Abstract
Aims
The spread of invasive weeds threatens biodiversity and stability of ecosystems. Jacobaea vulgaris is an invasive weed in some countries and an outbreak species in its native European range. Although biological control using specialist herbivores is available, controlling with soil microorganisms remains far less explored.
Methods
Twenty bacteria strains isolated from roots of J. vulgaris were used to examine bacterial effects on seed germination, root morphology and early plant growth. Moreover, we tested direct effects of the bacteria on a specialist herbivore of J. vulgaris, the leaf chewing caterpillar (Tyria jacobaeae), commonly used in biocontrol. We also tested indirect effects of bacteria, via the plant, on the performance of T. jacobaeae and the aphid species Aphis jacobaeae. Lastly, we examined the host specificity of two tested bacteria on three other forbs.
Results
Two Gammaproteobacteria, Pseudomonas brassicacearum and Serratia plymuthica, significantly reduced root growth of seedlings in-vitro, while seed germination was unaffected. However, these negative effects were observed across other forb species as well. Bacillus spp. injection led to the highest T. jacobaeae caterpillar mortality, while ingestion had no effect. Inoculation of the plants with bacteria did not affect aphid performance, but significantly affected T. jacobaeae preference. Specifically, P. syringae and one Bacillus sp. strain significantly increased T. jacobaeae preference.
Conclusions
Our results show that two root-associated bacteria inhibit J. vulgaris growth, but their lack of host specificity restricts their potential for biocontrol. Our study also highlights that belowground microorganisms can hamper or enhance the performance of aboveground insects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There has been a significant increase in the number of invasive alien plant species (weeds) due to globalization (Seebens et al. 2017, 2021). Invasive alien plant species can cause the loss of global biodiversity, degradation of ecosystem services and public health problems (Vilà et al. 2011; Simberloff et al. 2013; Blackburn et al. 2019; Schaffner et al. 2020; Liendo et al. 2023). For example, Ambrosia artemisiifolia is an invasive weed in Europe. It has been reported that 13.5 million persons suffer from Ambrosia pollen-induced allergies in Europe (Schaffner et al. 2020). Rosa multiflora is considered an invasive plant species in North America. Several studies have shown that sites with R. multiflora are concentrated with ticks, hence this invasive plant is linked to an increased tick-borne diseases risk (Adalsteinsson et al. 2016, 2018). Many methods have been employed to reduce the abundance of invasive weeds, including mechanical (i.e. removal of individuals and their propagules), chemical, and biological control (Pearson et al. 2016). Over the past decades, there has been increasing concerns about the negative effects of chemical herbicides on the environment, and this has led to an increasing demand for biological control agents of weeds (Pearson et al. 2016; Abbas et al. 2018; Schaffner et al. 2020). Introduction of host-specific herbivorous insects has become a classic and sustainable tool in biological control (Catton et al. 2015; Hinz et al. 2019). Alternatively, application of microorganisms (i.e. fungi, bacteria, and viruses) in invasive weed control is receiving more attention (Kennedy et al. 2001; Harding and Raizada 2015; Bo Bo et al. 2020; de Souza Barros et al. 2021), although the majority of biological control applications still make use of insects and mites (2191 releases of insects and mites vs. 166 releases of microorganisms in the past two centuries based on the online database in ibiocontrol.org (Winston et al. 2023)).
Since the 1990s, scientists have attempted to screen for plant-associated bacteria that have inhibitory effects on weeds (Johnson et al. 1996; Imaizumi et al. 1999; Weissmann and Gerhardson 2001; Trognitz et al. 2016; Fang et al. 2022). For example, a phyllosphere pathogen, Xanthomonas campestris pv. poannua, has been used as a biological agent to control the annual bluegrass Poa annua, and it has been commercialized as “Camperico” in Japan (Imaizumi et al. 1999). Besides the phyllosphere, plant roots are colonized by a multitude of soil-borne bacteria that influence plant health (Birt et al. 2022). Although beneficial effects of rhizosphere bacteria on plants are often reported (i.e. increased nutrient availability, production of growth hormones and suppression of pathogens) (Hurek et al. 2002; Sessitsch et al. 2002; Rolli et al. 2017; Carrión et al. 2019), the potential ability of rhizosphere bacteria as biological control agents of weeds should not be overlooked (Flores-Vargas and O’Hara 2006; Trognitz et al. 2016). Many pseudomonad rhizobacteria have been studied in detail for their potential ability to control weeds (Bo Bo et al. 2020; Fang et al. 2022). For example, Pseudomonas fluorescens strain D7, a rhizosphere bacteria isolated from winter wheat and downy brome, suppresses seed germination and plant growth of a number of weeds (Kennedy et al. 2001; Ibekwe et al. 2010). Another soil-borne Pseudomonas strain, Pseudomonas syringae pv. tagetis (PST), has been used for biological control of Canada thistle, and caused apical chlorosis in 67% of plants in a field experiment (Johnson et al. 1996; Gronwald et al. 2002; Tichich and Doll 2006; Sciegienka et al. 2011).
An increasing body of literature is highlighting that the application of bacteria to roots can also affect aboveground herbivorous insects and plant–herbivore interactions (Pangesti et al. 2013; Friman et al. 2021a, b). Bacteria can interfere both directly and indirectly with plant–insect interactions (Sugio et al. 2015). Rhizosphere bacteria can induce systemic resistance (ISR) against herbivorous insects and pathogens in the host plant via the induction of plant defense genes and/or phytohormone pathways (i.e. jasmonic acid and ethylene pathways) (van Loon et al. 1998; Pineda et al. 2010, 2017; Pangesti et al. 2013). The effects of microbe-induced plant resistance on aboveground insect herbivores can vary greatly between insect species. For example, P. fluorescens inoculated on Arabidopsis roots has been found to increase the resistance of the host plant against a generalist chewing insect (Mamestra brassicae), but it made plants more susceptible to a generalist sap-feeding aphid (Myzus persicae) and had no effects on a specialist aphid Brevicoryne brassicae (Pangesti et al. 2013; Pineda et al. 2013). Alternatively, rhizosphere bacteria can directly influence herbivorous insects by producing metabolic toxins and/or via colonization of the herbivores (Sugio et al. 2015; Flury et al. 2016). For example, Flury et al. (2016) showed that 15 strains of P. fluorescens had direct insecticidal activities after feeding or injection, and eight of these were isolated from roots or the rhizosphere. Interestingly, several recent studies showed that aboveground insects can acquire microbes directly from the soil in which the host plant is growing (Hannula et al. 2019; Zhang et al. 2022b). Therefore, an important step in the process of selecting bacteria for biological control of weeds is to examine their impact on other biocontrol agents such as aboveground insect herbivores. However, as far as we are aware, little attention has been paid to examining the consequences of soil-dwelling bacteria on aboveground insects in the context of biological control.
Jacobaea vulgaris is a monocarpic perennial that is native in Europe (Harper and Wood 1957). This plant species has invaded North America, Australia and New Zealand (Roberts and Pullin 2007). Common ragwort is an early successional species, and also in its native area its abundance is increasing and it is considered an unwanted outbreak species (Harper and Wood 1957; van de Voorde et al. 2012). Pyrrolizidine alkaloids (PAs) in the shoots are toxic to cattle, horses and generalist insects (Macel 2011). In invaded areas, the cinnabar moth (Tyria jacobaeae) and ragwort flea beetle (Longitarsus jacobaeae), which are both common herbivores in the native area, have been introduced for biological control of this plant (McEvoy et al. 1991; Leiss 2011). Release of both herbivore species can considerably reduce the density of common ragwort in North America, but the application has failed in other areas (McEvoy et al. 1991; Roberts and Pullin 2007). Aphis jacobaeae is another specialized insect herbivore that is frequently found on common ragwort (Heie 1986; Vrieling et al. 1991). The plant is a monocarpic perennial and the damage of these herbivores typically occurs during the growth season that the plant is flowering. J. vulgaris can produce a large number of seeds (1000 to 30,000 achenes per plant; van der Meijden and van der Waals-Kooi 1979), and suppression of ragwort populations can be achieved via reducing seed production but also via reducing survival at the early stages (i.e. seed germination and seedling development). As the herbivorous insects primarily used for biological control mainly feed on large ragwort plants, incorporating microbes that have significant effects during early plant stages could prove to be a valuable addition in controlling common ragwort (Lahlali et al. 2022).
In this study, we isolated 20 bacterial strains from J. vulgaris roots to test their effects on seed germination, root growth patterns and early plant growth of J. vulgaris plants. We also study the direct effects of the root-associated bacteria on the performance of the specialized chewing caterpillar T. jacobaeae and on the wax moth larvae (Galleria mellonella), a model insect often used in entomopathogenic tests (Pereira et al. 2020). Finally, we test whether infection of the plant with these bacteria influences the preference and performance of two specialized aboveground insect herbivores, T. jacobaeae and the phloem sucking aphid A. jacobaeae, via microbe-induced changes in phloem exudates in the host plant. The research questions that we address are: (i) do root-associated bacteria influence seed germination, seedling health, root structure and plant growth of J. vulgaris? (ii) do these bacteria influence the mortality of larvae of T. jacobaeae after injection and ingestion? (iii) do these bacteria influence aphid performance and the preference of caterpillars of T. jacobaeae via microbe-induced changes in the plant? and (iv) are the tested bacteria specific to J. vulgaris?
Materials and methods
Isolation and identification of bacteria from roots of J. vulgaris
Jacobaea vulgaris plants were collected from October to December 2021 from the coastal dunes of Meijendel (52°07ʹ N, 4°20ʹ E) and Texel (53°0ʹ N, 4°46ʹ E), The Netherlands. Whole plants together with rhizosphere soil were stored individually in plastic zip-bags at 4 °C until processing in the laboratory. Standard microbiological methods were used to isolate bacteria from roots. Milli-Q water, 10 mM MgSO4 solution, mortars and pestles were sterilized by autoclaving in advance. For each plant, we removed soil by handshaking and then washed the roots with tap water. We then randomly collected parts of the root system including the primary root, lateral roots and fine roots. Immediately afterwards, in a UV cabinet, the root parts were washed 5 times with 10 mM MgSO4 solution in 50 ml falcon tubes until the buffer was clear. Samples were then soaked and rinsed in 10 mM MgSO4 with 0.01% Tween 20 (1 min), 10 mM MgSO4 (1 min), 1% of bleach (3 min) and 70% ethanol (3 min). Each sample was then rinsed immediately five-times with Milli-Q water (adapted from Pangesti et al. 2020). The surface sterilized samples were ground in 1 ml of 10 mM MgSO4 buffer with mortars and pestles. Each ground sample was then plated on three Tryptic soy agar (TSA, 30 g TSA powder per 1 L and sterilized by autoclaving, Sigma) plates (50 μl per plate). Plates were incubated at 28 °C for 3 to 7 days and isolated colonies were used for sub-culturing onto plates with TSA media and single colonies were selected and stored. Care was taken to collect different strains based on morphology. The selected strains were cultured overnight at 28 °C in an Erlenmeyer with liquid TSA medium. From each strain glycerol stocks (40% glycerol) were made and stored at -80 °C. In total, there were 20 bacterial strains isolated from different J. vulgaris plants [6 bacterial strains isolated from 4 plants from Meijendel and 14 bacterial strains isolated from 8 plants from Texel (Table S1)]. Detailed information of DNA extraction, 16S amplicon, Sanger sequencing and classification can be found in the supplementary materials.
Inhibitory effects of bacteria on seed germination
Jacobaea vulgaris seeds were collected from plants growing in Meijendel in September 2019. Seeds were surface-sterilized by soaking in 5% sodium hypochlorite for 20 min and rinsing three times with sterilized Milli-Q water. Seeds were dried on sterilized filter paper and sets of 25 seeds were put into a sterilized Eppendorf tubes (1.5 ml). The 20 bacteria were grown in overnight cultures in TSB at 28 °C and 180 rpm. For inoculation, cultures were diluted to OD600 = 1. Subsequently bacterial solutions were spun down in a centrifuge and immediately resuspended in sterilized 10 mM MgCl2. Either 300 μL of the 20 bacterial solutions or 300 μL sterilized 10 mM MgCl2 (control) was added to the Eppendorf tube containing the sterilized seeds. The Eppendorf tubes were covered with aluminum foil and put on a shaker at 200 rpm and room temperature for 1 h (Król et al. 2014). In total, there were 65 tubes (20 bacterial solution × 3 replicates + 1 sterilized control × 5). Then the 25 seeds from each tube were placed on a 0.5 MS agar poured on Petri dishes (92 × 16 mm) with a sterilized tooth pick. All plates were sealed with parafilm. In total, we tested 1625 seeds on 65 plates (3 replicated plates × 20 bacteria × 25 seeds + 5 control plates × 25 seeds). The plates were incubated in a growth cabinet at a 16/8 h light–dark regime and a 25/20 °C temperature regime and RH = 70%. Seed germination of each seed was recorded at two-day intervals. After 12 days the experiment was ended and photographs were taken from each plate at a fixed height. The root length of each germinated seedling was measured by the plugin “ObjectJ” (Vischer and Nastase 2022) in imageJ (the software can be downloaded at https://fiji.sc/.).
Inhibitory effects of bacteria on seedling growth
Jacobaea vulgaris seeds were surface-sterilized as described above. Sterilized seeds were germinated on 0.5 MS media in plates in a growth cabinet with the same conditions as mentioned above. Seven days after germination, equally sized seedlings (Table S2) were randomly picked with a sterilized soft tweezer and 3 seedlings were put on a square plate (10 cm × 10 cm × 2 cm) with 0.5 MS medium. Bacteria were cultured and bacterial solutions (OD600 = 1) were prepared as described above. Either 2 μl of the bacterial solution or 2 μl sterilized 10 mM MgCl2 (control treatment) was added to the root tip of each seedling (Jeon et al. 2021). There were 9 seedlings (3 plates × 3 seedlings) per bacterial strain and 15 seedlings (5 plates × 3 seedlings) for the control treatment. The plates were placed vertically on racks and transferred to a growth cabinet at a 16/8 h light–dark regime and a 25/20 °C temperature regime and RH = 70%. Photos were taken from each plate at a fixed height on days 0, 2, 4, 6, 8, 10 and 13. On day 12, photographs of the root tip of the primary root of each seedling were taken using a stereo-microscope (LEICA MZ16 FA) at a magnification of × 7.95. All seedlings were removed from the plates and fresh root and shoot biomass were determined on day 13. To examine the effects of bacterial inoculation on root morphology and growth, a suite of root traits was measured from the photographs taken on day 13 (13 dpi, days post inoculation) and microscopy photographs taken on day 12 (12 dpi) including total root length, primary root length, lateral root length, number of lateral roots, length–width ratio, specific root length and root hair length. Detailed information regarding root trait measurements can be found in the supplementary materials.
Inhibitory effects of bacteria on plant performance in soil
Jacobaea vulgaris seeds were surface-sterilized and germinated on 0.5 MS media as described above. Bacterial strains were cultured and bacterial solutions (OD600 = 1) were prepared as described above. Seven days after germination, seedlings were dipped for 30 min either in a bacterial solution (20 strains) or in sterilized 10 mM MgCl2 (control). After dipping, each seedling was planted in a pot (6 cm × 6 cm × 7 cm) filled with sterilized soil. The soil was collected from a grassland at Driebergen, The Netherlands and was sterilized with gamma irradiation (> 25KGray, Isotron, Ede, The Netherlands). The soil is characterized as holtpodzol sandy loam with a particle size distribution: 2% < 0.002 mm, 11% 0.002–0.063 mm, 84% > 0.063 mm, with ~ 3% organic matter, 1,150 mg kg−1 N, 61 mg P2O5 100 g−1, 2.4 mmol K kg−1 and pH 5.9, and was sieved (0.5 cm mesh size) and homogenized prior to gamma irradiation. After planting the seedlings, we poured the bacterial or control solution (1 ml) used for dipping of the roots next to the stem of the seedling to improve the effectiveness of the inoculation. We used five plants for each of 20 bacterial strains and 20 plants were dipped in sterilized 10 mM MgCl2 as control. In total, there were 120 pots. One week after planting, each seedling received 1 ml of the same solution as a booster inoculum. The pots were placed individually in plastic containers, and randomly positioned in a walk-in climate-controlled growth chamber (relative humidity RH = 70%, light 16 h at 20 °C, dark 8 h at 20 °C). Plants were watered when needed.
Photographs of the rosettes were taken 13, 20 and 27 days after planting at a fixed height. To examine the effects of bacterial inoculation on plant growth, we calculated the total leaf area of each plant from the photographs for the 3 measurement points using “analyze_objects” and “get_measures” functions from the “pliman” package (Olivoto 2022) in R. For photographs where R software did not fully identify the leaves of rosettes, we instead used the plugin “Simple Interactive Object Extraction (SIOX)” in ImageJ to measure the total leaf area. These two methods produced comparable results in a test dataset (linear regression slope = 1.01, R2 = 0.99). Leaf yellowness of rosettes was measured from the photographs taken the final time (27 days after planting) with the plugin “Simple Interactive Object Extraction (SIOX)” in ImageJ (Madhavi et al. 2022) (detailed information regarding this method can be found in the supplementary materials). Twenty-eight days after planting, chlorophyll content was measured for each plant on a mature leaf twice with a chlorophyll meter (SPAD-502, KONICA-MINOLTA). Chlorophyll values were then averaged for each plant.
To examine the effects of inoculation of bacteria on alkaloid and amino acid concentrations in phloem of J. vulgaris plants, phloem exudates were collected from plants in the experiment. The third youngest fully developed leaf of each plant was collected following the procedure described in Kos et al. (2011). Detailed information regarding this method and LC-QTOF-MS analysis of amino acids and pyrrolizidine alkaloids in phloem samples can be found in the supplementary materials. The remaining leaves of each plant were then clipped and roots were carefully washed from the soil. Plant material was oven-dried (50 °C) and root and shoot dry weight (including the weight of the detached leaf) of each plant was then measured.
Direct effects of bacteria on wax moth larvae and T. jacobaeae caterpillars
Injection assay
To examine direct effects of bacteria on insects, the larvae of the wax moth, G. mellonella, and larvae of T. jacobaeae, an aboveground feeding insect used for biological control of J. vulgaris, were used. The larvae of the wax moth, G. mellonella, are often used as an infection model to test for virulence of bacteria and the effectiveness of antimicrobial agents (Pereira et al. 2020). Insect larvae (final larval stage) were ordered from Kreca entofeed B.V. (Ermelo, The Netherlands). Larvae of T. jacobaeae were cultured on J. vulgaris plants grown in potting soil in a climate-controlled chamber at 16/8 h light–dark regime and a 25/20 °C temperature regime. The culture originated from adult cinnabar moths collected from Meijendel. Bacterial strains were cultured as described above and bacterial solutions (OD600 = 1) were spun down and resuspended in sterilized 0.9% saline solution. Each larva was injected with 10 μl of the bacterial or saline solution with an insulin syringe on the position between two segments of the last two pairs of abdominal prolegs (Flury et al. 2016). For the wax moth, we injected 6 larvae per treatment (20 bacteria and control) and this was replicated 3 times. In total, 378 larvae were used. After injection, sets of 6 larvae were put in Petri dishes (20 bacterial solution × 6 larvae × 3 replicated plates + 1 control treatment × 6 larvae × 3 replicated plates) and placed on a laboratory table at room temperature. We recorded the death of the wax moth larvae after 24, 48 and 72 h and the percentage mortality was calculated for each Petri-dish. For T. jacobaeae, we injected 5 caterpillars (final larval stage) for each treatment as described above. In total, 105 caterpillars (20 bacteria × 5 + 1 control × 5) were used. After injection, each T. jacobaeae caterpillar was put in a separate Petri dish with a filter paper and ample fresh leaves as food. The Petri dishes were then placed in a climate cabinet at a 16/8 h light–dark regime and a 25/20 °C day/night temperature regime. We recorded survival of each T. jacobaeae caterpillar, removed frass and replaced leaves for each caterpillar after 24, 48 and 72 h.
Feeding assay
To examine the direct effects of bacteria on T. jacobaeae larval performance after ingestion, we conducted a feeding assay. In the feeding assay, leaf discs (16.5 mm diameter) were made from fresh leaves from 8-week-old J. vulgaris plants. A single leaf disc was placed in a Petri dish (92 × 16 mm) on a filter paper that had a wetted area roughly equal in size to the leaf disc area to prevent the leaf disc from drying too fast. Bacterial solutions were prepared as we described before. We applied either 1 ml of bacterial solution or l ml of sterilized saline solution on the surface of the leaf discs. Immediately afterwards, a 24 h starved larva which was pre-weighed (second to third larval stage) was placed in the Petri dish. There were 5 larvae per treatment, and a total of 105 larvae (20 bacteria × 5 larvae + 1 control × 5 larvae) were used. Each larva was allowed to forage on the leaf disc with bacteria for six hours. Then photographs of the remains of each leaf disc were made in order to calculate the leaf area (and bacteria) that was consumed (immediate consumption). We then daily replaced the leaf disc by a fresh leaf disc, that was not inoculated, and allowed the larvae to feed for five days. Each day mortality of larvae was recorded, and frass was removed. After 72 h of feeding, the weight of each surviving larva was measured and data was used to calculate the weight gain of each larva. Immediately afterwards, we removed the old leaf disc and replaced a new leaf disc in the petri dish of each surviving larva, and photographs of the remains of the leaf disc were then made after 1 h of feeding to calculate food consumption (later consumption). We used the plugin “Simple Interactive Object Extraction (SIOX)” in ImageJ to measure the consumed leaf area. After 120 h, final mortality was recorded.
Indirect effects of bacteria on the herbivorous insects Tyria jacobaeae and Aphis jacobaeae
T. jacobaeae and A. jacobaeae are both specialist insect herbivores of J. vulgaris and were used to test for indirect plant-mediated effects of rhizosphere bacteria on aboveground herbivorous insects. For this experiment, final instar larvae of T. jacobaeae were collected from the coastal dune area at Meijendel in June 2022. A. jacobaeae was cultured on J. vulgaris plants growing in potting soil in a climate chamber at 16/8 h light–dark regime and a 25/20 °C temperature regime. The culture originates from aphids collected from Meijendel.
Preference of T. jacobaeae
J. vulgaris seeds were surface sterilized and germinated on 0.5 MS media as described above. Bacteria strains were cultured as described above and bacterial solutions (OD600 = 1) were spun down and resuspended in sterilized 10 mM MgCl2. Seven days after germination, seedlings were dipped for 30 min either in a bacterial solution (20 strains) or in sterilized 10 mM MgCl2 (control). After dipping, seedlings were planted in pots filled with gamma-irradiated sterilized sandy soil (see above for details) in pots (13 cm × 13 cm × 13 cm). After planting the seedlings, we poured the bacterial solution (1 ml) used for dipping the roots next to seedling to improve the effectiveness of inoculation. There were 5 replicates for each of the 20 bacterial strains, and each plant from each bacterial treatment was paired, a priori, with a control plant that received 10 mM MgCl2 that was sterilized. In total, there were 100 plants that received bacterial solution (20 strains × 5 replicates) and 100 a priori paired control plants that received 10 mM MgCl2. The pots were randomly assigned a place in a climate-controlled chamber at a 16/8 h light–dark regime and a 20/15 °C temperature regime. Each plant was inoculated again with 1 ml of the same bacterial solution 8 days and 13 days after planting and the control plants received 1 ml of 10 mM MgCl2. The plants were watered when needed. The leaf area, yellowness and Chlorophyll content of each plant were measured as well (Detailed information about these measurements can be found in the supplementary materials).
Sixty days after planting, plants were harvested and a bioassay with T. jacobaeae larvae was carried out. Two leaf discs (12.5 mm diameter) were collected from each bacteria-inoculated plant and from a priori paired control plant for the herbivore assay. Two Petri dishes lined with wet filter paper were prepared and each contained one leaf disc from the control plant and one leaf disc from the paired bacterial-treated plant. To both Petri dishes one 24-h starved final instar of T. jacobaeae was added. The caterpillar was allowed to forage on the leaf discs for two hours. In the rare occasions (3 times in total) where the caterpillar had not eaten from either disc, the larva was replaced. The choice test was repeated twice for each plant, so there were two pseudo replicates per plant pair. Two hours after introduction, caterpillars were removed and a photograph was taken from both leaf discs in each petri dish. The area of the leftover of each leaf disc was then determined using the “analyze_objects” and “get_measures” functions from the “pliman” package (Olivoto 2022). Food consumption (area eaten) was then calculated as the initial area of the leaf disc minus the remaining area of the leaf disc. After collection of the leaf discs, shoot biomass was clipped and plant roots were taken from the soil and washed. The shoot and roots of each plant were oven dried at 50 °C for four days, and shoot and root dry weight of each plant were determined.
Aphid performance
To determine the effects of bacterial inoculation on aphid performance, J. vulgaris seedlings and bacteria solutions were prepared as described before. Seven days after germination, each seedling was inoculated and then planted in gamma-irradiated soil in pots (13 cm × 13 cm × 13 cm) as described for the T. jacobaeae preference experiment. The pots were randomly placed in a climate chamber at a 16/8 h light–dark regime and a 20/15 °C temperature regime, and the plants were watered when needed. The leaf area and yellowness and Chlorophyll content of each plant were also measured (Detailed information about these measurement can be found in the supplementary materials).
After 27 days of plant growth, we introduced one adult aphid on the surface of the largest leaf of each plant with a fine painting brush and trapped the aphid with a clip-on cage (2.5 cm diameter; Fig. S1 a). Each aphid was checked several times per day and settling time of each introduced adult aphid was determined as the number of days from introduction until the first nymph was born. After the first nymph was born, the adult aphid and possible other nymphs were removed from the plant, and the development of the remaining nymph was observed and recorded. Development time was determined as time (days) until this nymph produced the first offspring. Aphid fecundity was measured as the total number of nymphs produced by this new adult over a period of 5 days. After 5 days of reproduction, we removed the founder aphid and weighed it on an analytical balance. After the aphid experiment was finished, for all plants, leaves were clipped at soil level, the soil was removed from the pot and the roots were washed. All plant material was dried at 50 °C, and the shoot and root dry weight of each plant was determined.
Inhibitory effects of two bacteria on seedling growth of J. vulgaris and three other forbs
To examine the host specificity of two of the tested bacteria, P. brassicacearum and S. plymuthica, which exhibited negative effects on J. vulgaris, their effects after inoculation on three other forb species: Plantago lanceolata, Tripleurospermum maritimum, Achillea millefolium were examined. These plant species commonly co-occur with J. vulgaris in natural grasslands. Seeds of these three species were purchased from Cruydt-Hoeck (Nijeberkoop, The Netherlands), a supplier of seeds obtained from wild plants. Further details regarding this experiment can be found in the supplementary materials.
Data analysis
Seed germination and root length of germinated seedlings: The effects of bacterial inoculation on seed germination (yes/no) and on the speed of germination (days to germination) were examined with a generalized linear mixed effect model with a binomial distribution and a Poisson distribution, respectively. Petri dish ID was included as random effect. We applied model diagnosis and checked whether generalized linear models were over-dispersed using the function “check_overdispersion” from the package “performance” (Lüdecke et al. 2021). Significance of factors was assessed by comparing models with and without the factor using a Wald Chi-squared test on the residual deviance. To examine the effects of bacterial inoculation on the root length of germinated seedlings, a linear mixed effect model was used with treatment as the fixed effect and with petri dish ID and seedling age as random effects. Seedlings that germinated late (first appeared at the final record) had insufficient time for root development (32 out of 765), these seedlings were excluded from this analysis. The root length of germinated seedlings were square-root transformed to fulfil requirements of normality. Significance of factors was assessed by comparing models with and without the factor using a F-test on the residual deviance. A Dunnett post-hoc test was then used to compare seed germination, the speed of germination and root length of the germinated seedlings in each bacterial treatment with that in the control. We also analyzed temporal germination patterns of J. vulgaris in different bacterial treatments (Romano and Stevanato 2020), detailed information regarding this method can be found in the supplementary materials.
Root growth patterns in-vitro
The effects of bacterial inoculation on fresh root biomass, total root length, primary root length, lateral root length, length–width ratio, specific root length and root hair length were tested with a linear mixed effect model with the petri dish ID as the random effect. A generalized linear mixed effect model with a Poisson distribution and petri dish ID as random effect was used to test the effects of bacterial inoculation on the number of lateral roots. A Dunnett post-hoc test was then used to compare root traits of seedlings in the bacterial treatment and in the control. During the experiment, one seedling inoculated with bacteria Ps6 appeared contaminated and the seedlings on this plate were excluded from the analysis. To examine the host specificity of two bacteria, P. brassicacearum and S. plymuthica, the effects of bacterial inoculation on primary root length, total root length, fresh shoot and root biomass of the different plant species were tested with a linear mixed effect model with plant species identity as random effect. An one way-ANOVA was then used for each plant species, respectively. A Dunnett post-hoc test was then used to compare the difference between bacterial treatment and the control. Linear mixed effect models and generalized linear mixed effect models were performed using the “lmer” and “glmer” functions with the “lme4” package (Bates et al. 2015).
Dissimilarities in root traits
Nonmetric multidimensional scaling analysis (NMDS) was used to analyze dissimilarities in root traits (fresh root biomass was also included in these analyses) of seedlings. The NMDS was performed using the “metaMDS” function from the package “vegan” (Oksanen et al. 2022). To determine if dissimilarities in root traits could be attributed to bacterial inoculation, a permutational multivariate analysis of variance (PERMANOVA) with 999 permutations was performed.
Plant performance in soil
To examine the effects of bacterial inoculation on plant performance in soil, we tested for the effects of bacterial inoculation on shoot and root dry mass, relative growth rate, leaf yellowness and chlorophyll content. The relative growth rate of plants was calculated with the following formula:
where leaf area tn (cm2) represents the total leaf area based on the photographs taken the final time, and leaf area t0 (cm2) represented the total leaf area of rosettes based on the photographs taken the first time. Because we measured the same sets of plant performance indices, in the plant growth experiment, in the experiment testing the preference of T. jacobaeae and in the experiment measuring aphid performance, there were three parallel experiments for these tests. Hereafter we refer to these three parallel experiments as: plant performance experiment, plant performance in preference of T. jacobaeae experiment, and plant performance in aphid performance experiment. One-way ANOVA was used to test for the effects of bacterial inoculation on shoot and root dry mass, relative growth rate, leaf yellowness and chlorophyll content of J. vulgaris. A Dunnett post-hoc test was then used to compare plant performance of J. vulgaris in the bacterial treatments and in the control. ANOVA was carried out with the “aov” function and post-hoc tests were performed using the “glht” function of the “multcomp” package (Hothorn et al. 2008). Correlations among indices of plant performance were examined with the “cor” function in R. In all analyses, residuals were checked for homogeneity of variance using a Levene’s test and normality by a Shapiro Wilk test. The Levene’s test and Shapiro Wilk test were performed using the “levene_test” and “shapiro_test” function of the “rstatix” package (Kassambara 2021).
Insect mortality in the injection assay: Insect mortality of wax moth larvae was calculated as the proportion of dead larvae per Petri dish, and therefore the effects of bacterial inoculation on insect mortality was examined with generalized linear models with a negative binomial distribution. The effects of bacterial inoculation on T. jacobaeae survival (yes/no) was examined with a generalized linear model with a binomial distribution. Then we applied model diagnosis and checked whether generalized linear models were over-dispersed as described above. Significance of factors was assessed using a Chi-square Likelihood Ratio (LR) test on the residual deviance as described above. A Tukey’s post hoc test was used for pair-wise comparisons between bacterial treatments and the control treatment.
T. jacobaeae mortality and consumption in the feeding assay: To examine the preference of T. jacobaeae on leaf discs with bacterial inoculation, we tested the area of consumption on leaf disc after 6 h with one-way Anova. In addition, we counted the number of vomit spots by T. jacobaeae on filter paper from photographs and tested the effects of bacterial inoculation on it with a generalized linear model with a Poisson distribution. To examine the longer term effects of bacterial inoculation on T. jacobaeae fitness (72 h later), the weight gain, and the area of consumption after 1 h were analyzed by one-way Anova.
Preference of T. jacobaeae
To examine the effects of bacterial inoculation on preference of T. jacobaeae, we calculated preference of T. jacobaeae with the following formula:
Consumption.prop.1 is the proportion of consumption from the leaf disc collected from bacteria inoculated plants, and Consumption.prop.2 is the proportion of consumption from the leaf disc from control plants. Positive values indicate that T. jacobaeae preferred to feed on leaves from bacterial inoculated plants, while the negative values indicate that T. jacobaea preferred to feed on leaves from the control plants. As there were two pseudo replicates for each a priori paired bacteria-inoculated plant and control plant, we used a linear mixed effect model with the factor replicate nested into plant ID as a random effect. Significance of the inoculation treatment was assessed by comparing models with and without the factor using a F-test on the residual deviance. A Tukey’s post hoc test was used for pair-wise comparisons between bacteria treatments.
Aphid performance
As the settling time, the developing time and aphid fecundity are count data, we used generalized linear models with a Poisson distribution to test for the effects of bacterial inoculation. Then we applied model diagnosis and checked whether generalized linear models were over-dispersed as described above. If there was over-dispersion in the model, we instead used generalized linear models with a negative binomial distribution. Significance of factors was assessed using a Chi-square Likelihood Ratio (LR) test on the residual deviance. A Tukey’s post-hoc test was used for pair-wise comparisons between bacteria treatments and the control treatment. One-way ANOVA was used to test for the effects of bacterial inoculation on aphid weight. A Dunnett post-hoc test was then used to compare aphid performance in bacterial treatments and in the control.
Phloem exudates
Unconstrained principal component analysis (PCA) was used to analyze the composition of amino acids and pyrrolizidine alkaloids using the “prcomp” function in R. In order to test whether changes in the composition of amino acids and pyrrolizidine alkaloids could be explained by bacterial strains, we conducted a constrained redundancy analysis (RDA) and a permutational multivariate analysis of variance (PERMANOVA, 999 permutations) with plant total dry mass as a factor as well. The permutational multivariate analysis was carried out with the “rda” and the “adonis” function from the “vegan” package (Oksanen et al. 2022). All analyses were performed using the R statistical language (R Core Team 2022).
Results
Identification of root-associated bacteria
Based on Sanger sequencing, the 20 strains were assigned to known bacteria belonging to the genera Pseudomonas (seven isolates), Micrococcus (five isolates), Bacillus (four isolates), Pedobacter (one isolate), Curtobacterium (one isolate), Pantoea (one isolate) and Serratia (one isolate) (Table S1).
Seed germination and root length of germinated seedlings in-vitro
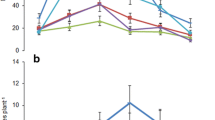
The proportion of germinated seeds ranged from 0.39 (SE: ± 0.05) to 0.57 (SE: ± 0.01) among treatments at the final measurement (12 dpi) (Fig. S2). There was no significant difference in the proportion of germinated seeds across the different bacterial inoculations (F20, 44 = 1.73, p = 0.32; Fig. S2, Table S3). However, root length of germinated seedlings was significantly affected by bacterial inoculation (F20, 42 = 3.23, p < 0.001; Fig. 1). Only inoculation with bacterium Ps4 resulted in significantly shorter roots when compared to the control group (Fig. 1).
Effects of bacterial inoculation on the root length of germinated seedlings 12 days post inoculation of seeds (a) and representative pictures of the seed germination assay (b) on 0.5 MS plates at the final measurement. In (a), Mean ± SE of the root length of germinated seedlings is shown, and mean ± SE of the plants of the control treatment is shown with the horizontal solid line and dashed lines respectively. Symbols: hexagon: Pedobacter alluvionis; square: Bacillus sp.; inverted triangle: Curtobacterium flaccumfaciens; diamond: Micrococcus sp.; circle: Pseudomonas sp.; cross: Pantoea agglomerans; triangle: Serratia plymuthica. Black symbols indicate significant differences between bacterial treatments and the control treatment in a Dunnett post-hoc test. * indicates significant differences between bacterial and control treatment at P < 0.05
Root growth in-vitro
The root morphology of seedlings exhibited significant variation depending on which bacterial inoculum was applied (Fig. 2; PERMANOVA: F20, 160 = 2.19, p < 0.001, R2 = 0.21). Notably, the treatment had a significant impact on the length of the primary root and of root hairs, and the number of lateral roots (Fig. 2A, B and C, Table S4). However, there was no significant difference in total root length between the bacterial treatments and the control (Fig. 2D, Table S4). Specifically, seedlings inoculated with bacteria Ps4 and Se1 developed a shorter primary root, but with a higher number of lateral roots (Fig. 2A, C, and F, Table S4). Inoculation with bacteria Se1 also resulted in longer root hairs at the tip of the primary root (Fig. 2B and G, Table S4), while bacteria Ba3 promoted root growth with an increased number of lateral roots (Fig. 2C, Table S4).
Effects of bacterial inoculation on primary root length (a), root hair length (b), number of lateral roots (c), total root length (d), dissimilarities in root traits of J. vulgaris in a Nonmetric multidimensional scaling analysis (e). Representative pictures of J. vulgaris seedlings on 0.5 MS plates 13 d post inoculation (dpi) (f), and stereo-microscopy pictures of root hairs in the primary roots of J. vulgaris seedlings 12 days post inoculation of seedlings (g) are also presented. In (a, b, c, d) Mean ± SE of the plants of the control treatment are shown with a horizontal solid line and dashed lines respectively. Symbols of bacteria are described in the legend of Fig. 1, and black symbols indicate significant differences between bacterial treatments and the control treatment in a Dunnett post-hoc test. *, **, *** indicates significant differences between bacterial and control treatment at P < 0.05, 0.01 or 0.001, respectively
Plant performance in soil
There were no significant effects of bacterial inoculation on the shoot and root dry mass, leaf yellowness, and chlorophyll content in three parallel pot experiments (Fig. 3, Fig. S3 and S4, Table S5). Relative growth rate of plants varied significantly depending on which bacterial inoculation they received only in the preference of T. jacobaea experiment (Fig. S4, Table S5). Plants inoculated with bacteria Se1 exhibited relatively low shoot and root dry mass in the plant performance experiment (Fig. 3), a significant reduction in relative growth rate and a higher prevalence of yellowish leaves in the other two parallel experiments (Fig. S4). There was no significant correlation between plant dry mass in the three parallel pot experiments (p > 0.05; Fig. S5).
Effects of bacterial inoculation on shoot (a) and root dry mass (b) of J. vulgaris 28 days after planting in the soil, and representative pictures of the experiment (c). In (a, b), Mean ± SE of the shoot and root dry mass of plants of the control treatment is shown with the horizontal solid line and dashed lines. Symbols of bacteria are described in the legend of Fig. 2. Treatment had no effects on the shoot and root dry mass in a one-way ANOVA
Direct effects of bacterial isolates on G. mellonella and T. jacobaeae
The mortality of G. mellonella varied significantly depending on the bacterial inoculum that was injected (df = 20, χ2 = 307.79, p < 0.001; Fig. 4). Ten strains showed insecticidal activities on larvae of G. mellonella (Fig. 4). The mortality of T. jacobaeae also varied significantly depending on which bacterial strain was injected and on the genus the bacterial strains belonged to (Table S6). Overall, Bacillus spp. resulted in a higher probability of mortality in T. jacobaeae than Pseudomonas spp. and Micrococcus spp. In the feeding assay, where bacteria were introduced on leaf discs, bacterial inoculation had no significant effects on consumption after 6 h, and on weight gain of caterpillars and mortality 5 days after (Fig. 5, Table S7). Feeding on leaf discs inoculated with bacteria caused caterpillars to vomit, and T. jacobaeae caterpillars vomited more when feeding on the leaf discs with bacteria Pa1 (Fig. 5, Table S7).
Effects of bacterial inoculation on insect mortality (a), and pictures (b) of model insect larvae, G. mellonella, 3 days after injection. The mortality of the control treatment is shown with the solid line. Symbols are described in the legend of Fig. 1, and black symbols indicate significant differences between bacterial treatments and the control treatment in a Dunnett post-hoc test. *, **, *** Indicates significant differences between bacteria and control treatment at P < 0.05, 0.01 or 0.001, respectively
Effects of bacterial inoculation on the proportion of consumption by T. jacobaeae after 6 h (a), the number of vomit spots by T. jacobaeae after 6 h of feeding on bacterial inoculated leaf discs (b), weight gain of T. jacobaeae after 72 h of feeding (c) and representative pictures showing the vomit spots of T. jacobaeae after 6 h of feeding on bacterial inoculated leaf discs (d). Mean ± SE of the control treatment are shown with the horizontal solid line and the dashed lines. Symbols are described in the legend of Fig. 1, and black symbols indicate significant differences between bacterial treatments and the control treatment in a Dunnett post-hoc test. * Indicates significant differences between bacteria and control treatment at P < 0.05
Aphid performance and T. jacobaeae preference
There were no significant effects of bacterial inoculations on setting time, development time, aphid fecundity per 5 days and aphid weight (Fig. 6, Table S8). Aphid fecundity was positively correlated with aphid weight (p < 0.001, R2 = 0.12; Fig S6). The preference of T. jacobaea was significantly affected by bacterial inoculation (df = 19, χ2 = 40.36, p < 0.01; Fig. 7). Specifically, T. jacobaeae preferred to feed on leaf discs of plants inoculated with bacteria Ba2 and Ps3 over leaf discs from control plants (Fig. 7).
Effects of bacterial inoculation on aphid setting time (a), development time (b), fecundity over 5 days (c), and adult weight (d) of A. jacobaeae and pictures of the aphid performance experiment (e). In (a, b, c, d), Mean ± SE of the aphid performance under the control treatment are shown with the horizontal solid line and the dashed lines. In (a, b, c, d), the effects of bacteria Se1 (S. plymuthica) on aphid performance were tested in a separate experiment. Symbols of bacteria are described in the legend of Fig. 1. Treatments had no effects on aphid performance
Effects of bacterial inoculation on T. jacobaeae preference. T. jacobaeae preference is presented as the proportion of consumption on leaf discs from bacteria inoculated plants relative to the proportion of consumption on leaf discs from control plants. The effect of bacteria Se1 (Serratia plymuthica) was examined in a separate experiment. Symbols of bacteria are described in the legend of Fig. 1. * Indicates significant differences between bacterial and control treatment at P < 0.05 in a pairwise t-test
Alkaloid concentrations in the phloem of leaves
Four pyrrolizidine alkaloids and twelve amino acids were identified in the phloem exudates (Fig. S7). Notably, amino acid composition in plants with bacterial inoculation differed considerably from that of control plants (Fig. S7 a and b, Table S9). Concentrations of pyrrolizidine alkaloids were too low to be detected in samples, and there were no clear effects of bacterial inoculation on the composition of pyrrolizidine alkaloids (Fig. S7 c and d, Table S9).
Host specificity of two tested bacteria
The inoculation treatment significantly influenced primary root length, total root length, shoot and root fresh biomass of the different plant species (Fig. 8, Table S10). In particular, inoculation with S. plymuthica significantly reduced root length and fresh biomass of seedlings across all species, while seedlings inoculated with P. brassicacearum did not differ from the control (Table S10).
Mean(± SE) primary root length (a), total root length (b), shoot (c) and root fresh mass (d) of J. vulgaris and three other forb speciesin the presence and absence of one of the two bacteria (Ps4 and Se1) in-vitro. In (a, b, c and d) asterisks indicate significant differences between bacterial inoculum and control treatment based on a Dunnett post hoc test of a one-way ANOVA for each plant species. *, ** indicates significant differences at P < 0.05 or 0.01, respectively.Abbreviations of species: JV = Jacobaea vulgaris, AM = Achillea millefolium, TM = Tripleurospermum maritimum, PL = Plantago lanceolata
Discussion
Inhibitory effects of bacteria on the growth of weeds at the early stages
In our study, bacteria isolated from roots of J. vulgaris directly affected the root length of germinated seedlings and root morphology in-vitro, but had no effects on seed germination. Several studies have found that rhizosphere bacteria can suppress seed germination (Ahonsi et al. 2002; Chahtane et al. 2018). For example, plant-associated bacterial strains have been used to suppress seed germination of Striga hermonthica (Ahonsi et al. 2002; Masteling et al. 2019). Phytotoxic compounds produced by these strains such as L-2-amino-4-methoxy-trans-3-butenoic acid (AMB), dimethyldisulfide and hydrogen cyanide (HCN) have been found to inhibit seed germination (Chahtane et al. 2018; Dahiya et al. 2019). In contrast, bacterial inoculation had no effect on seed germination in our study. The way that the bacteria were introduced to J. vulgaris seeds and the growth medium (0.5 MS without sugar) that we used for germination may have inhibited the bacteria population to build up large enough to cause an effect on germination (Friman 2021). After seeds germinated, bacteria can utilize carbon from root exudates and this can result in a rapid increase in bacterial density. This might explain why rather than detecting effects on seed germination, we observed significant effects of bacterial inoculation on the root length of germinated seedlings. Specifically, we observed a clear negative effect of bacteria Ps4, P. brassicacearum, on the root length of germinated seedlings. This is in line with our results of the root growth experiment where two bacteria, Ps4 and Se1 (P. brassicacearum and S. plymuthica), restricted growth of the primary roots, resulting in thicker and shorter roots. Bacteria with the potential to control weeds often do so by producing phytotoxic metabolites (Bo Bo et al. 2020; Fang et al. 2022). For example, rhizosphere pseudomonads have been found to produce hydrogen cyanide (HCN) that inhibit the root growth of two weeds (Axonopus affins and Lens esculenta) (Blom et al. 2011; Nandi et al. 2017). In our study, the inhibitory effect of Ps4 (P. brassicacearum) may also be due to the production of HCN; further experiments are required to examine this. Serratia spp. are known to produce a number of important secondary metabolites, and some compounds exhibit antagonistic effects on plant pathogens (i.e., Botryosphaeria dothidea) and have plant growth promoting effects (Rybakova et al. 2016; De Vleesschauwer and Höfte 2007; Sun et al. 2022). However, in our study, plants inoculated with Se1 (S. plymuthica) exhibited shorter primary roots with more lateral roots and longer root hairs. A previous study reported that volatiles of S. plymuthica can reduce plant growth, leading to chlorosis in Arabidopsis plants (Wenke et al. 2012). In our root growth experiment we did not observe chlorosis in J. vulgaris plants. Further studies are needed to examine the mechanisms behind the inhibitory effects of these two bacterial strains and especially focus on the effects of secondary metabolites of the two bacteria on plant growth.
Plants are typically susceptible to microorganisms such as pathogenic bacteria at the early growth stages (Ailstock et al. 2010; Bezemer et al. 2018). The inhibitory effects of bacteria at the early stages in our study are in line with this. However, bacterial inoculation did not significantly affect plant performance in soil when plant growth was measured over a longer growth period. There was a trend showing that plants inoculated with S. plymuthica produced relatively little dry mass. Previous studies have found that the negative effects of soil microorganisms on J. vulgaris growth are only detectable at the early stages (i.e. first weeks of growth) and that the effects diminish over time (Bezemer et al. 2018; Zhang et al. 2022a). In a field experiment, the effects of application of P. syringae pv. tagetis (Pst) on Canada thistle also decreased over time (Gronwald et al. 2002). This may be because plants are less sensitive to bacteria when larger, but it can also be that the density of bacteria decreases gradually over time.
The bacteria that were used in this study were isolated from J. vulgaris roots, which is a species with high alkaloid concentrations in root tissues. These alkaloids are known to exhibit negatively effects on many bacterial species (Joosten and van Veen 2011). Hence we expected that these bacteria that apparently can survive in an environment with relatively high alkaloid concentrations, would be specialized to J. vulgaris. However, contrary to our expectations, the negative effects of the two bacteria, P. brassicacearum and S. plymuthica, were not specific to J. vulgaris, but were also observed for the other forbs. The lack of host specificity also suggests that the negative effects may due to secondary compounds secreted by the bacteria which can negatively influence the growth of different plant species. Future studies should screen for host-specific microorganisms that can be used for biocontrol purposes (Trognitz et al. 2016).
Direct effects of bacteria on insects
Many of the bacterial strains that we tested exhibited direct insecticidal activities on a model insect, G. mellonella. In contrast to our expectation, with the same set of bacteria and with the same experimental settings, inoculation by injection of bacterial suspensions did not lead to a higher mortality in T. jacobaeae caterpillars. Interestingly, at the genus level, we did observe that mortality of T. jacobaeae caterpillars was higher after injection with Bacillus spp. than Pseudomonas spp. and Micrococcus spp. This result indicates that G. mellonella as a model insect to test infectivity is more sensitive to bacterial inoculations than T. jacobaeae. Therefore, putative insecticidal activity in a model insect does not necessarily correlate with insecticidal activity in herbivorous insects. A higher probability of T. jacobaeae larval mortality by injection with Bacillus strains was observed in our study, in agreement with other studies that have found that many Bacillus strains can be used for pest control and plant protection (Gomis-Cebolla and Berry 2023). For example, B. thuringiensis can inhibit the larval growth of insects (i.e. Leptinotarsa decemlineata Say.) by producing the alkaloid thiamethoxam (Sorokan et al. 2020). However, direct feeding for six hours on leaf discs that were inoculated with bacteria had no effect on the survival of T. jacobaeae larvae or on weight gain, though we observed that bacterial inoculation resulted in an increase in vomiting of larvae. It is important to note that T. jacobaeae is specialist herbivore on J. vulgaris and is able to digest alkaloids which are often toxic to generalist herbivores. Also, T. jacobaeae from different habitats have been found to host highly stable microbiomes (Gomes et al. 2020). T. jacobaeae may first ingest the exogenous bacteria but then regurgitate and vomit. If feeding on inoculated leaves would had been longer than six hours this might have resulted in higher mortality rates and reduced larval growth but this remains to be tested. Testing the direct effects of bacteria on aboveground herbivorous insects is often overlooked in biological control of weeds. Our study highlights that in weed control approaches with bacteria or other microorganisms, we should be aware that the application of bacteria (i.e., spraying) can affect other biocontrol agents such as insects negatively. Further studies should assess the risk of microorganisms on herbivorous insects of weeds before the application in the field.
Indirect effects of bacteria on aboveground insects via the host plant
It is well established that soil bacteria can affect aboveground plant–insect interactions, but that this depends on the mode of feeding of the insects and that it can vary between specialist and generalist insects (Pineda et al. 2010; Pangesti et al. 2013; Sugio et al. 2015). In our study, aphids performed equally well on host plants independent of whether the roots of the plants were inoculated or not, while for two bacterial strains, T. jacobaeae preferred leaf discs of plants that were inoculated over those from of plants that were mock inoculated. Aphids feed on the phloem of plants, and concentrations of amino acids and PAs are often plant-size dependent (i.e., J. vulgaris plants with larger root biomass often have higher PA concentrations; Hol 2011). A previous study found that in presence of soil microbes PA and amino acid concentrations in the phloem of J. vulgaris were higher and that there is a positive relationship between A. jacobaeae performance and concentrations of PAs and amino acid (Kos et al. 2015). In our study, although bacterial inoculation changed the amino acid composition in the phloem considerably, aphid performance did not differ between plants with bacterial inoculation and control plants. This may be because aphids were able to acquire sufficient nutrients from the phloem, although the composition of the amino acids was changed in the phloem.
A herbivorous insect can respond differently to various plant pathogens (Tack and Dicke 2013). Similarly, T. jacobaeae caterpillars do not show preference on host plant leaves infected by the fungus Puccinia lagenophorae, while they dislike leaves infected by another fungus, Coleosporium tussilaginis (Tinney et al. 1998). Our results of T. jacobaeae preference confirms this specificity and suggests that specialized leaf chewing insects respond differently to root bacteria-mediated differences in leaf quality. Further studies should examine how leaf chemistry and nutrients (i.e. sugar content) were affected by bacterial inoculation in the soil, and how this correlates with foliar feeding insect performance.
In conclusion, our study demonstrates that two of the tested root-associated bacteria, P brassicacearum and S. plymuthica, can negatively affect root growth of J. vulgaris. However, these two species also induce negative effects on other forb species and this limits the potential use of these bacteria for biological control purposes. We also show that introduction of bacteria can affect other weed biocontrol agents such as insects directly and indirectly via the plant. Hence, we highlight that studies on the application of bacteria in weed control also need to pay attention on the direct and indirect effects of these applications on aboveground plant-feeding insects.
Data availability
All data will be available on Mendeley Data. https://doi.org/10.17632/jrxp93nfh9.1.
References
Abbas T, Zahir ZA, Naveed M, Kremer RJ (2018) Limitations of existing weed control practices necessitate development of alternative techniques based on biological approaches. In: Sparks DL (ed) Advances in Agronomy. Academic Press, Cambridge, MA, pp 239–280
Adalsteinsson SA, D’Amico V, Shriver WG, Brisson D, Buler JJ (2016) Scale-dependent effects of nonnative plant invasion on host-seeking tick abundance. Ecosphere 7:e01317. https://doi.org/10.1002/ecs2.1317
Adalsteinsson SA, Shriver WG, Hojgaard A, Bowman JL, Brisson D, D’Amico V, Buler JJ (2018) Multiflora rose invasion amplifies prevalence of Lyme disease pathogen, but not necessarily Lyme disease risk. Parasit Vectors 11:1–10. https://doi.org/10.1186/s13071-018-2623-0
Ahonsi MO, Berner DK, Emechebe AM, Lagoke ST (2002) Selection of rhizobacterial strains for suppression of germination of Striga hermonthica (DEL.) Benth. seeds. Biol Control 24:143–152. https://doi.org/10.1016/S1049-9644(02)00019-1
Ailstock MS, Shafer DJ, Magoun AD (2010) Effects of planting depth, sediment grain size, and nutrients on Ruppia maritima and Potamogeton perfoliatus seedling emergence and growth. Restor Ecol 18:574–583. https://doi.org/10.1111/j.1526-100X.2010.00697.x
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67. https://doi.org/10.18637/jss.v067.i01
Bezemer TM, Jing J, Bakx-Schotman JMT, Bijleveld EJ (2018) Plant competition alters the temporal dynamics of plant-soil feedbacks. J Ecol 106:2287–2300. https://doi.org/10.1111/1365-2745.12999
Birt HWG, Tharp CL, Custer GF, Dini-Andreote F (2022) Root phenotypes as modulators of microbial microhabitats. Front Plant Sci 13:1003868. https://doi.org/10.3389/fpls.2022.1003868
Blackburn TM, Bellard C, Ricciardi A (2019) Alien versus native species as drivers of recent extinctions. Front Ecol Environ 17:203–207. https://doi.org/10.1002/fee.2020
Blom D, Fabbri C, Connor EC, Schiestl FP, Klauser DR, Boller T, Eberl L, Weisskopf L (2011) Production of plant growth modulating volatiles is widespread among rhizosphere bacteria and strongly depends on culture conditions. Environ Microbiol 13:3047–3058. https://doi.org/10.1111/j.1462-2920.2011.02582.x
Bo Bo A, Khaitov B, Umurzokov M, Cho KM, Park KW, Choi JS (2020) Biological control using plant pathogens in weed management. Weed Turf Sci 9:11–19. https://doi.org/10.5660/WTS.2020.9.1.11
Carrión VJ, Perez-Jaramillo J, Cordovez V, Tracanna V, de Hollander M, Ruiz-Buck D, Mendes LW, van Ijcken WFJ, Gomez-Exposito R, Elsayed SS, Mohanraju P, Arifah A, van der Oost J, Paulson JN, Mendes R, van Wezel GP, Medema MH, Raaijmakers JM (2019) Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science 366:606–612.https://doi.org/10.1126/science.aaw9285
Catton HA, Lalonde RG, De Clerck-Floate RA (2015) Nontarget herbivory by a weed biocontrol insect is limited to spillover, reducing the chance of population-level impacts. Ecol Appl 25:517–530. https://doi.org/10.1890/14-0250.1
Chahtane H, Füller TN, Allard PM, Marcourt L, Ferreira Queiroz E, Shanmugabalaji V, Falquet J, Wolfender JL, Lopez-Molina L (2018) The plant pathogen Pseudomonas aeruginosa triggers a DELLA-dependent seed germination arrest in Arabidopsis. Elife 7:1–34. https://doi.org/10.7554/eLife.37082
Dahiya A, Chahar K, Sindhu SS (2019) The rhizosphere microbiome and biological control of weeds: a review. Spanish J Agric Res 17:1–13. https://doi.org/10.5424/sjar/2019174-15073
de Souza Barros VM, Pedrosa JLF, Gonçalves DR, de Medeiros FCL, Carvalho GR, Gonçalves AH, Teixeira PVVQ (2021) Herbicides of biological origin: a review. J Hortic Sci Biotechnol 96:288–296. https://doi.org/10.1080/14620316.2020.1846465
Fang W, Liu F, Wu Z, Zhang Z, Wang K (2022) Plant-associated bacteria as sources for the development of bioherbicides. Plants 11:1–24. https://doi.org/10.3390/plants11233404
Flores-Vargas RD, O’Hara GW (2006) Isolation and characterization of rhizosphere bacteria with potential for biological control of weeds in vineyards. J Appl Microbiol 100:946–954. https://doi.org/10.1111/j.1365-2672.2006.02851.x
Flury P, Aellen N, Ruffner B, Péchy-Tarr M, Fataar S, Metla Z, Dominguez-Ferreras A, Bloemberg G, Frey J, Goesmann A, Raaijmakers JM, Duffy B, Höfte M, Blom J, Smits TH, Keel C, Maurhofer M (2016) Insect pathogenicity in plant-beneficial pseudomonads: phylogenetic distribution and comparative genomics. ISME J 10:2527–2542. https://doi.org/10.1038/ismej.2016.5
Friman J, Karssemeijer PN, Haller J, de Kreek K, van Loon JJA, Dicke M (2021a) Shoot and root insect herbivory change the plant rhizosphere microbiome and affects cabbage–insect interactions through plant–soil feedback. New Phytol 232:2475–2490. https://doi.org/10.1111/NPH.17746
Friman J, Pineda A, Gershenzon J, Dicke M, van Loon JJA (2021b) Differential effects of the rhizobacterium Pseudomonas simiae on above- and belowground chewing insect herbivores. J Appl Entomol 145:250–260. https://doi.org/10.1111/jen.12842
Friman J (2021) Tripartite interactions between Brassica oleracea, soil microbes, and shoot- and root feeding insects [Doctoral dissertation]. Wageningen University
Gomes SIF, Kielak AM, Hannula SE, Heinen R, Jongen R, Keesmaat I, De Long JR, Bezemer TM (2020) Microbiomes of a specialist caterpillar are consistent across different habitats but also resemble the local soil microbial communities. Anim Microbiome 2:37. https://doi.org/10.1186/s42523-020-00055-3
Gomis-Cebolla J, Berry C (2023) Bacillus thuringiensis as a biofertilizer in crops and their implications in the control of phytopathogens and insect pests. Pest Manag Sci 79:2992–3001. https://doi.org/10.1002/ps.7560
Gronwald JW, Plaisance KL, Ide DA, Wyse DL (2002) Assessment of Pseudomonas syringae pv. tagetis as a biocontrol agent for Canada thistle. Weed Sci 50:397–404. https://doi.org/10.1614/0043-1745(2002)050[0397:aopspt]2.0.co;2
Hannula SE, Zhu F, Heinen R, Bezemer TM (2019) Foliar-feeding insects acquire microbiomes from the soil rather than the host plant. Nat Commun 10:1254. https://doi.org/10.1038/s41467-019-09284-w
Harding DP, Raizada MN (2015) Controlling weeds with fungi, bacteria and viruses: a review. Front Plant Sci 6. https://doi.org/10.3389/fpls.2015.00659
Harper JL, Wood WA (1957) Senecio Jacobaea L. J Ecol 45:617. https://doi.org/10.2307/2256946
Heie OE (1986) The Aphidoidea (Hemiptera) of Fennoscandia and Denmark. III. Fauna Entomologica Scandinavica 17:180–181. E.J. Brill/Scandinavian Science Press Ltd. Leiden
Hinz HL, Winston RL, Schwarzländer M (2019) How safe is weed biological control? A global review of direct nontarget attack. Q Rev Biol 94:1–27. https://doi.org/10.1086/702340
Hol WHG (2011) The effect of nutrients on pyrrolizidine alkaloids in Senecio plants and their interactions with herbivores and pathogens. Phytochem Rev 10:119–126. https://doi.org/10.1007/s11101-010-9188-7
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biometrical J 50:346–363. https://doi.org/10.1002/bimj.200810425
Hurek T, Handley LL, Reinhold-Hurek B, Piché Y (2002) Azoarcus grass endophytes contribute fixed nitrogen to the plant in an unculturable state. Mol Plant-Microbe Interact 15:233–242. https://doi.org/10.1094/MPMI.2002.15.3.233
Ibekwe AM, Kennedy AC, Stubbs TL (2010) An assessment of environmental conditions for control of downy brome by Pseudomonas fluorescens D7. Int J Environ Technol Manag 12:27–46. https://doi.org/10.1504/IJETM.2010.029959
Imaizumi S, Honda M, Fujimori T (1999) Effect of temperature on the control of annual bluegrass (Poa annua L.) with Xanthomonas campestris pv. poae (JT-P482). Biol Control 16:13–17. https://doi.org/10.1006/bcon.1999.0728
Jeon JS, Carreno-Quintero N, van Eekelen HDLM, De Vos RCH, Raaijmakers JM, Etalo DW (2021) Impact of root-associated strains of three Paraburkholderia species on primary and secondary metabolism of Brassica oleracea. Sci Rep 11:2781. https://doi.org/10.1038/s41598-021-82238-9
Johnson DR, Wyse DL, Jones KJ (1996) Controlling weeds with phytopathogenic bacteria. Weed Technol 10:621–624. https://doi.org/10.1017/s0890037x00040549
Joosten L, van Veen JA (2011) Defensive properties of pyrrolizidine alkaloids against microorganisms. Phytochem Rev 10:127–136. https://doi.org/10.1007/s11101-010-9204-y
Kassambara A (2021) rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R package version 0.7.0. https://CRAN.R-project.org/package=rstatix. Accessed 01 May 2024
Kennedy AC, Johnson BN, Stubbs TL (2001) Host range of a deleterious rhizobacterium for biological control of downy brome. Weed Sci 49:792–797. https://doi.org/10.1614/0043-1745(2001)049[0792:HROADR]2.0.CO;2
Kos M, Kabouw P, Noordam R, Hendriks K, Vet LEM, van Loon JJA, Dicke M (2011) Prey-mediated effects of glucosinolates on aphid predators. Ecol Entomol 36:377–388. https://doi.org/10.1111/j.1365-2311.2011.01282.x
Kos M, Tuijl MAB, De Roo J, Mulder PPJ, Bezemer TM (2015) Plant-soil feedback effects on plant quality and performance of an aboveground herbivore interact with fertilisation. Oikos 124:658–667. https://doi.org/10.1111/oik.01828
Król P, Adamska J, Kępczyńska E (2014) Enhancement of Festuca rubra L. germination and seedling growth by seed treatment with pathogenic Agrobacterium rhizogenes. Acta Physiol Plant 36:3263–3274. https://doi.org/10.1007/s11738-014-1692-8
Lahlali R, Ezrari S, Radouane N, Kenfaoui J, Esmaeel Q, El Hamss H, Belabess Z, Barka EA (2022) Biological control of plant pathogens: a global perspective. Microorganisms 10:596. https://doi.org/10.3390/microorganisms10030596
Leiss KA (2011) Management practices for control of ragwort species. Phytochem Rev 10:153–163. https://doi.org/10.1007/s11101-010-9173-1
Liendo D, Campos JA, Gandarillas A (2023) Cortaderia selloana, an example of aggressive invaders that affect human health, yet to be included in binding international invasive catalogues. NeoBiota 89:229–237. https://doi.org/10.3897/neobiota.89.110500
Lüdecke D, Ben-Shachar M, Patil I, et al (2021) Performance: an R package for assessment, comparison and testing of statistical models. J Open Source Softw 6:3139. https://doi.org/10.21105/joss.03139
Macel M (2011) Attract and deter: a dual role for pyrrolizidine alkaloids in plant-insect interactions. Phytochem Rev 10:75–82. https://doi.org/10.1007/s11101-010-9181-1
Madhavi BGK, Basak JK, Paudel B, Kim NE, Choi GM, Kim HT (2022) Prediction of strawberry leaf color using RGB mean values based on soil physicochemical parameters using machine learning models. Agronomy 12:981. https://doi.org/10.3390/agronomy12050981
Masteling R, Lombard L, de Boer W, Raaijmakers JM, Dini-Andreote F (2019) Harnessing the microbiome to control plant parasitic weeds. Curr Opin Microbiol 49:26–33. https://doi.org/10.1016/j.mib.2019.09.006
McEvoy P, Cox C, Coombs E (1991) Successful biological control of ragwort, Senecio jacobaea, by introduced insects in Oregon. Ecol Appl 1:430–442. https://doi.org/10.2307/1941900
Nandi M, Selin C, Brawerman G, Dilantha Fernando WG, de Kievit T (2017) Hydrogen cyanide, which contributes to Pseudomonas chlororaphis strain PA23 biocontrol, is upregulated in the presence of glycine. Biol Control 108:47–54. https://doi.org/10.1016/j.biocontrol.2017.02.008
Oksanen J, Simpson G, Blanchet F, Kindt R, Legendre P, Minchin P, O'Hara R, Solymos P, Stevens M, Szoecs E, Wagner H, Barbour M, Bedward M, Bolker B, Borcard D, Carvalho G, Chirico M, De Caceres M, Durand S, Evangelista H, FitzJohn R, Friendly M, Furneaux B, Hannigan G, Hill M, Lahti L, McGlinn D, Ouellette M, Ribeiro Cunha E, Smith T, Stier A, Ter Braak C, Weedon J (2022) Vegan: community ecology package. R package version 2.5–5. https://CRAN.R-project.org/package=vegan. Accessed 01 May 2024
Olivoto T (2022) Lights, camera, pliman! An R package for plant image analysis. Methods Ecol Evol 13:789–798. https://doi.org/10.1111/2041-210X.13803
Pangesti N, Pineda A, Pieterse CMJ, Dicke M, van Loon JJ (2013) Two-way plant-mediated interactions between root-associated microbes and insects: from ecology to mechanisms. Front Plant Sci 4:1–11. https://doi.org/10.3389/fpls.2013.00414
Pangesti N, Pineda A, Hannula SE, Bezemer TM (2020) Soil inoculation alters the endosphere microbiome of chrysanthemum roots and leaves. Plant Soil 455:107–119. https://doi.org/10.1007/s11104-020-04655-5
Pearson DE, Ortega YK, Runyon JB, Butler JL (2016) Secondary invasion: the bane of weed management. Biol Conserv 197:8–17. https://doi.org/10.1016/j.biocon.2016.02.029
Pereira MF, Rossi CC, Da Silva GC, Rosa JN, Bazzolli DMS (2020) Galleria mellonella as an infection model: an in-depth look at why it works and practical considerations for successful application. Pathog Dis 78:1–15. https://doi.org/10.1093/femspd/ftaa056
Pineda A, Zheng SJ, van Loon JJA, Pieterse CM, Dicke M (2010) Helping plants to deal with insects: the role of beneficial soil-borne microbes. Trends Plant Sci 15:507–514. https://doi.org/10.1016/j.tplants.2010.05.007
Pineda A, Dicke M, Pieterse CMJ, Pozo MJ (2013) Beneficial microbes in a changing environment: are they always helping plants to deal with insects? Funct Ecol 27:574–586. https://doi.org/10.1111/1365-2435.12050
Pineda A, Kaplan I, Bezemer TM (2017) Steering soil microbiomes to suppress aboveground insect pests. Trends Plant Sci 22:770–778. https://doi.org/10.1016/j.tplants.2017.07.002
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 01 May 2024
Roberts PD, Pullin AS (2007) The effectiveness of management interventions used to control ragwort species. Environ Manage 39:691–706. https://doi.org/10.1007/s00267-006-0039-7
Rolli E, Marasco R, Saderi S, Corretto E, Mapelli F, Cherif A, Borin S, Valenti L, Sorlini C, Daffonchio D (2017) Root-associated bacteria promote grapevine growth: from the laboratory to the field. Plant Soil 410:369–382. https://doi.org/10.1007/s11104-016-3019-6
Romano A, Stevanato P (2020) Germination data analysis by time-to-event approaches. Plants 9:617. https://doi.org/10.3390/plants9050617
Rybakova D, Schmuck M, Wetzlinger U, Varo-Suarez A, Murgu O, Müller H, Berg G (2016) Kill or cure? The interaction between endophytic Paenibacillus and Serratia strains and the host plant is shaped by plant growth conditions. Plant Soil 405:65–79. https://doi.org/10.1007/s11104-015-2572-8
Schaffner U, Steinbach S, Sun Y, Skjøth CA, de Weger LA, Lommen ST, Augustinus BA, Bonini M, Karrer G, Šikoparija B, Thibaudon M, Müller-Schärer H (2020) Biological weed control to relieve millions from Ambrosia allergies in Europe. Nat Commun 11:1–7. https://doi.org/10.1038/s41467-020-15586-1
Sciegienka JK, Keren EN, Menalled FD (2011) Interactions between two biological control agents and an herbicide for Canada thistle (Cirsium arvense) suppression. Invas Plant Sci Mana 4:151–158. https://doi.org/10.1614/IPSM-D-10-00061.1
Seebens H, Blackburn TM, Dyer EE, Genovesi P, Hulme PE, Jeschke JM, Pagad S, Pyšek P, Winter M, Arianoutsou M, Bacher S, Blasius B, Brundu G, Capinha C, Celesti-Grapow L, Dawson W, Dullinger S, Fuentes N, Jäger H, Kartesz J, Kenis M, Kreft H, Kühn I, Lenzner B, Liebhold A, Mosena A, Moser D, Nishino M, Pearman D, Pergl J, Rabitsch W, Rojas-Sandoval J, Roques A, Rorke S, Rossinelli S, Roy HE, Scalera R, Schindler S, Štajerová K, Tokarska-Guzik B, van Kleunen M, Walker K, Weigelt P, Yamanaka T, Essl F (2017) No saturation in the accumulation of alien species worldwide. Nat Commun 8:1–9. https://doi.org/10.1038/ncomms14435
Seebens H, Bacher S, Blackburn TM, Capinha C, Dawson W, Dullinger S, Genovesi P, Hulme PE, van Kleunen M, Kühn I, Jeschke JM, Lenzner B, Liebhold AM, Pattison Z, Pergl J, Pyšek P, Winter M, Essl F (2021) Projecting the continental accumulation of alien species through to 2050. Glob Chang Biol 27:970–982. https://doi.org/10.1111/gcb.15333
Sessitsch A, Howieson JG, Perret X et al (2002) Advances in rhizobium research. CRC Crit Rev Plant Sci 21:323–378. https://doi.org/10.1080/0735-260291044278
Simberloff D, Martin JL, Genovesi P, Maris V, Wardle DA, Aronson J, Courchamp F, Galil B, García-Berthou E, Pascal M, Pyšek P, Sousa R, Tabacchi E, Vilà M (2013) Impacts of biological invasions: what’s what and the way forward. Trends Ecol Evol 28:58–66. https://doi.org/10.1016/j.tree.2012.07.013
Sorokan A, Cherepanova E, Burkhanova G, Veselova S, Rumyantsev S, Alekseev V, Mardanshin I, Sarvarova E, Khairullin R, Benkovskaya G, Maksimov I (2020) Endophytic Bacillus spp. as a prospective biological tool for control of viral diseases and non-vector Leptinotarsa decemlineata Say. in Solanum tuberosum L. Front Microbiol 11:1–13. https://doi.org/10.3389/fmicb.2020.569457
Sugio A, Dubreuil G, Giron D, Simon JC (2015) Plant-insect interactions under bacterial influence: ecological implications and underlying mechanisms. J Exp Bot 66:467–478. https://doi.org/10.1093/jxb/eru435
Sun M, Liu J, Li J, Huang Y (2022) Endophytic bacterium Serratia plymuthica from Chinese leek suppressed apple ring rot on postharvest apple fruit. Front Microbiol 12:1–14. https://doi.org/10.3389/fmicb.2021.802887
Tack AJM, Dicke M (2013) Plant pathogens structure arthropod communities across multiple spatial and temporal scales. Funct Ecol 27:633–645. https://doi.org/10.1111/1365-2435.12087
Tichich RP, Doll JD (2006) Field-based evaluation of a novel approach for infecting Canada thistle (Cirsium arvense) with Pseudomonas syringae pv. tagetis. Weed Sci 54:166–171. https://doi.org/10.1614/ws-03-144r3.1
Tinney GW, Hatcher PE, Ayres PG, Ayres PG, Paul ND, Whittaker JB (1998) Inter- and intra-species differences in plants as hosts to Tyria jacobaeae. Entomol Exp Appl 88:137–145. https://doi.org/10.1046/j.1570-7458.1998.00355.x
Trognitz F, Hackl E, Widhalm S, Sessitsch A (2016) The role of plant-microbiome interactions in weed establishment and control. FEMS Microbiol Ecol 92:1–15. https://doi.org/10.1093/femsec/fiw138
van de Voorde TFJ, van der Putten WH, Bezemer TM (2012) The importance of plant-soil interactions, soil nutrients, and plant life history traits for the temporal dynamics of Jacobaea vulgaris in a chronosequence of old-fields. Oikos 121:1251–1262. https://doi.org/10.1111/j.1600-0706.2011.19964.x
van der Meijden E, van der Waals-Kooi RE (1979) The population ecology of Senecio Jacobaea in a sand dune system: I. Reproductive strategy and the biennial habit. J Ecol 67:131. https://doi.org/10.2307/2259341
van Loon LC, Bakker PAHM, Pieterse CMJ (1998) Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol 36:453–483. https://doi.org/10.1146/annurev.phyto.36.1.453
Vilà M, Espinar JL, Hejda M, Hulme PE, Jarošík V, Maron JL, Pergl J, Schaffner U, Sun Y, Pyšek P (2011) Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol Lett 14:702–708. https://doi.org/10.1111/j.1461-0248.2011.01628.x
Vischer N, Nastase S (2022) ObjectJ. http://simon.bio.uva.nl/objectj/index.html. Accessed 01 May 2024
De Vleesschauwer D, Höfte M (2007) Using Serratia plymuthica to control fungal pathogens of plants. CAB Rev Perspect Agric Vet Sci Nutr Nat Resour 2. https://doi.org/10.1079/PAVSNNR20072046
Vrieling K, Smit W, van der Meijden E (1991) Tritrophic interactions between aphids (Aphis jacobaeae Schrank), ant species, Tyria jacobaeae L., and Senecio jacobaea L. lead to maintenance of genetic variation in pyrrolizidine alkaloid concentration. Oecologia 86:177–182. https://doi.org/10.1007/BF00317529
Weissmann R, Gerhardson B (2001) Selective plant growth suppression by shoot application of soil bacteria. Plant Soil 234:159–170. https://doi.org/10.1023/A:1017916717160
Wenke K, Wanke D, Kilian J, Berendzen K, Harter K, Piechulla B (2012) Volatiles of two growth-inhibiting rhizobacteria commonly engage AtWRKY18 function. Plant J 70:445–459. https://doi.org/10.1111/j.1365-313X.2011.04891.x
Winston RL, Schwarzländer M, Hinz HL, Day MD, Cock MJW, Julien MH (2023) Biological control of weeds: a world catalogue of agents and their target weeds. USDA Forest Service, Forest Health Technology Enterprise Team, Morgantown, WV, USA. https://www.ibiocontrol.org/catalog/. Accessed 24 Dec 2023
Zhang J, Klinkhamer PGL, Vrieling K, Bezemer TM (2022a) The negative effects of soil microorganisms on plant growth only extend to the first weeks. J Plant Ecol 15:854–863. https://doi.org/10.1093/jpe/rtac022
Zhang S, Li Z, Shu J, Xue H, Guo K, Zhou X (2022b) Soil-derived bacteria endow Camellia weevil with more ability to resist plant chemical defense. Microbiome 10:1–19. https://doi.org/10.1186/s40168-022-01290-3
Acknowledgements
We thank Dr. Qian Huang for helping X.Y.L to collect J. vulgaris in Texel, The Netherlands. We thank Jelmer van Lieshout for assistance in measuring leaf yellowness. We thank Dr. Young Hae Choi for his support on chemical analysis and comments on the manuscript. We thank three anonymous reviewers for insightful comments and helpful suggestions. X.Y.L. was funded by a Chinese Scholarship Council (CSC) grant (No.201906140116).
Author information
Authors and Affiliations
Contributions
X.Y.L., T.M.B., K.V. and S.T.E.L. designed and developed the study. X.Y.L. collected plant samples and isolated bacteria from roots. X.Y.L. carried out experiments, analyzed and presented the data with helpful inputs from S.I.F.G. and A.O. T.X.M.D.S and L.S.E. performed the aphid performance experiment. M.C.H.V.D.D. and F.A.Z. performed the T. jacobaeae preference experiment. Ö.E. measured and analyzed exudate samples. X.Y.L., T.M.B., K.V. and S.T.E.L. wrote the manuscript. All authors revised and improved subsequent versions of the manuscript.
Corresponding author
Additional information
Responsible Editor: Gaowen Yang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, X., Vrieling, K., Gomes, S.I.F. et al. Exploring the potential of root-associated bacteria to control an outbreak weed. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06726-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06726-3