Summary
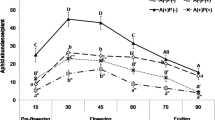
We hypothesize that the tritrophic interaction between ants, the aphid Aphis jacobaeae, the moth Tyria jacobaeae, and the plant Senecio jacobaea can explain the genetic variation observed in pyrrolizidine alkaloid concentration in natural populations of S. jacobaea. The ant Lasius niger effectively defends S. jacobaea plants infested with A. jacobaeae against larvae of T. jacobaeae. S. jacobaea plants with A. jacobaeae which are defended by ants escape regular defoliation by T. jacobaeae. Plants with aphids and ants have a lower pyrrolizidine alkaloid concentration than plants without aphids and ants. When these data are fitted to an existing theoretical model for temporal variation in fitness it is shown that varying herbivore pressure by T. jacobaeae in interaction with ants defending aphid-infested plants with a low pyrrolizidine alkaloid concentration can lead to a stable polymorphism in pyrrolizidine alkaloid concentration. Costs of the production and maintenance of pyrrolizidine alkaloids are not accounted for in the model.
Similar content being viewed by others
References
Aplin RT, Rothschild M (1972) Poisonous alkaloids in the body tissues of the Garden Tiger Moth (Arctia caja L.) and the Cinnabar Moth (Tyria (=Callimorpha), jacobaeae L.) (Lepidoptera) In: Vries A de, Kochva E (eds), Toxins of animal and plant origin. Gordon & Breach, London, pp 579–595
Aplin RT, Benn MH, Rothschild M (1968) Poisonous alkaloids in the body tissues of the Cinnabar Moth (Callimorpha jacobaeae L.) Nature 219:747–748
Bentley MD, Leonard DE, Stoddard WF, Zalkow LH (1984) Pyrrolizidine alkaloids as larval feeding deterrents for Spruce Budworm, Choristoneura fumiferana (Lepidoptera: Tortricidae). Ann Entomol Soc Am 77: 393–397
Berenbaum MR, Zangerl AR, Nitao JK (1986) Constraints on chemical coevolution: Wild Parsnips and the Parnsnip Webworm. Evolution: 40:1215–1228
Berenbaum MR, Zangerl AR, Lee K (1989) Chemical barriers to adaptation by a specialist herbivore. Oecologia 80:501–506
Brown DG (1988) The cost of plant defense: an experimentel analysis with inducible proteinase inhibitors in Tomato. Oecologia 76:467–470
Buckley R (1987) Ant — plant — homopteran interactions. Adv Ecol Res 16:53–85
Cates RG, Redak RA (1989) Variation in terpene chemistry of Douglas-fir and its relationship to western Spruce Budworm success. In: Spencer KC (ed.) Chemical mediation of coevolution. Acad Press Inc., San Diego, pp 317–344
Coley PD (1983) Herbivory and defensive characteristics of tree species in a lowland tropical forest. Ecol Monogr 53:209–223
Coley PD (1986) Costs and benefits of defense by tannins in a neotropical tree. Oecologia 70:238–241
Crawley MJ, Gillman MP (1989) Population dynamics of Cinnabar Moth and Ragwort in grassland. J Anim Ecol 58:1035–1050
Dempster JP, Lakhani KH (1979) A population model for Cinnabar Moth and its foodplant, Ragwort. J Anim Ecol 48:143–163
Dreyer DL, Jones KC, Molyneux RJ (1985) Feeding deterrency of some pyrrolizidine, indolizidine, and quinolizidine alkaloids towards Pea Aphid (Acyrtosiphon pisum) and evidence for phloem transport of indolizidine alkaloid swainsonine. J Chem Ecol 11:1045–1051
Ernst WHO (1987) Impact of the aphid Aulocorthum solani KLTB. on growth and reproduction of winter and summer annual life forms of Senecio sylvaticus L. Acta Oecol/Oecol Gener 8:537–547
Gould F (1983) Genetics of plant-herbivore systems: interactions between applied and basic study. — In: Denno RF, McClure MS (eds), Variable plants and herbivores in natural and managed systems. Acad Press, New York pp 599–653
Haldane JBS, Jayakar SD (1963) Polymorphism due to selection of varying direction. J Genet 58:237–242
Hanover JW (1966) Genetics of terpenes. I. Gene control of monoterpene levels in Pinus monticola Dougl. Heredity 21:73–84
Hartmann T, Zimmer M (1986) Organ specific distribution and accumulation of pyrrolizidine alkaloids during the life history of two annual Senecio species. J Plant Physiol 112:67–80
Hartmann T, Ehmke A, Eilert U, Borstel K von, Theuring C (1989) Sites of synthesis, translocation and accumulation of pyrrolizidine alkaloid N-oxides in Senecio vulgaris L. Planta 177:98–107
Heie OE (1986) The aphidoidea (Hemiptera of Fennoscandia and Denmark. III) — Fauna Entomologica Scandinavica 17:180–181 E.J. Brill/Scandinavian Science Press Ltd. Leiden Copenhagen
Johnson AE, Molyneux RJ, Merrill GB (1985) Chemistry of toxic range plants. Variation in pyrrolizidine alkaloid content of Senecio, Amsinckia and Crotolaria species. J Agric Food Chem 33:50–55
Kakes P (1989) An analysis of the costs and benefits of the cyanogenic system in Trifolium repens L. Theor Appl Gen 77:111–118
Krischik VA, Denno RF (1983) Individual, population, and geographic patterns in plant defense. In: Denno RF, McClure MS (eds). Variable plants and herbivores in natural and managed systems. Acad Press, New York, pp 463–512
Mattocks AR (1967) Spectrophotometric determination of unsaturated pyrrolizidine alkaloids. Anal Chem 34:443–447
Messina FJ (1981) Plant protection as a consequence of an antmembracid mutualism: interactions on Goldenrod (Solidago sp.) Ecology 62:1433–1440
Myers JH (1980) Is the insect or the plant the driving force in the Cinnabar Moth — Tansy Ragwort system? Oecologia 47:16–21
Pieters LAC, Zoelen AM van, Vrieling K, Vlietinck AJ (1989) Determination of the pyrrolizidine alkaloids from Senecio jacobaeae by 1H and 13C NMR spectroscopy. Mag Res Chem 27:754–759
Prins AH (1990) Herbivory and plant performance of Senecio jacobaea L. and Cynoglossum officinale L. Thesis Leiden
Østrem L (1987) Studies on genetic variation in reed canarygrass, Phalaris arundinacea L. I. Alkaloid type and concentration. Hereditas 107:235–248
Rausher M, Simms EL (1989) The evolution of resistance to herbivory in Ipomoea purpurea. I. Attempts to detect selection. Evolution 43:563–572
Roughgarden J (1979) Theory of population genetics and evolutionary ecology: An introduction. Macmillan Publ Co, New York
Segall HJ (1978) Pyrrolizidine alkaloids derived from Senecio jacobaea. Toxic Let 1:279–284
Segall HJ, Krick TP (1979) Pyrrolizidine alkaloids: organohalogen derivative isolated from Senecio jacobaeae (Tansy Ragwort). Toxic Let 4:193–198
Simms EL, Rausher MD (1987) Costs and benefits of plant resistance to herbivory. Am Nat 130:570–581
Simms EL, Rausher MD (1989) The evolution of resistance to herbivory in Ipomoea purpurea. II. Natural selection by insects and costs of resistance. Evolution 43:573–585
Steiner AA (1968) Soilless culture. Proc 6th Coll Int Potash Inst, Florence, Italy:324–341. International Potash Institute, Berne, Switzerland
Van der Meijden E (1973) Experiments on dispersal, late-larval predation, and pupation in the Cinnabar Moth (Tyria jacobaeae L.) with a radioactive label (192Ir). Neth J Zool 23:430–445
Van der Meijden E (1979) Herbivore exploitation of a fugitive plant species: local survival and extinction of the Cinnabar Moth and Ragwort in a heterogeneous environment. Oecologia 42:307–323
Van der Meijden E, Bemmelen M van, Kooi R, Post BJ (1984) Nutritional quality and chemical defence in the Ragwort-Cinnabar Moth interaction. J Anim Ecol 53:443–453
Van der Meijden E, Zoelen AM van, Soldaat LL (1989) Oviposition by the Cinnabar Moth, Tyria jacobaeae, in relation to nitrogen, sugars and alkaloids of Ragwort, Senecio jacobaea. Oikos 54:337–344
Von Borstel K, Witte L, Hartmann T (1989) Pyrrolizidine alkaloid patterns in populations of Senecio vulgaris, S. vernalis and their hybrids. Phytochem 28:1635–1638
Wink M (1987) Chemical ecology of quinolizidine alkaloids. In: Waller GR (ed) Allelochemicals: Role in agriculture and forestry. ACS Symp Ser 330:524–533
Wink M, Witte L (1984) Turnover and transport of quinolizidine alkaloids. Diurnal fluctuations of lupanine in the phloem sap, leaves and fruits of Lupinus alba L. Planta 161:519–524
Zúñiga GE, Corcuera GE (1986) Effect of gramine in the resistance of barley seedlings to the aphid Rhopalosiphum padi. Entomol Exp Appl 40:259–262
Zúñiga GE, Salgado MS, Corcuera LJ (1985) Role of an indole alkaloid in the resistance of barley seedlings to aphids. Phytochem 24:945–947
Author information
Authors and Affiliations
Additional information
Publication of the “Meijendel-comité”, new series no. 114
Rights and permissions
About this article
Cite this article
Vrieling, K., Smit, W. & van der Meijden, E. Tritrophic interactions between aphids (Aphis jacobaeae Schrank), ant species, Tyria jacobaeae L., and Senecio jacobaea L. lead to maintenance of genetic variation in pyrrolizidine alkaloid concentration. Oecologia 86, 177–182 (1991). https://doi.org/10.1007/BF00317529
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00317529