Abstract
Background
Phosphorus (P) is an essential nutrient for plant growth, taking part in primary cellular metabolic processes as a structural component of key biomolecules. Soil processes as adsorption, precipitation, and coprecipitation can affect P bioavailability, leading to limited plant growth and excessive use of P fertilizers, with adverse impacts on the environment and progressive depletion of P reserves. To cope with P stress, plants undergo several growth, development, and metabolic adjustments, aimed at increasing P-acquisition and -utilization efficiency. Recently, strigolactones (SLs) have emerged as newly defined hormones that mediate multiple levels of morphological, physiological and biochemical changes in plants as part of the P acclimation strategies to optimize growth. Therefore, understanding the soil processes affecting P availability and P acquisition strategies by plants can contribute to improved agronomical practices, resources optimization and environmental protection, and the development of plants with high P use efficiency for enhanced agricultural productivity.
Scope
In this review, we discuss the range of abiotic processes that control P retention in soil and how different concentrations or degrees of P bioavailability can trigger various responses in plants, while critically highlighting the inconsistent conditions under which experiments evaluating aspects of P nutrition in plants have been conducted. We also present recent advances in elucidating the role of SLs in the complex P signalling pathway, with a special focus on what has been discovered so far in the model plant tomato (Solanum lycopersicum L.).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is one of the essential macronutrients required by plants for their growth and development (Vance et al. 2003). It constitutes about 0.2% of the plant’s dry matter, participates in primary cellular metabolic processes like photosynthesis and respiration, and is a structural component of key biomolecules such as sugar phosphates, nucleotides and nucleic acids, phospholipids, and energy-rich compounds, in particular adenosine triphosphate (ATP) (Abel et al. 2002; Aziz et al. 2014; Czarnecki et al. 2013; Marschner 1995; Schachtman et al. 1998; Vance et al. 2003). It also contributes to cellular signalling cascades by functioning as a mediator of signal transduction (Ha and Tran 2014).
The extensive use of P-based fertilizers in conventional agronomic practices has significantly fostered plant productivity, enabling agricultural production to support the exponential growth of the human population (Cordell et al. 2009). However, the majority of the P supplied through fertilizers is no longer bioavailable after application due to soil abiotic processes such as adsorption, immobilization, precipitation and coprecipitation, making P one of the most immobile, inaccessible, and unavailable among nutrients (Holford 1997; Niu et al. 2013; Richardson et al. 2009). Phosphorus limitation is estimated to affect crop productivity in over 40% of arable land (Vance 2001). Excessive use of P fertilizers to overcome this problem, coupled with low P utilization efficiency by plants, has resulted in large reserves of residual P in many arable soils (Bouwman et al. 2017). This has led to the progressive exhaustion of phosphate rock reserves and increased costs of P fertilizers, on one hand, and, on the other hand, to detrimental effects on the environment. In fact, a considerable amount of P applied to soil is subjected to erosion and runoff, with transfer from soil to waterbodies, causing algal blooms in lakes, river and coastal waters, as well as the formation of dead zones. All of these issues highlight the unsustainability of the current use of P (Ha and Tran 2014; Rothwell et al. 2020).
Plants have developed a plurality of strategies to cope with P deficiency, including a more efficient use of internal P through P recycling, modifications of the root system architecture traits, physiological adjustments and symbiotic interactions with arbuscular mycorrhizal fungi (AMF) intended to increase P acquisition from the soil (Dixon et al. 2020 and references therein). In recent years, a number of new signalling players and networks have emerged to regulate these responses (Rouached et al. 2010). In particular, several studies have established a major role for strigolactones (SLs), a group of carotenoid-derived compounds, as signalling molecules that trigger morphological, physiological and biochemical responses associated with plant acclimation to P shortage (Czarnecki et al. 2013 and references therein; Trasoletti et al. 2022). Experimental evidence supports the role of SLs as early modulators of plant response to low P availability. They are able to influence the expression of key regulatory genes and P transporters, and alter the metabolic profile of plants to improve their resilience to P limitation (Gamir et al. 2020). Low or suppressed transcription of SL biosynthetic or signalling genes can affect several plant processes, such as leaf senescence, root growth, and shoot branching (Ito et al. 2015; Koltai et al. 2010b; Yamada et al. 2014). Additionally, SL-deficient or SL-insensitive mutants are hypersensitive to a number of adverse conditions like salinity, nutrient deficiency, drought and osmotic stress, meaning that SLs positively regulate abiotic stress tolerance (Chi et al. 2021; Ito et al. 2015; Santoro et al. 2020, 2021; Yamada et al. 2014; Zheng et al. 2021; Zulfiqar et al. 2021). In this context, it has been shown that exogenous SL act as a biostimulant by increasing plant tolerance to combined water and nutrient stress (Kalozoumis et al. 2021). These effects are associated with the differential activation of other hormone signalling pathways, highlighting the pivotal and interconnected regulatory role of SLs in the adaptive response to plant stresses (Barbier et al. 2021; de Saint Germain et al. 2013; Ito et al. 2017; Jiu et al. 2022; Koltai et al. 2010a, b; Koltai 2011; López‐Ráez et al. 2008; Puga et al. 2017; Wang et al. 2022; Yang et al. 2019; Yoneyama et al. 2007a, b, 2012).
To address the challenges of climate change and environmental pollution, a better understanding of the regulatory network behind the activation of plant responses to P limitation can provide novel strategies for developing crops with improved P use efficiency. Among horticultural crops, tomato (Solanum lycopersicum L.) is one of the most valuable and widely consumed globally, and represents the cornerstone for biological research on genetic improvement of solanaceous species (Ercolano et al. 2012; López-Ráez and Bouwmeester 2008). In recent years, tomato has emerged as an important model species for research on SLs, leading to the generation of several tomato mutants with altered SL traits. The major SLs produced by this crop have been identified, and it has been established that there is a rapid surge in SL biosynthesis within its roots as a response to P starvation (Gamir et al. 2020; Kohlen et al. 2012; López‐Ráez et al. 2008; Marro et al. 2022; Rial et al. 2019; Vogel et al. 2010). Furthermore, tomato displays a strong P starvation response, characterized by the increase of root/shoot ratio and the secretion of acid phosphatases.
The targets of tomato breeding programs vary widely by location and individual needs, but often focus on enhancing yield, fruit quality, and resistance to pests and pathogens rather than improving nutrient use efficiency (Bai and Lindhout 2007). Nevertheless, using tomato cultivars with high P-use efficiency could allow for the same yield with a lower P input or higher yield with the same amount of P (Bera et al. 2020). Optimizing traits associated with or selecting P-efficient genotypes may promote better P mobilization in soil that would otherwise remain unavailable (Bera et al. 2020).
The purpose of this review is (i) to provide a comprehensive overview of the P processes within the soil–plant system, encompassing a wide range of abiotic processes responsible for the retention of inorganic and organic P in soil, including novel mechanistic insights into the coprecipitation phenomenon, and (ii) to discuss how these processes form soil P fractions that are largely unavailable for plants, thereby eliciting distinct plant responses to cope with P deficiency. Indeed, variation of P concentration in the soil solution during plant growth can lead to remarkably diverse outcomes even within the same plant species. Within the context of this review, we focused our attention on tomato as it serves as an ideal model for investigating the intricate relationship between SLs and the plant's response to P starvation. By examining these responses in tomato, we can gain valuable insights that can be extrapolated to other crop species. Moreover, through the evaluation of the specific mechanisms by which SLs participate in the signaling pathways triggered by P deficiency, we aim to shed light on their fundamental role as early modulators in the adaptive response of plants to P deprivation.
Soil abiotic processes controlling P bioavailability
The distribution, dynamics, and availability of P in soil are controlled by a combination of biological, chemical, and physical processes. It is crucial to understand these processes since a significant proportion of the P applied to croplands undergoes transformation into forms that are either unavailable or sparingly accessible (Giles et al. 2011). Indeed, soils globally contain a large amount of total P (roughly 90–200 × 1015 g, Liu et al. 2008 and references therein), but only a small proportion of it is immediately available for plant uptake under most soil conditions (Dixon et al. 2020), accounting for around 2500 × 1012 g of P globally (Liu et al. 2008 and references therein). Fertilizers contribute to a minor fraction, approximately 15–20%, of the total P content in plants, while the majority of P is derived from soil P reserves (Johnston and Steen 2000).
The primary source of P for plants is inorganic phosphate (Pi), in the form of orthophosphate ion (Fig. 1). The typical Pi concentration in the soil solution (0.1 to 10 µM) is substantially lower than the P requirement of plants (Frossard et al. 2000; Hinsinger 2001; Raghothama 1999), which varies across different species, ranging from several µM to tens of µM for highly demanding species, such as tomato (Hinsinger 2001 and references therein). Thus, at any given time, the soil solution holds only about 1% of the P required to sustain optimal plant growth, highlighting the need for a continuous replenishment of the element from the inorganic, organic, and microbial P pools in the soil, as P is continuously taken up by plants and microorganisms (Emsley 1980).
Orthophosphate (inorganic phosphate, Pi) structure and generic structures of organic P forms (based on Cade-Menun 2005). For inositol phosphate, the stereoisomer myo-inositol hexakisphosphate (myo-InsP6) is represented, in the 5-equatorial-1-axial conformation
Primary P minerals, such as apatites, strengite, and variscite, constitute a are very stable pool of P, and therefore the release of P from them is generally insufficient to meet crop demand (Shen et al. 2011). Nevertheless, research has demonstrated that the direct application of phosphate rocks can be relatively efficient in sustaining crop growth, particularly in acidic soils (Liu et al. 2008; Shen et al. 2011). Secondary P minerals including calcium (Ca), iron (Fe), and aluminum (Al) phosphates vary in their dissolution rates depending on mineral particles size and soil pH, and serve as an alternative source of P for plants (Shen et al. 2011).
In addition to P derived from primary and secondary minerals, which make up 35 to 70% of soil total P (Harrison 1987), various organic forms of P (Po) are also present (Fig. 1). The amount of Po depends on factors like vegetation type and climate, and it can account for 20 to 80% of the total P in the soil (Jarosch et al. 2015). Consequently, the Po pool holds the potential to serve as a significant source of P for plants, despite the slow release of phosphate by hydrolysis. However, this process can be accelerated by phosphatase and phytase enzymes present in the soil (Liu et al. 2008 and references therein).
Acidic soils tend to accumulate more total Po than alkaline soils due to the reaction of organic phosphates with Fe and Al, which renders them insoluble (Harrison 1987). The resulting salts or metal complexes of phosphate esters release phosphate slowly through hydrolysis. At later stages of soil development, P is transformed progressively into less-soluble Fe and Al associated forms, causing the Po contents of the soil to decline (Stewart and Tiessen 1987).
Recent advances in analytical chemistry, especially synchrotron-based X-ray absorption near-edge structure (XANES) and solution nuclear magnetic resonance (NMR) spectroscopies have greatly enhanced our understanding of P speciation at the molecular level (Fig. 1, Liu et al. 2017). A considerable portion of Po in many soils exists as stereoisomers of inositol hexakisphosphate (InsP6), with most inputs from plants being in the form of myo-inositol hexakisphosphate (myo-InsP6, Fig. 1), a storage compound of P in seeds. It is believed that the neo- and scyllo-stereoisomers are of microbial origin (Jørgensen et al. 2011; Liu et al. 2018), while the origins, dynamics, and biological function of the D-chiro-stereoisomer remain unknown, due in large part to analytical limitations in its measurement in environmental samples (Turner et al. 2012). Orthophosphate diesters, labile orthophosphate monoesters, and organic polyphosphates (Fig. 1), represent less stable forms of Po, that are likely present in the soil solution, where they can be readily degraded by biological processes (Celi and Barberis 2005; Condron et al. 2015; Turner et al. 2002).
All organic forms of P exist in complex equilibria with Pi (Fig. 2), forming a mixture of stable, sparingly available P, and plant-available P pools, such as labile P and soluble P. The relative sizes of the sources and stocks of P in the soil change as a function of soil development and, over time, the Po pool becomes the primary reservoir, especially in tropical soils, due to the insolubility and chemical stability of organic phosphates (Condron and Tiessen 2005). Inositol phosphates, in particular, make up over 60% of the soil orthophosphate monoesters and are the most common organic P species in mineral soils (Celi and Barberis 2007; Sims and Pierzynski 2018; Turner et al. 2002). This enrichment can also be attributed to soil microorganisms driving P immobilization in organic P compounds, and their subsequent mineralization to Pi (Dixon et al. 2020; Hinsinger 2001; Turner 2006). As a result, inositol phosphates are selectively enriched in soil relative to more labile P monoesters and diesters, such as glucose-1-phosphate, DNA fragments and phosphoglycerides (Darch et al. 2014; George et al. 2018; Magid et al. 1996).
Adsorption
Phosphorus strongly binds to the surfaces of Fe and Al (hydr)oxides, which are present in soil as positively charged nano- to micro-particles across a wide range of pH (3–8). Adsorption is a major process controlling P speciation and one of the most important rate-limiting factors of P release in soils. With aging, the adsorbed P may be occluded within nanopores of Al and Fe (hydr)oxides, further becoming unavailable to plants (Arai and Sparks 2007; Giaveno et al. 2008; Hauduc et al. 2015; Luengo et al. 2006).
The degree of P adsorption strongly depends on environmental factors (pH, ionic strength, P concentration in solution) and on the chemical and physical properties of the sorbent phase. These properties include mineral composition, functional groups, stability, particle size, porosity, specific surface area. One crucial parameter that determines the adsorption efficiency is the distance between the singly coordinated –OH groups on the adsorbent surface, which should match the –OH distance in the P molecule (Celi et al. 2020). From the point of view of the adsorbed molecule, it was shown that the adsorption affinity of different P compounds for soil mineral surfaces can be ranked in the following order: inositol phosphates > phosphate > glucose phosphates > DNA > phospholipids (Arai and Sparks 2007; Celi and Barberis 2007; Elzinga and Sparks 2007; Giles et al. 2011; Johnson et al. 2012; Li et al. 2016; Mallet et al. 2013).
In acidic soils, inorganic and organic P are adsorbed on the surface of Al and Fe (hydr)oxides, such as gibbsite, ferrihydrite, hematite, and goethite, by forming various surface complexes (Celi et al. 1999, 2020; Parfitt 1989). Iron and Al (hydr)oxides, in particular, are known to retain more inositol phosphates than other minerals, and poorly crystalline forms are responsible for retaining the majority of P in soils (Celi and Barberis 2007; Jiang et al. 2015; Turner et al. 2007). Ferrihydrite, a poorly crystalline Fe oxide, is one of the most efficient P-retaining Fe (hydr)oxides, due to the small size of its particles, which provides a large surface area for P adsorption (Celi et al. 1999, 2003; Chen et al. 2020; Giaveno et al. 2008; Johnson et al. 2012). In the case of Pi, the non-protonated and protonated bidentate complexes on the surface of ferrihydrite (Fig. 3) may coexist at pH 4 to 9, while the protonated bidentate inner-sphere complex is predominant under acidic soil conditions (Atkinson et al. 1974; Arai and Sparks 2001; Elzinga and Kretzschmar 2013; Persson et al. 1996). The formation of bidentate mononuclear and monodentate mononuclear complexes has also been proposed, with varying degrees of protonation but weak bond energy (Persson et al. 1996). In the case of Al (hydr)oxides, Pi adsorption is characterized by a maximum at pH 4.0 (Huang et al. 2009 and references therein; Tanada et al. 2003), and the process is highly selective for Pi even in complex solutions and involves ion-exchange between Pi and the –OH groups on the surface of the mineral (Tanada et al. 2003).

Adapted from Celi et al. 2022
Adsorption mechanisms of P on Fe (hydr)oxides via i) a bidentate binuclear ligand exchange mechanism (orthophosphate (Pi) and organic P monoesters), and ii) a monodentate mononuclear ligand exchange mechanism (organic P diesters). The retention of organic P compounds on mineral surfaces reduces phosphatase activity and inhibits phytase activity. Plant and microorganisms can produce protons (H+), organic acid anions and electron donors to release the organic P compounds from minerals for enzymatic hydrolysis and plant uptake.
Both inner- and outer-sphere surface complexes can play a role in the stabilization of Po in soil (Celi and Barberis 2005; Chen et al. 2020; Johnson et al. 2012; Ognalaga et al. 1994; Ruyter-Hooley et al. 2015; Yan et al. 2014). For a long time, the association between inositol phosphates and poorly crystalline Fe (hydr)oxides minerals was considered the most important reason for the stabilization of inositol phosphates in soils (Fig. 3, Celi et al. 1999, 2001, 2003; Celi and Barberis 2005). Indeed, the high anionic charge density of these molecules enables their interaction with positively charged surfaces (Fuentes et al. 2014; Giaveno et al. 2008). In this regard, Chen and Arai (2019) observed that the phosphate groups P1, P3 and P2 of myo-InsP6 (cfr. Figure 1) contribute to the creation of inner-sphere complexes at the ferrihydrite-water interface, whereas adsorption on Al hydroxides involves a combination of both outer- and inner-sphere processes (Guan et al. 2006; Ruyter-Hooley et al. 2015). Since adsorption is believed to take place through only some of the six phosphate groups, the remaining groups can affect the mineral surface characteristics through steric hindrance or altered charge, or can change particle dispersion/flocculation equilibria (Giles et al. 2011 and references therein).
The retention of P can be also affected by the crystallinity and stability of metal oxides. According to Celi et al. (2020), hematite showed lower retention of both Pi and Po compared to goethite and maghemite. Maghemite exhibited greater P retention than goethite, but was found to be less stable and more easily dissolved by organic acid anions like oxalate (Celi et al. 2020). As a result, highly weathered and Fe-rich soils may experience a reduction in inositol phosphate accumulation due to weaker binding of the molecule to the oxide surface, as well as increased efficiency of plant exudates in removing P from the solid phase (Fig. 3) (Celi et al. 2020; Giaveno et al. 2008). Studies on the adsorptive effects of the combined solution of Pi and inositol phosphates showed that the latter inhibited Pi adsorption and caused the desorption of Pi adsorbed on ferrihydrite, an effect which was also observed in soil (Anderson et al. 1974; Berg and Joern 2006; Bowman and Moir 1993; De Groot and Golterman 1993).
The type of interaction of P diesters with soil minerals can vary considerably depending on their molecular properties. Iron (hydr)oxides, for example, adsorb DNA via the backbone phosphate (Fig. 3), but are influenced by the steric hindrance of the nucleotides (Liu and Liu 2014, 2015; Yiu et al. 2013). Extracellular polymeric substances secreted by microorganisms, lipopolysaccharides and membrane-bound proteins located on the outer surface of bacterial cells were found to be involved in bacterial adhesion to Fe (hydr)oxides by forming inner-sphere complexes (Cagnasso et al. 2010; Parikh and Chorover 2006). In a recent study, Santoro et al. (2019) observed that the phospholipid phosphatidylcholine was retained on the surface of ferrihydrite only through a solid/liquid partitioning, possibly forming a bilayer configuration around the Fe core, supporting results from previous studies (De Cuyper and Joniau 1991; Denizot et al. 1999). Conversely, Cagnasso et al. (2010) reported that the interaction of phosphatidylcholine with the surface of goethite and hematite occurs only through electrostatic attraction, which explains why these compounds are more easily biodegraded and do not accumulate in the environment (Magid et al. 1996).
Organic P adsorption on Al minerals has been recently reviewed by Yan et al. (2023), who reported that the maximum adsorption increases with decreasing crystallinity of Al (hydr)oxides, following the order: α-Al2O3 < boehmite < amorphous Al hydroxide. Adsorption density is as well affected by the particle size of the minerals and the Po molecular size, in the order: InsP6 < adenosine triphosphate < glucose-6-phosphate < glycerophosphate, i.e., the maximum adsorption densities increased with decreasing Po molecular size (Yan et al. 2023). Despite having the largest molecular size, InsP6 has been shown to adsorb in greater amounts on amorphous Al hydroxide than other Po compounds because of the transformation of surface complexes to surface precipitates (Yan et al. 2023 and references therein).
In neutral-to-calcareous soils, P can also be adsorbed on the surface of Ca carbonate, clay minerals and organic matter (OM) (Shen et al. 2011). Calcite exhibits a high capacity for P retention, with a stronger effect observed for InsP6 than for Pi. The reaction involves adsorption followed by precipitation of Ca salts (Celi and Barberis 2007). Clay minerals adsorb less organic P than Fe and Al oxides, but with a higher affinity (Celi and Barberis 2005). The adsorption reaction of Pi and Po on clays is not readily reversible, although some P can be released into the solution. The desorption phenomenon depends on factors as pH, percentage of P saturation and presence of competing ligands such as citrate, oxalate or carbonate (Celi and Barberis 2007 and references therein).
Finally, soil OM can participate in the retention of organic P in soil. This can occur through physical or chemical incorporation of organic P in the OM fraction, direct adsorption on the organic surfaces by ionic or hydrophobic interactions or indirect adsorption through polyvalent cations that act as bridges to form ternary complexes (Celi and Barberis 2007 and references therein).
Precipitation and coprecipitation
At low concentrations in the soil solution, P tends to interact with soil components through adsorption or anion exchange, forming weak and reversible electrostatic bonds. High P concentrations, mainly resulting from the application of P fertilizers, can instead cause the precipitation of metal phosphates, following a partial dissolution of soil minerals (Celi et al. 2000, 2020; Celi and Barberis 2005; Sample et al. 2015; Yan et al. 2014). pH values below 6 can in fact promote the dissolution of Al and Fe oxides and hydroxides, and the resulting Fe and Al ions can precipitate directly with P in solution. Phosphorus from precipitated Fe and Al phosphates is apparently more available to plants than most of the P fixed on soil particles, as discussed later in this review.
In calcareous soils, the formation of Ca phosphates is favored by the presence of soluble Ca, which occurs in soil as Ca ions bound to cation exchange sites, different Ca minerals, or residual limestone (Penn and Camberato 2019). Many chemical fertilizers also contain highly soluble Ca phosphates, which can saturate the soil solution with both Ca and P, leading to the precipitation of sparingly soluble minerals such as brushite, monetite and amorphous forms. Over time, these minerals may transform into more stable minerals, such as hydroxyapatite (Essington 2021; Lindsay 1979).
Complexation and precipitation of organic P with polyvalent cations enhance its retention in the colloidal phase. These processes result in a significant portion of organic P being stabilized by mineral components, thereby rendering it unavailable to plants (Celi and Barberis 2007; Giles et al. 2011; Jiang et al. 2015). The ability to form complexes or precipitates mainly depends on the number of phosphate groups present in the organic P molecule (Celi and Barberis 2005). For example, the six phosphate groups of InsP6 make it a strong ligand in soils, with a high ability to bind metal cations producing complexes that are soluble at low pH and insoluble at mid-range pH values (Chen et al. 2020; Martin 1987; Nolan et al. 1987). In soils and sediments, the complexation of P, especially with Fe and Ca and their minerals, can increase Po stabilization, thus turning most of the labile and moderately labile organic P compounds into more resistant forms (Harrison 1987; House and Denison 2002; Zhang et al. 1994).
Apart from adsorption and precipitation, P coprecipitation with Fe resulting from changes in pH, redox potential or ionic strength is a common process in waters and sediments. To date, only a few studies have investigated this process and the interactions between P and Fe dynamics under alternate redox conditions, and even fewer have distinguished between inorganic and organic P coprecipitation. More attention was instead posed to the effect of dissolved OM on the coprecipitation with Fe, which could in any case have indirect effects on P accumulation (Angelico et al. 2014; Colombo et al. 2015; Jones et al. 2015; Kashyap et al. 2014; Mikutta et al. 2008, 2014; Pédrot et al. 2011; Pham and Waite 2008; Shimizu et al. 2013; Sodano et al. 2017; Sundman et al. 2016).
If the soil solution contains Fe(II) as a result of Fe (hydr)oxides reductive dissolution, the occurrence of oxidizing conditions can lead to its oxidation and subsequent precipitation as Fe(III) (hydr)oxides. These minerals have the ability to effectively retain many inorganic and organic anions, including both Pi and Po (Gorra et al. 2012; Huang et al. 2013; Mikutta et al. 2014). When phosphate is incorporated into the nanoparticles of the newly formed precipitate, its retention is significantly enhanced compared to a simple adsorption mechanism (Châtellier et al. 2004, 2013; Luo et al. 2022; Santoro et al. 2019; Senn et al. 2015; Thibault et al. 2009; van der Grift et al. 2016; Voegelin et al. 2013). The presence and concentration of Pi play a significant role in influencing the kinetics and mechanisms of Fe oxidation and precipitation, promoting the formation of Fe(III) phosphates instead of crystalline Fe(III) oxides (Santoro et al. 2019; Thibault et al. 2009; van der Grift et al. 2016; Voegelin et al. 2013). In this regard, Thibault et al. (2009) and Voegelin et al. (2013) observed that even low Pi concentrations inhibit the formation of crystalline Fe (hydr)oxides in favor of less crystalline forms. In support of these studies, Santoro et al. (2019) demonstrated the formation of Fe (hydr)oxides nanoparticles with short-range crystalline order at low Pi concentration, and of amorphous Fe(III) phosphate at increasing Pi loadings. These results suggest that Pi disrupts the overall structural order of the oxide, leading to smaller sizes of the precipitated particles due to Pi surface poisoning (Châtellier et al. 2004; Thibault et al. 2009; van der Grift et al. 2016; Voegelin et al. 2013). The maximum amount of Pi associated with ferrihydrite particles was observed at a P/Fe ratio of approximately 0.5–0.6, as beyond this point any surplus Pi did not cause additional distortion to the crystal lattice or change to the crystal size (Santoro et al. 2019; Thibault et al. 2009; Voegelin et al. 2013).
Once formed, the stability of the coprecipitated phases could further impact on the bioavailability of the occluded P for plants and microbial communities after the dissolution of Fe oxides (Jiang et al. 2015). The lack of crystallinity of the formed phases suggests their lower stability and higher solubility compared to Fe oxide phases (Châtellier et al. 2013). Mayer and Jarrell (2000) in fact observed the release of over 75% of the retained Pi after coprecipitation, probably due to coagulation and crystalline growth of Fe oxides which expelled P back into solution. Aging of ferrihydrite has been shown to decrease the surface area and the concentration of surface sites per mass unit, releasing P into solution and, as sorption of Pi reduces the positive charge of particles, this can induce aggregation and release of retained ions into solution (Mayer and Jarrell 2000).
Organic P coprecipitation may exhibit different behavior. Inositol phosphate can be retained within the forming system up to a P/Fe ratio of 1, with rapid precipitation kinetics due to its strong affinity for Fe (Santoro et al. 2019). This results in the rapid formation of Fe-InsP6 species that act as crystallization nuclei, accelerating crystal growth on lateral planes while incorporating the organic P compound within the structure. The coprecipitation process involves both adsorption on the newly-formed surfaces of ferrihydrite with subsequent particle aggregation due to electrostatic interactions, and precipitation of the Fe-InsP6 salt at higher P concentrations (Santoro et al. 2019). Chen et al. (2020) reported similar results, showing that coprecipitated Fe-InsP6 material did not cause the transformation of the background ferrihydrite into goethite or hematite during aging. In contrast, the coprecipitation of P diesters, such as phosphatidylcholine with Fe, did not modify the mineral properties of ferrihydrite (Santoro et al. 2019), and resulted in the formation of a phospholipid bilayer on the oxide surface, retained by weak non-specific forces, which may contribute to the fast turnover of this class of molecules in the soil. Similarly, coprecipitation experiments using bacterial cells, whose outer structure consists of a phospholipid bilayer with protein inclusions, did not alter the mineralogy of the forming secondary Fe-oxides. However, the presence of bacterial cells (and the macromolecules they can release) can affect the size, shape, and spatial organization of the mineral particles (Châtellier et al. 2004).
Plant strategies for unlocking unavailable soil P fractions: a focus on strigolactones and the model plant tomato
The abiotic processes described so far, i.e., adsorption, precipitation and coprecipitation, can lead to soil P fractions that are strongly unavailable for plants. In response to this P-deficiency, plants activate complex regulatory mechanisms known as P starvation responses (PSRs) that include genetic, physiological, and morphological changes aimed at optimizing the so-called P use efficiency (PUE) (de Souza Campos et al. 2019). It encompasses strategies of P acquisition from soil, translocation from root to shoot, allocation, utilization, and remobilization within the plant (He et al. 2019). These strategies typically involve modifications in plants that enhance either P acquisition or P utilization efficiency (PAE and PUtE, respectively) (Dixon et al. 2020; Vance et al. 2003). Nutrient acquisition efficiency is an indicator of the plant’s ability to absorb poorly available nutrients as P, while nutrient utilization efficiency estimates the capacity of a plant to produce maximum yield per unit of nutrient absorbed (Aziz et al. 2014). Phosphorus starvation responses aimed at increasing PUE include reduced growth rate, remobilization of internal P through scavenging and recycling enzymes, alteration of carbon (C) metabolism, utilization of alternative respiratory pathways, and biosynthesis of low-P membranes (Aziz et al. 2014 and references therein). To enhance P uptake, plants can exude P-solubilizing enzymes and low molecular weight organic acids, acidify the rhizosphere, modify root architecture and root hair development, engage in symbiotic interactions with mycorrhizae or increase the expression and activity of high-affinity P transporters (Aziz et al. 2014; de Souza Campos et al. 2019). Overall, the PSRs and PUE mechanisms play important roles in helping plants to adapt to P-deficient soils and can help improve plant growth and yield in low-P environments.
The various strategies harbored by plants to increase P acquisition and respond to P deficiency have been extensively reviewed in the past (e.g., Aziz et al. 2014; Bais et al. 2006; Hinsinger 2001; Hodge 2004; Lambers et al. 2006; Niu et al. 2013; Péret et al. 2011, 2014; Shen et al. 2011; Ticconi and Abel 2004; Vance et al. 2003). The following sections will therefore focus on the recent discoveries regarding PSRs in the model plant tomato and the influence of SLs on such responses.
Before going deeper in the following sections, we do have to highlight the important need of appropriate conditions to mimic the real P availability in soil. Indeed, in existing literature, most of the in vitro and field experiments have primarily focused on studying the mechanisms of plant adaptation to a steady low P concentration (Wissuwa et al. 2005). However, it is important to recognize that these conditions may not accurately reflect the natural variation of P availability in soil, which can undergo temporal and spatial changes. Consequently, the strategies employed by plants to adapt to fluctuating P availability have received poor attention. Typically, small-scale studies employ "P deficiency" concentrations that exceed the optimal amount of P required for plant growth, as noted by de Groot et al. (2003). This can lead to plants acclimating to high P concentrations, potentially concealing or delaying the onset of the canonical P starvation responses. Table 1 highlights the broad range of P concentrations used in research papers, along with the specific roles of SL in plant responses to P deficiency. Therefore, when studying plant P starvation responses in soil-less systems, it is crucial to establish appropriate P conditions that support optimal plant growth prior to imposing P shortage. This approach is essential to prevent an excessive accumulation of P that plants may later rely on as a reserve.
Furthermore, plant responses may not solely depend on the initial P concentration, but also on the temporal variation of P availability throughout the experiment. In fact, it must be recognized that in field the amount of P available in the soil solution can fluctuate according to seasonality and fertilization practices, leading the plants to adapt to gradual or sudden decreases in P availability (Fan et al. 2014; Rubaek and Sibbesen 1995). An additional factor to consider is that a significant proportion of P in soil exists as sparingly available sources, which accumulate in the soil, as highlighted in the previous section, forming the so called legacy-P (Lun et al. 2018; Shen et al. 2011).
From a research perspective, these considerations translate into the need to overcome the use of nutrient solutions (e.g., Hoagland) routinely adopted in most studies investigating plant nutrition responses conducted in soil-less systems, where a soluble source of P such as ammonium, sodium or potassium phosphate is provided (Gerloff 1987). These hydroponic solutions are unbuffered, contain high soluble P concentrations and cannot simulate a real soil solution, which is strongly buffered, chemically heterogeneous, and complex (Bera et al. 2020). As a result, recent research works include a sparingly available P source to represent the conditions affecting P availability in soil more realistically. For example, Bera et al. (2020) formulated a simulated soil solution using insoluble phosphate, which contained high total P but low plant-available P. Other authors added soluble organic P forms (see for instance Zhou et al. 2021), P forms adsorbed on (e.g., Martin et al. 2004) or precipitated with (e.g., Santoro et al. 2022) Fe oxides, Fe/Al/Ca phosphates (e.g., Edayilam et al. 2020), or phosphate rocks (e.g., Rezakhani et al. 2019) in soil-less systems. Table 2 reports a list of papers with related experimental details in which scarcely available P forms were used to study plant responses to P deprivation.
Strigolactones: chemistry, biosynthesis, and production by plants
The collective term “strigolactones” was coined to designate a small group of compounds that are secreted from the roots of various plant species and induce seed germination in root parasitic plants of the genus Striga, commonly referred to as witchweeds (Butler 1995). After the discovery of strigol in cotton root exudates as a germination stimulant of Striga lutea (Cook et al. 1966), other SLs have been identified in exudates of a number of plant species, and have been shown to stimulate seed germination in root parasitic plants also of the genera Orobanche, Phelipanche and Alectra, resulting in significant losses in agricultural production worldwide (reviewed in Xie et al. 2010). Among the plant secondary metabolites known to induce seed germination of root parasites, SLs are the most active, functioning at concentrations of 10–7–10–15 M (Xie et al. 2010). For over 40 years SLs were known only as germination stimulants and were therefore considered to be waste or harmful metabolites until their function as a host-recognition signal for AMF was discovered by Akiyama et al. (2005). About 80% of land plants engage in symbiosis with these soil-borne microorganisms, in which organic C from the plant is exchanged for minerals absorbed by the fungus through an extensive network of hyphae (Gutjahr and Parniske 2013; Schmitz and Harrison 2014). This symbiotic relationship enables plants to access and acquire P more efficiently. Interestingly, the production of SLs in non-mycotrophic plant such as Arabidopsis, white lupin and spinach suggests that these molecules may have other roles in plant biology beyond their involvement in the AMF symbiotic pathway (Yoneyama et al. 2008). In 2008, Gomez-Roldan et al. (2008) and Umehara et al. (2008) independently classified SLs, or their downstream metabolites, as a novel class of phytohormones that function as long-distance branching factors regulating shoot branching by suppressing the growth of preformed axillary shoot buds. In their respective studies, both research groups demonstrated the effect of SLs on shoot branching through the application of synthetic SL analogue GR24 (Fig. 4d). In the study by Gomez-Roldan et al. (2008), the application of GR24 to shoot branching mutants of pea resulted in the restoration of the wild-type branching phenotype. Similarly, Umehara et al. (2008) observed the same phenomenon in rice mutants upon treatment with GR24.
Chemical structure of strigolactones (SLs). Naturally occurring SLs can be divided into two families: a) the strigol family and b) the orobanchol family, based on stereochemistry around the BC rings. Chemical differences within a family are related to substitutions on the A or B rings. All naturally occurring SLs found to date display C2'-(R) stereochemistry via the enol-ether bridge that connects the C and D rings; c) ‘Non-canonical’ SLs (lacking the tricyclic lactone); d) GR24, shown as its two stereoisomers, is the most used synthetic SL. Based on Bürger and Chory 2020
In addition to their role in controlling shoot branching, SLs are relevant molecules that modulate the coordinated development of roots (e.g., primary and lateral root development, root hair elongation) and shoots (Akiyama et al. 2005; Gomez-Roldan et al. 2008; Kapulnik et al. 2011; Koltai 2011). Moreover, SLs have been implicated in the regulation of leaf senescence, secondary growth, reproduction (including flower and seed setting), and protection against pathogens and root-knot nematodes (Decker et al. 2017; Xu et al. 2019). Numerous studies have also proved that SLs mediate part of the molecular, biochemical and morphological responses needed for plants to acclimate to nutritionally poor environments (i.e., P deficiency) (Andreo-Jimenez et al. 2015; Brewer et al. 2013; de Souza Campos et al. 2019; Decker et al. 2017; Ito et al. 2017; Koltai 2013; Marzec et al. 2013; Sun et al. 2016; Yamada et al. 2014), water deprivation (Cardinale et al. 2018; Visentin et al. 2016), low light stress (Mayzlish-Gati et al. 2012), and heat and/or cold stress (Chi et al. 2021).
To date, 30 canonical SLs have been characterized from plant root exudates (Yoneyama and Brewer 2021), with a tricyclic lactone (ABC ring) and 2’R configured butenolide ring (D ring), being these two structural features required for biological activity (Fig. 4) (Flematti et al. 2016; Scaffidi et al. 2014). Strigol- and orobanchol-like SLs differ for the stereochemistry of the B-C-ring junction: the C ring of the strigol-like SLs is in the β orientation, whereas that of orobanchol-like SLs is in the α orientation (Fig. 4a,b) (Al-Babili and Bouwmeester 2015). The AB part in both families can be modified through methylation, hydroxylation, epoxidation or ketolation, giving rise to the diversity of SLs.
Strigolactones are mainly biosynthesized in the roots, where they are produced at very low concentrations (in the pico- and nanomolar range), typically as a blend of molecules specific to the species. The biosynthesis occurs via the carotenoid pathway (Matusova et al. 2005), with the core biosynthetic module comprising the DWARF27 (D27) isomerase and carotenoid cleavage dioxygenase 7 (CCD7) and 8 (CCD8) that work sequentially to convert β-carotene to SLs universal precursor carlactone (Alder et al. 2012; Jia et al. 2018; Machin et al. 2020). Supporting the role of CCD7 and CCD8 in SL biosynthesis, tomato plants with antisense SlCCD7 construct exhibit increased branching and their root extracts induced 90% less germination of Orobanche ramosa seed compared to their wild-type (Koltai et al. 2010b; Vogel et al. 2010). On the other hand, tomato plants with RNAi-silenced SlCCD8 showed increased shoot branching, reduced plant height and reduced SL exudation (Kohlen et al. 2012). These SL mutants represent a powerful tool to unravel the capacity of SLs to influence and control plants physiological processes, and in particular the responses to nutritional stresses. By catalyzing repeated oxygenation reactions that can be coupled to ring closure, the CYP711A subfamily of cytochrome P450 oxygenases then convert carlactone into tricyclic-ring-containing canonical (Fig. 4a,b) and non-canonical (Fig. 4c) SLs (Kyozuka et al. 2022). Modifying enzymes further increase the diversity of SLs (as reviewed in Mashiguchi et al. 2021). In tomato, SlCYP722C was demonstrated to convert carlactone to orobanchol, probably via 18-hydroxy-carlactone (Wakabayashi et al. 2019).
Strigolactones function in plants through a signal transduction pathway mediated by receptor proteins (Xu et al. 2021). In SL-insensitive dwarf 14 (d14) rice mutants, an α/β-fold hydrolase family protein was found to be implicated in SL signalling for the regulation of shoot branching (Arite et al. 2009; Ruyter-Spira et al. 2013), together with the F-box protein D3/MORE AXILLARY GROWTH2 (MAX2), which is part of the complex with the Skp1-Cullin-F-box (SCF) E3 ubiquitin ligase complex (Barbier et al. 2019 and references therein; Smith and Waters 2012; Xu et al. 2021).
F-box genes, including MAX2, also play a pivotal role in P starvation responses in tomato and are plausible targets of the transcription factor PHR1, which regulates most typical P starvation responses (Akash et al. 2021; Srivastava et al. 2021). In parallel, karrikins (KARs), smoke-derived butanolide signalling compounds, are perceived by a D14 homolog, KARRIKIN INSENSITIVE2 (KAI2) (Ahmad et al. 2020; Yang et al. 2019). Although only three mutations seem to be required to turn the non-SL receptor KAI2 into a receptor able to recognize SL (Arellano-Saab et al. 2021), the biological functions of KARs and SLs in plant growth regulation are different. Karrikins, for instance, are not active in hyphal branching of AMF or in inhibition of shoot branching (Xie et al. 2010; Yang et al. 2019), and in the model plant Arabidopsis thaliana, KAI2-mediated signalling alone regulates root hair density and length as well as root skewing, straightness and diameter, seed germination and stress response, while both KAI2 and the SL receptor D14 pathways regulate shoot branching, lateral root density and epidermal cell length (Kramna et al. 2019; Kyozuka et al. 2022; Villaecija-Aguilar et al. 2019).
Many studies suggest that the production of SLs is species-specific and, within individual species, different varieties can generate different SLs and/or mixtures of them (as reviewed in Ćavar et al. 2015). In tomato, orobanchol, solanacol and the two isomers of didehydro-orobanchol are the main SLs produced, but several additional SLs have been detected in root exudates and extracts, including strigol-type SLs (Kohlen et al. 2013; López‐Ráez et al. 2008; Zhang et al. 2018a). Since SLs have a dual role in plant development adjustments in response to nutritional stresses and in the establishment of symbiosis with AMF, their levels in roots and root exudates are strongly influenced by the availability of nutrients, particularly P. When P becomes scarce, SL biosynthesis and exudation increase (López‐Ráez et al. 2008; Yoneyama et al. 2007b, 2012; Umehara et al. 2010). In tomato, SL exudation is enhanced by the lack of external P, not nitrogen (N) (Yoneyama et al. 2012). Rial et al. (2019) and Marro et al. (2022) reported the presence of orobanchol (2 μg L−1) and solanacol (60 μg L−1) in the exudates of P-starved tomato plants. Notably, these compounds were not found in the exudates or root extracts of P-sufficient plants. Similarly, Gamir et al. (2020) observed a significant increase in the quantities of orobanchol and solanacol in tomato roots after one week of P starvation.
It is not surprising that SLs are produced in low concentrations in plants, given their potent activity at concentrations as low as 10–13 M for the establishment of the symbiosis with AMF (Akiyama and Hayashi 2006), 10–7 to 10–15 M for the germination of seeds of parasitic weeds (Xie et al. 2010), and 10–8 M for GR24 effect on lateral buds (Gomez-Roldan et al. 2008). An intriguing aspect of SL production is that the relative amount of SLs varies between root exudates and tissues, and different results can be obtained even under similar plant growing conditions (Zhang et al. 2018a). For example, Floková et al. (2020) detected similar amounts of orobanchol and solanacol (around 1 μg L−1) in root exudates of P-deficient tomato plants. However, the concentrations differed considerably in root tissue extracts, with solanacol being more than twice orobanchol. In a previous similar experiment, Rial et al. (2019) reported very different amounts of orobanchol and solanacol in tomato root exudates and extracts. Specifically, solanacol was found to be 35 times more abundant than orobanchol in root exudates, whereas similar concentrations for each SL were determined in root exudates and extracts of the same sample. Overall, the divergences observed in these studies underscore the need for an improved and standardized strategy for extracting, concentrating and quantifying these rapidly degradable molecules, despite significant advances in the development and optimization of highly sensitive UHPLC-MS/MS methods (Floková et al 2020; Rial et al 2019). In particular, the use of appropriate solvents and extraction conditions seems crucial to achieve maximum recovery and stability of SLs for accurate quantification.
Plant responses to P deficiency: a matter of concentrations
Root architecture modifications
Modifications of root growth and architecture are the best-documented responses of plants to P starvation (Czarnecki et al. 2013; Lynch 2007). To implement a prompt reaction to P shortage, plants must first sense the P status both locally and systemically in order to orchestrate the appropriate responses, with a large set of genes (> 1000) being regulated (de Souza Campos et al. 2019). In fact, P-deficiency-induced remodeling of root development is an active cellular process, mainly determined by an internal genetic program rather than being a consequence of reduced metabolic activity due to nutrient shortage (Péret et al. 2014). Svistoonoff et al. (2007) provided evidence for an important role of the primary root tip in sensing P deficiency and/or responding to it in Arabidopsis, highlighting how external P supply rather than internal P status triggers the local root growth response to P availability (Abel 2011). The spatial configuration of plant roots, also referred to as root system architecture, is highly plastic in response to low P conditions, and is characterized by a modular structure which enables exceptional flexibility and allows root deployment in nutrient-rich zones (Hodge 2004). Common root system architecture responses to P deficiency are high root/shoot ratio, topsoil foraging, highly branched root system with species-dependent modification of primary root, increased number and length of lateral roots and root hairs, and formation of cluster roots (Hodge 2004; López-Bucio et al. 2003; Lynch 2007; Niu et al. 2013; Ramaekers et al. 2010; Péret et al. 2014; Williamson et al. 2001). Many species such as maize and Arabidopsis adopt the strategy of lateral root modification under P deficiency (Dixon et al. 2020 and references therein). In contrast, in tomato plants, high P levels have been shown to increase lateral root number (Jiang et al. 2015). However, like many other species, tomato plants can respond to P deficiency by increasing root surface area and decreasing total root weight and average root diameter (Garcia and Ascencio 1992).
It has been widely demonstrated that SLs play a critical role in regulating the coordinate development of roots and shoots in response to P shortage (De Cuyper et al. 2015; Kapulnik et al. 2011; Koltai 2011; Ruyter-Spira et al. 2011; Shindo et al. 2020; Sun et al. 2014). Recently, Santoro et al. (2020) suggested that SLs contribute to adjusting the root traits of tomato plants which may aid soil exploration under low P availability. Specifically, SLs were found to increase the primary root length, the number of lateral roots, and biomass allocation to the roots. Results from the same set of experiments but with different P concentrations and timing of P deficiency establishment indicate that plant responses can be highly dependent on the level of P stress applied and on the occurrence of an acclimation period before total P deprivation. Constant P provision or sudden P depletion are not common conditions for plants in open field environments. Phosphorus availability in soils can gradually decrease due to plant uptake or fixation processes, leading to P deficiency. Therefore, a low synthesis of SLs can impact the ability of tomato roots to respond to a gradual P decrease when plants are allowed to acclimate before becoming completely P-deprived. According to Santoro et al. (2020), under conditions of P starvation, SL-depleted plants presented a decrease in total root length compared to plants that have sufficient P levels. This reduction was not due to inhibition of primary root elongation, but to a decline in the number and length of lateral roots, and was attributed to the extensive cell and tissue alterations observed in the root tips of P-starved SL-depleted plants, possibly caused by the depleted synthesis of SLs. These findings reinforce the prominent role of SLs in regulating lateral root formation and development in tomato plants, as previously observed in Arabidopsis and rice mutants (Kapulnik et al. 2011; Ruyter-Spira et al. 2011; Ito et al. 2015; Mayzlish-Gati et al. 2012; Sun et al. 2019), but also the inability of other hormones controlling cell division and differentiation processes at the root apex to completely overcome alterations in root morphology when SLs are depleted (Niu et al. 2013).
A common response of plants to low P levels is also the stimulation of root hair formation (Niu et al. 2013). Root hairs can occupy up to 90% of the root surface, facilitating water and nutrient uptake and allowing for soil exploration at reduced metabolic costs (Aziz et al. 2014; Czarnecki et al. 2013; Hodge 2004; Lynch 2007; Ramaekers et al. 2010). The hair length of tomato roots was shown to increase from 0.1 to 0.2–0.7 mm as the external P concentration decreased from 100 to 2 μM (Foehse and Jungk 1983). By enhancing root hair length and density, roots can significantly increase the soil volume explored for P absorption, resulting in increased P availability as root hairs may assist in the exudation of P-mobilizing compounds such as organic acid anions, protons, and phosphatases (Ramaekers et al. 2010). Root hair development, like other root traits, is under hormonal control (Omoarelojie et al. 2019). The SL-auxin crosstalk, in particular, was initially proposed to regulate root hair formation and elongation, with SLs triggering increased auxin accumulation in root epidermal cells by modulating auxin flux from the root (Czarnecki et al. 2013; López-Bucio et al. 2003; Koltai et al. 2010a; Kapulnik et al. 2011). However, these reports have been recently revised, leading to the conclusion that the KAI2-initiated sibling pathway and not SLs is responsible for root hair changes in Arabidopsis (Villaecija-Aguilar et al. 2019). The KAI2 receptor and its unidentified SL-like ligand appear to ultimately regulate root hair length and density under normal P growth conditions in this species, while SLs regulate lateral root density (Villaecija-Aguilar et al. 2019). Whether the same holds true for tomato plants has yet to be thoroughly investigated. Thus, further research is needed to elucidate and differentiate the respective contributions of SLs and KAI2 in driving root adaptations in tomato plants. Nonetheless, decreased SL levels were observed to impede root hair elongation in P-deficient tomato roots, possibly by altering auxin levels in epidermal cells (Santoro et al. 2020).
Morphological changes in roots upon P starvation are not only the result of hormonal control but also of complex interactions between P and other nutrients, especially Fe (Rouached et al. 2010). In Arabidopsis, Fe accumulates more in P-starved plants than in plants with sufficient P and inhibits primary root elongation in a concentration-dependent manner (Svistoonoff et al. 2007; Ward et al. 2008). Similarly, inhibition of root growth in tomato plants treated with P-Fe coprecipitates has been attributed to Fe toxicity at the root tip (Santoro et al. 2022). Root tip inhibition is a key determinant of root system architecture remodeling triggered by low P, and this Fe-requiring response involves the LPR1 and LPR2 ferroxidases, leading to apoplastic accumulation of Fe(III) and a concomitant increase in reactive oxygen species and callose deposition in the root meristem (Müller et al. 2015; Puga et al. 2017; Svistoonoff et al. 2007). As a result, the intracellular symplastic movement of Fe is reduced, which might interfere with the intercellular movement of the SHORT ROOT protein, a key regulator of radial root patterning, impairing root growth (Puga et al. 2017).
Root exudation
Under normal growing conditions, plant roots exude a variety of substances into the rhizosphere, significantly influencing its chemistry, soil microorganisms, and plant growth (reviewed in Aziz et al. 2014; Baetz and Martinoia 2014; Bais et al. 2006; Bertin et al. 2003; Hinsinger 2001; Vance et al. 2003; Wang and Lambers 2020). The nature of plant root exudates varies significantly in response to P deficiency. It might involve proton release to acidify the rhizosphere, organic acid anion exudation to mine sparingly available P and the release of phosphatases or phytases to mobilize organic P through hydrolysis (Aziz et al. 2014; Neumann and Römheld 1999; Wang and Lambers 2020 and references therein).
Proton (H+)-ATPase activity is the driving force behind rhizosphere acidification, coupling ATP hydrolysis with proton transport and establishing electrochemical gradients across the plasma membrane (Fig. 5a) (Duby and Boutry 2009). Root-induced acidification can decrease the rhizosphere pH by 2 to 3 units compared to the bulk soil, which may result in significant dissolution of sparingly available soil P, particularly in alkaline to mildly acidic soils where Ca phosphates are present (Hinsinger 2001; Marschner 1995). Furthermore, the release of H+ in the rhizosphere could also increase the availability of P adsorbed on metal oxides (Bertrand et al. 1999), and promote the hydrolysis of organic P forms by P-hydrolyzing enzymes, as phytases have an optimal working pH close to 5 (Giaveno et al. 2010). These hydrolytic reactions can produce additional protons, therefore contributing to the acidification of the soil solution.
Root exudates and their influence on plant phosphorous (P) uptake. a) Protons (H+), b) organic acid anions and c) polyphenols cooperate to solubilize the inorganic (Pi) and organic (Po) P pools by dissolving, reducing and chelating P-(hydr)oxides and Fe/Al/Ca-phosphates. d) Strigolactones (SLs) in the exudates indirectly influence P acquisition by favoring the symbiosis with arbuscular mycorrhizal fungi (AMF), while e) acid phosphatases (APases) and phytases liberate P from P-containing organic compounds. The transporters responsible for phenolics and acid phosphatase efflux are not known. f) High-affinity phosphate transporters (PHT) are responsible for P uptake at the root level, cotransporting protons. ALMT, aluminum-activated malate transporter; MATE, multidrug and toxic compound extrusion; InsP6, inositol hexaphosphate. Based on Chai and Schachtman 2022
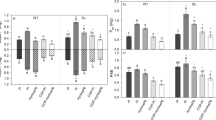
Proton exudation as a mechanism for coping with P deficiency has been observed in various plant species including bean, tea, white lupin, and tomato (Dixon et al. 2020 and references therein). When exposed to low P concentration, tomato plant release approximately 80 nM H+ h−1 g−1 fresh weight whereas at high P level the release is around 30 nM H+ h−1 g−1 fresh weight (Zhang et al. 2018b). In tomato plants, the release of protons in root exudates is influenced by SLs, as reported by Santoro et al. (2021, 2022), who noted that exogenous application of GR24 triggers rhizosphere acidification in P sufficient tomato plants, resembling the response observed under P deprivation. In a hydroponic experiment, the same authors found that wild-type tomato plants showed a prompt increase in H+ release in response to P starvation, whereas SL-depleted mutants exhibited a delayed response (Santoro et al 2021). Similar findings were obtained in a long-lasting experiment, where P was provided to wild-type and SL-depleted tomato plants in the form of coprecipitated Pi or no P (Santoro et al. in review, personal communication). Under both conditions, the acidifying activity of roots started to be evident in both genotypes after 5 days since the beginning of the experiment, and remained equivalent until day 12, after which proton exudation increased severely in wild-type plants and only slightly in SL-depleted ones. These results underscore the importance of timing and process kinetics in influencing and differentiating the adaptative responses of the two genotypes, which depend on SL production. Factors operating alongside SLs in the signalling pathway induced by P deficiency could balance the dysregulated response of SL-depleted plants to compensate for and restore the exudation to wild-type plants’ levels, as observed in rice and Arabidopsis mutants in terms of root architecture modifications (Mayzlish-Gati et al. 2012; Sun et al. 2014). In a recent study however, P was provided in hydroponics as, among others, sparingly available organic forms (soluble and Fe-coprecipitated InsP6), and in that case proton exudation appeared to be more related to the type of P source than to SL production (Santoro et al. 2022).
Besides proton release, increasing C exudation is a common strategy to enhance P acquisition. Among the pool of C compounds, organic acid anions are important for plant mineral nutrition and microbial growth in the rhizosphere environment (Fig. 5b) (Jones 1998), and can reach the concentration of 70 to 200 µM in the soil solution near the root surface under P deficiency (Jones et al. 1996). Organic acid anions include acetate, aconitate, citrate, malate, fumarate, lactate, oxalate, and succinate (Aziz et al. 2014; Lambers et al. 2006), with citrate and oxalate being by far the most effective in solubilizing unavailable P forms (Hinsinger 2001). Organic anions increase the availability of Pi and Po for plants by complexing and chelating cations bound to P, such as Fe, Al, and Ca (Gerke 2015; Hinsinger 2001; Römheld and Marschner 1990), competing with Pi for sorption sites (Fink et al. 2016) and mobilizing P bound in humic-metal complexes (Adeleke et al. 2017; Gerke 1994). Organic acid anions can also have a synergistic effect on secreted P-solubilizing enzymes by changing the chemical structure or molecular size of the extracted organic P to make it more accessible to enzymatic action (Hayes et al. 2000a,b; Wang and Lambers 2020).
Johnson and Loeppert (2006) observed that the order of effectiveness of P release from ferrihydrite by organic acid anions at pH 4 was citrate > malate > tartrate > > oxalate = malonate = succinate. Ferrihydrite released more P (and Fe) than goethite, a result that indicates that under low P conditions the exudation of these anions could be more effective at increasing P availability in soils dominated by poorly than high crystalline Fe oxides.
Although it is commonly accepted that the exudation of organic acid anions leads to rhizosphere acidification (Hinsinger 2001), emerging studies have shown that, in the context of tomato plants, these two processes are biochemically separate and exhibit a weak correlation, despite their spatial coordination (Wang and Lambers 2020). Moreover, in order to maintain charge equilibrium, cations as K+, Na+ and Mg2+ are concurrently excreted alongside the organic acid anions (Lambers et al. 2006; Wang and Lambers 2020).
The dominant organic acid anions released by exudation can vary between genotypes and are species-specific. In the case of tomato, citrate and oxalate are the main anions exuded, followed by succinate and fumarate (Dixon et al. 2020; Santoro et al. 2021). Enhanced organic acid anion exudation following P starvation has been reported in many plant species, such as white lupin (Johnson et al. 1996; Kihara et al. 2003; Neumann 2000), alfalfa (Lipton et al. 1987), rice (Tawaraya et al. 2013) and oilseed rape (Hoffland 1992), but it appears that P deficiency does not trigger any substantial increase in organic anions exudation in tomato plants (Dixon et al. 2020; Neumann and Römheld 1999; Santoro et al. 2021). Nevertheless, exogenous SL application was found to have an impact on the metabolic profile of tomato roots, including the production of organic acids (Gamir et al. 2020). Notably, when tomato plants were grown under optimal P supply, low doses of 2’-epi-GR24 resulted in an alteration of malate and citrate production, partly resembling the effect observed under P limitation (Gamir et al. 2020). Responses to P deficiency involving organic acid anion release from roots however seem to depend on the specific anion. Santoro et al. (2021) observed a slight increase in succinate exudation by both SL-producing and SL-silenced tomato plants under low P conditions, while oxalate exudation increased only in the latter. In contrast, under P-repleted conditions, enhanced exudation of organic acid anions by SL-depleted tomato plants was observed and ascribed to unnecessary activation of molecular, physiological and biochemical responses to sustain an elevated P uptake with a high C cost (Santoro et al. 2021).
Genetic improvement of organic acid anion exudation could be useful in selecting more P-efficient tomato genotypes, which would reduce the application of costly P-containing fertilizers while protecting against Al toxicity and low P availability that typically constrains plant growth in acidic soils (as exemplified by Wang et al. 2013). Oxalic acid, in particular, is the simplest dicarboxylic acid, with high acidity (pKa1 = 1.23) and strong chelating capacity for Ca, Al and Fe (Ryan et al. 2001). Due to its chemical properties, it could be an optimal target molecule for plants breeding as it may be more easily released by tomato roots than other metabolic intermediates (Zhao and Wu 2014). Organic acid anions released by plants may also inhibit the crystallization of Al and Fe hydrous oxides, reducing the rate of P occlusion (Schlesinger 1991). At the same time, they can be rapidly adsorbed onto soil particles or metabolized by soil microorganisms (Wang and Lambers 2020). Despite the potential of organic acid anion exudation genetic improvement, some authors do not expect a close correlation between their quantities in the rhizosphere and plant P acquisition, as the plant may release only small amounts of them due to the C cost associated with their exudation (Dixon et al. 2020; Wang and Lambers 2020).
Plants may also enhance the exudation of isoflavonoids, phenolics and mucilage under P deficiency (Fig. 5c) (Lambers et al. 2006). For instance, roots of pigeon pea exude piscidic acid, alfalfa exudes alfafuran, and P-deficient cluster roots of white lupin exude isoflavonoids (Vance et al. 2003 and references therein). These compounds can act as chelators or electron donors, reducing Fe(III) to Fe(II) and leading to oxide dissolution with the possible release of bound P. However, they are reportedly less effective than organic acid anions (Neumann and Römheld 2002). On the other hand, phospholipids may increase P concentration in the soil solution by directly competing with phosphate ions for adsorption sites or by altering the energetics of the interaction between the phosphate ion and the adsorption site (Read et al. 2003).
Finally, to overcome P deficiency in soil, plants enhance the release and activity of intracellular and extracellular acid phosphatase and phytase (Fig. 5e) (Dixon et al. 2020; Vance et al. 2003). This mechanism is particularly important when plants grow in soils containing high amounts of organic P pools. Soil organic P compounds sorbed onto soil particles are first mobilized by organic acid anions and then hydrolyzed to release plant available Pi (Lambers et al. 2006). Acid phosphatases can hydrolyze a wide range of organic P compounds, such as ATP, phosphoenolpyruvate, and phosphoproteins (Tarafdar et al. 2001), while phytases specifically hydrolyze phytates (myo-inositol penta- and hexa-phosphate), which are fairly resistant to other phosphatases (Hayes et al. 2000b). The increase of phytase activity serves as a crucial mechanism for efficiently recycling the internal P pools within the plant, as phytate is a major storage form of P in many plant tissues, especially the seeds (Konietzny and Greiner 2002; Srivastava et al. 2020). In tomato, two monomeric secreted acid phosphatase isoenzymes have been identified (SAP1 and SAP2), which mobilize external organophosphates (Bozzo et al. 2006). In addition, a P-starvation induced (PSI) gene, LePS2, coding for an internal acid phosphatase has been characterized, whose transcripts are rapidly induced in tomato plants and cell cultures in the absence of P, while being repressed by P supply (Baldwin et al. 2001).
The manipulation of acid phosphatases has been proposed as an efficient strategy to improve P acquisition efficiency. For instance, in soybean and rice the overexpression of purple acid phosphatases AtPAP15 and OsPAP21b, respectively, increased P acquisition, utilization, and remobilization (Srivastava et al. 2020 and references therein). To date, this approach has not been extended to tomato, but research has shown that most of the 25 purple acid phosphatases members coded by the tomato genome are activated under P deficiency, which could fast-track the biotechnological efforts for improving P use efficiency by tomato in the future (Srivastava et al. 2020).
Exogenous application of racGR24 has been reported to promote anthocyanin accumulation and activate acid phosphatases, suggesting a role for SLs in modulating early PSRs in plants (Ito et al. 2015). Recent studies by Gamir et al. (2020), Marro et al. (2022) and Santoro et al. (2020, 2021, 2022) have demonstrated that the deficiency in SL synthesis can impact the susceptibility to P shortage and related regulatory mechanisms in tomato. Notably, SL-deficient plants harbor higher root phytase and phosphatase activity than SL-producing plants under normal P conditions (Santoro et al. 2021, 2022). The increased phosphatase activity may indicate a more intense release of P from P-containing cellular constituents, such as membrane phospholipids, to facilitate P recycling in the plant and metabolism reprogramming to avoid P-requiring C metabolic pathways (Bozzo et al. 2006; Vance et al. 2003). The elevated activity of phytases suggests instead that plants may be attempting to mine P from phytate or using the internal phytate-derived P (Baldwin et al. 2001; Brinch-Pedersen et al. 2002; Hayes et al. 2000b). This difference in the activity of P-mobilizing enzymes was observed only under P sufficient conditions, as it remained consistent between the SL-depleted and the wild-type genotypes during P depletion (Santoro et al. 2021, 2022). Based on these findings, the authors inferred that while the typical PSRs were activated in P-sufficient SL-depleted tomato plants perhaps because of altered P signalling and perception, the onset of these responses in the same genotype when P-starved was not as intense as conceivable given their elevated P requirement for supporting optimal growth, concluding that the negative effects of the lack of SL control on plant responses appear to occur under all P conditions. Similarly, Ito et al. (2015) observed that the application of SL induced various P starvation events in Arabidopsis plants under both high and low P conditions, such as anthocyanin accumulation, acid phosphatase production, and root hair elongation. These results suggest that SL signalling is involved in multiple responses to P availability, not only under low P, possibly by mediating and/or compensating for signalling defects caused by P deficiency.
Soluble InsP6 was found to stimulate the production of phytases by tomato plants, especially when SL-depleted (Santoro et al. 2022). High phytase activity was also determined in tomato plants supplied with InsP6 coprecipitated with Fe, albeit with little effect on P acquisition (Santoro et al. 2022). This is likely because the efficacy of phytases and in general of P-solubilizing enzymes can be reduced when InsP6 is retained on a surface (Fig. 3, George et al. 2007). In support of this, Giaveno et al. (2010) stated that phytases are unable to hydrolyze inositol phosphates when these are adsorbed on soil particles, highlighting that the efficacy of these phosphohydrolases can be greatly altered by the substrate availability, microbial activity, and soil physical and chemical properties (George et al. 2007; Giaveno et al. 2010; Violante and Caporale 2015), and that the effect of root exudation on P bioavailability and acquisition should be systematically evaluated, also as a function of the P source.
Mycorrhizal associations
The association between AMF and plant roots is considered one of the most important symbiotic relationships between plants and microorganisms (Redecker and Raab 2006). This symbiosis occurs in the roots of more than 80% of plants with fungi from the phylum Glomeromycota (Smith and Read 2008). In exchange for photosynthetically fixed C, root colonizing AMF translocate water and nutrients, primarily P and N, to the host plants, effectively increasing the surface area of plant roots and enabling greater soil exploration and P uptake (Smith and Read 2008). Specifically, the hyphae of AMF can exploit either soil Pi by mining for distant pools of available P, or the soil organic P pool by producing phosphatase enzymes (Ezawa et al. 2005; Nasto et al. 2014; Sato et al. 2015). They can also increase the local soil P availability, improving the utilization of sparingly soluble P forms and reducing the risks of soil erosion and P losses (Cavagnaro et al. 2006; Gianinazzi et al. 2010; Parihar et al. 2019). The ability of AMF to mobilize P from less-accessible sources can lead to higher shoot P content, photosynthetic capacity, and P use efficiency. This is again related to the release of organic acid anions, which can dissolve or exchange ligands with components retaining P in the soil (Andrino et al. 2021). For all these reasons, this type of symbiosis is maximized under P deficiency, while high soil P levels generally reduce AMF colonization of the roots (Nagahashi and Douds 2004).
Mycorrhizal P uptake is generally regulated by the plant P status. In tomato, for instance, when P concentration in the plant is high the mycorrhizal uptake pathway is repressed almost completely (10% P taken up via mycorrhiza, Nagy et al. 2005). Conversely, when plant P concentrations are low the mycorrhizal uptake pathway becomes dominant (75% P taken up via mycorrhiza). In addition, recent field experiments have revealed that AMF symbiosis increases tomato fruit biomass and nutrient content to the same degree of fertilizers (Tran et al. 2022).
The establishment of AMF symbiosis relies on molecular communication between the host plant and AMF, which is conveyed by root-exuded metabolites (Fig. 5d) (Guillotin et al. 2017). Plant root exudates produced under P-limited conditions are more stimulating towards the hyphal elongation and branching of AMF than exudates produced under adequate P supply (Mashiguchi et al. 2021). Strigolactones are the key symbiotic signals that can induce hyphal branching, stimulate cell proliferation, and spore germination in AMF (Akiyama et al. 2005). Analyses of root exudates have shown that the content of SLs increases significantly under P deficiency in many plant species, including tomato, to promote AMF symbiosis, and that AMF symbiosis in turn induces changes in the transcriptional and hormonal profiles of tomato roots, reducing SL production (Andreo-Jimenez et al. 2015; López-Ráez et al. 2011). Among the various natural and synthetic SLs, (+)-orobanchol was found to have the highest activity, followed by 5-deoxystrigol, while the synthetic analogue GR24 had a high activity comparable to that of (+)-strigol. A tight regulated hormonal interplay is however responsible for the successful interaction between AMF and plant roots. Specifically, auxin has been identified as playing a key role in regulating the early stages of AMF formation by controlling the amount of SLs in the roots (Guillotin et al. 2017). This molecular dialogue offers a promising starting point for the development of new strategies to enhance P uptake by plants, as AMF have shown to be effective biostimulants for the sustainable management of agricultural ecosystems (Andreo-Jimenez et al. 2015 and references therein; Arcidiacono et al. 2023; Chandrasekaran et al. 2021; Yoneyama et al. 2019): understanding the hormonal mechanisms underlying their interactions with plants can lead to the development of more efficient and sustainable agricultural practices.
Internal P remobilization, gene expression, and metabolic changes
Phosphorus deficiency triggers a process of within-plant remobilization of P, where the element is moved from structures and processes where it is least required, such as from senescent to developing tissues (Dixon et al. 2020; Maillard et al. 2015; Vance et al. 2003). Phosphorus is mainly stored in vacuoles, where 85 to 95% of P reserves are normally located. Unlike cytoplasmic P concentration, which remains relatively constant (5–10 mM) and independent of external P supply, vacuolar P concentration varies widely (Schachtman et al. 1998 and references therein). Vacuolar P could be almost undetectable under P starvation, but when plants have an adequate P supply, Pi is converted into organic storage compounds, primarily InsP6 (Haran et al. 2000; Schachtman et al. 1998). Internal P is fairly mobile, which allows for the temporary mobilization and maximum utilization of P reserves in response to P deficiency. This strategy may result in reduced growth rates, decreased vacuolar P content, reuse of P from membrane lipids and reduced nucleic acid P pools (Rausch and Bucher 2002). Besford (1979) observed that P deficiency induced a rapid export of P from tomato plant leaves after the transfer of plants from a P-rich to a P-free medium. More recently, the identification of phosphate transporters such as Pht1;5 in Arabidopsis and OsPht1;8 in rice, involved in P redistribution from source to sink organs according to the P status of the plant, has shed light on the molecular mechanisms underlying P remobilization in plants (Li et al. 2015; Nagarajan et al. 2011).
Intracellular acid phosphatases play a crucial role in the recycling of P from expendable intracellular organophosphate pools. A study with tomato suspension cells revealed that low P induced the expression of a phosphatase involved in internal P remobilization. This explains the ability of tomato seedlings, as observed in a parallel experiment, to use stores of phytate and prevent the onset of the typical morphological and biochemical symptoms of P deficiency during the first 10 days of growth (Bozzo et al. 2006).
Adaptation to P deficiency also implies important changes in gene expression. Wang et al. (2021a) observed that two days of P starvation resulted in 57 up-regulated genes and the downregulation of only one gene compared to control P conditions in tomato. The number of differentially expressed genes increased to 331 and 406 at 3 and 4 days of P starvation, respectively. The maximum number of differentially expressed genes observed after 4 days was likely due to the plant acclimation to P depletion. One day of P resupply reduced the expression of 139 P starvation upregulated genes, while increasing the expression of 28 downregulated genes, indicating that these genes were highly responsive to variation in P availability (Wang et al. 2021a).
Sugars play a critical role in regulating the transcription of PSI genes in plants (Khurana et al. 2021 and references therein). Sucrose non-fermenting 1-related protein kinases (SnRK) genes mediate plant signalling pathways in response to various abiotic and biotic stresses by phosphorylating target proteins (Khurana et al. 2021). Khurana et al. (2021) identified 40 SnRK members in the tomato genome, grouped into three subfamilies (SnRK1, SnRK2, and SnRK3) and found that SlSnRK3.10a, SlSnRK3.15a and SlSnRK3.26 were activated at varying levels under P starvation. Although the functional role of PSI SlSnRK genes are still unknown in tomato, SnRK1 members in Arabidopsis interact with PHT1;4 and PHO1 (Carianopol et al. 2020).
De Groot et al. (2001) observed significant metabolic changes in P-depleted tomato plants, which revealed important effects on plant growth and physiology. Severe P limitation resulted in a decreased rate of photosynthesis and reduced production of assimilates, whereas the application of increasing P concentrations led to improved growth rather than increased plant P concentration. The authors also found that the importance of morphological changes versus physiological responses varied with the severity of P limitation, with mild P limitation mainly affecting plant morphology and severe P limitation influencing physiological responses (de Groot et al. 2001). These findings highlight the significant impact that even slight differences in P provision can have on plant responses at different levels. Therefore, careful consideration of the level of P-deficiency is crucial when setting up P stress experiments.
Phosphorus deficiency can have far-reaching consequences on the metabolic processes that control crucial fruit quality traits. Li et al. (2021) observed that low P treatment during tomato fruit ripening caused an overaccumulation of main pigments and key organic acids linked to tomato fruit sourness, while the content of soluble organic sugars responsible for fruit sweetness was reduced. These changes were attributed to alterations in enzyme activities in relevant metabolic pathways, such as the γ–aminobutyric acid shunt (Li et al. 2021).
The massive transcriptional reprogramming that plants undergo under P deficiency can lead to increased production of SLs (López-Ráez et al. 2008; Gamir et al. 2020; Marro et al. 2022). Phosphorus starvation upregulates the SL biosynthetic genes such as D27, CCD8, MAX1 and CYP722C in tomato, while their expression is repressed upon P replenishment (Wang et al. 2021a). A recent research by Wang et al. (2021a) sheds light on the functional link between increased SL biosynthesis and transcriptional modifications. This study shows that SLs play a crucial role in regulating the PSR as plant hormones, beyond being just the end products of the response. In the tomato CCD8 RNAi line (SL-depleted), about 96% of the PSR genes were less affected by P deficiency than in the wild-type. The presence of SLs was found to be essential for two thirds of the transcriptional changes referred to genes involved in the suppression of phospholipid biosynthesis, degradation of phospholipids and biosynthesis of sulfolipids and galactolipids under P starvation (Wang et al. 2021a). Other biosynthetic pathways that are reprogrammed under P starvation and as a function of SL production include those involved in the production of phenylpropanoids and carotenoids, pantothenate and CoA, and alkaloids (Wang et al. 2021a). Gamir et al. (2020) also reported that some metabolites (e.g., azelaic, linoleic and alpha-linolenic acids) related to plant immunity were accumulated in tomato under P limitation and exogenous SL application, while SL-deficient mutants were more susceptible to pathogens due to the low accumulation of these metabolites. Such results clearly indicate that SLs are not just the end-product of the PSR in plants, but play a major role in the regulation of the PSR itself.
High-affinity phosphate transporters and P uptake regulation
Phosphorus is absorbed by plants as the orthophosphate anion in the form of H2PO4− and HPO42− (Fig. 3). This process is driven by a proton gradient generated by plasma membrane H+-ATPases (Liu et al. 1998; Victor Roch et al. 2019) and, after uptake, P can remain as Pi or can be incorporated into organic molecules forming an ester with a hydroxyl group of a carbon chain such as sugar phosphates, or by attaching to another Pi via an energy-rich pyrophosphate bond (Fig. 3, Marschner 1995). The root is the primary organ for nutrient uptake from soil, and P acquisition depends on rapid root growth and large root area to increase soil exploration. While the root cap contributes to about 20% of total P uptake, P acquisition at the elongation zone is marginal (Kanno et al. 2016a, b). The concentration of P in root cells can be up to 1000-fold higher than in the soil solution, making P uptake an energy-mediated process that involves multiple epidermally located transport systems (Czarnecki et al. 2013; Duby and Boutry 2009; Hinsinger 2001; Schachtman et al. 1998). Plants have both low- and high-affinity P uptake systems to facilitate this process (Furihata et al. 1992). The low-affinity system is constitutive in plants and operates at the mM range (Raghothama 1999), whereas the high-affinity system is induced under low P conditions in the μM range, thus increasing the potential for enhanced P uptake from low P soils (Fig. 5f) (Chen et al. 2014). It is important to note that only the high-affinity system operates in the rhizosphere, where P concentrations are typically low (Hinsinger 2001 and references therein).
Most of the genes encoding for membrane-bound phosphate transporters are expressed in the roots and are categorized into five families (He et al. 2019; Victor Roch et al. 2019). Among these, the PHOSPHATE TRANSPORTER 1 (PHT1) gene family's high-affinity Pi/H+ symporters are primarily responsible for P uptake from soil and subsequent redistribution in plants. A total of eight putative PHT1 genes (LePT1 to 8) have been identified in the tomato genome (Chen et al. 2014), with LePT1 and LePT2 consisting of 12 membrane-spanning regions and sharing a high sequence similarity with other high-affinity P transporters (Dixon et al. 2020). The expression of these genes varies depending on the plant P status and developmental stage. LePT1 and LePT7 are ubiquitously expressed in roots, stems, young leaves, flowers, and fruits, and their transcripts are abundantly accumulated during P starvation (Chen et al. 2014; Zhang et al. 2018b). LePT2 and LePT6 are predominantly expressed in the roots of P-deficient plants, while LePT3, LePT4 and LePT5 are strongly upregulated in roots colonized by AMF under low P conditions (Chen et al. 2014; Gómez-Ariza et al. 2009; Zhang et al. 2018b). Conversely, LePT1, LePT2, LePT6, and LePT7 are downregulated in mycorrhized tomato roots under low P supply (Chen et al. 2014).
Liu et al. (1998) demonstrated that the transcript levels of LePT1 and LePT2 in tomato roots significantly increased within 1 day of transferring plants to a P-deficient medium, likely due to the depletion of internal P reserves and/or lack of P supply to the roots. This effect was most prominent after 5 days of P starvation, and resupplying P to P-starved tomato plants repressed LePT1 and LePT2 transcripts to their uninduced levels within 2 days. In addition, based on the findings of a split-root experiment, the authors hypothesized that the signals responsible for the P starvation response may arise internally due to changes in the cellular P concentration (Liu et al. 1998). Earlier reports have shown that providing P to a portion of the root system can partly or completely compensate for P deficiency in other parts of the root system by increasing nutrient uptake rates and derepressing the expression of P transporters genes (Drew and Saker 1984).
The expression of high-affinity P transporters can be affected by exogenous SL application, as shown in some studies (Gamir et al. 2020; Marro et al. 2022; Santoro et al. 2021, 2022). For example, Gamir et al. (2020) found that short-term application of 2’-epi-GR24 enhanced the expression of LePT2. Interestingly, the SL-deficient tomato line SlCCD8-RNAi displayed altered transcript levels of key regulatory elements of phosphate transporters, such as PHO2, which were also less sensitive to P starvation (Gamir et al. 2020). Marro et al. (2022) confirmed these findings, supporting the role of SLs in regulating the PSR response. However, SL deficient lines can apparently modulate their responses based on the level of P deficiency. Santoro et al. (2021, 2022) found that LePT1, LePT2 and LePT4 were up-regulated by P starvation in wild-type tomato plants, but not in the SlCCD7-silenced line, for which in turn these genes were up-regulated when P was sufficient. Discrepancies in these outcomes have been ascribed to difference in P concentrations used for treating tomato plants. The concentration of P used by Santoro et al. (2022) for the P-sufficient treatment (80 µM) should simulate the typical range of P concentration in the field (Hinsinger 2001), and was sufficient for wild-type tomato plants, but revealed a defect in SL-depleted plants, likely due to altered perception and/or production of internal P stocks. Furthermore, Santoro et al. (2022) investigated the PSR activation when different P forms (soluble and sparingly available), both inorganic and organic, were supplied. In this case, SL-depleted plants tended to be more effective in accessing sparingly available P forms than wild-type plants, thanks to higher PHT transcription and phytase activity. Therefore, the differences in external P concentration and P source may have triggered diverse regulatory signals of PHT transporters in the two genotypes, highlighting the fine-tuned regulation of SLs in response to differential external P availability and the need to consider the specific applied conditions, which makes generalizations difficult at this stage of SL research.
Complex signalling network underlying plant responses to P deficiency: which role for strigolactones?
The activation of PSRs requires a fine-tuned coordination and integration of local and systemic (or long distance) signalling pathways, involving a number of genes and signalling molecules (Ham et al. 2018; Puga et al. 2017). Phosphorus homeostasis is regulated systemically, with most of the genes related to P transport, recycling, and signalling being controlled at the systemic level, including those involved in phospholipid remobilization and galactolipid and sulfolipid synthesis (Thibaud et al. 2010). It is also mediated by the perception of Pi at the cellular level in different organs, followed by inter-organ communication of P concentration via long-distance systemic signalling (Ham et al. 2018; Marro et al. 2022; Puga et al 2017). Even though Pi is known to act as a signal in regulating diverse responses to P limitation, it does not appear to be a long-distance signal from root to shoot (Ticconi and Abel 2004). Instead, the allocation or recycling of P between shoots and roots is believed to provide the systemic signal (Lin et al. 2008 and references therein). Changes in root development are instead locally regulated by P availability in the external medium (Péret et al. 2011), with sensing of external P concentrations likely occuring at the root tip and inducing modifications in root architecture (Müller et al. 2015; Ticconi and Abel 2004; Svistoonoff et al. 2007). This process is dependent on the auxin receptor TRANSPORT INHIBITOR RESPONSE1 (TIR1) and MORE AXILLARY GROWTH 2 (MAX2), a F-box protein that also functions as a key signalling component in the SL pathway (Al-Babili and Bouwmeester 2015; Mayzlish-Gati et al. 2012; Wang and Smith 2016; Wang et al. 2021b).
Much of the P starvation signalling pathway centers on the transcriptional activator PHOSPHATE STARVATION RESPONSE 1 (PHR1) and related transcription factors that regulate the main PSRs. These include induction of P scavenging and transport activities, membrane lipid remodelling, increase in root/shoot ratio, and repression of photosynthesis and photorespiration (Fig. 6b) (Puga et al. 2017 and references therein). PHR1 is expressed constitutively in Arabidopsis, and its activity is regulated by the plant P status. Recent studies have highlighted the critical roles of SPX domain-containing proteins SPX1 and SPX2 in sensing external and internal Pi levels (Puga et al. 2017; Zhou et al. 2015). These proteins can detect inositol phosphates as a P signal and negatively regulate PHR1 activity. Inositol ottaphosphate (InsP8) has been recently identified as the intracellular P signalling molecule serving as the ligand of SPX1 for controlling P homeostasis in plants (Dong et al. 2019). A high level of intracellular P promotes the synthesis of InsP8, which binds to the SPX domain in SPX1 and promotes the binding of SPX1 to PHR1, blocking PHR1 transcriptional activity (Fig. 6a) (Dong et al. 2019). Under P deficiency conditions, the interaction between SPX1 and PHR1 is abolished due to a decrease in InsP8. This enables the PHR1-mediated activation of P starvation-induced gene expression, which promotes P uptake and relieves P-mediated negative post-translational control of PHT1 transporters by inducing the expression of microRNA399 (miR399). mir399 is transported through the phloem from the shoots to the roots, where it triggers post transcriptional inhibition of PHO2, a protein that controls PHT1 turnover via ubiquitination. This leads to decreased proteolytic degradation of PHT proteins and enhanced P uptake (Ham et al. 2018; Lin et al. 2008; Liu et al. 2012; Puga et al. 2017). As a consequence of this mechanism, Arabidopsis plants overexpressing miR399 or the pho2 mutant over accumulate P in the shoots (Lin et al. 2008). PHR1 also controls the induction of the genes that encode IPS1 (TPSl1 in tomato) and related non-coding RNAs that sequester and negatively modulate miR399 to prevent the interaction miR399-PHO2 and the degradation of PHO2 transcripts. Therefore, the triad IPS1-miR399-PHO2 has a central role in the regulation of P acquisition by plants and P homeostasis, particularly in long-term P deprivation (de Souza Campos et al. 2019; Puga et al. 2017). A P starvation response partially independent from the PHR1 pathway described above is root tip inhibition. The signalling pathway of this response involves extracellular P sensing and depends on local P levels (Puga et al. 2017). It is distinct from that centered around PHR1, which is controlled by the overall plant P status. For this reason, the two signalling pathways are qualified as operationally different, although they are integrated because they share crosstalk (Puga et al. 2017).
Schematic model of the main players involved in the regulation of P signalling pathway in plants. This is constituted of two branches, one centered around PHR1 and related transcription factors, that is primarily dependent on intracellular Pi sensing and is controlled by overall plant Pi status (systemically controlled), the other dependent on extracellular Pi sensing and controlled by local Pi, which has an effect on root tip growth and other root system architecture traits. (a) A high level of intracellular Pi promotes the synthesis of inositol ottaphosphate (InsP8), which binds to the SPX domain in SPX1 and promotes the binding of SPX1 to PHR1, blocking PHR1 transcriptional activity and the activation of P starvation-induced (PSI) genes. (b) Under Pi deficiency, strigolactone (SL) biosynthesis is promoted, which affects the complex SPX‐PHR1. The complex becomes unstable and releases the regulator PHR1, which in turn promotes the expression of P transporters from the PHT1 family in the roots, favoring Pi uptake. In addition, by inducing the expression of microRNA miR399, PHR1 indirectly inhibits PHO2, which mediate post-translational negative control of PHT1 and/or PHO1 (involved in xylem loading of Pi). On the other hand, IPS1 can interact and block miR399 transcripts, preventing miR399‐PHO2 binding and degradation of PHO2. Low Pi-triggered root tip inhibition requires iron (Fe) and involves LPR1 and LPR2 ferroxidases, leading to apoplastic Fe3+ accumulation and a concomitant increase in reactive oxygen species and callose deposition in the root meristem. InsP7, inositol pyropolyphosphate. Based on Gamir et al. 2020; Marro et al. 2022; Puga et al. 2017
Recent discoveries suggest that SLs are involved in regulating P signalling in plants, as the expression of the triad IPS1-miR399-PHO2 was modified following exogenous SL application or in SL-biosynthesis mutants (Gamir et al. 2020; Marro et al. 2022; Santoro et al. 2021, 2022). Short-term application of 2’-epi-GR24 to tomato plants increased the expression of PHO2, IPS1, and miR399 genes, which were already promoted by P starvation (Gamir et al. 2020). Exogenous SL application induced the expression of IPS1 and miR399 genes under optimal P conditions, partly mimicking the effect observed under P starvation, and increased SL concentration under both P deficiency and optimal P conditions, when SLs levels in plants are usually very low. This supports the evidence that SLs act as signals that trigger plant responses to P deficiency (Gamir et al. 2020). Interestingly, the authors found that the SL-deficient line SlCCD8-RNAi displayed altered levels of these key regulatory elements, especially PHO2, and was less sensitive to P starvation (Gamir et al. 2020). Marro et al. (2022) have obtained similar results, confirming that PSR regulation is deficient in the SL-defective line and supporting the role of SLs in this response. However, the type and severity of the P deficiency condition may elicit different responses, particularly when SL production is inhibited or silenced, as highlighted in the case of PHTs. At 80 μM Pi, which is a lower concentration than that used by Gamir et al. (2020) and Marro et al. (2022) for P-sufficient conditions, the miR399-PHO2 module was dysregulated in SL-deficient tomato plants (Santoro et al. 2021). This suggests that the defective trait is conditioned to sublimiting P availability, and is not evident at higher P concentrations, where a less intense PSR was observed in SL-deficient plants (Santoro et al. 2021). However, initial investigations have revealed that the signalling cascade may be activated differently when P is provided to plants in forms other than Pi. Although PHO2 transcripts were found to inversely correlate with P uptake and shoot P content of wild-type and SL-depleted plants grown with Pi or without P, such correlation was lost when P was supplied in other forms (Santoro et al. 2021, 2022). Such multifaceted result highlight that there is still much to be discovered regarding the effect of SLs on the signalling pathway activated by scarcely available P forms.
Concluding remarks
In recent years, the significant roles of SLs in the adaptation of plants to environmental constraints, particularly low P availability in agricultural soils, have gained great attention. This review tried to combine the extending knowledge on abiotic P processes occurring in soil that limit P availability to plants with the emerging roles of SLs in tomato plant P nutrition and responses to P scarcity, as summarized in Fig. 7. While acting as germination stimulants for parasitic weeds, SLs also control plant development and enhance symbioses, making them an important but to date potential tool to improve crop productivity and resilience. Field trials have validated the potential use of SLs (as well as SL analogs or inhibitors) as agrochemicals or genetic targets in breeding programmes, with applications in improving nutrient uptake, drought tolerance, increasing yield and controlling parasitic weed germination. However, many of the activities of SLs remain unexplored, and their commercial deployment is currently hindered by difficulties related to the absence of an affordable source of SLs or SL analogues and their low stability. The development and use of specific and stable SL analogues may allow cost-effective and efficient agricultural use, as well as the identification or generation of under-producing SL mutants/transgenic plants, including tomato, with an increased ability to scavenge P from scarcely available forms in the soil. In addition to this, a comprehensive understanding of the P processes at the soil–plant interface can open new opportunities to optimize P recycling and circularity and increase plant P acquisition efficiency while minimizing crop requirements. This would help to reduce P losses to the environment and mitigate the ecologically damaging impact of P on water resources, ultimately leading to more sustainable agriculture. For this to happen, it is necessary to consider the specific factors under which experiments are carried out that may influence P acquisition: diverse plant species and age, growth medium composition, especially type and concentration of provided P, duration of exposure, and SL concentration (in the case of SL treatments). The detailed control of these factors and the adoption of conditions that best mimic the soil abiotic processes may provide a deeper understanding of the fine-tuned action of SLs in regulating plant responses to different crop scenarios and find new solutions to improve agriculture sustainability.
Conclusive conceptual summary of how strigolactones (SLs) are involved P-deficiency responses in tomato plants. Belowground, they influence root architecture, root exudation, enzymatic activity and the expression of P transporters and their regulators. Their exudation can indirectly increase P uptake through the instauration of symbiosis with arbuscular mycorrhizal fungi (AMF). In the shoots, they have an effect on P remobilization and on transcriptional and metabolic reprogramming. InsP6, inositol hexaphosphate; LR, lateral root; PRL, primary root length; RD, root diameter; RH, root hair; RSA, root system architecture; RV, root volume
Abbreviations
- AMF:
-
Arbuscular mycorrhizal fungi
- InsP6:
-
Inositol hexaphosphate
- Pi:
-
Inorganic phosphate
- Po:
-
Organic phosphorus
- PSR:
-
Phosphorus starvation response
- PSI:
-
Phosphorus starvation induced
- SL:
-
Strigolactone
References
Abel S (2011) Phosphate sensing in root development. Curr Opin Plant Biol 14:303–309. https://doi.org/10.1016/j.pbi.2011.04.007
Abel S, Ticconi CA, Delatorre CA (2002) Phosphate sensing in higher plants. Physiol Plant 115:1–8. https://doi.org/10.1034/j.1399-3054.2002.1150101.x
Adeleke R, Nwangburuka C, Oboirien B (2017) Origins, roles and fate of organic acids in soils: A review. South Afr J Bot 108:393–406. https://doi.org/10.1016/j.sajb.2016.09.002
Ahmad MZ, ur Rehman N, Yu S, Zhou Y, ul Haq B, Wang J, Li P, Zeng Z, Zhao J (2020) GmMAX2–D14 and –KAI interaction-mediated SL and KAR signaling play essential roles in soybean root nodulation. Plant J 101:334–351. https://doi.org/10.1111/tpj.14545
Akash PAP, Srivastava A, Mathur S, Sharma AK, Kumar R (2021) Identification, evolutionary profiling, and expression analysis of F-box superfamily genes under phosphate deficiency in tomato. Plant Physiol Biochem 162:349–362. https://doi.org/10.1016/j.plaphy.2021.03.002
Akhtar MS, Oki Y, Adachi T (2008) Genetic variability in phosphorus acquisition and utilization efficiency from sparingly soluble P-Sources by Brassica cultivars under P-stress environment. J Agron Crop Sci 194:380–392. https://doi.org/10.1111/j.1439-037X.2008.00326.x
Akhtar MS, Oki Y, Adachi T (2009a) Mobilization and acquisition of sparingly soluble P-sources by Brassica cultivars under p-starved environment i. differential growth response, P-efficiency characteristics and P-remobilization. J Integr Plant Biol 51:1008–1023. https://doi.org/10.1111/j.1744-7909.2009.00874.x
Akhtar MS, Oki Y, Adachi T (2009b) Mobilization and acquisition of sparingly soluble P-sources by Brassica cultivars under P-starved environment II. Rhizospheric pH changes, Redesigned Root Architecture and Pi-Uptake Kinetics. J Integr Plant Biol 51:1024–1039. https://doi.org/10.1111/j.1744-7909.2009.00873.x
Akiyama K, Hayashi H (2006) Strigolactones: Chemical signals for fungal symbionts and parasitic weeds in plant roots. Ann Bot 97:925–931. https://doi.org/10.1093/aob/mcl063
Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435:824–827. https://doi.org/10.1038/nature03608
Al-Babili S, Bouwmeester HJ (2015) Strigolactones, a novel carotenoid-derived plant hormone. Annu Rev Plant Biol 66:161–186. https://doi.org/10.1146/annurev-arplant-043014-114759
Alder A, Jamil M, Marzorati M, Bruno M, Vermathen M, Bigler P, Ghisla S, Bouwmeester H, Beyer P, Al-Babili S (2012) The path from β-Carotene to Carlactone, a strigolactone-like plant hormone. Science 335:1348–1351. https://doi.org/10.1126/science.1218094
Anderson G, Williams EG, Moir JO (1974) A comparison of the sorption of inorganic orthophosphate and inositol hexaphosphate by six acid soils. J Soil Sci 25:51–62. https://doi.org/10.1111/j.1365-2389.1974.tb01102.x
Andreo-Jimenez B, Ruyter-Spira C, Bouwmeester HJ, Lopez-Raez JA (2015) Ecological relevance of strigolactones in nutrient uptake and other abiotic stresses, and in plant-microbe interactions below-ground. Plant Soil 394:1–19. https://doi.org/10.1007/s11104-015-2544-z
Andrino A, Guggenberger G, Kernchen S et al (2021) Production of organic acids by Arbuscular Mycorrhizal fungi and their contribution in the mobilization of phosphorus bound to iron oxides. Front Plant Sci 12:661842. https://doi.org/10.3389/fpls.2021.661842
Angelico R, Ceglie A, He J-Z, Yu-Rong L, Palumbo G, Colombo C (2014) Particle size, charge and colloidal stability of humic acids coprecipitated with Ferrihydrite. Chemosphere 99:239–247. https://doi.org/10.1016/j.chemosphere.2013.10.092
Arai Y, Sparks DL (2007) Phosphate reaction dynamics in soils and soil components: A multiscale approach. Adv Agron 94:135–179. https://doi.org/10.1016/S0065-2113(06)94003-6
Arai Y, Sparks DL (2001) ATR–FTIR spectroscopic investigation on phosphate adsorption mechanisms at the ferrihydrite–water interface. J Colloid Interface Sci 241:317–326. https://doi.org/10.1006/jcis.2001.7773
Arcidiacono M, Pellegrino E, Nuti M, Ercoli L (2023) Field inoculation by arbuscular mycorrhizal fungi with contrasting life-history strategies differently affects tomato nutrient uptake and residue decomposition dynamics. Plant Soil. https://doi.org/10.1007/s11104-023-05995-8
Arellano-Saab A, Bunsick M, Al Galib H, Zhao W, Schuetz S, Bradley JM, Xu Z, Adityani C, Subha A, McKay H, de Saint Germain A, Boyer F-D, McErlean CSP, Toh S, McCourt P, Stogios PJ, Lumba S (2021) Three mutations repurpose a plant karrikin receptor to a strigolactone receptor. Proc Natl Acad Sci 118:e2103175118. https://doi.org/10.1073/pnas.2103175118
Arite T, Umehara M, Ishikawa S, Hanada A, Maekawa M, Yamaguchi S, Kyozukaet J (2009) d14, a Strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers. Plant Cell Physiol 50:1416–1424. https://doi.org/10.1093/pcp/pcp091
Atkinson RJ, Parfitt RL, Smart RStC (1974) Infra-red study of phosphate adsorption on goethite. J Chem Soc Faraday Trans 1 Phys Chem Condens Phases 70:1472. https://doi.org/10.1039/f19747001472
Aziz T, Sabir M, Farooq M, Maqsood MA, Ahmad HR, Warraich EA (2014) Phosphorus deficiency in plants: responses, adaptive mechanisms, and signaling. In: Hakeem KR, Rehman RU, Tahir I (eds) Plant signaling: Understanding the molecular crosstalk. Springer India, New Delhi, pp 133–148
Baetz U, Martinoia E (2014) Root exudates: the hidden part of plant defense. Trends Plant Sci 19:90–98. https://doi.org/10.1016/j.tplants.2013.11.006
Bai Y, Lindhout P (2007) Domestication and breeding of tomatoes: What have we gained and what can we gain in the future? Ann Bot 100:1085–1094. https://doi.org/10.1093/aob/mcm150
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57:233–266. https://doi.org/10.1146/annurev.arplant.57.032905.105159
Baldwin JC, Karthikeyan AS, Raghothama KG (2001) LEPS2, a phosphorus starvation-induced novel acid phosphatase from tomato. Plant Physiol 125:728–737. https://doi.org/10.1104/pp.125.2.728
Barbier FF, Dun EA, Kerr SC, Chabikwa TG, Beveridge CA (2019) An update on the signals controlling shoot branching. Trends Plant Sci 24:220–236. https://doi.org/10.1016/j.tplants.2018.12.001
Barbier FF, Cao D, Fichtner F, Weiste C, Perez-Garcia M-D, Caradeuc M, Le Gourrierec J, Sakr S, Beveridge CA (2021) HEXOKINASE1 signalling promotes shoot branching and interacts with cytokinin and strigolactone pathways. New Phytol 231:1088–1104. https://doi.org/10.1111/nph.17427
Bera T, Song F, Liu G (2020) Rapid identification of phosphorus-efficient genotypes from commercially grown tomato (Solanum lycopersicum L.) varieties in a simulated soil solution. J Hortic Sci Biotechnol 95:395–404. https://doi.org/10.1080/14620316.2019.1684210
Berg AS, Joern BC (2006) Sorption dynamics of organic and inorganic phosphorus compounds in soil. J Environ Qual 35:1855–1862. https://doi.org/10.2134/jeq2005.0420
Bertin C, Yang X, Weston LA (2003) The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 256:67–83. https://doi.org/10.1023/A:1026290508166
Bertrand I, Hinsinger P, Jaillard B, Arvieu JC (1999) Dynamics of phosphorus in the rhizosphere of maize and rape grown on synthetic, phosphated calcite and goethite. Plant Soil 211:111–119. https://doi.org/10.1023/A:1004328815280
Besford RT (1979) Uptake and distribution of phosphorus in tomato plants. Plant Soil 51:331–340. https://doi.org/10.1007/BF02197780
Bouwman AF, Beusen AHW, Lassaletta L et al (2017) Lessons from temporal and spatial patterns in global use of N and P fertilizer on cropland. Sci Rep 7:40366. https://doi.org/10.1038/srep40366
Bowman RA, Moir JO (1993) Basic EDTA as an extractant for soil organic phosphorus. Soil Sci Soc Am J 57:1516–1518. https://doi.org/10.2136/sssaj1993.03615995005700060020x
Bozzo GG, Dunn EL, Plaxton WC (2006) Differential synthesis of phosphate-starvation inducible purple acid phosphatase isozymes in tomato (Lycopersicon esculentum) suspension cells and seedlings. Plant Cell Environ 29:303–313. https://doi.org/10.1111/j.1365-3040.2005.01422.x
Brewer PB, Koltai H, Beveridge CA (2013) Diverse roles of strigolactones in plant development. Mol Plant 6:18–28. https://doi.org/10.1093/mp/sss130
Brinch-Pedersen H, Sørensen LD, Holm PB (2002) Engineering crop plants: getting a handle on phosphate. Trends Plant Sci 7:118–125. https://doi.org/10.1016/S1360-1385(01)02222-1
Bürger M, Chory J (2020) The many models of strigolactone signaling. Trends Plant Sci 25:395–405. https://doi.org/10.1016/j.tplants.2019.12.009
Butler L (1995) Chemical communication between the parasitic weed Striga and its crop host. A new dimension in allelochemistry. In: Allelopathy, Organisms, Processes and Applications. Inderjit KM, Dakshini M, Enhelling FA, Washington, DC: Am Chem Soc pp 158–66
Cade-Menun B (2005) Characterizing phosphorus in environmental and agricultural samples by 31P nuclear magnetic resonance spectroscopy. Talanta 66:359–371. https://doi.org/10.1016/j.talanta.2004.12.024
Cagnasso M, Boero V, Franchini MA, Chorover J (2010) ATR-FTIR studies of phospholipid vesicle interactions with α-FeOOH and α-Fe2O3 surfaces. Colloids Surf B Biointerfaces 76:456–467. https://doi.org/10.1016/j.colsurfb.2009.12.005
Cardinale F, Korwin Krukowski P, Schubert A, Visentin I (2018) Strigolactones: mediators of osmotic stress responses with a potential for agrochemical manipulation of crop resilience. J Exp Bot 69:2291–2303. https://doi.org/10.1093/jxb/erx494
Carianopol CS, Chan AL, Dong S, Provart NJ, Lumba S, Gazzarrini S (2020) An abscisic acid-responsive protein interaction network for sucrose non-fermenting related kinase1 in abiotic stress response. Commun Biol 3:145. https://doi.org/10.1038/s42003-020-0866-8
Cavagnaro TR, Jackson LE, Six J, Ferris H, Goyal S, Asami D, Scow KM (2006) Arbuscular mycorrhizas, microbial communities, nutrient availability, and soil aggregates in organic tomato production. Plant Soil 282:209–225. https://doi.org/10.1007/s11104-005-5847-7
Ćavar S, Zwanenburg B, Tarkowski P (2015) Strigolactones: occurrence, structure, and biological activity in the rhizosphere. Phytochem Rev 14:691–711. https://doi.org/10.1007/s11101-014-9370-4
Celi L, Barberis E (2005) Abiotic stabilization of organic phosphorus in the environment. In: Turner BL, Frossard E, Baldwin DS (eds) Organic phosphorus in the environment, 1st edn. CABI Publishing, UK, pp 113–132
Celi L, Barberis E (2007) Abiotic reactions of inositol phosphates in soil. In: Turner BL, Richardson AE, Mullaney EJ (eds) Inositol phosphates: linking agriculture and the environment, 1st edn. CABI, UK, pp 207–220
Celi L, Lamacchia S, Ajmore-Marsan F, Barberis E (1999) Interaction of inositol hexaphosphate on clays: adsorption and charging phenomena. Soil Sci 164(8):574–585
Celi L, Lamacchia S, Barberis E (2000) Interaction of inositol phosphate with calcite. Nutr Cycl Agroecosyst 57:271–277. https://doi.org/10.1023/A:1009805501082
Celi L, Presta M, Ajmore-Marsan F, Barberis E (2001) Effects of pH and electrolytes on inositol hexaphosphate interaction with goethite. Soil Sci Soc Am J 65:753–760. https://doi.org/10.2136/sssaj2001.653753x
Celi L, De Luca G, Barberis E (2003) Effects of interaction of organic and inorganic P with ferrihydrite and kaolinite-iron oxide systems on iron release. Soil Sci 168:479–488. https://doi.org/10.1097/01.ss.0000080333.10341.a4
Celi L, Prati M, Magnacca G, Santoro V, Martin M (2020) Role of crystalline iron oxides on stabilization of inositol phosphates in soil. Geoderma 374:114442. https://doi.org/10.1016/j.geoderma.2020.114442
Celi L, Martin M, Barberis E (2022) Phosphorus in soil. Encyclopedia of Soils in the Environment, Second Edition. Elsevier. https://doi.org/10.1016/B978-0-12-822974-3.00137-3
Chai YN, Schachtman DP (2022) Root exudates impact plant performance under abiotic stress. Trends Plant Sci 27:80–91. https://doi.org/10.1016/j.tplants.2021.08.003
Chandrasekaran M, Boopathi T, Manivannan P (2021) Comprehensive assessment of ameliorative effects of amf in alleviating abiotic stress in tomato plants. J Fungi 7:303. https://doi.org/10.3390/jof7040303
Châtellier X, West MM, Rose J, Fortin D, Leppard GG, Ferris G (2004) Characterization of iron-oxides formed by oxidation of ferrous ions in the presence of various bacterial species and inorganic ligands. Geomicrobiol J 21:99–112. https://doi.org/10.1080/01490450490266343
Châtellier X, Grybos M, Abdelmoula M, Kemner KM, Leppard GG, Mustin C, West MM, Paktunc D (2013) Immobilization of P by oxidation of Fe(II) ions leading to nanoparticle formation and aggregation. Appl Geochem 35:325–339. https://doi.org/10.1016/j.apgeochem.2013.04.019
Chen A, Arai Y (2019) Functional group specific phytic acid adsorption at the ferrihydrite–water interface. Environ Sci Technol 53:8205–8215. https://doi.org/10.1021/acs.est.9b01511
Chen A, Chen X, Wang H, Liao D, Gu M, Qu H, Sun S, Xu G (2014) Genome-wide investigation and expression analysis suggest diverse roles and genetic redundancy of Pht1 family genes in response to Pi deficiency in tomato. BMC Plant Biol 14:61. https://doi.org/10.1186/1471-2229-14-61
Chen A, Li Y, Shang J, Arai Y (2020) Ferrihydrite transformation impacted by coprecipitation of phytic acid. Environ Sci Technol 54:8837–8847. https://doi.org/10.1021/acs.est.0c02465
Chi C, Xu X, Wang M, Zhang H, Fang P, Zhou J, Xia X, Shi K, Zhou Y, Yu J (2021) Strigolactones positively regulate abscisic acid-dependent heat and cold tolerance in tomato. Hortic Res 8:237. https://doi.org/10.1038/s41438-021-00668-y
Colombo C, Palumbo G, Sellitto VM, Cho HG, Amalfitano C, Adamo P (2015) Stability of coprecipitated natural humic acid and ferrous iron under oxidative conditions. J Geochem Explor 151:50–56. https://doi.org/10.1016/j.gexplo.2015.01.003
Condron LM, Tiessen H (2005) Interactions of organic phosphorus in terrestrial ecosystems. In: Turner BL, Frossard E, Baldwin DS (eds) Organic phosphorus in the environment, 1st edn. CABI Publishing, UK, pp 295–307
Condron LM, Turner BL, Cade-Menun BJ (2015) Chemistry and dynamics of soil organic phosphorus. In: Thomas Sims J, Sharpley AN (eds) American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America. American Society, Madison, WI, USA, pp 87–121
Cook CE, Whichard LP, Turner B, Wall ME, Egley GH (1966) Germination of witchweed (Striga lutea Lour.): Isolation and properties of a potent stimulant. Science 154:1189–1190. https://doi.org/10.1126/science.154.3753.1189
Cordell D, Drangert J-O, White S (2009) The story of phosphorus: Global food security and food for thought. Glob Environ Change 19:292–305. https://doi.org/10.1016/j.gloenvcha.2008.10.009
Czarnecki O, Yang J, Weston D, Tuksan GA, Chen J-G (2013) A dual role of strigolactones in phosphate acquisition and utilization in plants. Int J Mol Sci 14:7681–7701. https://doi.org/10.3390/ijms14047681
Darch T, Blackwell MSA, Hawkins JMB, Haygarth PM, Chadwick D (2014) A meta-analysis of organic and inorganic phosphorus in organic fertilizers, soils, and water: Implications for water quality. Crit Rev Environ Sci Technol 44:2172–2202. https://doi.org/10.1080/10643389.2013.790752
De Cuyper M, Joniau M (1991) Mechanistic aspects of the adsorption of phospholipids onto lauric acid stabilized magnetite nanocolloids. Langumir 7:647–652
De Groot CJ, Golterman HL (1993) On the presence of organic phosphate in some Camargue sediments: evidence for the importance of phytate. Hydrobiologia 252:117–126. https://doi.org/10.1007/BF00000133
De Cuyper C, Fromentin J, Yocgo RE, De Keyser A, Guillotin B, Kunert K, Boyer F-D, Goormachtig S (2015) From lateral root density to nodule number, the strigolactone analogue GR24 shapes the root architecture of Medicago truncatula. J Exp Bot 66:4091–4091. https://doi.org/10.1093/jxb/erv227
de Groot CC, Marcelis LFM, Van Den Boogaard R, Lambers H (2001) Growth and dry-mass partitioning in tomato as affected by phosphorus nutrition and light: Growth and dry-mass partitioning. Plant Cell Environ 24:1309–1317. https://doi.org/10.1046/j.0016-8025.2001.00788.x
de Groot CC, Marcelis LFM, van den Boogaard R, Kaiser WM, Lambers H (2003) Interaction of nitrogen and phosphorus nutrition in determining growth. Plant Soil 248:257–268. https://doi.org/10.1023/A:1022323215010
de Saint G, Ligerot Y, Dun EA, Pillot J-D, Ross JJ, Beveridge CA, Rameau C (2013) Strigolactones stimulate internode elongation independently of gibberellins. Plant Physiol 163:1012–1025. https://doi.org/10.1104/pp.113.220541
de Souza Campos PM, Cornejo P, Rial C, Borie F, Varela RM, Seguel A, López-Ráez JA (2019) Phosphate acquisition efficiency in wheat is related to root:shoot ratio, strigolactone levels, and PHO2 regulation. J Exp Bot 70:5631–5642. https://doi.org/10.1093/jxb/erz349
Decker EL, Alder A, Hunn S, Ferguson J, Lehtonen MT, Scheler B, Kerres KL, Wiedemann G, Safavi-Rizi V, Nordzieke S, Balakrishna A, Baz L, Avalos J, Valkonen JPT, Reski R, Al-Babili S (2017) Strigolactone biosynthesis is evolutionarily conserved, regulated by phosphate starvation and contributes to resistance against phytopathogenic fungi in a moss, Physcomitrella patens. New Phytol 216:455–468. https://doi.org/10.1111/nph.14506
Denizot B, Tanguy G, Hindre F, Rump E, Le Jeune JJ, Jallet P (1999) Phosphorylcholine coating of iron oxide nanoparticles. J Colloid Interface Sci 209:66–71. https://doi.org/10.1006/jcis.1998.5850
Dixon M, Simonne E, Obreza T, Liu G (2020) Crop response to low phosphorus bioavailability with a focus on tomato. Agronomy 10:617. https://doi.org/10.3390/agronomy10050617
Dong D, Peng X, Yan X (2004) Organic acid exudation induced by phosphorus deficiency and/or aluminium toxicity in two contrasting soybean genotypes. Physiol Plant 122:190–199. https://doi.org/10.1111/j.1399-3054.2004.00373.x
Dong J, Piñeros MA, Li X, Yang H, Liu Y, Murphy AS, Kochian LV, Liu D (2017) An Arabidopsis ABC transporter mediates phosphate deficiency-induced remodeling of root architecture by modulating iron homeostasis in roots. Mol Plant 10:244–259. https://doi.org/10.1016/j.molp.2016.11.001
Dong J, Ma G, Sui L, Wei M, Satheesh V, Zhang R, Ge S, Li J, Zhang T-E, Wittwer C, Jessen HJ, Zhang H, An G-Y, Chao D-Y, Liu D, Lei M (2019) Inositol pyrophosphate InsP8 Acts as an intracellular phosphate signal in Arabidopsis. Mol Plant 12:1463–1473. https://doi.org/10.1016/j.molp.2019.08.002
Drew MC, Saker LR (1984) Uptake and long-distance transport of phosphate, potassium and chloride in relation to internal ion concentrations in barley: evidence of non-allosteric regulation. Planta 160:500–507. https://doi.org/10.1007/BF00411137
Duby G, Boutry M (2009) The plant plasma membrane proton pump ATPase: a highly regulated P-type ATPase with multiple physiological roles. Pflüg Arch - Eur J Physiol 457:645–655. https://doi.org/10.1007/s00424-008-0457-x
Edayilam N, Ferguson B, Montgomery D, Al Mamun A, Martinez N, Powell BA, Tharayil N (2020) Dissolution and vertical transport of uranium from stable mineral forms by plants as influenced by the co-occurrence of uranium with phosphorus. Environ Sci Technol 54:6602–6609. https://doi.org/10.1021/acs.est.9b06559
Elzinga EJ, Kretzschmar R (2013) In situ ATR-FTIR spectroscopic analysis of the co-adsorption of orthophosphate and Cd(II) onto hematite. Geochim Cosmochim Acta 117:53–64. https://doi.org/10.1016/j.gca.2013.04.003
Elzinga EJ, Sparks DL (2007) Phosphate adsorption onto hematite: An in situ ATR-FTIR investigation of the effects of pH and loading level on the mode of phosphate surface complexation. J Colloid Interface Sci 308:53–70. https://doi.org/10.1016/j.jcis.2006.12.061
Emsley J (1980) The phosphorus cycle. In: Hutzinger O (ed) The handbook of environmental chemistry: The natural environment and the biogeochemical cycles. Springer Verlag Berlin Heidelberg, New York, pp 147–167
Ercolano MR, Sanseverino W, Carli P, Ferriello F, Frusciante L (2012) Genetic and genomic approaches for R-gene mediated disease resistance in tomato: retrospects and prospects. Plant Cell Rep 31:973–985. https://doi.org/10.1007/s00299-012-1234-z
Essington ME (2021) Soil and water chemistry: an integrative approach, Second edition, first issued in paperback. CRC Press, Taylor & Francis Group, Boca Raton London New York
Ezawa T, Hayatsu M, Saito M (2005) A new hypothesis on the strategy for acquisition of phosphorus in arbuscular mycorrhiza: up-regulation of secreted acid phosphatase gene in the host Plant. Mol Plant-Microbe Interactions 18:1046–1053. https://doi.org/10.1094/MPMI-18-1046
Fan J, Wang J-Y, Hu X-F, Chen F-S (2014) Seasonal dynamics of soil nitrogen availability and phosphorus fractions under urban forest remnants of different vegetation communities in Southern China. Urban Urban Green 13:576–585. https://doi.org/10.1016/j.ufug.2014.03.002
Fink JR, Inda AV, Bavaresco J, Barrón V, Torrent J, Bayer C (2016) Adsorption and desorption of phosphorus in subtropical soils as affected by management system and mineralogy. Soil Tillage Res 155:62–68. https://doi.org/10.1016/j.still.2015.07.017
Flematti GR, Scaffidi A, Waters MT, Smith SM (2016) Stereospecificity in strigolactone biosynthesis and perception. Planta 243:1361–1373. https://doi.org/10.1007/s00425-016-2523-5
Floková K, Shimels M, Andreo Jimenez B, Bardaro N, Strnad M, Novák O, Bouwmeester HJ (2020) An improved strategy to analyse strigolactones in complex sample matrices using UHPLC–MS/MS. Plant Methods 16:125. https://doi.org/10.1186/s13007-020-00669-3
Foehse D, Jungk A (1983) Influence of phosphate and nitrate supply on root hair formation of rape, spinach and tomato plants. Plant Soil 74:359–368. https://doi.org/10.1007/BF02181353
Foo E, Yoneyama K, Hugill CJ, Quittenden LJ, Reid JB (2013) Strigolactones and the regulation of pea symbioses in response to nitrate and phosphate deficiency. Mol Plant 6:76–87. https://doi.org/10.1093/mp/sss115
Frossard E, Condron LM, Oberson A, Sinaj S, Fardeau JC (2000) processes governing phosphorus availability in temperate soils. J Environ Qual 29:15–23. https://doi.org/10.2134/jeq2000.00472425002900010003x
Fuentes B, de la Luz Mora M, Bol R, San Martin F, Pérez E, Cartes P (2014) Sorption of inositol hexaphosphate on desert soils. Geoderma 232–234:573–580. https://doi.org/10.1016/j.geoderma.2014.06.016
Furihata T, Suzuki M, Sakurai H (1992) Kinetic characterization of two phosphate uptake systems with different affinities in suspension-cultured Catharanthus roseus protoplasts. Plant Cell Physiol. https://doi.org/10.1093/oxfordjournals.pcp.a078367
Furlani AMC, Clark RB, Maranville JW, Ross WM (1987) Organic and inorganic sources of phosphorus on growth and phosphorus uptake in sorghum genotypes. J Plant Nutr 10:163–186. https://doi.org/10.1080/01904168709363565
Gamir J, Torres-Vera R, Rial C, Berrio E, de Souza Campos PM, Varela R, Macías FA, Pozo MJ, Flors V, López-Ráez JA (2020) Exogenous strigolactones impact metabolic profiles and phosphate starvation signalling in roots. Plant Cell Environ 43:1655–1668. https://doi.org/10.1111/pce.13760
Garcia M, Ascencio J (1992) Root morphology and acid phosphatase activity in tomato plants during development of and recovery from phosphorus stress. J Plant Nutr 15:2491–2503. https://doi.org/10.1080/01904169209364489
Garcia-Lopez AM, Aviles M, Delgado A (2015) Plant uptake of phosphorus from sparingly available P- sources as affected by Trichoderma asperellum T34. Agric Food Sci 24:249–260. https://doi.org/10.23986/afsci.49532
Gaume A, Mächler F, De León C, Narro L, Frossard E (2001) Low-P tolerance by maize (Zea mays L.) genotypes: Significance of root growth, and organic acids and acid phosphatase root exudation. Plant Soil 228:253–264. https://doi.org/10.1023/A:1004824019289
George TS, Simpson RJ, Hadobas PA, Marshall DJ, Richardson AE (2007) Accumulation and phosphatase-lability of organic phosphorus in fertilised pasture soils. Aust J Agric Res 58:47. https://doi.org/10.1071/AR06167
George TS, Giles CD, Menezes-Blackburn D et al (2018) Organic phosphorus in the terrestrial environment: a perspective on the state of the art and future priorities. Plant Soil 427:191–208. https://doi.org/10.1007/s11104-017-3391-x
Gerke J (1994) Kinetics of soil phosphate desorption as affected by citric acid. Z Für Pflanzenernähr Bodenkd 157:17–22. https://doi.org/10.1002/jpln.19941570104
Gerke J (2015) The acquisition of phosphate by higher plants: effect of carboxylate release by the roots. A critical review. J Plant Nutr Soil Sci 178:351–364. https://doi.org/10.1002/jpln.201400590
Gerloff GC (1987) Intact-plant screening for tolerance of nutrient-deficiency stress. Plant Soil 99:3–16. https://doi.org/10.1007/BF02370149
Gianinazzi S, Gollotte A, Binet M-N, van Tuinen D, Redecker D, Wipf D (2010) Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 20:519–530. https://doi.org/10.1007/s00572-010-0333-3
Giaveno C, Celi L, Cessa RMA, Prati M, Bonifacio E, Barberis E (2008) Interaction of organic phosphorus with clays extracted from oxisols. Soil Sci 173:694–706. https://doi.org/10.1097/SS.0b013e3181893b59
Giaveno C, Celi L, Richardson AE, Simpson RJ, Barberis E (2010) Interaction of phytases with minerals and availability of substrate affect the hydrolysis of inositol phosphates. Soil Biol Biochem 42:491–498. https://doi.org/10.1016/j.soilbio.2009.12.002
Giles C, Cade-Menun B, Hill J (2011) The inositol phosphates in soils and manures: abundance, cycling, and measurement. Can J Soil Sci 91:397–416. https://doi.org/10.4141/cjss09090
Gómez-Ariza J, Balestrini R, Novero M, Bonfante P (2009) Cell-specific gene expression of phosphate transporters in mycorrhizal tomato roots. Biol Fertil Soils 45:845–853. https://doi.org/10.1007/s00374-009-0399-2
Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot J-P, Letisse F, Matusova R, Danoun S, Portais J-C, Bouwmeester H, Bécard G, Beveridge CA, Rameau C, Rochange SF (2008) Strigolactone inhibition of shoot branching. Nature 455:189–194. https://doi.org/10.1038/nature07271
Gorra R, Webster G, Martin M, Celi L, Mapelli F, Weightman AJ (2012) Dynamic microbial community associated with Iron-Arsenic co-precipitation products from a groundwater storage system in Bangladesh. Microb Ecol 64:171–186. https://doi.org/10.1007/s00248-012-0014-1
Guan X-H, Shang C, Zhu J, Chen G-H (2006) ATR-FTIR investigation on the complexation of myo-inositol hexaphosphate with aluminum hydroxide. J Colloid Interface Sci 293:296–302. https://doi.org/10.1016/j.jcis.2005.06.070
Guillotin B, Etemadi M, Audran C, Bouzayen M, Bécard G, Combier J-P (2017) Sl-IAA27 regulates strigolactone biosynthesis and mycorrhization in tomato (var. MicroTom). New Phytol 213:1124–1132. https://doi.org/10.1111/nph.14246
Gutjahr C, Parniske M (2013) Cell and developmental biology of arbuscular mycorrhiza symbiosis. Annu Rev Cell Dev Biol 29:593–617. https://doi.org/10.1146/annurev-cellbio-101512-122413
Ha S, Tran LS (2014) Understanding plant responses to phosphorus starvation for improvement of plant tolerance to phosphorus deficiency by biotechnological approaches. Crit Rev Biotechnol 34:16–30. https://doi.org/10.3109/07388551.2013.783549
Ham B-K, Chen J, Yan Y, Lucas WJ (2018) Insights into plant phosphate sensing and signaling. Curr Opin Biotechnol 49:1–9. https://doi.org/10.1016/j.copbio.2017.07.005
Haran S, Logendra S, Seskar M, Bratanova M, Raskin I (2000) Characterization of arabidopsis acid phosphatase promoter and regulation of acid phosphatase expression. Plant Physiol 124:615–626. https://doi.org/10.1104/pp.124.2.615
Harrison AF (1987) Soil organic phosphorus: A review of world literature. CAB International, Wallingford
Hauduc H, Takács I, Smith S, Szabo A, Murthy A, Daigger GT, Spérandio M (2015) A dynamic physicochemical model for chemical phosphorus removal. Water Res 73:157–170. https://doi.org/10.1016/j.watres.2014.12.053
Hayes JE, Richardson AE, Simpson RJ (2000a) Components of organic phosphorus in soil extracts that are hydrolysed by phytase and acid phosphatase. Biol Fertil Soils 32:279–286. https://doi.org/10.1007/s003740000249
Hayes JE, Simpson RJ, Richardson AE (2000b) The growth and phosphorus utilisation of plants in sterile media when supplied with inositol hexaphosphate, glucose 1-phosphate or inorganic phosphate. Plant Soil 220:165–174. https://doi.org/10.1023/A:1004782324030
He Q, Wang F, Wang Y, Lu H, Yang Z, Lv Q, Mao C (2019) Molecular control and genetic improvement of phosphorus use efficiency in rice. Mol Breed 39:162. https://doi.org/10.1007/s11032-019-1059-3
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237:173–195. https://doi.org/10.1023/A:1013351617532
Hodge A (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162:9–24. https://doi.org/10.1111/j.1469-8137.2004.01015.x
Hoffland E (1992) Quantitative evaluation of the role of organic acid exudation in the mobilization of rock phosphate by rape. Plant Soil 140:279–289. https://doi.org/10.1007/BF00010605
Holford ICR (1997) Soil phosphorus: its measurement, and its uptake by plants. Soil Res 35:227. https://doi.org/10.1071/S96047
House WA, Denison FH (2002) Total phosphorus content of river sediments in relationship to calcium, iron and organic matter concentrations. Sci Total Environ 282–283:341–351. https://doi.org/10.1016/S0048-9697(01)00923-8
Huang X, Foster GD, Honeychuck RV, Schreifels JA (2009) The maximum of phosphate adsorption at pH 4.0: why it appears on aluminum oxides but not on iron oxides. Langmuir 25:4450–4461. https://doi.org/10.1021/la803302m
Huang L-M, Zhang G-L, Thompson A, Rossiter DG (2013) Pedogenic transformation of Phosphorus during paddy soil development on calcareous and acid parent materials. Soil Sci Soc Am J 77:2078–2088. https://doi.org/10.2136/sssaj2013.01.0033
Ito S, Nozoye T, Sasaki E, Imai M, Shiwa Y, Shibata-Hatta M, Ishige T, Fukui K, Ito K, Nakanishi H, Nishizawa NK, Yajima S, Asami T (2015) Strigolactone regulates anthocyanin accumulation, acid phosphatases production and plant growth under low phosphate condition in Arabidopsis. PLoS One 10:e0119724. https://doi.org/10.1371/journal.pone.0119724
Ito S, Yamagami D, Umehara M, Hanada A, Yoshida S, Sasaki Y, Yajima S, Kyozuka J, Ueguchi-Tanaka M, Matsuoka M, Shirasu K, Yamaguchi S, Asami T (2017) Regulation of strigolactone biosynthesis by gibberellin signaling. Plant Physiol 174:1250–1259. https://doi.org/10.1104/pp.17.00301
Jarosch KA, Doolette AL, Smernik RJ, Tamburini F, Frossard E, Bünemann EK (2015) Characterisation of soil organic phosphorus in NaOH-EDTA extracts: A comparison of 31P NMR spectroscopy and enzyme addition assays. Soil Biol Biochem 91:298–309. https://doi.org/10.1016/j.soilbio.2015.09.010
Jia K-P, Baz L, Al-Babili S (2018) From carotenoids to strigolactones. J Exp Bot 69:2189–2204. https://doi.org/10.1093/jxb/erx476
Jiang X, Chen W, Xu C, Zhu H-H, Yao Q (2015) Influences of arbuscular mycorrhizal fungus and phosphorus level on the lateral root formation of tomato seedlings. J Appl Ecol 26:1186–1192
Jiu S, Xu Y, Xie X, Wang J, Xu J, Liu X, Sun W, Xu W, Wang S, Zhang C (2022) Strigolactones affect the root system architecture of cherry rootstock by mediating hormone signaling pathways. Environ Exp Bot 193:104667. https://doi.org/10.1016/j.envexpbot.2021.104667
Johnson SE, Loeppert RH (2006) Role of organic acids in phosphate mobilization from iron oxide. Soil Sci Soc Am J 70:222–234. https://doi.org/10.2136/sssaj2005.0012
Johnson JF, Allan DL, Vance CP, Weiblen G (1996) Root carbon dioxide fixation by phosphorus-deficient lupinus albus (contribution to organic acid exudation by proteoid roots). Plant Physiol 112:19–30. https://doi.org/10.1104/pp.112.1.19
Johnson BB, Quill E, Angove MJ (2012) An investigation of the mode of sorption of inositol hexaphosphate to goethite. J Colloid Interface Sci 367:436–442. https://doi.org/10.1016/j.jcis.2011.09.066
Johnston AE, Steen I (2000) Understanding phosphorus and its use in agriculture. European Fertilizer Manufacturers Association (EFMA), Brussels. https://fertiliser-society.org/wp-content/uploads/2019/11/EFMA-Phosphorus-booklet-2000.pdf
Jones DL (1998) Organic acids in the rhizosphere – a critical review. Plant Soil 205:25–44. https://doi.org/10.1023/A:1004356007312
Jones DL, Darah PR, Kochian LV (1996) Critical evaluation of organic acid mediated iron dissolution in the rhizosphere and its potential role in root iron uptake. Plant Soil 180:57–66. https://doi.org/10.1007/BF00015411
Jones AM, Griffin PJ, Waite TD (2015) Ferrous iron oxidation by molecular oxygen under acidic conditions: the effect of citrate, EDTA and fulvic acid. Geochim Cosmochim Acta 160:117–131. https://doi.org/10.1016/j.gca.2015.03.026
Jørgensen C, Jensen HS, Andersen FØ et al (2011) Occurrence of orthophosphate monoesters in lake sediments: significance of myo- and scyllo-inositol hexakisphosphate. J Environ Monit 13:2328. https://doi.org/10.1039/c1em10202h
Kalozoumis P, Savvas D, Aliferis K, Ntatsi G, Marakis G, Simou E, Tampakaki A, Karapanos I (2021) Impact of plant growth-promoting rhizobacteria inoculation and grafting on tolerance of tomato to combined water and nutrient stress assessed via metabolomics analysis. Front Plant Sci 12:670236. https://doi.org/10.3389/fpls.2021.670236
Kanno S, Arrighi J-F, Chiarenza S, Bayle V, Berthomé R, Péret B, Javot H, Delannoy E, Marin E, Nakanishi TM, Thibaud M-C, Nussaume L (2016a) A novel role for the root cap in phosphate uptake and homeostasis. eLife 5:e14577. https://doi.org/10.7554/eLife.14577
Kanno S, Cuyas L, Javot H, Bligny R, Gout E, Dartevelle T, Hanchi M, Nakanishi TM, Thibaud M-C, Nussaume L (2016b) Performance and limitations of phosphate quantification: Guidelines for plant biologists. Plant Cell Physiol 57:690–706. https://doi.org/10.1093/pcp/pcv208
Kapulnik Y, Delaux P-M, Resnick N, Mayzlish-Gati E, Wininger S, Bhattacharya C, Séjalon-Delmas N, Combier J-P, Bécard G, Belausov E, Beeckman T, Dor E, Hershenhorn J, Koltai H (2011) Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis. Planta 233:209–216. https://doi.org/10.1007/s00425-010-1310-y
Kashyap S, Woehl TJ, Liu X, Mallapragada SK, Prozorov T (2014) Nucleation of iron oxide nanoparticles mediated by Mms6 protein in situ. ACS Nano 8:9097–9106. https://doi.org/10.1021/nn502551y
Khurana A, Akash RA (2021) Identification of phosphorus starvation inducible SnRK genes in tomato (Solanum lycopersicum L.). J Plant Biochem Biotechnol 30:987–998. https://doi.org/10.1007/s13562-021-00701-0
Kihara T, Wada T, Suzuki Y, Hara T, Koyama H (2003) Alteration of citrate metabolism in cluster roots of white lupin. Plant Cell Physiol 44:901–908. https://doi.org/10.1093/pcp/pcg115
Kohlen W, Charnikhova T, Lammers M, Pollina T, Tóth P, Haider I, Pozo MJ, de Maagd RA, Ruyter-Spira C, Bouwmeester HJ, López-Ráez JA (2012) The tomato CAROTENOID CLEAVAGE DIOXYGENASE8 (SlCCD8) regulates rhizosphere signaling, plant architecture and affects reproductive development through strigolactone biosynthesis. New Phytol 196:535–547. https://doi.org/10.1111/j.1469-8137.2012.04265.x
Kohlen W, Charnikhova T, Bours R, López-Ráez JA, Bouwmeester HJ (2013) Tomato strigolactones: a more detailed look. Plant Signal Behav 8:e22785. https://doi.org/10.4161/psb.22785
Koltai H (2011) Strigolactones are regulators of root development. New Phytol 190:545–549. https://doi.org/10.1111/j.1469-8137.2011.03678.x
Koltai H (2013) Strigolactones activate different hormonal pathways for regulation of root development in response to phosphate growth conditions. Ann Bot 112:409–415. https://doi.org/10.1093/aob/mcs216
Koltai H, Dor E, Hershenhorn J, Joel DM, Weininger S, Lekalla S, Shealtiel H, Bhattacharya C, Eliahu E, Resnick N, Barg R, Kapulnik Y (2010a) Strigolactones’ effect on root growth and root-hair elongation may be mediated by auxin-efflux carriers. J Plant Growth Regul 29:129–136. https://doi.org/10.1007/s00344-009-9122-7
Koltai H, LekKala SP, Bhattacharya C, Mayzlish-Gati E, Resnick N, Wininger S, Dor E, Yoneyama K, Yoneyama K, Hershenhorn J, Joel DM, Kapulnik Y (2010b) A tomato strigolactone-impaired mutant displays aberrant shoot morphology and plant interactions. J Exp Bot 61:1739–1749. https://doi.org/10.1093/jxb/erq041
Konietzny U, Greiner R (2002) Molecular and catalytic properties of phytate-degrading enzymes (phytases). Int J Food Sci Technol 37:791–812. https://doi.org/10.1046/j.1365-2621.2002.00617.x
Kramna B, Prerostova S, Vankova R (2019) Strigolactones in an experimental context. Plant Growth Regul 88:113–128. https://doi.org/10.1007/s10725-019-00502-5
Kruse J, Abraham M, Amelung W, Baum C, Bol R, Kühn O, Lewandowski H, Niederberger J, Oelmann Y, Rüger C, Santner J, Siebers M, Siebers N, Spohn M, Vestergren J, Vogts A, Leinweber P (2015) Innovative methods in soil phosphorus research: a review. J Plant Nutr Soil Sci 178:43–88. https://doi.org/10.1002/jpln.201400327
Kumar M, Pandya-Kumar N, Dam A, Haor H, Mayzlish-Gati E, Belausov E, Wininger S, Abu-Abied M, McErlean CSP, Bromhead LJ, Prandi C, Kapulnik Y, Koltai H (2015) Arabidopsis response to low-phosphate conditions includes active changes in actin filaments and PIN2 polarization and is dependent on strigolactone signalling. J Exp Bot 66:1499–1510. https://doi.org/10.1093/jxb/eru513
Kyozuka J, Nomura T, Shimamura M (2022) Origins and evolution of the dual functions of strigolactones as rhizosphere signaling molecules and plant hormones. Curr Opin Plant Biol 65:102154. https://doi.org/10.1016/j.pbi.2021.102154
Lambers H, Shane MW, Cramer M, Pearse SJ, Veneklaas EJ (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot 98:693–713. https://doi.org/10.1093/aob/mcl114
Li L, Tang C, Rengel Z, Zhang F (2003) Chickpea facilitates phosphorus uptake by intercropped wheat from an organic phosphorus source. Plant Soil 248:297–303. https://doi.org/10.1023/A:1022389707051
Li Y, Zhang J, Zhang X, Fan H, Gu M, Qu H, Xu G (2015) Phosphate transporter OsPht1;8 in rice plays an important role in phosphorus redistribution from source to sink organs and allocation between embryo and endosperm of seeds. Plant Sci 230:23–32. https://doi.org/10.1016/j.plantsci.2014.10.001
Li M, Liu J, Xu Y, Qian G (2016) Phosphate adsorption on metal oxides and metal hydroxides: a comparative review. Environ Rev 24:319–332. https://doi.org/10.1139/er-2015-0080
Li Z, Qiu Q, Chen Y, Lin D, Huang J, Huang T (2021) Metabolite alteration in response to low phosphorus stress in developing tomato fruits. Plant Physiol Biochem 159:234–243. https://doi.org/10.1016/j.plaphy.2020.12.023
Lin S-I, Chiang S-F, Lin W-Y, Chen J-W, Tseng C-Y, Wu P-C, Chiou T-J (2008) Regulatory network of microRNA399 and PHO2 by systemic signaling. Plant Physiol 147:732–746. https://doi.org/10.1104/pp.108.116269
Lindsay WL (1979) Chemical equilibria in soils. Wiley, New York
Lipton DS, Blanchar RW, Blevins DG (1987) Citrate, malate, and succinate concentration in exudates from P-sufficient and P-stressed Medicago sativa L. Seedlings Plant Physiol 85:315–317. https://doi.org/10.1104/pp.85.2.315
Liu B, Liu J (2014) DNA adsorption by magnetic iron oxide nanoparticles and its application for arsenate detection. Chem Commun 50:8568. https://doi.org/10.1039/C4CC03264K
Liu B, Liu J (2015) Comprehensive screen of metal oxide nanoparticles for DNA adsorption, fluorescence quenching, and anion discrimination. ACS Appl Mater Interfaces 7:24833–24838. https://doi.org/10.1021/acsami.5b08004
Liu C, Muchhal US, Uthappa M, Kononowicz AK, Raghothama KG (1998) Tomato phosphate transporter genes are differentially regulated in plant tissues by phosphorus. Plant Physiol 116:91–99. https://doi.org/10.1104/pp.116.1.91
Liu Y, Mi G, Chen F, Zhang J, Zhang F (2004) Rhizosphere effect and root growth of two maize (Zea mays L.) genotypes with contrasting P efficiency at low P availability. Plant Sci 167:217–223. https://doi.org/10.1016/j.plantsci.2004.02.026
Liu Y, Villalba G, Ayres RU, Schroder H (2008) Global phosphorus flows and environmental impacts from a consumption perspective. J Ind Ecol 12:229–247. https://doi.org/10.1111/j.1530-9290.2008.00025.x
Liu T-Y, Huang T-K, Tseng C-Y, Lai Y-S, Lin S-I, Lin W-Y, Chen Y-W, Chiou T-J (2012) PHO2-dependent degradation of PHO1 modulates phosphate homeostasis in Arabidopsis. Plant Cell 24:2168–2183. https://doi.org/10.1105/tpc.112.096636
Liu J, Yang J, Cade-Menun BJ et al (2017) Molecular speciation and transformation of soil legacy phosphorus with and without long-term phosphorus fertilization: Insights from bulk and microprobe spectroscopy. Sci Rep 7:15354. https://doi.org/10.1038/s41598-017-13498-7
Liu J, Cade-Menun BJ, Yang J et al (2018) Long-term land use affects phosphorus speciation and the composition of phosphorus cycling genes in agricultural soils. Front Microbiol 9:1643. https://doi.org/10.3389/fmicb.2018.01643
López-Bucio J, Cruz-Ramı́rez A, Herrera-Estrella L (2003) The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol 6:280–287. https://doi.org/10.1016/S1369-5266(03)00035-9
López-Ráez JA, Bouwmeester H (2008) Fine-tuning regulation of strigolactone biosynthesis under phosphate starvation. Plant Signal Behav 3:963–965. https://doi.org/10.4161/psb.6126
López-Ráez JA, Charnikhova T, Gómez-Roldán V, Matusova R, Kohlen W, De Vos R, Verstappen F, Puech-Pages V, Bécard G, Mulder P, Bouwmeester H (2008) Tomato strigolactones are derived from carotenoids and their biosynthesis is promoted by phosphate starvation. New Phytol 178:863–874. https://doi.org/10.1111/j.1469-8137.2008.02406.x
López-Ráez JA, Charnikhova T, Fernández I, Bouwmeester H, Pozo MJ (2011) Arbuscular mycorrhizal symbiosis decreases strigolactone production in tomato. J Plant Physiol 168:294–297. https://doi.org/10.1016/j.jplph.2010.08.011
Luengo C, Brigante M, Antelo J, Avena M (2006) Kinetics of phosphate adsorption on goethite: Comparing batch adsorption and ATR-IR measurements. J Colloid Interface Sci 300:511–518. https://doi.org/10.1016/j.jcis.2006.04.015
Lun F, Liu J, Ciais P, Nesme T, Chang J, Wang R, Goll D, Sardans J, Peñuelas J, Obersteiner M (2018) Global and regional phosphorus budgets in agricultural systems and their implications for phosphorus-use efficiency. Earth Syst Sci Data 10:1–18. https://doi.org/10.5194/essd-10-1-2018
Luo C, Wen S, Lu Y, Dai J, Du Y (2022) Coprecipitation of humic acid and phosphate with Fe(III) enhances the sequestration of carbon and phosphorus in sediments. Chem Geol 588:120645. https://doi.org/10.1016/j.chemgeo.2021.120645
Lynch JP (2007) Roots of the second green revolution. Aust J Bot 55:493. https://doi.org/10.1071/BT06118
Ma Z, Baskin TI, Brown KM, Lynch JP (2003) Regulation of root elongation under phosphorus stress involves changes in ethylene responsiveness. Plant Physiol 131:1381–1390. https://doi.org/10.1104/pp.012161
Machin DC, Hamon-Josse M, Bennett T (2020) Fellowship of the rings: a saga of strigolactones and other small signals. New Phytol 225:621–636. https://doi.org/10.1111/nph.16135
Magid J, Tiessen H, Condron LM (1996) Dynamics of organic phosphorus in soils under natural and agricultural ecosystems. In: Piccolo A (ed) Humic Substances in Terrestrial Ecosystems. Elsevier Science, Amsterdam, pp 429–466. https://doi.org/10.1016/B978-044481516-3/50012-8
Maillard A, Diquélou S, Billard V, Laîné P, Garnica M, Prudent M, Garcia-Mina J-M, Yvin J-C, Ourry A (2015) Leaf mineral nutrient remobilization during leaf senescence and modulation by nutrient deficiency. Front Plant Sci 6. https://doi.org/10.3389/fpls.2015.00317
Mallet M, Barthélémy K, Ruby C, Renard A, Naille S (2013) Investigation of phosphate adsorption onto ferrihydrite by X-ray Photoelectron Spectroscopy. J Colloid Interface Sci 407:95–101. https://doi.org/10.1016/j.jcis.2013.06.049
Marro N, Lidoy J, Chico MÁ, Rial C, García J, Varela RM, Macías FA, Pozo MJ, Janoušková M, López-Ráez JA (2022) Strigolactones: New players in the nitrogen–phosphorus signalling interplay. Plant Cell Environ 45:512–527. https://doi.org/10.1111/pce.14212
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, Boston, MA, USA
Martin C (1987) Phytic acid:divalent cation interactions. V. titrimetric, calorimetric, and binding studies with cobalt(ii) and nickel(ii) and their comparison with ot. J Inorg Biochem 30:101–119. https://doi.org/10.1016/0162-0134(87)80047-8
Martin M, Celi L, Barberis E (2004) Desorption and plant availability of myo-inositol hexaphosphate adsorbed on goethite. Soil Sci 169:115–124. https://doi.org/10.1097/01.ss.0000117787.98510.9d
Marzec M, Muszynska A, Gruszka D (2013) The role of strigolactones in nutrient-stress responses in plants. Int J Mol Sci 14:9286–9304. https://doi.org/10.3390/ijms14059286
Mashiguchi K, Seto Y, Yamaguchi S (2021) Strigolactone biosynthesis, transport and perception. Plant J 105:335–350. https://doi.org/10.1111/tpj.15059
Matusova R, Rani K, Verstappen FWA, Franssen MCR, Beale MH, Bouwmeester HJ (2005) The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol 139:920–934. https://doi.org/10.1104/pp.105.061382
Mayer TD, Jarrell WM (2000) Phosphorus sorption during iron(II) oxidation in the presence of dissolved silica. Water Res 34:3949–3956. https://doi.org/10.1016/S0043-1354(00)00158-5
Mayzlish-Gati E, De-Cuyper C, Goormachtig S, Beeckman T, Vuylsteke M, Brewer PB, Beveridge CA, Yermiyahu U, Kaplan Y, Enzer Y, Wininger S, Resnick N, Cohen M, Kapulnik Y, Koltai H (2012) Strigolactones are involved in root response to low phosphate conditions in Arabidopsis. Plant Physiol 160:1329–1341. https://doi.org/10.1104/pp.112.202358
Mikutta C, Mikutta R, Bonneville S, Wagner F, Voegelin A, Christl I, Kretzschmar R (2008) Synthetic coprecipitates of exopolysaccharides and ferrihydrite. Part I: Characterization. Geochim Cosmochim Acta 72:1111–1127. https://doi.org/10.1016/j.gca.2007.11.035
Mikutta R, Lorenz D, Guggenberger G, Haumaier L, Freund A (2014) Properties and reactivity of Fe-organic matter associations formed by coprecipitation versus adsorption: Clues from arsenate batch adsorption. Geochim Cosmochim Acta 144:258–276. https://doi.org/10.1016/j.gca.2014.08.026
Müller J, Toev T, Heisters M, Teller J, Moore KL, Hause G, Dinesh DC, Bürstenbinder K, Abel S (2015) Iron-dependent callose deposition adjusts root meristem maintenance to phosphate availability. Dev Cell 33:216–230. https://doi.org/10.1016/j.devcel.2015.02.007
Nagahashi G, Douds DD (2004) Isolated root caps, border cells, and mucilage from host roots stimulate hyphal branching of the arbuscular mycorrhizal fungus, Gigaspora gigantea. Mycol Res 108:1079–1088. https://doi.org/10.1017/S0953756204000693
Nagarajan VK, Jain A, Poling MD, Lewis AJ, Raghothama KG, Smith AP (2011) Arabidopsis Pht1;5 mobilizes phosphate between source and sink organs and influences the interaction between phosphate homeostasis and ethylene signaling. Plant Physiol 156:1149–1163. https://doi.org/10.1104/pp.111.174805
Nagy R, Karandashov V, Chague V, Kalinkevich K, Tamasloukht M, Xu G, Jakobsen I, Levy AA, Amrhein N, Bucher M (2005) The characterization of novel mycorrhiza-specific phosphate transporters from Lycopersicon esculentum and Solanum tuberosum uncovers functional redundancy in symbiotic phosphate transport in solanaceous species: phosphate transport in solanaceae. Plant J 42:236–250. https://doi.org/10.1111/j.1365-313X.2005.02364.x
Nasto MK, Alvarez-Clare S, Lekberg Y, Sullivan BW, Townsend AR, Cleveland CC (2014) Interactions among nitrogen fixation and soil phosphorus acquisition strategies in lowland tropical rain forests. Ecol Lett 17:1282–1289. https://doi.org/10.1111/ele.12335
Neumann G (2000) Physiological aspects of cluster root function and development in phosphorus-deficient white lupin (Lupinus albus L.). Ann Bot 85:909–919. https://doi.org/10.1006/anbo.2000.1135
Neumann G, Römheld V (1999) Root excretion of carboxylic acids and protons in phosphorus-deficient plants. Plant Soil 211:121–130. https://doi.org/10.1023/A:1004380832118
Neumann G, Römheld V (2002) Root-induced changes in the availability of nutrients in the rhizosphere. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots: the hidden half, 3rd edn. Marcel Dekker Inc., New York, pp 617–649
Ngwene B, George E, Claussen W, Neumann E (2010) Phosphorus uptake by cowpea plants from sparingly available or soluble sources as affected by nitrogen form and arbuscular-mycorrhiza-fungal inoculation. J Plant Nutr Soil Sci 173:353–359. https://doi.org/10.1002/jpln.200900203
Niu YF, Chai RS, Jin GL, Wang H, Tang CX, Zhang YS (2013) Responses of root architecture development to low phosphorus availability: a review. Ann Bot 112:391–408. https://doi.org/10.1093/aob/mcs285
Nolan KB, Duffin PA, McWeeny DJ (1987) Effects of phytate on mineral bioavailability.in vitro studies on Mg2+, Ca2+, Fe3+, Cu2+ and Zn2+ (also Cd2+) solubilities in the presence of phytate. J Sci Food Agric 40:79–85. https://doi.org/10.1002/jsfa.2740400110
Ognalaga M, Frossard E, Thomas F (1994) Glucose-1-phosphate and myo-inositol hexaphosphate adsorption mechanisms on Goethite. Soil Sci Soc Am J 58:332–337. https://doi.org/10.2136/sssaj1994.03615995005800020011x
Omoarelojie LO, Kulkarni MG, Finnie JF, Van Staden J (2019) Strigolactones and their crosstalk with other phytohormones. Ann Bot 124:749–767. https://doi.org/10.1093/aob/mcz100
Parfitt RL (1979) The availability of P from phosphate-goethite bridging complexes. Desorption and uptake by ryegrass. Plant Soil 53:55–65. https://doi.org/10.1007/BF02181879
Parfitt RL (1989) Phosphate reactions with natural allophane, ferrihydrite and goethite. J Soil Sci 40:359–369. https://doi.org/10.1111/j.1365-2389.1989.tb01280.x
Parihar M, Meena VS, Mishra PK, Rakshit A, Choudhary M, Yadav RP, Rana K, Bisht JK (2019) Arbuscular mycorrhiza: a viable strategy for soil nutrient loss reduction. Arch Microbiol 201:723–735. https://doi.org/10.1007/s00203-019-01653-9
Parikh SJ, Chorover J (2006) ATR-FTIR spectroscopy reveals bond formation during bacterial adhesion to iron oxide. Langmuir 22:8492–8500. https://doi.org/10.1021/la061359p
Pearse SJ, Veneklaas EJ, Cawthray G, Bolland MDA, Lambers H (2007) Carboxylate composition of root exudates does not relate consistently to a crop species’ ability to use phosphorus from aluminium, iron or calcium phosphate sources. New Phytol 173:181–190. https://doi.org/10.1111/j.1469-8137.2006.01897.x
Pédrot M, Boudec AL, Davranche M, Dia A, Henin O (2011) How does organic matter constrain the nature, size and availability of Fe nanoparticles for biological reduction? J Colloid Interface Sci 359:75–85. https://doi.org/10.1016/j.jcis.2011.03.067
Penn C, Camberato J (2019) A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 9:120. https://doi.org/10.3390/agriculture9060120
Péret B, Clément M, Nussaume L, Desnos T (2011) Root developmental adaptation to phosphate starvation: better safe than sorry. Trends Plant Sci 16:442–450. https://doi.org/10.1016/j.tplants.2011.05.006
Péret B, Desnos T, Jost R, Berkowitz O, Nussaume L (2014) Root architecture responses: in search of phosphate. Plant Physiol 166:1713–1723. https://doi.org/10.1104/pp.114.244541
Pérez Corona ME, Van Der Klundert I, Verhoeven JTA (1996) Availability of organic and inorganic phosphorus compounds as phosphorus sources for Carex species. New Phytol 133:225–231. https://doi.org/10.1111/j.1469-8137.1996.tb01889.x
Persson P, Nilsson N, Sjöberg S (1996) Structure and bonding of orthophosphate ions at the iron oxide–aqueous interface. J Colloid Interface Sci 177:263–275. https://doi.org/10.1006/jcis.1996.0030
Pham AN, Waite TD (2008) Oxygenation of Fe(II) in the presence of citrate in aqueous solutions at pH 6.0−8.0 and 25 °C: Interpretation from an Fe(II)/citrate speciation perspective. J Phys Chem A 112:643–651. https://doi.org/10.1021/jp077219l
Puga MI, Rojas-Triana M, de Lorenzo L, Leyva A, Rubio V, Paz-Ares J (2017) Novel signals in the regulation of Pi starvation responses in plants: facts and promises. Curr Opin Plant Biol 39:40–49. https://doi.org/10.1016/j.pbi.2017.05.007
Raghothama KG (1999) Phosphate acquisition. Annu Rev Plant Physiol Plant Mol Biol 50:665–693. https://doi.org/10.1146/annurev.arplant.50.1.665
Ramaekers L, Remans R, Rao IM, Blair MW, Vanderleyden J (2010) Strategies for improving phosphorus acquisition efficiency of crop plants. Field Crops Res 117:169–176. https://doi.org/10.1016/j.fcr.2010.03.001
Rausch C, Bucher M (2002) Molecular mechanisms of phosphate transport in plants. Planta 216:23–37. https://doi.org/10.1007/s00425-002-0921-3
Read DB, Bengough AG, Gregory PJ, Crawford JW, Robinson D, Scrimgeour CM, Young IM, Zhang K, Zhang X (2003) Plant roots release phospholipid surfactants that modify the physical and chemical properties of soil. New Phytol 157:315–326. https://doi.org/10.1046/j.1469-8137.2003.00665.x
Redecker D, Raab P (2006) Phylogeny of the Glomeromycota (arbuscular mycorrhizal fungi): recent developments and new gene markers. Mycologia 98:885–895. https://doi.org/10.1080/15572536.2006.11832618
Rezakhani L, Motesharezadeh B, Tehrani MM, Etesami H, Hosseini HM (2019) Phosphate–solubilizing bacteria and silicon synergistically augment phosphorus (P) uptake by wheat (Triticum aestivum L.) plant fertilized with soluble or insoluble P source. Ecotoxicol Environ Saf 173:504–513. https://doi.org/10.1016/j.ecoenv.2019.02.060
Rial C, Varela RM, Molinillo JMG, López-Ráez JA, Macías FA (2019) A new UHPLC-MS/MS method for the direct determination of strigolactones in root exudates and extracts. Phytochem Anal 30:110–116. https://doi.org/10.1002/pca.2796
Richardson AE, Hocking PJ, Simpson RJ, George TS (2009) Plant mechanisms to optimise access to soil phosphorus. Crop Pasture Sci 60:124. https://doi.org/10.1071/CP07125
Römheld V, Marschner H (1990) Genotypical differences among graminaceous species in release of phytosiderophores and uptake of iron phytosiderophores. Plant Soil 123:147–153. https://doi.org/10.1007/BF00011260
Rothwell SA, Doody DG, Johnston C, Forber KJ, Cencic O, Rechberger H, Withers PJA (2020) Phosphorus stocks and flows in an intensive livestock dominated food system. Resour Conserv Recycl 163:105065. https://doi.org/10.1016/j.resconrec.2020.105065
Rouached H, Arpat AB, Poirier Y (2010) Regulation of phosphate starvation responses in plants: signaling players and cross-talks. Mol Plant 3:288–299. https://doi.org/10.1093/mp/ssp120
Rubaek GH, Sibbesen E (1995) Soil phosphorus dynamics in a long-term field experiment at Askov. Biol Fertil Soils 20:86–92. https://doi.org/10.1007/BF00307847
Ruyter-Hooley M, Larsson A-C, Johnson BB, Antzutkin ON, Angove MJ (2015) Surface complexation modeling of inositol hexaphosphate sorption onto gibbsite. J Colloid Interface Sci 440:282–291. https://doi.org/10.1016/j.jcis.2014.10.065
Ruyter-Spira C, Kohlen W, Charnikhova T, van Zeijl A, van Bezouwen L, de Ruijter N, Cardoso C, Lopez-Raez JA, Matusova R, Bours R, Verstappen F, Bouwmeester H (2011) Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: another belowground role for strigolactones? Plant Physiol 155:721–734. https://doi.org/10.1104/pp.110.166645
Ruyter-Spira C, Al-Babili S, van der Krol S, Bouwmeester H (2013) The biology of strigolactones. Trends Plant Sci 18:72–83. https://doi.org/10.1016/j.tplants.2012.10.003
Ryan P, Delhaize E, Jones D (2001) Function and mechanism of organic anion exudation from plant roots. Annu Rev Plant Physiol Plant Mol Biol 52:527–560. https://doi.org/10.1146/annurev.arplant.52.1.527
Sample EC, Soper RJ, Racz GJ (2015) Reactions of phosphate fertilizers in soils. In: Khasawneh FE, Sample EC, Kamprath EJ (eds) ASA, CSSA, and SSSA Books. American society of agronomy crop science society of america, soil science society of america, Madison, pp 263–310
Sánchez-Calderón L, López-Bucio J, Chacón-López A, Cruz-Ramírez A, Nieto-Jacobo F, Dubrovsky JG, Herrera-Estrella L (2005) Phosphate starvation induces a determinate developmental program in the roots of Arabidopsis thaliana. Plant Cell Physiol 46:174–184. https://doi.org/10.1093/pcp/pci011
Santoro V, Martin M, Persson P, Lerda C, Said-Pullicino D, Magnacca G, Celi L (2019) Inorganic and organic P retention by coprecipitation during ferrous iron oxidation. Geoderma 348:168–180. https://doi.org/10.1016/j.geoderma.2019.04.004
Santoro V, Schiavon M, Gresta F, Ertani A, Cardinale F, Sturrock CJ, Celi L, Schubert A (2020) strigolactones control root system architecture and tip anatomy in Solanum lycopersicum L. plants under P starvation. Plants 9:612. https://doi.org/10.3390/plants9050612
Santoro V, Schiavon M, Visentin I, Constán-Aguilar C, Cardinale F, Celi L (2021) Strigolactones affect phosphorus acquisition strategies in tomato plants. Plant Cell Environ 44:3628–3642. https://doi.org/10.1111/pce.14169
Santoro V, Schiavon M, Visentin I, Martin M, Said-Pullicino D, Cardinale F, Celi L (2022) Tomato plant responses induced by sparingly available inorganic and organic phosphorus forms are modulated by strigolactones. Plant Soil 474:355–372. https://doi.org/10.1007/s11104-022-05337-0
Sas L, Rengel Z, Tang C (2001) Excess cation uptake, and extrusion of protons and organic acid anions by Lupinus albus under phosphorus deficiency. Plant Sci 160:1191–1198. https://doi.org/10.1016/S0168-9452(01)00373-9
Sato T, Ezawa T, Cheng W, Tawaraya K (2015) Release of acid phosphatase from extraradical hyphae of arbuscular mycorrhizal fungus Rhizophagus clarus. Soil Sci Plant Nutr 61:269–274. https://doi.org/10.1080/00380768.2014.993298
Scaffidi A, Waters MT, Sun YK, Skelton BW, Dixon KW, Ghisalberti EL, Flematti GR, Smith SM (2014) Strigolactone hormones and their stereoisomers signal through two related receptor proteins to induce different physiological responses in Arabidopsis. Plant Physiol 165:1221–1232. https://doi.org/10.1104/pp.114.240036
Schachtman DP, Reid RJ, Ayling SM (1998) Phosphorus uptake by plants: from soil to cell. Plant Physiol 116:447–453. https://doi.org/10.1104/pp.116.2.447
Schlesinger WH (1991) Biogeochemistry: an analysis of global change. Academic Press, San Diego
Schmitz AM, Harrison MJ (2014) Signaling events during initiation of arbuscular mycorrhizal symbiosis. J Integr Plant Biol 56:250–261. https://doi.org/10.1111/jipb.12155
Senn A-C, Kaegi R, Hug SJ, Hering JG, Mangold S, Voegelin A (2015) Composition and structure of Fe(III)-precipitates formed by Fe(II) oxidation in water at near-neutral pH: Interdependent effects of phosphate, silicate and Ca. Geochim Cosmochim Acta 162:220–246. https://doi.org/10.1016/j.gca.2015.04.032
Shen J, Yuan L, Zhang J, Li H, Bai Z, Chen X, Zhang W, Zhang F (2011) Phosphorus dynamics: from soil to plant. Plant Physiol 156:997–1005. https://doi.org/10.1104/pp.111.175232
Shi T, Zhao D, Li D, Wang N, Meng J, Xu F, Shi L (2012) Brassica napus root mutants insensitive to exogenous cytokinin show phosphorus efficiency. Plant Soil 358:61–74. https://doi.org/10.1007/s11104-012-1219-2
Shimizu M, Zhou J, Schröder C, Obst M, Kappler A, Borch T (2013) Dissimilatory reduction and transformation of ferrihydrite-humic acid coprecipitates. Environ Sci Technol 47:13375–13384. https://doi.org/10.1021/es402812j
Shindo M, Yamamoto S, Shimomura K, Umehara M (2020) Strigolactones decrease leaf angle in response to nutrient deficiencies in rice. Front Plant Sci 11:135. https://doi.org/10.3389/fpls.2020.00135
Sims JT, Pierzynski GM (2018) Chemistry of Phosphorus in Soils. In: Tabatabai MA, Sparks DL (eds) SSSA Book Series. Soil Science Society of America, Madison, pp 151–192
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic Press Ltd, Cambridge
Smith SM, Waters MT (2012) Strigolactones: destruction-dependent perception? Curr Biol 22:R924–R927. https://doi.org/10.1016/j.cub.2012.09.016
Sodano M, Lerda C, Nisticò R, Martin M, Magnacca G, Celi L, Said-Pullicino D (2017) Dissolved organic carbon retention by coprecipitation during the oxidation of ferrous iron. Geoderma 307:19–29. https://doi.org/10.1016/j.geoderma.2017.07.022
Srivastava R, Akash PAP, Chauhan PK, Kumar R (2020) Identification, structure analysis, and transcript profiling of purple acid phosphatases under Pi deficiency in tomato (Solanum lycopersicum L.) and its wild relatives. Int J Biol Macromol 165:2253–2266. https://doi.org/10.1016/j.ijbiomac.2020.10.080
Srivastava R, Sirohi P, Chauhan H, Kumar R (2021) The enhanced phosphorus use efficiency in phosphate-deficient and mycorrhiza-inoculated barley seedlings involves activation of different sets of PHT1 transporters in roots. Planta 254:38. https://doi.org/10.1007/s00425-021-03687-0
Stewart JWB, Tiessen H (1987) Dynamics of soil organic phosphorus. Biogeochemistry 4:41–60. https://doi.org/10.1007/BF02187361
Sun H, Tao J, Liu S, Huang S, Chen S, Xie X, Yoneyama K, Zhang Y, Xu G (2014) Strigolactones are involved in phosphate- and nitrate-deficiency-induced root development and auxin transport in rice. J Exp Bot 65:6735–6746. https://doi.org/10.1093/jxb/eru029
Sun H, Bi Y, Tao J, Huang S, Hou M, Xue R, Liang Z, Gu P, Yoneyama K, Xie X, Shen Q, Xu G, Zhang Y (2016) Strigolactones are required for nitric oxide to induce root elongation in response to nitrogen and phosphate deficiencies in rice. Plant Cell Environ 39:1473–1484. https://doi.org/10.1111/pce.12709
Sun H, Xu F, Guo X et al (2019) A strigolactone signal inhibits secondary lateral root development in rice. Front Plant Sci 10:1527. https://doi.org/10.3389/fpls.2019.01527
Sundman A, Karlsson T, Sjöberg S, Persson P (2016) Impact of iron–organic matter complexes on aqueous phosphate concentrations. Chem Geol 426:109–117. https://doi.org/10.1016/j.chemgeo.2016.02.008
Svistoonoff S, Creff A, Reymond M, Sigoillot-Claude C, Ricaud L, Blanchet A, Nussaume L (2007) Root tip contact with low-phosphate media reprograms plant root architecture. Nat Genet 39:792–796. https://doi.org/10.1038/ng2041
Tanada S, Kabayama M, Kawasaki N, Sakiyama T, Nakamura T, Araki M, Tamura T (2003) Removal of phosphate by aluminum oxide hydroxide. J Colloid Interface Sci 257:135–140. https://doi.org/10.1016/S0021-9797(02)00008-5
Tarafdar JC, Yadav RS, Meena SC (2001) Comparative efficiency of acid phosphatase originated from plant and fungal sources. J Plant Nutr Soil Sci 164:279–282. https://doi.org/10.1002/1522-2624(200106)164:3%3c279::AID-JPLN279%3e3.0.CO;2-L
Tarafdar JC, Claassen N (1988) Organic phosphorus compounds as a phosphorus source for higher plants through the activity of phosphatases produced by plant roots and microorganisms. Biol Fertil Soils 5:. https://doi.org/10.1007/BF00262137
Tawaraya K, Horie R, Saito A, Shinano T, Wagatsuma T, Saito K, Oikawa A (2013) Metabolite profiling of shoot extracts, root extracts, and root exudates of rice plant under phosphorus deficiency. J Plant Nutr 36:1138–1159. https://doi.org/10.1080/01904167.2013.780613
Thibaud M-C, Arrighi J-F, Bayle V, Chiarenza S, Creff A, Bustos R, Paz-Ares J, Poirier Y, Nussaume L (2010) Dissection of local and systemic transcriptional responses to phosphate starvation in Arabidopsis: Local versus systemic responses to phosphate starvation. Plant J 64:775–789. https://doi.org/10.1111/j.1365-313X.2010.04375.x
Thibault P-J, Rancourt DG, Evans RJ, Dutrizac JE (2009) Mineralogical confirmation of a near-P:Fe=1:2 limiting stoichiometric ratio in colloidal P-bearing ferrihydrite-like hydrous ferric oxide. Geochim Cosmochim Acta 73:364–376. https://doi.org/10.1016/j.gca.2008.10.031
Ticconi C, Abel S (2004) Short on phosphate: plant surveillance and countermeasures. Trends Plant Sci 9:548–555. https://doi.org/10.1016/j.tplants.2004.09.003
Tiziani R, Pii Y, Celletti S, Cesco S, Mimmo T (2020) Phosphorus deficiency changes carbon isotope fractionation and triggers exudate reacquisition in tomato plants. Sci Rep 10:15970. https://doi.org/10.1038/s41598-020-72904-9
Tran CTK, Watts-Williams SJ, Smernik RJ, Cavagnaro TR (2022) Arbuscular mycorrhizas increased tomato biomass and nutrition but did not affect local soil P availability or 16S bacterial community in the field. Sci Total Environ 819:152620. https://doi.org/10.1016/j.scitotenv.2021.152620
Trasoletti M, Visentin I, Campo E, Schubert A, Cardinale F (2022) Strigolactones as a hormonal hub for the acclimation and priming to environmental stress in plants. Plant Cell Environ 45:3611–3630. https://doi.org/10.1111/pce.14461
Turner BL (2006) Organic phosphorus in Madagascan rice soils. Geoderma 136:279–288. https://doi.org/10.1016/j.geoderma.2006.03.043
Turner BL, McKelvie ID, Haygarth PM (2002) Characterisation of water-extractable soil organic phosphorus by phosphatase hydrolysis. Soil Biol Biochem 34:27–35. https://doi.org/10.1016/S0038-0717(01)00144-4
Turner BL, Condron LM, Richardson SJ, Peltzer DA, Allison VJ (2007) Soil organic phosphorus transformations during pedogenesis. Ecosystems 10:1166–1181. https://doi.org/10.1007/s10021-007-9086-z
Turner BL, Cheesman AW, Godage HY et al (2012) Determination of neo- and d-chiro-Inositol Hexakisphosphate in Soils by Solution 31P NMR Spectroscopy. Environ Sci Technol 46:4994–5002. https://doi.org/10.1021/es204446z
Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, Kyozuka J, Yamaguchi S (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455:195–200. https://doi.org/10.1038/nature07272
Umehara M, Hanada A, Magome H, Takeda-Kamiya N, Yamaguchi S (2010) Contribution of strigolactones to the inhibition of tiller bud outgrowth under phosphate deficiency in rice. Plant Cell Physiol 51:1118–1126. https://doi.org/10.1093/pcp/pcq084
van der Grift B, Behrends T, Osté LA, Schot PP, Wassen MJ, Griffioen J (2016) Fe hydroxyphosphate precipitation and Fe(II) oxidation kinetics upon aeration of Fe(II) and phosphate-containing synthetic and natural solutions. Geochim Cosmochim Acta 186:71–90. https://doi.org/10.1016/j.gca.2016.04.035
Vance CP (2001) Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable resources. Plant Physiol 127:390–397. https://doi.org/10.1104/pp.010331
Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157:423–447. https://doi.org/10.1046/j.1469-8137.2003.00695.x
Victor Roch G, Maharajan T, Ceasar SA, Ignacimuthu S (2019) The role of PHT1 family transporters in the acquisition and redistribution of phosphorus in plants. Crit Rev Plant Sci 38:171–198. https://doi.org/10.1080/07352689.2019.1645402
Villaecija-Aguilar JA, Hamon-Josse M, Carbonnel S, Kretschmar A, Ljung K, Bennett T, Gutjahr C (2019) KAI2 regulates root and root hair development by modulating auxin distribution. https://doi.org/10.1101/539734
Violante A, Caporale AG (2015) Biogeochemical processes at soil-root interface. J Soil Sci Plant Nutr. https://doi.org/10.4067/S0718-95162015005000038
Visentin I, Vitali M, Ferrero M, Zhang Y, Ruyter-Spira C, Novák O, Strnad M, Lovisolo C, Schubert A, Cardinale F (2016) Low levels of strigolactones in roots as a component of the systemic signal of drought stress in tomato. New Phytol 212:954–963. https://doi.org/10.1111/nph.14190
Voegelin A, Senn A-C, Kaegi R, Hug SJ, Mangold S (2013) Dynamic Fe-precipitate formation induced by Fe(II) oxidation in aerated phosphate-containing water. Geochim Cosmochim Acta 117:216–231. https://doi.org/10.1016/j.gca.2013.04.022
Vogel JT, Walter MH, Giavalisco P, Lytovchenko A, Kohlen W, Charnikhova T, Simkin AJ, Goulet C, Strack D, Bouwmeester HJ, Fernie AR, Klee HJ (2010) SlCCD7 controls strigolactone biosynthesis, shoot branching and mycorrhiza-induced apocarotenoid formation in tomato. Plant J 61:300–311. https://doi.org/10.1111/j.1365-313X.2009.04056.x
Wakabayashi T, Hamana M, Mori A, Akiyama R, Ueno K, Osakabe K, Osakabe Y, Suzuki H, Takikawa H, Mizutani M, Sugimoto Y (2019) Direct conversion of carlactonoic acid to orobanchol by cytochrome P450 CYP722C in strigolactone biosynthesis. Sci Adv 5:eaax906. https://doi.org/10.1126/sciadv.aax9067
Wang Y, Lambers H (2020) Root-released organic anions in response to low phosphorus availability: recent progress, challenges and future perspectives. Plant Soil 447:135–156. https://doi.org/10.1007/s11104-019-03972-8
Wang L, Smith SM (2016) Strigolactones redefine plant hormones. Sci China Life Sci 59:1083–1085. https://doi.org/10.1007/s11427-016-0259-5
Wang X, Tang C, Guppy CN, Sale PWG (2010) Cotton, wheat and white lupin differ in phosphorus acquisition from sparingly soluble sources. Environ Exp Bot 69:267–272. https://doi.org/10.1016/j.envexpbot.2010.04.007
Wang X, Guppy CN, Watson L, Sale PWG, Tang C (2011) Availability of sparingly soluble phosphorus sources to cotton (Gossypium hirsutum L.), wheat (Triticum aestivum L.) and white lupin (Lupinus albus L.) with different forms of nitrogen as evaluated by a 32P isotopic dilution technique. Plant Soil 348:85–98. https://doi.org/10.1007/s11104-011-0901-0
Wang Y, Xu H, Kou J, Shi L, Zhang C, Xu F (2013) Dual effects of transgenic Brassica napus overexpressing CS gene on tolerances to aluminum toxicity and phosphorus deficiency. Plant Soil 362:231–246. https://doi.org/10.1007/s11104-012-1289-1
Wang Y, Duran HGS, van Haarst JC, Schijlen EGWM, Ruyter-Spira C, Medema MH, Dong L, Bouwmeester HJ (2021a) The role of strigolactones in P deficiency induced transcriptional changes in tomato roots. BMC Plant Biol 21:349. https://doi.org/10.1186/s12870-021-03124-0
Wang Y, Yao R, Du X, Guo L, Chen L, Xie D, Smith SM (2021b) Molecular basis for high ligand sensitivity and selectivity of strigolactone receptors in Striga. Plant Physiol 185:1411–1428. https://doi.org/10.1093/plphys/kiaa048
Wang Q, Smith SM, Huang J (2022) Origins of strigolactone and karrikin signaling in plants. Trends Plant Sci 27:450–459. https://doi.org/10.1016/j.tplants.2021.11.009
Ward JT, Lahner B, Yakubova E, Salt DE, Raghothama KG (2008) The effect of iron on the primary root elongation of Arabidopsis during phosphate deficiency. Plant Physiol 147:1181–1191. https://doi.org/10.1104/pp.108.118562
Williamson LC, Ribrioux SPCP, Fitter AH, Leyser HMO (2001) phosphate availability regulates root system architecture in Arabidopsis. Plant Physiol 126:875–882. https://doi.org/10.1104/pp.126.2.875
Wissuwa M, Gamat G, Ismail AM (2005) Is root growth under phosphorus deficiency affected by source or sink limitations? J Exp Bot 56:1943–1950. https://doi.org/10.1093/jxb/eri189
Xie X, Yoneyama K, Yoneyama K (2010) The Strigolactone story. Annu Rev Phytopathol 48:93–117. https://doi.org/10.1146/annurev-phyto-073009-114453
Xu X, Fang P, Zhang H, Chi C, Song L, Xia X, Shi K, Zhou Y, Zhou J, Yu J (2019) Strigolactones positively regulate defense against root-knot nematodes in tomato. J Exp Bot 70:1325–1337. https://doi.org/10.1093/jxb/ery439
Xu Y, Wang J, Wang R, Wang L, Zhang C, Xu W, Wang S, Jiu S (2021) The role of strigolactones in the regulation of root system architecture in grapevine (Vitis vinifera L.) in response to root-restriction cultivation. Int J Mol Sci 22:8799. https://doi.org/10.3390/ijms22168799
Yamada Y, Furusawa S, Nagasaka S, Shimomura K, Yamaguchi S, Umehara M (2014) Strigolactone signaling regulates rice leaf senescence in response to a phosphate deficiency. Planta 240:399–408. https://doi.org/10.1007/s00425-014-2096-0
Yan Y, Li W, Yang J, Zheng A, Liu F, Feng X, Sparks DL (2014) Mechanism of myo-inositol hexakisphosphate sorption on amorphous aluminum hydroxide: Spectroscopic evidence for rapid surface precipitation. Environ Sci Technol 48:6735–6742. https://doi.org/10.1021/es500996p
Yan Y, Wan B, Jiang R, Wang X, Wang H, Lan S, Zhang Q, Feng X (2023) Interactions of organic phosphorus with soil minerals and the associated environmental impacts: A review. Pedosphere 33:74–92. https://doi.org/10.1016/j.pedsph.2022.08.001
Yang T, Lian Y, Wang C (2019) Comparing and contrasting the multiple roles of butenolide plant growth regulators: strigolactones and karrikins in plant development and adaptation to abiotic stresses. Int J Mol Sci 20:6270. https://doi.org/10.3390/ijms20246270
Yiu HHP, Bouffier L, Boldrin P, Long J, Claridge JB, Rosseinsky MJ (2013) Comprehensive study of DNA binding on Iron(II, III) oxide nanoparticles with a positively charged polyamine three-dimensional coating. Langmuir 29:11354–11365. https://doi.org/10.1021/la400848r
Yoneyama K, Brewer PB (2021) Strigolactones, how are they synthesized to regulate plant growth and development? Curr Opin Plant Biol 63:102072. https://doi.org/10.1016/j.pbi.2021.102072
Yoneyama K, Yoneyama K, Takeuchi Y, Sekimoto H (2007a) Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta 225:1031–1038. https://doi.org/10.1007/s00425-006-0410-1
Yoneyama K, Xie X, Kusumoto D, Sekimoto H, Sugimoto Y, Takeuchi Y, Yoneyama K (2007b) Nitrogen deficiency as well as phosphorus deficiency in sorghum promotes the production and exudation of 5-deoxystrigol, the host recognition signal for arbuscular mycorrhizal fungi and root parasites. Planta 227:125–132. https://doi.org/10.1007/s00425-007-0600-5
Yoneyama K, Xie X, Sekimoto H, Takeuchi Y, Ogasawara S, Akiyama K, Hayashi H, Yoneyama K (2008) Strigolactones, host recognition signals for root parasitic plants and arbuscular mycorrhizal fungi, from Fabaceae plants. New Phytol 179:484–494. https://doi.org/10.1111/j.1469-8137.2008.02462.x
Yoneyama K, Xie X, Kim HI, Kisugi T, Nomura T, Sekimoto H, Yokota T, Yoneyama K (2012) How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta 235:1197–1207. https://doi.org/10.1007/s00425-011-1568-8
Yoneyama K, Xie X, Kisugi T, Nomura T, Yoneyama K (2013) Nitrogen and phosphorus fertilization negatively affects strigolactone production and exudation in sorghum. Planta 238:885–894. https://doi.org/10.1007/s00425-013-1943-8
Yoneyama K, Xie X, Yoneyama K, Nomura T, Takahashi I, Asami T, Mori N, Akiyama K, Kusajima M, Nakashita H (2019) Regulation of biosynthesis, perception, and functions of strigolactones for promoting arbuscular mycorrhizal symbiosis and managing root parasitic weeds. Pest Manag Sci 5401. https://doi.org/10.1002/ps.5401
Zhang YS, Werner W, Scherer HW, Sun X (1994) Effect of organic manure on organic phosphorus fractions in two paddy soils. Biol Fertil Soils 17:64–68. https://doi.org/10.1007/BF00418674
Zhang FS, Ma J, Cao YP (1997) Phosphorus deficiency enhances root exudation of low-molecular weight organic acids and utilization of sparingly soluble inorganic phosphates by radish (Raghanus satiuvs L.) and rape (Brassica napus L.) plants. Plant Soil 196:261–264. https://doi.org/10.1023/A:1004214410785
Zhang H, Huang Y, Ye X, Xu F (2011) Genotypic variation in phosphorus acquisition from sparingly soluble P sources is related to root morphology and root exudates in Brassica napus. Sci China Life Sci 54:1134–1142. https://doi.org/10.1007/s11427-011-4254-y
Zhang Y, Cheng X, Wang Y, Díez-Simón C, Flokova K, Bimbo A, Bouwmeester HJ, Ruyter-Spira C (2018a) The tomato MAX1 homolog, SlMAX1, is involved in the biosynthesis of tomato strigolactones from carlactone. New Phytol 219:297–309. https://doi.org/10.1111/nph.15131
Zhang L, Li G, Li Y, Min J, Kronzucker HJ, Shi W (2018b) Tomato plants ectopically expressing Arabidopsis GRF9 show enhanced resistance to phosphate deficiency and improved fruit production in the field. J Plant Physiol 226:31–39. https://doi.org/10.1016/j.jplph.2018.04.005
Zhao K, Wu Y (2014) Rhizosphere calcareous soil P-extraction at the expense of organic carbon from root-exuded organic acids induced by phosphorus deficiency in several plant species. Soil Sci Plant Nutr 60:640–650. https://doi.org/10.1080/00380768.2014.934191
Zheng X, Li Y, Xi X, Ma C, Sun Z, Yang X, Li X, Tian Y, Wang C (2021) Exogenous Strigolactones alleviate KCl stress by regulating photosynthesis, ROS migration and ion transport in Malus hupehensis Rehd. Plant Physiol Biochem 159:113–122. https://doi.org/10.1016/j.plaphy.2020.12.015
Zhou Z, Wang Z, Lv Q, Shi J, Zhong Y, Wu P, Mao C (2015) SPX proteins regulate Pi homeostasis and signaling in different subcellular level. Plant Signal Behav 10:e1061163. https://doi.org/10.1080/15592324.2015.1061163
Zhou W, Chen D, Zeng Q, Tahir MA, Wu Q, Huang Y, Jiang Y, Li Q, Ao J, Huang Z (2021) Differential physiological behavior of sugarcane genotypes in response to sparingly soluble phosphorus-sources. J Plant Nutr Soil Sci 184:187–197. https://doi.org/10.1002/jpln.202000333
Zulfiqar H, Shahbaz M, Ahsan M, Nafees M, Nadeem H, Akram M, Maqsood A, Ahmar S, Kamran M, Alamri S, Siddiqu MH, Saud S, Fahad S (2021) Strigolactone (GR24) induced salinity tolerance in sunflower (Helianthus annuus L.) by ameliorating morpho-physiological and biochemical attributes under in vitro conditions. J Plant Growth Regul 40:2079–2091. https://doi.org/10.1007/s00344-020-10256-4
Funding
Open access funding provided by Università degli Studi di Torino within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by Veronica Santoro and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no conflicts of interest to declare.
Additional information
Responsible Editor: Tim S. George.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Santoro, V., Schiavon, M. & Celi, L. Role of soil abiotic processes on phosphorus availability and plant responses with a focus on strigolactones in tomato plants. Plant Soil 494, 1–49 (2024). https://doi.org/10.1007/s11104-023-06266-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06266-2