Abstract
Background and aims
Partitioning the measured net ecosystem carbon dioxide (CO2) exchange into gross primary productivity (GPP) and ecosystem respiration remains a challenge, which scientists try to tackle by using the properties of the trace gas carbonyl sulfide (COS). Its similar pathway into and within the leaf makes it a potential photosynthesis proxy. The application of COS as an effective proxy depends, among other things, on a robust inventory of potential COS sinks and sources within ecosystems. While the soil received some attention during the last couple of years, the role of plant roots is mostly unknown. In our study, we investigated the effects of live roots on the soil COS exchange.
Methods
An experimental setup was devised to measure the soil and the belowground plant parts of young beech trees observed over the course of 9 months.
Results
During the growing season, COS emissions were significantly lower when roots were present compared to chambers only containing soil, while prior to the growing season, with photosynthetically inactive trees, the presence of roots increased COS emissions. The difference in the COS flux between root-influenced and uninfluenced soil was fairly constant within each month, with diurnal variations in the COS flux driven primarily by soil temperature changes rather than the presence or absence of roots.
Conclusion
While the mechanisms by which roots influence the COS exchange are largely unknown, their contribution to the overall ground surface COS exchange should not be neglected when quantifying the soil COS exchange.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The trace gas carbonyl sulfide (COS) has kept the scientific community interested in its properties for decades. Earlier COS research concentrated on its contribution to stratospheric aerosol formation (Crutzen 1976), newer studies focus on its potential as a tracer for plant photosynthesis and stomatal conductance (Sandoval-Soto et al. 2005; Seibt et al. 2010; Whelan et al. 2018). Leaves take up COS in the same way they take up CO2, through their stomata, and both gases pass through the mesophyll to their respective endpoints, carbonic anhydrase (CA) for COS and Rubisco for CO2 (Seibt et al. 2010). The key difference between COS and CO2, and the advantage scientists want to leverage during recent years, is that there is no known diffusion of COS out of the leaf, resulting in an unidirectional flux (Seibt et al. 2010; Stimler et al. 2010) in parallel with gross photosynthesis. Attempts have been made to use the unidirectional nature of the COS flux to estimate the gross CO2 uptake (Asaf et al. 2013; Berkelhammer et al. 2014; Berry et al. 2013; Billesbach et al. 2014; Blonquist et al. 2011; Maseyk et al. 2014; Whelan et al. 2018), especially as the later cannot be directly measured. Fluxes with opposing signs result in the net ecosystem productivity, which can be observed, but is hard to decompose into its component fluxes. The problem caused by multiple sinks and sources contributing to the observable net CO2 exchange is particularly relevant on scales larger than the leaf level (Anav et al. 2015).
In order to use COS as a proxy for the CO2 exchange of leaves, a robust understanding of other COS sinks and sources is needed. At the ecosystem level, the soil has received increased attention in regard to its COS exchange potential during the last couple of years (Bunk et al. 2017; Kaisermann et al. 2018; Kitz et al. 2020; Meredith et al. 2018; Ogee et al. 2016; Sun et al. 2015, 2018; Whelan et al. 2016), considering it harbors a multitude of organisms that could potentially contribute to the net ecosystem COS flux, via emission or uptake, and thus obscure or exaggerate part of the plant-mediated COS uptake. Other potential contributors to the ecosystem-scale COS exchange include plant litter, living non-leaf plant parts, and abiotic COS sources and sinks.
Oxic soils are usually thought to act as sinks for COS, which has been shown in the laboratory (Kesselmeier et al. 1999; Meredith et al. 2019; Van Diest and Kesselmeier 2008; Whelan et al. 2016) and in situ experiments (Steinbacher et al. 2004; Yi et al. 2007). The suspected mechanism underlying COS consumption in oxic soils is the destruction of COS by enzymes from the carbonic anhydrase family (Conrad 1996; Meredith et al. 2018; Ogawa et al. 2016; Seibt et al. 2006; Wingate et al. 2008), nitrogenase (Seefeldt et al. 1995), CO dehydrogenase (Ensign, 1995) and CS2 hydrolase (Smeulders et al. 2013). While COS uptake seems to outweigh COS emission in oxic soils in most cases, some evidence suggests that under dry, hot, or high-light conditions processes that emit COS can turn soil into a net source for COS (Kitz et al. 2017; Maseyk et al. 2014). The mechanisms underlying emissions from oxic soils are not very well understood and are thought to be a combination of abiotic and biotic processes. Among the abiotic factors, soil temperature (Meredith et al. 2018; Whelan and Rhew 2015), soil moisture (Bunk et al. 2017; Kaisermann et al. 2018) and light level (Kitz et al. 2020; Whelan and Rhew 2015) are considered to have the biggest impact on soil COS emission.
One component of the soil COS flux neglected so far is the impact of roots. At this point, we are not aware of any published study which would have investigated whether and how the presence of living plant roots affects the soil COS exchange. Two studies which measured the COS exchange of roots not connected to living plants are Maseyk et al. (2014), showing consistent COS emission from roots of multiple species of an agricultural field, and Whelan and Rhew (2015), observing increased COS emission from roots mixed with soil when exposed to light. But roots also contain the COS-consuming enzyme CA (Demir et al. 2009; Dimou et al. 2009; Yu et al. 2007) and are able to take up gases, like CO2 (Milton et al. 1998; Ota and Tanaka 2019; Rao and Wu 2017), from the surrounding soil, which could lead to COS uptake by living roots. In addition, plants can influence the surrounding soil and as a consequence alter gas exchange, be it biotic or abiotic in nature, from the soil. While in situ measurements, from ecosystem-scale eddy covariance to chamber measurements, of the COS exchange in many cases, inevitably include the root contribution and plant-soil interactions, lab experiments usually use soil samples uncoupled from the plants they are associated with in situ measurements. This separation of plant and soil might bias the data gained from those experiments, since for decades the scientific community is aware that plants influence the soil they grow in, primarily via their roots, which led to the creation of the term rhizosphere (Hiltner 1904). While there are different conceptual frameworks of the rhizosphere, its importance is undisputed (Canto et al. 2020; Philippot et al. 2013; York et al. 2016).
In this study we thus investigated the influence of living plant roots on the soil COS exchange, using young beech trees grown in potting soil under controlled laboratory conditions over the course of nine months. We hypothesized that.
-
(I)
the soil COS flux would change when plant roots are present considering the multitude of metabolites released into the soil via root exudates.
-
(II)
soil COS fluxes in root-influenced soil will exhibit a diurnal cycle following light availability and thus photosynthetic activity of the corresponding beech trees.
-
(III)
the soil COS flux will change throughout the year, with environmental conditions in the lab kept constant, following changes in the phenological development of the investigated beech trees.
Answering these questions are a first step to bridge the gap between soil observations under laboratory conditions and field observations of root-influence soil.
Materials and methods
Trees and potting soil
18 young (~ two years old) beech trees (Fagus sylvatica L.) from a local tree nursery were root-washed and potted, with the same potting soil used later in the experiment, 7 months prior to the start of the experiment in October 2020. Average tree height in October 2020 was 32.8 cm and average maximum stem width was 8.4 cm (see Table S1 for more details including initial soil moisture). The potting soil (Hawita Fruhstorfer Erde – Aussaat- und Stecklingserde, HAWITA Gruppe GmbH, Germany) was peat-based with a pH of 5.9, a nitrogen content of 80 mg/l, a phosphorus content of 60 mg/l, a potassium content of 90 mg/l and a magnesium content of 100 mg/l.
The trees were grown in a greenhouse, which had only frost protection heating and was therefore considerably cooler in February. The lab temperature was set to the same value (25 °C) in all months, which meant that the soil in February would experience an unusually high temperature, especially in comparison to the temperature the soil experienced in the greenhouse, in which the pots were prepared in.
Chamber design
Six glass desiccators (Duran Mobilex with GL 32 thread, DN 200, DWK Life Sciences, Germany) with a volume of 5.8 l were used as chambers (see Fig. 1), three of them holding one beech tree each, two containing only potting soil and one control chamber without soil and/or plant (but otherwise identical to the treatment chambers). For each month a set of three new trees from the 18 trees in the greenhouse was used. Multiport connector caps (GL32, Duran) with a PTFE insert and four stainless steel tubing connectors were used to connect the PFA tubing with the chambers. Two layers of PTFE plates, with each layer consisting of two plates that could be connected and sealed via a tongue and groove system, were placed on top of the chamber with a hole in the middle for the stem of the beech tree (see Fig. 1). The PTFE plates together with a silicone ring closed off the inside of the chamber and allowed controlled circulation of the air through the PFA tubing. The airflow through the system was realized with two pumps, one pushing air through the system and one driving air to the instrument (see the sketch in Fig. 2). Room air was pushed through a nafion dryer (PD-100T-48MSS, Perma Pure, New Jersey, United States) and self-build charcoal filters, which have proven to be efficient in removing COS (Kesselmeier et al. 1999), towards a flow controller set to 2.175 l/min. A second flow controller set to 0.0085 l/min was connected to a gas cylinder containing a known COS concentration in nitrogen (Linde Gas GmbH, Stadl-Paura, Austria). Air with a concentration of 500 ppt was mixed by adjusting the flow controllers accordingly. The mixed air was pushed to the bottom of the soil column in the chambers at a flow rate of 0.36 l/min. Air-permeable, hydrophobic polypropylene tubes (Gut et al. 1998) (Accurel), arranged in a loop, on the top of the soil columns were used to sample air that has passed through the soil. The air was then either directed to the instrument measuring gas concentrations or to a common exhaust. The flow through all the chambers was therefore constant, with one being measured and the remaining five being flushed at any given time.
The chambers used in the experiment were desiccators with a multiport connector on the side, which allowed two PFA tubes (an inlet and an outlet) and one thermocouple to be inserted into the potting soil. The top PFA tube was connected to a semi-permeable Accurel tube. The top of the desiccator was sealed off using two PTFE panels and a silicone seal. Arrows indicate the direction of the air flow
Schematic representation of the experimental setup. Purple arrows indicate airflow direction. The instrument measuring the COS and CO2 concentrations is denoted as QCL (Quantum cascade laser). Room air is entering the setup from the right-hand side (right-bottom) and is mixed with a known COS concentration from a gas bottle after all COS was removed from the room air stream using activated carbon filters. The resulting air mixture is supplied to the bottom of six chambers, one of them is measured, while the other five are flushed with the same flow rate
Gas measurements
The Accurel tubes were connected to PFA tubing leading to a rotary valve (VICI 16 port valve, VICI Valco Instruments, Houston, USA), which was controlled by the instrument measuring the gas concentrations – an Aerodyne Dual QCL (Aerodyne Research, Billerica, MA, USA). The Aerodyne software TDLWintel controlled the rotary valve and calculated the COS and CO2 concentrations in real-time. The optical bench of the instrument was heated to a temperature of 35 °C and the absorption spectra were fitted at a frequency of 1 Hz. Air from one selected position on the rotary valve was pushed through another flow controller set to 0.36 l/min to the laser, while the rest was pushed to a common exhaust. In addition to pushing the air through the valve, a vacuum pump was sucking the air to the laser. To avoid pressure oscillations, the pressure lock was deactivated in TDLWintel and the cell pressure (~ 4 kPa) was adjusted manually at the beginning of the experiment by setting a fixed valve position and flow rate to the instrument. To test for leaks, the instrument including the vacuum pump was disconnected from the setup, and the flow at the third flow controller, which was directly in front of the Dual-QCL, was checked before each run of the experiment. In addition, a manometer was connected to the empty chamber to check if the overpressure in the system was constant throughout the experiment.
Environmental variable measurements
During the measurements, the chambers were placed on self-build balances using 10 kg load cells (B6N-C4-10 kg-1B6, Zemic Europe B.V., Etten-Leur, Netherlands) and connected to a datalogger (CR5000, Campbell Scientific, Logan, US) to monitor weight loss, due to water loss via plant transpiration, by the chambers. The aboveground part of the trees received light from growth lamps (ViparSpectra 600 W Reflector LED with both modes switched on) for 10 h a day, the light intensity (PAR) at the top of the trees was approximately 1500 µmol m−2 s−1. A stainless-steel thermal sensor Typ K (RS Pro) with a rod length of 150 mm connected to a datalogger (CR5000, Campbell Scientific, Logan, United States) was used to measure the temperature inside the chambers.
COS flux measurement
Measurements were carried out in 2021, with a set of measurements in February, April, May, June, and October. One week before the start of each measurement, three young beech trees were moved from their pots, with as little potting soil clinging to the roots as possible without severe disturbance of the roots, to the experimental chambers described above. The remaining volume was filled with potting soil, whereby a new package of potting soil was used for each month to avoid colonization by microbes once the steamed potting soil bags were opened. The same potting soil was used for the soil only chambers. After repotting all experimental chambers, the ones with trees and the ones with only potting soil, they were watered with 1 l of tap water.
For each measurement, one empty (control), two soil only, and three tree-containing (soil + root) chambers were used. In each half-hourly measurement cycle, every soil only and tree chamber was measured for three minutes preceded and followed by measurements of the control chamber. The temperature in the lab was set to 25 °C in all months.
After three days of measurements, leaves were harvested and scanned to get the tree leaf area and roots washed, dried and weighed to gain the root dry weight. The leaf area was used as an indicator for the phenological stages of the trees. The potting soil was sampled before and after the three-day measurement period, the soil samples were dried and weighed to obtain the fresh and dry weight, with which the gravimetric soil moisture was calculated, at the start and end of the experiment.
Flux calculation and statistics
Fluxes were calculated using Python 3.7.13, IPython 7.22.0, Spyder 4.1.3, and the pandas library (McKinney 2010). Errors in air temperature, air pressure, flow rate and weight were propagated through flux calculations via the “uncertainties” package (Lebigot n.d.). The COS and CO2 fluxes were calculated according to Eq. 1.
F is the soil flux in pmol kg−2 s−1 for COS and µmol kg−2 s−1 for CO2, q is the flow rate (mol s−1), Ce is the mean concentration in the control (empty) chamber before and after the measurement of the current chamber, Cc is the mean concentration in the current chamber and DW is the dry weight in kilograms (kg) of the potting soil.
Statistical analyses were carried out using the software R 4.2.2 (R Core Team 2018) and RStudio (RStudio Team 2016) and the following packages: data.table (Dowle and Srinivasan 2022), ggplot2 (Wickham 2016), lme4 (Bates et al. 2022), relaimpo (Groemping 2021), randomForest (Liaw and Wiener 2002), car (Fox et al. 2022), tsutils (Kourentzes 2022), stats (R Core Team 2018 ) and permimp (Debeer et al. 2021). No soil temperature data was available for the soil only chambers in May due to a thermocouple failure. No outliers were removed from the dataset. To test for significant differences between groups, Kruskal-Wallis rank sum test were performed. The flux data was decomposed, to separately investigate the daily variations and the 3-day trend, with the decompose function from the stats package using a moving average and a frequency of 24 h for the seasonal component (Suppl. Fig. 2). To characterize the temporal pattern of the daily variation and trend k-Means clustering was performed using the kmeans function from the stats package, providing the function with a value of three for the number of centers and 100 for the number of random sets (nstart). The relative importance of the predictors in the linear models were calculated using the boot.relimp function in the relaimpo package with b = 10,000, type = c(“lmg”, “last”), rank = TRUE, diff = TRUE and rela = TRUE. The relative importance values presented in the paper are the ones yielded by the lmg (Lindemann, Merenda and Gold) method (the “last” method did not yield a different ordering, see Suppl. Fig. 1). In order to calculate the root-only COS fluxes the means of the soil only chambers for each month were calculated and subtracted from each beech tree measurement. In order to investigate the variable importance of the root-only COS data, a random forest regression with 1000 trees was performed followed by an analysis for retrieving the conditional permutation importance (permimp function).
Results
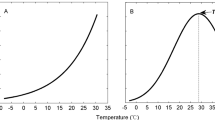
Mean soil COS emission, CO2 emission, and soil temperature were highest in April for both soil (COS: 0.693 pmol kg−1 s−1, CO2: 0.233 µmol kg−1 s−1, 26.8 °C) and soil + root (COS: 0.534 pmol k−1 s−1, CO2: 0.372 µmol kg−1 s−1, 27.0 °C) and lowest in February, again for both soil (COS: 0.047 pmol kg−1 s−1, CO2: 0.050 µmol kg−1 s−1, 21.5 °C) and soil + root (COS: 0.026 pmol kg−1 s−1, CO2: 0.108 µmol kg−1 s−1, 21.8 °C). For each month, the differences between soil and soil + root for COS and CO2 were highly significant (Kruskal-Wallis rank sum test – p < 2.2e−16), with higher CO2 and lower COS emissions in the soil + root chambers (except for February) (Fig. 3). COS fluxes were moderately to highly (R2 > 0.3) correlated to soil temperature, except for the soil treatment in April (see Table 1; Fig. 4). The COS to CO2 flux ratio was higher in the soil treatment compared to the root treatment, that is to say, the presence of roots reduced the emission of COS per unit of emitted CO2, except in February. The soil moisture decrease from the start to the end of each 3-day experiment as a mean over all months was 1.65% in the beech chambers and 0.54% in the soil chambers. The mean soil moisture decrease over both treatments was highest (2.83%) in June and lowest in October (0.48%).
The change of the COS flux over the course of one experiment (henceforth referred to as temporal change) varied between months, but could be organized, using kmeans clustering, into three distinct groups (Fig. 5). In April there was an overall decrease in the COS flux over the course of the experiment, with a steep decline at the beginning and only marginal changes towards the end of the measurements. In June and May the slope of the COS flux trend was close to zero (Fig. 5). In February and October, the COS flux increased slightly throughout the experiment. The kmeans clustering did only group the COS flux trends by month and did not separate the different treatments from each other.
The mean (+ sd) COS temporal change for each of the three clusters created by kmeans clustering. Cluster 1: February, October; Cluster 2: April (one beech replicate), June, May; Cluster 3: April (remaining replicates)). The first cluster contains the pre-season and late season measurements, while the second cluster contains measurements taken during the peak growing season (except for one replicate in April, which was assigned to its own cluster)
The “periodic” component with a frequency of 24 h (from now on called “daily component” or just “daily” in variable names) was also clustered using kmeans. Group three contained the daily components of most replicates in April and two of the replicates in October and had a mean amplitude of 0.091 pmol kg−1 s−1. Group two contained the daily components from June with an amplitude of 0.114 pmol kg−1 s−1. The daily components of the remaining replicates were clustered together in group three with an amplitude of 0.024 pmol kg−1 s−1 (Suppl. Fig. 3).
Two multiple linear regressions were performed on the daily component data, one for each of the two treatments. For the multiple linear regressions COS-daily-soil, \(cosfl\widehat{ux}dailysoil={\widehat\beta}_0+{\widehat\beta}_1soiltemperaturedaily+{\widehat\beta}_2CO2fluxdaily+{\widehat\beta}_3light+{\widehat\beta}_4rootweight+{\widehat\beta}_5soilmoisture+{\widehat\beta}_6month\), and COS-daily-root, \({cosfl\widehat{ux}dailyroot}=\widehat{\beta}_{0}+\widehat{\beta}_{1}soiltemperaturedaily+\widehat{\beta}_{2}CO2fluxdaily+\widehat{\beta}_{3}light+\widehat{\beta}_{4}rootweight+\widehat{\beta}_{5 }soilmoisture+\widehat{\beta}_{6} month\), no multicollinearity (Variance inflation factors < 4) were detected and both models had the predictive capability (F Statistic: p < 0.05). The COS-daily-soil model explained 83% of the variability in the response variable (COS flux daily soil) and all predictors were highly significant (p < 0.001). The most important predictor in the model was the daily component of the CO2 flux (CO2 flux daily) followed by daily soil temperature and light (Fig. 6). The COS-daily-root model explained 78% of the variability in the response variable (COS flux daily root) with all predictors except the root weight being highly significant. The most important predictor in the model was the daily component of the soil temperature followed by the CO2 flux and light (Fig. 6).
The mean differences in the COS flux between the soil + root and soil treatments were 0.074 pmol kg−1 s−1 in February, -0.159 pmol kg−1 s−1 in April, -0.041 pmol kg−1 s−1 in May, -0.046 pmol kg−1 s−1 in June and − 0.036 pmol kg−1 s−1 in October. The mean difference in the CO2 flux was 0.058 µmol kg−1 s−1 in February, 0.139 µmol kg−1 s−1 in April, 0.086 µmol kg−1 s−1 in May, 0.093 µmol kg−1 s−1 in June and 0.217 µmol kg−1 s−1 in October (Fig. 7). The random forest regression performed on the COS difference data (root-only) as response and the time series independent variables (root weight, leaf area, starting soil moisture, month, stem length, stem weight, tree ID) as predictors explained 79.06% of the variance (out of the bag). The conditional permutation importance ranked month as the most important predictor closely followed by root weight and initial soil moisture (Fig. 8).
Discussion
Throughout most of the experiment (except in February), both treatments were a source of COS, which is in contrast to many previous lab and field studies (see Whelan et al. (2018) and Whelan et al. (2022) characterizing oxic soils as a COS sink. This is most likely due to the very nutrient rich potting soil used in the experiment, which is commonly used as a cultivation soil in gardening and cannot be compared to natural soils. The choice to use an “artificial soil” was made to provide an as much as possible homogeneous substrate across all replicates and throughout the duration of the experiment in order to focus on the root contribution and to use the same soil for the cultivation of the trees and the experiment itself. During the growing season, COS fluxes in chambers containing beech trees were significantly lower compared to the chambers containing only soil (Fig. 3), while higher CO2 emissions from those chambers indicate increased root and soil respiration. COS fluxes in February showed the reverse, with higher COS fluxes from the soil + roots chambers (Fig. 3). One explanation for the lower COS fluxes in chambers with higher biological activity, as indicated by increased CO2 emission, is the biotic uptake of COS by the ubiquitous enzymes of the carbonic anhydrase family, which have been shown to consume COS in microorganisms (Masaki et al. 2016; Ogawa et al. 2013, 2016) and can also be found in roots (Demir et al. 2009).
Since soil respiration was higher in the beech chambers in February compared to the soil chambers (Fig. 3), overall decreased activity and the resulting decrease in the transfer of metabolites to the soil, which could be hypothesized due to the missing leaves and therefore inability of the plant to photosynthesize, cannot be the sole explanation for the reverse behavior in February compared to the rest of the year. One reason might be differences in the microbial community composition. Izumi (2018) showed changes in rhizosphere species composition after the defoliation of birch and pine trees, which could lead to changes in COS emission and uptake behavior (Behrendt et al. 2019; Kitz et al. 2019; Meredith et al. 2019). A major process by which plants influence the soil is rhizodeposition (Haichar et al. 2014; Jones et al. 2009), a term encompassing root exudates, mucilage, border cells, and gases released by plant roots. The investment into the rhizosphere by plants is substantial, with an estimated 5–21% of the total carbon fixed by photosynthesis ending up being released into the rhizosphere via root exudates (Derrien et al. 2004; Lynch and Whipps 1990; Marschner et al. 2008; Nguyen 2003). Not only the quantity, but also the composition of root exudates varies greatly, including amino acids, sugars, enzymes, organic acids, fatty acids, sterols, growth factors, vitamins, flavonoids, alcohols, and nucleotides/purines (Dennis et al. 2010; Vives-Peris et al. 2020). Quantity and quality of root exudates not only depend on the species, but also on the time of the day (Hubbard et al. 2018; Staley et al. 2017), age, and external biotic and abiotic factors (Jones et al. 2004). Changes in the quality and quantity of rhizodeposition providing microorganisms with a different substrate could therefor change the COS flux (Behrendt et al. 2019; Conrad 1996). For example, compounds known to be involved in COS emission are thiocyanates (Conrad 1996), which can be exuded by roots (Halkier and Gershenzon 2006; Xu et al. 2017). But rhizodeposition during the off-season is poorly understood even though plant root activity during that period does not cease completely (Andresen and Michelsen 2005; Kaiser et al. 2010). The observed difference between root-influenced and pure soil in this experiment was primarily an offset, while the trend and diurnal cycle were not substantially different (see Figs. 4 and 5). Nevertheless, hypotheses I – a change in the COS flux, when roots are present – cannot be rejected by this experiment.
The observed diurnal cycle in COS exchange was primarily driven by other abiotic factors rather than light availability, which we hypothesized (hypothesis II) would influence the COS fluxes of the corresponding soil via photosynthetic activity of the plant. Soil temperature, which was identified in previous studies (Kitz et al. 2017; Meredith et al. 2018; Whelan and Rhew 2015) to be a decisive factor for the soil COS flux, especially COS emission, was the most important predictor for the diurnal variation in the soil COS flux (Fig. 6). While soil moisture, which linearly decreased over the duration of each measurement, due to the slow drying of the soil, since no water was added after the start of each experiment, was an important predictor in the random forest regression to explain differences in the root-only COS fluxes (Fig. 8). The COS flux trends further reveal that the faster drying by the soil due to transpiration by the beech trees did not affect the COS flux trend in comparison with the soil only chambers, even though soil moisture dropped faster and to a lower overall value at the end of the experiment. Previous lab studies (Bunk et al. 2017; Kaisermann et al. 2018; Whelan et al. 2016) have shown that soil moisture can have a strong impact on the soil COS flux, especially if the soil gets very dry. In this experiment the very organic potting soil was fairly moist and dried little over the course of the three-day measurements, resulting in a small range of observed soil moisture values, which most likely meant neither plant nor soil were water limited at any time. While both treatments were driven in a similar fashion by the abiotic drivers, the variable importance for the diurnal cycle was different, with COS fluxes in the soil + roots treatments primarily driven by soil temperature and COS fluxes in the soil only treatments primarily driven by biological activity. Even though those two variables, soil temperature and soil respiration, are closely linked, the observed difference might indicate a stronger contribution by soil microbiota, in the soil only chambers the only contributors to soil respiration, to the soil COS flux, in comparison to the direct root contribution. The lack of light as a significant predictor might be caused by a delayed impact on COS exchange, since the literature suggests a delayed response of root exudation to plant photosynthesis (Ekblad and Hogberg 2001; Nakayama and Tateno 2018), which could have further masked the response of the COS flux to soil temperature and plant light availability. The parallel diurnal cycle of the soil and the soil + roots treatments (Fig. 4) further supports the interpretation of a missing impact of plant light exposure to soil COS exchange, since the soil treatment was in opaque chambers and the potting soil therefore not exposed to light.
The differences in COS fluxes between the months from the soil + roots chambers throughout the year suggest a seasonal pattern, which confirms our third hypothesis, but the soil only chambers showed a strikingly similar seasonal pattern. For that reason, the root only component was calculated (Fig. 7), assuming COS uptake and emission would not change substantially with changing local COS concentrations in the soil pore space. The month of the experiment, a proxy for the different phenological stages of the beech trees, was still the second most important predictor in the random forest to differentiate between the root-only COS fluxes in the beech chambers. This suggests an impact by changing plant activity during the course of the season on the soil COS fluxes, especially earlier in the season, while the impact in summer and autumn was minimal in regard to the overall magnitude of the flux (Fig. 7). Differences in rhizodeposition quantity and quality throughout the season are well documented in the literature (Edwards et al. 2018; Kaiser et al. 2010; York et al. 2016). Plants change the allocation of their carbon throughout the year redistributing resources to meet their needs, which does not necessarily have to be recently fixed carbon (Klein and Hoch 2015). This ties into the dependence of the root-only COS flux on root weight (Fig. 7), which varies throughout the season, and is known to influence root exudate quantity, while the impact of root morphology, e.g. young vs. mature roots, identified by previous studies to influence root exudation quantity and composition (Iannucci et al. 2021; Proctor and He 2017), was not investigated in this study. COS flux trends throughout the year were similar between both treatments, indicating no clear impact of tree phenology on observed soil COS fluxes (Fig. 5). The observed difference in the COS flux trend between summer and spring/fall treatments might rather be due to the difference between greenhouse and lab temperature and as a consequence the soil temperature, which was largest in February, with an air temperature difference of approximately 15 °C. Possibly, the seasonal differences may also reflect associated changes in the microbial community composition (Devi and Yadava 2006; Edwards and Jefferies 2013).
Conclusion
Roots, a part of the ecosystem neglected so far, have an impact on the soil COS flux. In our study the presence of live roots decreased soil COS emissions, the suggested mechanisms are direct COS uptake by the roots themselves or root-mediated changes in microbiological composition or activity. But while the presence of live roots changed the overall magnitude of the COS flux, they did not, at least in this experiment, change the response of the soil to environmental parameters, like temperature and soil moisture. We have furthermore not seen an impact of the plant circadian clock on the soil COS flux. Research carried out so far that did use soil samples without live roots may have missed the influence of roots on the soil COS flux and might therefore not accurately reflect the COS flux regime in situ. Future research should try to disentangle the direct root contribution from the root-mediated contribution of the rhizosphere. Our experimental setup may also provide useful information for exploring the relationship between soil heterotrophic respiration and COS exchange, incorporated in some land surface models. Additional studies, exploring different plant species and (natural) soil types, are needed to further our understanding of the complex interactions, which lead to the surface COS exchange.
Data availability
The data used in the current study is available under the DOI https://doi.org/10.5281/zenodo.10021106.
References
Anav A, Friedlingstein P, Beer C, Ciais P, Harper A, Jones C, ..., Zhao MS (2015) Spatiotemporal patterns of terrestrial gross primary production: a review. Rev Geophys 53(3):785–818. https://doi.org/10.1002/2015rg000483
Andresen LC, Michelsen A (2005) Off-season uptake of nitrogen in temperate heath vegetation. Oecologia 144(4):585–597. https://doi.org/10.1007/s00442-005-0044-1
Asaf D, Rotenberg E, Tatarinov F, Dicken U, Montzka SA, Yakir D (2013) Ecosystem photosynthesis inferred from measurements of carbonyl sulphide flux. Nat Geosci 6(3):186–190. https://doi.org/10.1038/ngeo1730
Bates D, Maechler M, Bolker B, Walker S (2022) lme4: Linear Mixed-Effects Models using Eigen and S4. Retrieved from https://github.com/lme4/lme4/. Accessed 24 Feb 2023
Behrendt T, Catao ECP, Bunk R, Yi ZG, Schweer E, Kolb S, ..., Trumbore S (2019) Microbial community responses determine how soil-atmosphere exchange of carbonyl sulfide, Carbon Monoxide, and nitric oxide responds to soil moisture. Soil 5(1):121–135. https://doi.org/10.5194/soil-5-121-2019
Berkelhammer M, Asaf D, Still C, Montzka S, Noone D, Gupta M, Yakir D (2014) Constraining surface carbon fluxes using in situ measurements of carbonyl sulfide and carbon dioxide. Glob Biogeochem Cycles 28(2):161–179. https://doi.org/10.1002/2013gb004644
Berry J, Wolf A, Campbell JE, Baker I, Blake N, Blake D, ..., Zhu Z (2013) A coupled model of the global cycles of carbonyl sulfide and CO2: a possible new window on the carbon cycle. J Geophys Res-Biogeosci 118(2):842–852. https://doi.org/10.1002/jgrg.20068
Billesbach DP, Berry JA, Seibt U, Maseyk K, Torn MS, Fischer ML, Campbell JE (2014) Growing season eddy covariance measurements of carbonyl sulfide and CO2 fluxes: COS and CO2 relationships in Southern Great Plains winter wheat. Agric for Meteorol 184:48–55. https://doi.org/10.1016/j.agrformet.2013.06.007
Blonquist JM Jr., Montzka SA, Munger JW, Yakir D, Desai AR, Dragoni D, ..., Bowling DR (2011) The potential of carbonyl sulfide as a proxy for gross primary production at flux tower sites. J Geophys Res-Biogeosci 116. https://doi.org/10.1029/2011jg001723
Bunk R, Behrendt T, Yi ZG, Andreae MO, Kesselmeier J (2017) Exchange of carbonyl sulfide (OCS) between soils and atmosphere under various CO2 concentrations. J Geophys Res-Biogeosci 122(6):1343–1358. https://doi.org/10.1002/2016jg003678
Canto CD, Simonin M, King E, Moulin L, Bennett MJ, Castrillo G, Laplaze L (2020) An extended root phenotype: the rhizosphere, its formation and impacts on plant fitness. Plant J 103(3):951–964. https://doi.org/10.1111/tpj.14781
Conrad R (1996) Soil microorganisms as controllers of atmospheric trace gases (H-2, CO, CH4, OCS, N2O, and NO). Microbiol Rev 60(4):609–640
Crutzen PJ (1976) The possible importance of CSO for the sulfate layer of the stratosphere. Geophys Res Lett 3(2):73–76. https://doi.org/10.1029/GL003i002p00073
Debeer D, Hothorn T, Strobl C (2021) permimp: Conditional Permutation Importance. Retrieved from https://CRAN.R-project.org/package=permimp. Accessed 24 Feb 2023
Demir N, Nadaroglu H, Demir Y (2009) Carbonic anhydrase from Potato (Solanum tuberosum) roots and leaves. Asian J Chem 21(7):5104–5116
Dennis PG, Miller AJ, Hirsch PR (2010) Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol Ecol 72(3):313–327. https://doi.org/10.1111/j.1574-6941.2010.00860.x
Derrien D, Marol C, Balesdent J (2004) The dynamics of Neutral sugars in the rhizosphere of wheat. An approach by C-13 pulse-labelling and GC/C/IRMS. Plant Soil 267(1–2):243–253. https://doi.org/10.1007/s11104-005-5348-8
Devi NB, Yadava PS (2006) Seasonal dynamics in soil microbial biomass C, N and P in a mixed-oak forest ecosystem of Manipur, North-East India. Appl Soil Ecol 31(3):220–227. https://doi.org/10.1016/j.apsoil.2005.05.005
Dimou M, Paunescu A, Aivalakis G, Flemetakis E, Katinakis P (2009) Co-localization of Carbonic anhydrase and phosphoenolpyruvate carboxylase and localization of pyruvate kinase in roots and hypocotyls of etiolated Glycine max seedlings. Int J Mol Sci 10(7):2896–2910. https://doi.org/10.3390/ijms10072896
Dowle M, Srinivasan A (2022) data.table: Extension of ‘data.frame’. Retrieved from https://CRAN.R-project.org/package=data.table. Accessed 24 Feb 2023
Edwards KA, Jefferies RL (2013) Inter-annual and seasonal dynamics of soil microbial biomass and nutrients in wet and dry low-Arctic sedge meadows. Soil Biol Biochem 57:83–90. https://doi.org/10.1016/j.soilbio.2012.07.018
Edwards KR, Kastovska E, Borovec J, Santruckova H, Picek T (2018) Species effects and seasonal trends on plant efflux quantity and quality in a spruce swamp forest. Plant Soil 426(1–2):179–196. https://doi.org/10.1007/s11104-018-3610-0
Ekblad A, Hogberg P (2001) Natural abundance of C-13 in CO2 respired from forest soils reveals speed of link between tree photosynthesis and root respiration. Oecologia 127(3):305–308. https://doi.org/10.1007/s004420100667
Ensign SA (1995) Reactivity of carbon-monoxide dehydrogenase from rhodospirillum-rubrum with carbon-dioxide, carbonyl sulfide, and carbon-disulfide. Biochemistry 34(16):5372–5381. https://doi.org/10.1021/bi00016a008
Fox J, Weisberg S, Price B (2022) car: Companion to Applied Regression. Retrieved from https://CRAN.R-project.org/package=car. Accessed 24 Feb 2023
Groemping U (2021) relaimpo: Relative Importance of Regressors in Linear Models. Retrieved from https://CRAN.R-project.org/package=relaimpo. Accessed 24 Feb 2023
Gut A, Blatter A, Fahrni M, Lehmann BE, Neftel A, Staffelbach T (1998) A new membrane tube technique (METT) for continuous gas measurements in soils. Plant Soil 198(1):79–88. https://doi.org/10.1023/a:1004277519234
Haichar FE, Santaella C, Heulin T, Achouak W (2014) Root exudates mediated interactions belowground. Soil Biol Biochem 77:69–80. https://doi.org/10.1016/j.soilbio.2014.06.017
Halkier BA, Gershenzon J (2006) Biology and biochemistry of glucosinolates. Annu Rev Plant Biol 57(1):303–333. https://doi.org/10.1146/annurev.arplant.57.032905.105228
Hiltner L (1904) Über Neuere Erfahrungen und Probleme Auf dem Gebiete Der Bodenbakteriologie unter besonderer Berücksichtigung Der Gründüngung Und Brache. Arb DLG 98:59–78
Hubbard CJ, Brock MT, van Diepen LT, Maignien L, Ewers BE, Weinig C (2018) The plant circadian clock influences rhizosphere community structure and function. ISME J 12(2):400–410. https://doi.org/10.1038/ismej.2017.172
Iannucci A, Canfora L, Nigro F, De Vita P, Beleggia R (2021) Relationships between root morphology, root exudate compounds and rhizosphere microbial community in durum wheat. Appl Soil Ecol 158:10. https://doi.org/10.1016/j.apsoil.2020.103781
Izumi H (2018) Contrasting responses of the bacterial communities in ectomycorrhizal roots and rhizosphere soils to defoliation or winter hardening. Rhizosphere 8:8–11. https://doi.org/10.1016/j.rhisph.2018.08.002
Jones DL, Hodge A, Kuzyakov Y (2004) Plant and mycorrhizal regulation of rhizodeposition. New Phytol 163(3):459–480. https://doi.org/10.1111/j.1469-8137.2004.01130.x
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil-root interface. Plant Soil 321(1–2):5–33. https://doi.org/10.1007/s11104-009-9925-0
Kaiser C, Koranda M, Kitzler B, Fuchslueger L, Schnecker J, Schweiger P, Richter A (2010) Belowground carbon allocation by trees drives seasonal patterns of extracellular enzyme activities by altering microbial community composition in a beech forest soil. New Phytol 187(3):843–858. https://doi.org/10.1111/j.1469-8137.2010.03321.x
Kaisermann A, Ogée J, Sauze J, Wohl S, Jones SP, Gutierrez A, Wingate L (2018) Disentangling the rates of carbonyl sulphide (COS) production and consumption and their dependency with soil properties across biomes and land use types. Atmos Chem Phys Discuss 2018:1–27. https://doi.org/10.5194/acp-2017-1229
Kesselmeier J, Teusch N, Kuhn U (1999) Controlling variables for the uptake of atmospheric carbonyl sulfide by soil. J Phys Res 104(D9):11577. https://doi.org/10.1029/1999jd900090
Kitz F, Gerdel K, Hammerle A, Laterza T, Spielmann FM, Wohlfahrt G (2017) In situ soil COS exchange of a temperate mountain grassland under simulated drought. Oecologia 183(3):851–860. https://doi.org/10.1007/s00442-016-3805-0
Kitz F, Gomez-Brandon M, Eder B, Etemadi M, Spielmann FM, Hammerle A, Wohlfahrt G (2019) Soil carbonyl sulfide exchange in relation to microbial community composition: insights from a managed grassland soil amendment experiment. Soil Biol Biochem 135:28–37. https://doi.org/10.1016/j.soilbio.2019.04.005
Kitz F, Spielmann FM, Hammerle A, Kolle O, Migliavacca M, Moreno G, Wohlfahrt G (2020) Soil COS exchange: a comparison of three European ecosystems. Glob Biogeochem Cycles 34(4):15. https://doi.org/10.1029/2019gb006202
Klein T, Hoch G (2015) Tree carbon allocation dynamics determined using a carbon mass balance approach. New Phytol 205(1):147–159. https://doi.org/10.1111/nph.12993
Kourentzes N (2022) tsutils: time series exploration, modelling and forecasting. Available from https://github.com/trnnick/tsutils/. Accessed 24 Feb 2023
Lebigot EO (n.d.) Uncertainties: a Python package for calculations with uncertainties (Version 2.4.6.1). Retrieved from http://pythonhosted.org/uncertainties/. Accessed 24 Feb 2023
Liaw A, Wiener M (2002) Classification and Regression by randomForest. R News 2 (3):18–22
Lynch JM, Whipps JM (1990) Substrate flow in the rhizosphere. Plant Soil 129(1):1–10. https://doi.org/10.1007/bf00011685
Marschner B, Brodowski S, Dreves A, Gleixner G, Gude A, Grootes PM, Wiesenberg GLB (2008) How relevant is recalcitrance for the stabilization of organic matter in soils? J Plant Nutr Soil Sci 171(1):91–110. https://doi.org/10.1002/jpln.200700049
Masaki Y, Ozawa R, Kageyama K, Katayama Y (2016) Degradation and emission of carbonyl sulfide, an atmospheric trace gas, by fungi isolated from forest soil. FEMS Microbiol Lett 363(18):7. https://doi.org/10.1093/femsle/fnw197
Maseyk K, Berry JA, Billesbach D, Campbell JE, Torn MS, Zahniser M, Seibt U (2014) Sources and sinks of carbonyl sulfide in an agricultural field in the Southern Great Plains. Proc Natl Acad Sci USA 111(25):9064–9069. https://doi.org/10.1073/pnas.1319132111
McKinney W (2010) Data structures for statistical computing in python. Proceedings of the 9th Python in Science Conference, 445:56–61. https://doi.org/10.25080/Majora-92bf1922-00a
Meredith L, Boye K, Youngerman C, Whelan M, Ogée J, Sauze J, Wingate L (2018) Coupled Biological and Abiotic mechanisms Driving Carbonyl Sulfide production in soils. Soil Syst 2(3):37
Meredith LK, Ogee J, Boye K, Singer E, Wingate L, von Sperber C, ...., Welander PV (2019) Soil exchange rates of COS and CO(18)o differ with the diversity of microbial communities and their carbonic anhydrase enzymes. ISME J 13(2):290–300. https://doi.org/10.1038/s41396-018-0270-2
Milton GM, King KJ, Sutton J, Enright S (1998) Tracer studies of carbon source utilization in a wetland on the Canadian shield. Appl Geochem 13(1):23–30. https://doi.org/10.1016/s0883-2927(97)00050-4
Nakayama M, Tateno R (2018) Solar radiation strongly influences the quantity of forest tree root exudates. Trees-Structure and Function 32(3):871–879. https://doi.org/10.1007/s00468-018-1685-0
Nguyen C (2003) Rhizodeposition of organic C by plants: mechanisms and controls. Agronomie 23(5–6):375–396. https://doi.org/10.1051/agro:2003011
Ogawa T, Noguchi K, Saito M, Nagahata Y, Kato H, Ohtaki A, Katayama Y (2013) Carbonyl sulfide hydrolase from Thiobacillus thioparus strain THI115 is one of the beta-carbonic anhydrase family enzymes. J Am Chem Soc 135(10):3818–3825. https://doi.org/10.1021/ja307735e
Ogawa T, Kato H, Higashide M, Nishimiya M, Katayama Y (2016) Degradation of carbonyl sulfide by Actinomycetes and detection of clade D of beta-class carbonic anhydrase. FEMS Microbiol Lett 363(19):9. https://doi.org/10.1093/femsle/fnw223
Ogee J, Sauze J, Kesselmeier J, Genty B, Van Diest H, Launois T, Wingate L (2016) A new mechanistic framework to predict OCS fluxes from soils. Biogeosciences 13(8):2221–2240. https://doi.org/10.5194/bg-13-2221-2016
Ota M, Tanaka T (2019) Importance of root uptake of (CO2)-C-14 on C-14 transfer to plants impacted by below-ground (CH4)-C-14 release. J Environ Radioact 201:5–18. https://doi.org/10.1016/j.jenvrad.2019.01.012
Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH (2013) Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol 11(11):789–799. https://doi.org/10.1038/nrmicro3109
Proctor C, He YH (2017) Quantifying root extracts and exudates of sedge and shrub in relation to root morphology. Soil Biol Biochem 114:168–180. https://doi.org/10.1016/j.soilbio.2017.07.006
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing (Version 3.5.0), Vienna. Retrieved from https://www.R-project.org/. Accessed 24 Feb 2023
Rao S, Wu YY (2017) Root-derived bicarbonate assimilation in response to variable water deficit in Camptotheca acuminate seedlings. Photosynth Res 134(1):59–70. https://doi.org/10.1007/s11120-017-0414-7
RStudio Team (2016) RStudio: Integrated Development for R. RStudio Inc., Boston. Retrieved from http://www.rstudio.com/. Accessed 24 Feb 2023
Sandoval-Soto L, Stanimirov M, von Hobe M, Schmitt V, Valdes J, Wild A, Kesselmeier J (2005) Global uptake of carbonyl sulfide (COS) by terrestrial vegetation: estimates corrected by deposition velocities normalized to the uptake of carbon dioxide (CO2). Biogeosciences 2(2):125–132
Seefeldt LC, Rasche ME, Ensign SA (1995) Carbonyl sulfide and carbon-dioxide as new substrates, and carbon-disulfide as a new inhibitor, of nitrogenase. Biochemistry 34(16):5382–5389. https://doi.org/10.1021/bi00016a009
Seibt U, Wingate L, Lloyd J, Berry JA (2006) Diurnally variableδ18O signatures of soil CO2fluxes indicate carbonic anhydrase activity in a forest soil. J Geophys Res: Biogeosci 111(G4). https://doi.org/10.1029/2006jg000177
Seibt U, Kesselmeier J, Sandoval-Soto L, Kuhn U, Berry JA (2010) A kinetic analysis of leaf uptake of COS and its relation to transpiration, photosynthesis and carbon isotope fractionation. Biogeosciences 7(1):333–341
Smeulders MJ, Pol A, Venselaar H, Barends TRM, Hermans J, Jetten MSM, Op den Camp HJM (2013) Bacterial CS2 hydrolases from Acidithiobacillus thiooxidans strains are homologous to the archaeal catenane CS2 Hydrolase. J Bacteriol 195(18):4046–4056. https://doi.org/10.1128/jb.00627-13
Staley C, Ferrieri AP, Tfaily MM, Cui YY, Chu RK, Wang P, Sadowsky MJ (2017) Diurnal cycling of rhizosphere bacterial communities is associated with shifts in carbon metabolism. Microbiome 5:13. https://doi.org/10.1186/s40168-017-0287-1
Steinbacher M, Bingemer H, Schmidt U (2004) Measurements of the exchange of carbonyl sulfide (OCS) and carbon disulfide () between soil and atmosphere in a spruce forest in central Germany. Atmos Environ 38(35):6043–6052. https://doi.org/10.1016/j.atmosenv.2004.06.022
Stimler K, Montzka SA, Berry JA, Rudich Y, Yakir D (2010) Relationships between carbonyl sulfide (COS) and CO2 during leaf gas exchange. New Phytol 186(4):869–878. https://doi.org/10.1111/j.1469-8137.2010.03218.x
Sun W, Maseyk K, Lett C, Seibt U (2015) A soil diffusion-reaction model for surface COS flux: COSSM v1. Geosci Model Dev 8(10):3055–3070. https://doi.org/10.5194/gmd-8-3055-2015
Sun W, Kooijmans LMJ, Maseyk K, Chen HL, Mammarella I, Vesala T, ..., Seibt U (2018) Soil fluxes of carbonyl sulfide (COS), Carbon Monoxide, and carbon dioxide in a boreal forest in southern Finland. Atmos Chem Phys 18(2):1363–1378. https://doi.org/10.5194/acp-18-1363-2018
Van Diest H, Kesselmeier J (2008) Soil atmosphere exchange of carbonyl sulfide (COS) regulated by diffusivity depending on water-filled pore space. Biogeosciences 5(2):475–483
Vives-Peris V, de Ollas C, Gomez-Cadenas A, Perez-Clemente RM (2020) Root exudates: from plant to rhizosphere and beyond. Plant Cell Rep 39(1):3–17. https://doi.org/10.1007/s00299-019-02447-5
Whelan ME, Rhew RC (2015) Carbonyl sulfide produced by abiotic thermal and photodegradation of soil organic matter from wheat field substrate. J Geophys Res-Biogeosci 120(1):54–62. https://doi.org/10.1002/2014jg002661
Whelan ME, Hilton TW, Berry JA, Berkelhammer M, Desai AR, Campbell JE (2016) Carbonyl sulfide exchange in soils for better estimates of ecosystem carbon uptake. Atmos Chem Phys 16(6):3711–3726
Whelan ME, Lennartz ST, Gimeno TE, Wehr R, Wohlfahrt G, Wang Y,. .., Campbell JE (2018) Reviews and syntheses: Carbonyl sulfide as a multi-scale tracer for carbon and water cycles. Biogeosciences 15(12):3625–3657. https://doi.org/10.5194/bg-15-3625-2018
Whelan ME, Shi MJ, Sun W, Vries LKD, Seibt U, Maseyk K (2022) Soil carbonyl sulfide (OCS) fluxes in Terrestrial ecosystems: an empirical model. J Geophys Res-Biogeosci 127(9):13. https://doi.org/10.1029/2022jg006858
Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York
Wingate L, Seibt U, Maseyk K, OgÉE J, Almeida P, Yakir DAN, Mencuccini M (2008) Evaporation and carbonic anhydrase activity recorded in oxygen isotope signatures of net CO2fluxes from a Mediterranean soil. Glob Change Biol 14(9):2178–2193. https://doi.org/10.1111/j.1365-2486.2008.01635.x
Xu DY, Hanschen FS, Witzel K, Nintemann SJ, Nour-Eldin HH, Schreiner M, Halkier BA (2017) Rhizosecretion of stele-synthesized glucosinolates and their catabolites requires GTR-mediated import in Arabidopsis. J Exp Bot 68(12):3205–3214. https://doi.org/10.1093/jxb/erw355
Yi ZG, Wang XM, Sheng GY, Zhang DQ, Zhou GY, Fu JM (2007) Soil uptake of carbonyl sulfide in subtropical forests with different successional stages in south China. J Geophys Res-Atmos 112(D8):11. https://doi.org/10.1029/2006jd008048
York LM, Carminati A, Mooney SJ, Ritz K, Bennett MJ (2016) The holistic rhizosphere: integrating zones, processes, and semantics in the soil influenced by roots. J Exp Bot 67(12):3629–3643. https://doi.org/10.1093/jxb/erw108
Yu S, Zhang XX, Guan QJ, Takano T, Liu SK (2007) Expression of a carbonic anhydrase gene is induced by environmental stresses in Rice (Oryza sativa L.). Biotechnol Lett 29(1):89–94. https://doi.org/10.1007/s10529-006-9199-z
Acknowledgements
We thank Monika Wille for helping us with the root washing during the experiment.
Funding
Open access funding provided by Austrian Science Fund (FWF). The research was funded by the Austrian Science Fund (FWF) under Project P 31,669.
Author information
Authors and Affiliations
Contributions
Florian Kitz, Herbert Wachter and Georg Wohlfahrt contributed to the study conception and design. Material preparation was performed by Herbert Wachter and Florian Kitz. Data collection was performed by Florian Kitz and Felix M. Spielmann. Data analysis was performed by Florian Kitz and Albin Hammerle. The first draft of the manuscript was written by Florian Kitz and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
The authors have no relevant financial or non-financial interests to disclose.
Conflict of Interest
The authors have no conflicts of interest to declare that are relevant to the content of this article. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. The authors have no financial or proprietary interests in any material discussed in this article.
Additional information
Responsible Editor: Amandine Erktan.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 572 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kitz, F., Wachter, H., Spielmann, F. et al. Root and rhizosphere contribution to the net soil COS exchange. Plant Soil 498, 325–339 (2024). https://doi.org/10.1007/s11104-023-06438-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06438-0