccarthy PO, Doherty JV (2005) Dietary manipulation post weaning to improve piglet performance and gastro-intestinal health. Anim Sci 81:347–356
Pires FA, Marnotes GN, Díaz Rubio O, Garcia Cobos A (2021) Dairy by-products: a review on the valorisation of whey and second cheese whey. Foods 10:1067. https://doi.org/10.3390/foods10051067
Pires A, Gomes D, Noronha J, Díaz O, Cobos A, Pereira CD (2023) Evaluation of the characteristics of sheep’s and goat’s ice cream, produced with uf concentrated second cheese whey and different starter cultures. Foods 11:4091. https://doi.org/10.3390/foods11244091
Pisponen A, Pajumägi S, Mootse H, Karus A, Poikalainen V (2013) The lactose from ricotta cheese whey: the effect of pH and concentration in size and morphology of lactose crystals. Dairy Sci Technol 93(4):477–486
Poništ J, Dubšíková V, Schvarz M, Samešová D (2021) Method of processing whey waste from dairies. A review. Environ Protect Eng 47(4):67–84
Pontonio E, Montemurro M, De Gennaro GV, Miceli V, Rizzello CG (2021) Antihypertensive peptides from ultrafiltration and fermentation of the ricotta cheese exhausted whey: design and characterization of a functional ricotta cheese. Foods 10:2573. https://doi.org/10.3390/foods10112573
Qureshi N, Maddox IS (1987) Continuous solvent production from whey permeate using cells of Clostridium acetobutylicum immobilized by adsorption onto bonechar. Enzyme Microb Technol 9:668–671
Qureshi N, Paterson AHJ, Maddox IS (1988) Model for continuous production of solvents from whey permeate in a packed bed reactor using cells of Clostridium acetobutylicum immobilizes by adsorption onto bonechar. Appl Microbiol Biotechnol 29:323–328
Qureshi N, Meagher MM, Hutkins RW (1999) Recovery of butanol from model solutions and fermentation broths using silicalite/silicone membrane. J Membr Sci 158:115–125. https://doi.org/10.1016/S0376-7388(99)00010-1
Qureshi N, Maddox IS (2005) Reduction in Butanol inhibition by Perstraction: Utilization of concentrated Lactose/whey permeate by Clostridium acetobutylicum to Enhance Butanol Fermentation Economics. Food Bioprod 83:43–52. https://doi.org/10.1205/fbp.04163
Qureshi N, Friedl A, Maddox IS (2014) Butanol production from concentrated lactose/whey permeate: use of pervaporation membrane to recover and concentrate product. Appl Microbiol Biotechnol 98(23):9859–9867
Rabaioli Rama G, Kuhn D, Beux S, Jachetti Maciel M, Volken de Souza CF (2019) Potential applications of dairy whey for the production of lactic acid bacteria cultures. Int Dairy J 98:25–37. https://doi.org/10.1016/j.idairyj.2019.06.012
Rabaioli Rama G, Kuhn D, Beux S, Jachetti Maciel M, Volken de Souza CF (2020) Cheese whey and ricotta whey for the growth and encapsulation of endogenous lactic acid bacteria. Food Bioprocess Technol 13:308–322. https://doi.org/10.1007/s11947-019-02395-8
Raganati F, Olivieri G, Procentese A, Russo ME, Salatino P, Marzocchella A (2013) Butanol production by bioconversion of cheese whey in a continuous packed bed reactor. Biores Technol 138:259–265
Raho S, Carofiglio VE, Montemurro M, Miceli V, Centrone D, Stufano P, Schioppa M, Pontonio E, Rizzello CG (2020) Production of the Polyhydroxyalkanoate PHBV from Ricotta Cheese Exhausted Whey by Haloferax mediterranei Fermentation. Foods 9(10):1459. https://doi.org/10.3390/foods9101459
Ramani R, Ramani V (2018) Probiotic microencapsulation techniques and coating materials. Int J Probiotics Prebiotics 13(4)
Ribeiro JES, Martini M, Altomonte I et al (2017) Production of Chlorella protothecoides biomass, chlorophyll and carotenoids using the dairy industry by-product scotta as a substrate. Biocatal Agric Biotechnol 11:207–213. https://doi.org/10.1016/j.bcab.2017.07.007
Ribeiro JES, Martini M, Andreucci A et al (2017b) Preliminary investigation of reuse of ricotta cheese whey (scotta) as substrate for the growth of Rhodotorula glutinis intended for the production of carotenoids. ARPN J Agric Biol Sci 12(10):302–306
Rigo J, Boehm G, Georgi G, Jelinek J, Nyambugabo K, Sawatzki G, Studzinski F (2001) An infant formula free of glycomacropeptide prevents hyperthreoninemia in formula-fed preterm infants. J Pediatr Gastroenterol Nutr 32(2):127–130. https://doi.org/10.1097/00005176-200102000-00006
Rizzolo A, Cortellino G (2017) Ricotta cheese whey–fruit based beverages: pasteurization effects on antioxidant composition and color. Beverages 3(1):15. https://doi.org/10.3390/beverages3010015
Rizzolo A, Cortellino G (2018) Beverages based on ricotta cheese whey and fruit juices. It J Food Sci 30:289–300
Rogosa M, Browne HH, Whittier EO (1947) Ethyl Alcohol from Whey. J Dairy Sci 30(4):263–269. https://doi.org/10.3168/jds.S0022-0302(47)92261-8]
Rong Y, Lu Z, Zhang H, Zhang L, Song D, Wang Y (2015) Effects of casein glycomacropeptide supplementation on growth performance, intestinal morphology, intestinal barrier permeability and inflammatory responses in Escherichia coli K88 challenged piglets. Anim Nutr 1(2):54–59
Roohina F, Mohammadi M, Najafpour GD (2016) Immobilized Kluyveromyces marxianus cells in carboxymethyl cellulose for production of ethanol from cheese whey: experimental and kinetic studies. Bioprocess Biosyst Eng 39(9):1341–1349. https://doi.org/10.1007/s00449-016-1607-8
Rosales-Colunga LM, Razo-Flores E, Ordoñez LG, Alatriste-Mondragón F, De León-Rodríguez A (2010) Hydrogen production by Escherichia coli ΔhycA ΔlacI using cheese whey as substrate. Int J Hydrog Energy 35:491–499. https://doi.org/10.1016/j.ijhydene.2009.10.097
Russo GL, Langellotti PM, Oliviero M, Baselice M, Sacchi R, Masi P (2021) Valorization of second cheese whey through cultivation of extremophile microalga Galdieria sulphuraria. AIMS Environ Sci 8(5):435–448. https://doi.org/10.3934/environsci.2021028
Sabapathy PC, Devaraj S, Meixner K, Anburajan P, Kathirvel P, Ravikumar Y, Zabed HM, Qi X (2020) Recent developments in Polyhydroxyalkanoates (PHAs) production–A review. Biores Technol 306:123132. https://doi.org/10.1016/j.biortech.2020.123132
Saddoud A, Hassaïri I, Sayadi S (2007) Anaerobic membrane reactor with phase separation for the treatment of cheese whey. Biores Technol 98(11):2102–2108. https://doi.org/10.1016/j.biortech.2006.08.023
Sáez-Orviz S, Marcet I, Rendueles M, Diaz M (2022) The antimicrobial and bioactive properties of lactobionic acid. J Sci Food Agric 102(9):3489–3924. https://doi.org/10.1002/jsfa.11823
Saini P, Beniwal A, Kokkiligadda A, Vij S (2017) Evolutionary adaptation of Kluyveromyces marxianus strain for efficient conversion of whey lactose to bioethanol. Proc Biochem 62:69–79. https://doi.org/10.1016/j.procbio.2017.09.009
Salehizadeh H, van Loosdrecht MCM (2004) Production of polyhydroxyalkanoates by mixed culture: recent trends and biotechnological importance. Biotechnol Adv 22:261–279. https://doi.org/10.1016/j.biotechadv.2004.03.005
Sampaio FC, De Faria JT, De Souza Oliveira RP, Converti A (2020) Cheese whey permeate fermentation by kluyveromyces lactis, a combined approach to waste water treatment and bioethanol production. Environ Technol 1:24
Sansonetti S, Curcio S, Calabro V, Iorio G (2009) Bio-ethanol production by fermentation of ricotta cheese whey as an effective alternative non-vegetable source. Biomass Bioen 33:1687–1692. https://doi.org/10.1016/j.biombioe.2009.09.002
Sansonetti S, Curcio S, Calabro V, Iorio G (2010a) Evaluation of the parameters effects on the bio-ethanol production process from ricotta cheese whey. Chem Eng Trans 20:13–18
Sansonetti S, Curcio S, Calabro V, Iorio G (2010b) Optimization of ricotta cheese whey (RCW) fermentation by response surface methodology. Biores Technol 101:9156–9162. https://doi.org/10.1016/j.biortech.2010.06.024
Santana O, Carneiro J, Motta SP, Arcuri D (2020) Energy Recovery from Ricotta Cheese Whey in Co-Digestion with Cattle Manure in Anaerobic Reactors. JBAB-D-20–00718 https://ssrn.com/abstract=3640184
Saraceno A, Curcio S, Calabrò V, Iorio G (2010) A hybrid neural approach to model batch fermentation of “ricotta cheese whey” to ethanol. Comput Chem Eng 34:1590–1596. https://doi.org/10.1016/j.compchemeng.2010.06.018
Sarenkova I, Ciprovica I (2018) The current status and future perspectives of lactobionic acid production: a review. Food Sci 1:233–239
Scano EA, Grosso M, Pistis A, Carboni G, Cocco D (2021) An in-depth analysis of biogas production from locally agro-industrial by-products and residues. An Italian Case Renewable Energy 179:308–318
Schoutens GH, Nieuwenhuizen MCH, Kossen NWF (1984) Butanol from whey ultrafiltrate: Batch experiments with Clostridium beijeinckii, LMD27.6. Appl Microbiol Biotechnol 19(4):203–206
Schultz N, Chang L, Hauck A, Reuss M, Syldatk C (2006) Microbial production of single-cell protein from deproteinized whey concentrates. Appl Microbiol Biotechnol 69:515–520. https://doi.org/10.1007/s00253-005-0012-z
Secchi N, Catzeddu P, García MR, Giunta D, Mannazzu I, Pretti L, Roggio T (2012) Bioconversion of ovine scotta into lactic acid with pure and mixed cultures of lactic acid bacteria. J Ind Microbiol Biotechnol 39:175–181. https://doi.org/10.1007/s10295-011-1013-9
Shirreffs S (2009) Hydration in sport and exercise: water, sports drinks and other drinks. Nutr Bull 34:374–379
Singh RS (2014) Biotechnological approaches for valorization of whey. In: Singh RS, Pandey A, Larroche C (Eds) Advances in Industrial Biotechnology, IK International Publishing House PVT. LTD, New Delhi, pp 443–478
Sommella E, Pepe G, Ventre G, Pagano F, Conte GM, Ostacolo C, Manfra M, Tenore GC, Russo M, Novellino E, Campiglia P (2016) Detailed peptide profiling of “Scotta”: from a dairy waste to a source of potential health-promoting compounds. Dairy Sci Technol 96:763–771. https://doi.org/10.1007/s13594-016-0297-y
Sudesh K, Abe H, Doi Y (2000) Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog Polym Sci 25:1503–1555. https://doi.org/10.1016/S0079-6700(00)00035-6
Susmel P, Spanghero M, Mills C, Stefanon B (1995) Rumen fermentation characteristics and digestibility of cattle diets containing different whey: maize ratios. Anim Feed Sci Technol 53(1):81–89
Taskin M, Aydogan SA, MN, Arslan NP, (2014) Microbial lipid production by cold-adapted oleaginous yeast Yarrowia lipolytica B9 in non-sterile whey medium. Biofuels Bioprod Biorefin 9:595–605. https://doi.org/10.1002/bbb.1560
Tsolcha ON, Tekerlekopoulou AG, Akratos C, Bellou S, Aggelis G, Katsiapi M, Moustaka-Gouni M, Vayenas DV (2016) Treatment of second cheese whey effluents using a Choricystis-based system with simultaneous lipid production. J Chem Technol Biotechnol 91(8):2349–2359. https://doi.org/10.1002/jctb.4846
Terpou A, Papadaki A, Lappa IK, Kachrimanidou V, Bosnea LA, Kopsahelis N (2019) Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients 11(7):1591. https://doi.org/10.3390/nu11071591
Tesfaw A (2023) The current tends of bioethanol production from cheese whey using yeasts: biological and economical perspectives. Front Energy Res. https://doi.org/10.3389/fenrg.2023.1183035
Thompkinson D, Kharb S (2007) Aspects of infant food formulation. Compr Rev Food Sci Food Saf 6(4):79–102. https://doi.org/10.1111/j.1541-4337.2007.00020.x
Tirloni E, Vasconi M, Cattaneo P, Moretti V, Bellagamba F, Bernardi C, Stella S (2020) A possible solution to minimise scotta as a food waste: a sports beverage. Int J Dairy Technol 73(2):421–428. https://doi.org/10.1111/1471-0307.12647
Vasilakis G, Karayannis D, Massouras T, Politis I, Papanikolaou S (2022) Biotechnological conversions of mizithra second cheese whey by wild-type non-conventional yeast strains: production of yeast cell biomass, single-cell oil and polysaccharides. Appl Sci 12:11471. https://doi.org/10.3390/app122211471
Vasmara C, Marchetti R (2017) Initial pH influences in-batch hydrogen production from scotta permeate. Int J Hydrogen Energy 42(21):14400–14408. https://doi.org/10.1016/j.ijhydene.2017.04.067
Venetsaneas N, Antonopoulou G, Stamatelatou K, Kornaros M, Lyberatos G (2009) Using cheese whey for hydrogen and methane generation in a two-stage continuous process with alternative pH controlling approaches. Biores Technol 100:3713–3717
Veniamakis E, Kaplanis G, Voulgaris P, Nikolaidis PT (2022) Effects of sodium intake on health and performance in endurance and ultra-endurance sports. Int J Environ Res Public Health 19:3651
Villegas Calvo M, Colombo B, Corno L, Eisele G, Cosentino C, Papa G, Scaglia B, Pilu R, Adani SB (2018) Bioconversion of giant cane for integrated production of biohydrogen, carboxylic acids, and polyhydroxyalkanoates (PHAs) in a multistage biorefinery approach. ACS Sustain Chem Eng 6:15361–15373. https://doi.org/10.1021/acssuschemeng.8b03794
Vincenzi A, Jachetti Maciel M, Burlani LE, Conceição Oliveira E, Volpato G, Lehn D, Volken de Souza CF (2014) Ethanol Bio-production from ricotta cheese whey by several strains of the yeast Kluyveromyces. Am J Food Technol 9(6):281–291
Visioli LJ, Enzweiler H, Kuhn RC, Schwaab M, Mazutti MA (2014) Recent advances on biobuthanol production. Sust Chem Proc 2:15
Webb BH, Whittier EO (1948) The utilization of whey: a review. J Dairy Sci 31(2):139–164
Whittier EO (1944) Lactose and its utilization: a review. J Dairy Sci 27(7):505–537
Xu J (1999) Effects of dietary lactose compared with ground corn on the growth rate of ruminal papillae and rate of valerate absorption from the rumen. Dissertation, Michigan State University
Yamada S, Takamatsu Y, Ikeda S, Kouzuma A, Watanabe K (2022) Towards application of electro-fermentation for the production of value-added chemicals from biomass feedstocks. Front Chem. https://doi.org/10.3389/fchem.2021.813011
Yang P, Zhang R, McGarvey JA, Benemann JR (2007) Biohydrogen production from cheese processing waste water by anaerobic fermentation using mixed microbial communities. Int J Hydrog Energy 32:4761–4771. https://doi.org/10.1016/j.ijhydene.2007.09.006
Yu Z-T, Chen C, Newburg DS (2013) Utilization of major fucosylated and sialylated human milk oligosaccharides by isolated human gut microbes. Glycobiology 23(11):1281–1292. https://doi.org/10.1093/glycob/cwt065
Zhang Z, O’Hara IM, Mundree S, Gao B, Ball AS, Bai ZhuN, Z, Jin B, (2016) Biofuels from food processing wastes. Curr Opin Biotechnol 38:97–105. https://doi.org/10.1016/j.copbio.2016.01.017
Zimermann JDF, Sydney EB, Cerri ML et al (2020) Growth kinetics, phenolic compounds profile and pigments analysis of Galdieria sulphuraria cultivated in whey permeate in shake-flasks and stirred-tank bioreactor. J Water Process Eng 38:101598. https://doi.org/10.1016/j.jwpe.2020.101598
Zoppellari F (2013) Production of bioethanol from effluents of the dairy industry by Kluyveromyces marxianus. J Biotechnol 30:6. https://doi.org/10.3923/biotech.2013.1.8
Zou J, Chang XJ (2022) Past, present, and future perspectives on whey as a promising feedstock for bioethanol production by yeast. J Fungi 8:395. https://doi.org/10.3390/jof8060395
Acknowledgements
This study was partially supported by the Agritech National Research Center and received funding from the European Union Next-GenerationEU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR) – MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4 – D.D. 1032 17/06/2022, CN00000022). This manuscript reflects only the authors’ views and opinions, neither the European Union nor the European Commission can be considered responsible for them.
Funding
Open access funding provided by Università degli Studi di Sassari within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fancello, F., Zara, G., Hatami, F. et al. Unlocking the potential of second cheese whey: a comprehensive review on valorisation strategies. Rev Environ Sci Biotechnol 23, 411–441 (2024). https://doi.org/10.1007/s11157-024-09687-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11157-024-09687-2