Abstract
Background and aims
In extremely low-phosphorus (P) environments, most Proteaceae exude carboxylates from cluster roots. These carboxylates mobilise inorganic P which leads to a relatively high leaf manganese concentration ([Mn]). However, we found that Adenanthos cygnorum (Proteaceae) in a low-P habitat did not invariably have a high leaf [Mn] in south-western Australia. We aimed to explore how A. cygnorum acquires P in severely P-impoverished habitats.
Methods
We determined soil P concentrations and leaf [Mn] of A. cygnorum growing within 1 m and more than 10 m away from other large Proteaceae. We also grew plants in a glasshouse to determine its root carboxylate exudation and rhizosheath phosphatase activity.
Results
Adenanthos cygnorum did not produce functional cluster roots. It depended on carboxylates released by a P-mobilising neighbour, Banksia attenuata (Proteaceae), to acquire P when growing in severely P-impoverished soil (< 8 mg P kg− 1 dry soil). In slightly less P-impoverished soil (> 11 mg P kg− 1 dry soil), phosphatases released by A. cygnorum hydrolysed sufficient organic P that was relatively mobile.
Conclusion
The reliance on facilitation of P acquisition in A cygnorum depended strongly on location. We demonstrated the exudation of phosphatases, which mobilise inorganic P; this P was adequate for growth when there was sufficient organic P in soil. Facilitation of P acquisition by B. attenuata allowed A. cygnorum to extend its range into severely P-impoverished habitats where it cannot exist without facilitation. This knowledge provides a better understanding of the diversity of P-acquisition strategies in severely P-impoverished environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is an essential plant nutrient, and frequently limits plant productivity, especially in biodiversity hotspots including south-western Australia (Kooyman et al. 2017). In response to low soil P availability, roots exhibit a variety of adaptive strategies. Many Fabaceae and Myrtaceae access soil P by being mycorrhizal, provided the soil P concentration ([P]) is not extremely low (Abbott et al. 1984; Parfitt 1979; Treseder and Allen 2002). Most Proteaceae are non-mycorrhizal and develop cluster roots as an adaptation for nutrient acquisition when soil P availability is extremely low (Lamont 1982; Shane et al. 2004a). At the morphological level, each cluster root comprises hundreds to thousands of rootlets, which are covered in abundant root hairs, greatly increasing their surface area (Shane et al. 2004a). At the physiological level, cluster roots release exudates (e.g., carboxylates, protons, phenolics, phosphatases, phytases) into the rhizosphere that mobilise soil P (Dinkelaker et al. 1995; Shane and Lambers 2005).
The large amounts of carboxylates released into the soil by cluster roots mobilise both inorganic P (Pi) and organic P (Po) that are sorbed onto soil particles into the soil solution. However, roots only absorb Pi, and access organic forms of P following hydrolysis by phosphatases (Lambers et al. 2008; Lambers 2022), which releases Pi. Not all Proteaceae have functional cluster roots that release large amounts of carboxylates. For example, Xylomelum occidentale (Proteaceae) produces tiny cluster roots that only live for three days, and do not release carboxylates into the soil (Zhong et al. 2021).
Carboxylates mobilise a variety of Po molecules. They can mobilise strongly sorbed Po molecules, such as phytate, which can then be hydrolysed by phytases (Giles et al. 2017). Carboxylates are not needed to mobilise all Po molecules, since not all Po is as strongly sorbed as phytate to soil particles (Turner 2007; Giles et al. 2017). Strongly sorbed Po includes phytate and glucose-6-phosphate (Amadou et al. 2022), while phospholipids and nucleic acids (RNA and DNA) are less subject to strong sorption (Jarosch et al. 2019). Roots of X. occidentale that do not exude carboxylates acquire P from weakly sorbed soil phospholipids and RNA using exuded phosphatases, without involvement of carboxylates (Zhong et al. 2021).
Plants occurring next to P-mobilising plants may utilise Pi mobilised by those neighbours to facilitate their P uptake (Li et al. 2007). Facilitation of nutrient acquisition refers to a positive effect on nutrient uptake for at least one of the interacting species which may occur as a result of an increase in the availability of a limiting nutrient, such as iron (Fe), zinc (Zn) and manganese (Mn) (Li et al. 2014). For example, Banksia attenuata (Proteaceae) facilitates Mn acquisition of neighbouring Scholtzia involucrata (Myrtaceae), because carboxylates released by its cluster roots increase Mn availability in soil (Muler et al. 2014).
The uptake of Mn, including that mobilised by carboxylates, is poorly controlled (Lambers et al. 2015; Alejandro et al. 2020). Therefore, Proteaceae with carboxylate-releasing cluster roots (Lambers et al. 2021) take up more Mn from soil than mycorrhizal plants, because mycorrhizal hyphae intercept Mn (Kothari et al. 1991; Canton et al. 2016). The Mn is then exported from the roots to the shoot, giving rise to relatively high mature leaf Mn concentrations ([Mn]) in most Proteaceae. An exception is X. occidentale, due to its ineffective cluster roots (Zhong et al. 2021). Thus, leaf [Mn] is a proxy for carboxylate concentrations in the rhizosphere (Lambers et al. 2015; Pang et al. 2018), and, therefore, can also be used to assess facilitation of P acquisition. When using leaf [Mn] as a proxy for belowground carboxylate release, both positive and negative reference species are needed (Zhou et al. 2020). A positive reference is a species known to depend on carboxylate release for its P acquisition and has a high leaf [Mn], as found in Banksia species. A negative reference is a species such as Xanthorrhoea preissii, which is known to not depend on carboxylates for P acquisition and always has a low leaf [Mn]. Sometimes, when a negative reference cannot be found nearby, young leaves of the same test species can be used as the negative reference (Shane and Lambers 2005; Zhou et al. 2020; Lambers et al. 2021). When P is a severely limiting resource, plants will use all the absorbed Pi for growth so that leaf [P] does not increase (de Groot et al. 2003; Shane et al. 2003). In this case, leaf [P] cannot be used as a proxy for belowground interactions, while leaf [Mn] can be used.
Native plants in south-western Australia generally occur on extremely P-impoverished soils; they not only exhibit efficient P-acquisition strategies, but also use P very efficiently (Lambers et al. 2010). Photosynthetic rates are relatively fast in these scleromorphic plants (Lambers et al. 2012), in contrast to those of crop plants growing in P-impoverished soil which tend to have very slow photosynthetic rates per unit leaf area (Brooks et al. 1988). Many Proteaceae from this region have low leaf [P] compared with global values (Wright et al. 2004) and a high leaf mass per area (LMA) (Wright and Westoby 2003). Relatively fast photosynthetic rates combined with low leaf [P] typically provide Proteaceae with very high photosynthetic P-use efficiencies (Denton et al. 2007).
Adenanthos Labill. (Proteaceae) is a genus of 33 species that are endemic to southern Australia, particularly in those parts with a Mediterranean-type climate (Nge et al. 2021). Adenanthos cygnorum Diels (common woolly bush) is endemic to south-western Australia (Fig. 1a; https://florabase.dpaw.wa.gov.au/browse/profile/1775). In Alison Baird Reserve (Fig. 1a and b and 32°01’10ʺS, 115°58’46ʺE), the main location of the present study, it has a low, but variable, leaf [Mn] compared with other Proteaceae (J. Wasaki et al. pers. obs.). We noticed during preliminary observations at this location that A. cygnorum only has tiny cluster roots compared with adjacent B. attenuata (Fig. S1). These observations suggested that cluster roots contribute little to its P acquisition, similar to what was found in Xylomelum species (Zhong et al. 2021), raising the question of how A. cygnorum survives on severely P-impoverished soils. We also noted that A. cygnorum only grows next to large Banksia species, B. attenuata and B. menziesii, on top of a two 2-million-year-old dune of highly weathered and severely nutrient-impoverished Bassendean sand (Turner et al. 2018) in the reserve. However, it can grow away from other Proteaceae at the bottom of the same dune. We hypothesise that soils at the bottom of the dune have a significantly higher [P] than those at the top of the dune, because P would have eroded down the slope towards the bottom of the dune.
a The distribution of Adenanthos cygnorum in south-western Australia. https://florabase.dpaw.wa.gov.au/browse/profile/1775. b Drone map of collection sites for leaf and soil samples of Adenanthos cygnorum in Alison Baird Reserve. On the top (yellow dot) of the taller dune (large red oval), A cygnorum grows next to Banksia attenuata. At the bottom of the taller dune (yellow dot) and on the low dune (small red oval), the sand profile is too shallow for B. attenuata to occur. At the bottom of the taller dune, A. cygnorum is able to grow in the absence of nearby Proteaceae, but it does not occur on the low dune. The locations for sampling A. cygnorum seedlings for phosphatase activity measurement are shown by blue lines. Bar = 75 m
Based on our preliminary observations, we wondered how A. cygnorum acquired P in extremely P-impoverished habitats. We developed the following hypotheses: (1) Unlike most Proteaceae, A. cygnorum does not produce functional cluster roots that release large amounts of carboxylates. (2) Its roots release phosphatases into soil to hydrolyse those Po compounds that are not tightly sorbed onto soil particles, as is the case for X. occidentale (Zhong et al. 2021). (3) At locations where Banksia attenuata dominates and makes soil P available, such on top of a dune of Bassendean sand in Alison Baird Reserve, where we expect the availability of soil P to be very low (Hayes et al. 2014), P acquisition by A. cygnorum is facilitated by this P-mobilising neighbour. (4) At adjacent sites, where A. cygnorum does not grow next to tall Proteaceae, such as at the base of a dune in Alison Baird Reserve, the availability of soil P will be higher and A. cygnorum can acquire sufficient P without facilitation by P-mobilising neighbours. (5) Adenanthos cygnorum uses P efficiently in photosynthesis as most Proteaceae in south-western Australia do. To test these hypotheses, we measured soil [P] and [Mn] and leaf [P] and [Mn] in A. cygnorum both near and away from large Banksia plants, at three locations to test for facilitation of P acquisition. We also measured photosynthesis rates of A. cygnorum both near and away from Banksia plants in Alison Baird Reserve.
Materials and methods
Study area
The field experiment was conducted at three locations. The main location was in Alison Baird Reserve, a 34.6-ha flora reserve located 20 km southeast of Perth in south-western Australia (Fig. 1a, Supplementary Material 1). The other locations were near Jurien Bay (30°10’58.8"S 115°07’58.8"E), 200 km north of Perth (Hayes et al. 2014) and at Melaleuca Park (31˚39′44ʺ S 115˚55′29ʺ E), 70 km north of Perth (Veneklaas and Poot 2003). The climate of the study area is Mediterranean, with hot dry summers (Dec to Feb) and cool wet winters (Jun to Aug). The mean annual rainfall (1994–2022) in the Perth region is 736.8 mm, around 80% of which falls between May and September. The mean daily temperature range is 12.9–24.8 °C (1994–2022), with the highest temperature in February (18.4–31.6 °C), and the lowest in July (8.0-18.5 °C). In Jurien Bay, the mean annual rainfall is 545 mm (1968–2022), and the mean daily temperature range is 13.1–25.0 °C (1969–2022), with the highest temperature in February (18.0-30.8 °C) and the lowest in July (9.4–19.6 °C). Unless mentioned otherwise, the results shown pertain to the ecosystem in Alison Baird Reserve. The ecosystem represented by the three locations differs from most well-studied systems outside Australia (Supplementary Material 1). Each location is dominated by 2-million-year-old dunes of Bassendean sand (Turner et al. 2018). The sands of the dunes are podosols (Isabell and NCST 2016) of a marine origin.
In Alison Baird Reserve, we selected a habitat on top of a tall dune (Fig. 1b). At this site, all A. cygnorum plants grow next to tall Banksia species such as B. attenuata. All A. cygnorum plants were within 5 m of a tall Banksia plant at this site, while roots of tall Banksia plants can extend over 10 m (Pate and Dixon 1996). We also selected a site at the bottom of the same tall dune, where tall Proteaceae other than A. cygnorum were absent and where A. cygnorum plants could be found away from all Proteaceae, except other individuals of A. cygnorum. All A. cygnorum plants in Alison Baird Reserve germinated after a fire in 2005, so plant size was similar. For comparison, we also selected a low dune (Fig. 1b) lacking both tall Banksia species and A. cygnorum. Banksia attenuata requires deep sand to access sufficient water during the Mediterranean summer (Canham et al. 2009); this habitat is available on top of the tall dune, but not on the low dune or at the bottom of the tall dune. Because the Bassendean dune is approximately two million years old, the seeds of both B. attenuata and A. cygnorum must have had sufficient time to arrive at the low dune from the tall dune which is less than 400 m away from the tall dune. Thus, absence of seeds of A. cygnorum on the low dune can be ruled out as an explanation of why it has not established there. At the Melaleuca Park and Jurien Bay locations, we also selected a habitat where all A. cygnorum grew next to tall Banksia species such as B. attenuata and another habitat where A. cygnorum grew away from all Proteaceae, except other individuals of A. cygnorum.
Leaf and soil sample collection
At each location, youngest fully-expanded undamaged mature leaves were collected from six individuals of A. cygnorum that were more than 10 m away from the nearest Proteaceae, except conspecifics, and from six individuals growing within 1 m of B. attenuata. For the sake of simplicity, the former plants will be referred to as A. cygnorum away from other Proteaceae. Leaves were collected in March, September and December 2019 in Alison Baird Reserve, in June 2020 near Jurien Bay and in June 2021 in Melaleuca Park. In Alison Baird Reserve, young and senesced leaves were also collected from the same A. cygnorum plants sampled for mature leaves. Senesced leaves were collected after spreading a white sheet under a bush and gently shaking the branches. In addition, at Alison Baird Reserve youngest fully-expanded undamaged mature leaves of B. attenuata were collected from randomly selected plants without considering their position relative to A. cygnorum. Leaf samples were oven-dried at 70 °C for two days, and finely ground into powder with zirconium beads (Geno/Grinder 2010, Spex SamplePrep, Metuchen, NJ, USA). Leaf samples were digested in hot concentrated HNO3:HClO4 (3:1) (Zarcinas et al. 1987) and total leaf [P] and [Mn] determined by inductively coupled plasma optical-emission spectrometry (ICP-OES, Model 5300DV, Perkin Elmer, Shelton, CT, USA).
Soil samples down to 10 cm depth and no more than 0.5 m from the base of all 12 A. cygnorum plants from each location were collected using a spade. We also randomly collected five soil samples from the low dune in Alison Baird Reserve down to 10 cm depth. Soil samples were air-dried at 40 °C for 72 h and sieved (< 2 mm) to remove roots and debris. Dried soil was finely ground for total element determination. Total [Mn] in sieved and ground soil was measured after an aqua regia (HNO3/HCl) digestion by ICP-OES as previously described (Rayment and Higginson 1992) (Optima 7300DV; Perkin Elmer Inc, Wellesley, MA, USA). Total and organic [P] was measured by the ignition method and extracted from soil by 1 M HCl (Saunders and Williams 1955). For readily plant-available soil P (measured as resin [P], readily exchangeable Pi), soil samples were shaken for 16 h with 30 mL deionized (DI) water and Pi extracted using anion-exchange membrane (AEM) strips (Turner and Romero 2009). The AEM strips were then washed free of soil particles with DI water. After washing, the strips were transferred to 10 mL 0.5 M HCl and shaken for 1 h to extract the Pi.
Leaf gas exchange
Leaf gas exchange was measured on youngest fully-expanded undamaged green leaves between 9:00 and 11:00 am on plants in Alison Baird Reserve (LI6400XT portable photosynthesis system, Li-Cor Inc., Lincoln, NE, USA). During measurements, leaf temperature and photosynthetic photon flux density were maintained at 25.0 ± 0.5oC and 1200 µmol photons m–2 s–1, respectively. The reference CO2 concentration, vapour pressure and relative humidity were 400 µmol mol− 1, 1.1 ± 0.05 kPa and 55–65%, respectively. Leaf area was measured by scanning at a resolution of 200 dpi (Epson Perfection V800, Seiko Epson, Nagano, Japan) and the images were analysed with WinRHIZO software (Regent Instruments, Quebec, Canada).
Glasshouse experiments
Glasshouse experiments were conducted at the University of Western Australia, Perth, Australia (31.57°S, 115.47°E) from September 2019 to June 2020 in a controlled glasshouse environment with min/max temperatures of 20/32˚C in summer and 13/23˚C in winter. Relative humidity was maintained at approximately 60% in the glasshouse. An overhead shade screen (~60% light transmission) automatically deployed if photosynthetically active radiation (PAR) exceeded 1650 μmol m−2 s−1.
Hydroponic plant growth for root carboxylate measurement
Adenanthos cygnorum seedlings were collected from a fire break (Supplementary Material 1) on a Bassendean dune near Jurien Bay, south-western Australia, in September 2019 and transferred into 4.5 L pots containing 4 L continuously aerated nutrient solution with one plant per pot. Pots were placed in a root-cooling tank to maintain the root temperature at 18–20˚C for plant growth. Nutrient solution (pH 5.8) had the following composition (in µM): 0.5 P (applied as KH2PO4), 0.12 Mn2+, 200 NO3–, 100 Ca2+, 100 K+, 77 SO42–, 27 Mg2+, 20 Cl–, 1.0 Fe-EDTA, 0.05 Zn2+, 0.02 Cu2+, 1.2 H3BO3, 0.15 Mo4+, and 50 SiO32−. Nutrient solution was changed twice a week.
Plants were harvested 30 weeks after transfer to hydroponics. Carboxylate exudation was measured on whole root systems that included cluster roots and non-cluster roots, and on mature cluster roots separately. After excision, whole root systems or mature cluster roots were soaked in 15 ml or 1.5 ml, respectively, of basal nutrient solution without P and calcium (Ca) for 20 min to avoid effects from root detachment. Roots were then transferred to another tube containing the same nutrient solution, aerated gently with a stream of air, covered with aluminium foil, and incubated for 90 min. After incubation, the solution containing root exudates was filtered using a 0.2 μm syringe filter and fresh weight of the root was determined. The filtered solution (1.0 ml) was transferred to an HPLC vial to which 20 µl concentrated ortho-phosphoric acid was added and mixed well. The carboxylates exuded from the root were then determined (Cawthray 2003) (Waters HPLC, 600E dual head pump, 717 plus autosampler, and 996 photodiode-array detector; Milford, MA, USA).
Soil experiments for root phosphatase measurement
Soil for potting was collected near the bottom of the tall dune in Alison Baird Reserve more than 10 m from the nearest tall Proteaceae, dried at 40˚C for one week, and sieved using a 2-mm strainer to remove large plant debris. The A. cygnorum seedlings collected from the field were grown in hydroponics for 30 weeks and then eleven seedlings of uniform size were transferred to large free-draining PVC pots that contained 3.0 kg sieved soil. These pots were watered with deionised water to maintain adequate soil moisture.
After six months, A. cygnorum root systems were harvested. Rhizosheath soil, the soil compartment adhering to the root surface (Hinsinger et al. 2009), was collected by shaking plants gently to remove bulk soil, and then using a paint brush to remove the rhizosheath soil. Acid phosphatase activity was measured spectrophotometrically using p-nitrophenol as substrate (Tabatabai 1994). As a comparison, phosphatase activity was also determined for 11 A. cygnorum seedlings collected from two sites (Fig. 1b) in Alison Baird Reserve in October 2020 using the same method described above.
Calculations
Leaf mass per area (LMA) was calculated by dividing leaf dry weight (DW) by leaf area. Photosynthetic rate on a leaf DW basis was calculated by dividing photosynthetic rate on a leaf area basis by LMA. Photosynthetic phosphorus-use efficiency (PPUE) was calculated by dividing photosynthetic rate on a leaf DW basis by leaf [P] on a leaf DW basis. Phosphorus-resorption was calculated by dividing senesced-leaf P content by mature-leaf P content. Weight loss was taken into consideration for senesced leaves to recalculate P content using mature leaf weight divided by senesced leaf weight.
Leaf anatomy
Cross-sections of fully expanded mature leaves of A. cygnorum were prepared using a microtome (Leica RM2125 RTS; Leica Biosystems Nussloch GmbH, Heidelberg, Germany). They were viewed under epifluorescence microscopy (Axioskop optical microscope, El-Einsatz; Carl Zeiss, Oberkochen, Germany), and photos were taken using an Axiocam digital camera (Carl Zeiss Microscopy, GmbH).
Statistics
Leaf and soil [Mn], leaf [P], leaf DW, photosynthetic rates, LMA, PPUE and phosphatase activity were analysed by unpaired Student’s t-test (SAS 8.1, USA). Analysis of variance (ANOVA) for soil [P] was conducted using the one-way non-block experiment ANOVA model in SAS. Homogeneity of variance among the variables was distributed normally according to the SAS algorithm. Significant differences between or among means were determined by LSD at P ≤ 0.05. All graphs were produced using SigmaPlot (SigmaPlot 10.0, USA).
Results
Leaf and soil manganese concentrations
Mature leaf [Mn] of A. cygnorum from both study sites on the tall dune at Alison Baird Reserve was around twice that of young leaves (Fig. 2a), while mature leaf [Mn] of B. attenuata was significantly higher than that of A. cygnorum growing at either site. In young A. cygnorum leaves on the tall dune, there was no significant difference in [Mn] next to B. attenuata or 10 m away from other Proteaceae. The mature leaf [Mn] at the other two locations, like that at Alison Baird Reserve, was two-fold greater in plants next to B. attenuata than that in plants 10 m away from other Proteaceae (Figs. 2a, S2). However, soil [Mn] under A. cygnorum at these locations was similar next to B. attenuata and 10 m away from other tall Proteaceae (Figs. 2b, S3).
a Manganese concentrations ([Mn]) of young and mature leaves of Adenanthos cygnorum growing more than 10 m away from the nearest large Proteaceae and within 1 m of Banksia attenuata and mature leaf [Mn] of B. attenuata. b Soil total [Mn] under A. cygnorum growing more than 10 m away from the nearest Proteaceae or within 1 m of B. attenuata. The bars represent means \(\pm\) SE (n = 6). Different letters indicate significant differences at p < 0.05 within a leaf type (young / mature leaves) (a) or between sites (b)
Leaf and soil phosphorus concentrations
In young leaves of A. cygnorum away from other Proteaceae at Alison Baird Reserve, [P] was 30% greater than that of plants next to B. attenuata (Fig. 3a). The [P] in young leaves was at least ten-fold higher than that in mature leaves at both sites (Fig. 3a). At each location, including Alison Baird Reserve, the [P] of mature leaves sampled next to B. attenuata and away from other Proteaceae was very low and the same (Figs. 3a, S4). Similarly, there was no significant difference in [P] between senesced leaves at the contrasting sites in Alison Baird Reserve. On average, [P] of senesced leaves was only 30% lower than that of mature leaves (Fig. 3a). However, on average, leaf dry weight (DW) of mature leaves was about 60% greater than that of senesced leaves (Fig. 3b). Taking into account the loss of DW in senescing leaves, showed that P resorption was about 55% for leaves sampled either next to B. attenuata or away from other Proteaceae (Fig. 3b).
a Phosphorus concentrations ([P]) of young, mature and senesced leaves, and b dry weight per mature or senesced leaf of Adenanthos cygnorum growing more than 10 m away from the nearest Proteaceae and within 1 m of Banksia attenuata. The percentage P resorption efficiency from senescing leaves is indicated above the bars for each location. The bars represent means \(\pm\) SE (n = 6). Different letters indicate significant differences at p < 0.05 within a leaf type (a), and between sites (b)
Soil organic P concentration ([Po]) was significantly greater than soil resin [P] at all three locations, irrespective of sampling site (Figs. 4, S5, S6, S7). At Alison Baird Reserve, soil resin [P] under A. cygnorum away from other Proteaceae, at 2.99 mg P kg− 1 dry soil, was significantly higher than that of soils under A. cygnorum next to B attenuata and on the low dune, at 1.75 and 1.12 mg P kg− 1 dry soil, respectively (Fig. 4). Similarly, soils under A. cygnorum growing away from other Proteaceae contained about 50% more Po than those under plants next to B. attenuata or soils sampled at the low dune (Fig. 4). Total [P] of soil under A. cygnorum growing away from other Proteaceae and of soil from the lower dune was about 50% greater than that under A. cygnorum next to B. attenuata (Fig. 4). At the other two sampling locations, soil total [P], resin [P] and organic [P] under A. cygnorum away from other Proteaceae was also significantly greater than that of soil under A. cygnorum next to B. attenuata (Figs. S5, S6, S7).
Total phosphorus (P), resin P and organic P concentration of soils sampled under Adenanthos cygnorum growing more than 10 m away from the nearest Proteaceae and within 1 m of Banksia attenuata, and on the lower dune lacking both species. The bars represent means \(\pm\) SE (n = 5). Different letters indicate significant differences at p < 0.05 among sites
Statistical comparisons were only made between the two sites at each location but not among the sites at all three locations because the topography of the locations was not equivalent. At Alison Baird Reserve, there is a distinct dune with a top and a bottom deposited on a substrate with a different soil composition to the dune (Leopold and Zhong 2019). The locations sampled in Melaleuca Park and in Jurien Bay were deep Bassendean sand over a wide area where we were able to find some A. cygnorum away from other large Proteaceae. Moreover, the substrate underlying the sand dunes at the three locations was not the same. The results we present are mainly based on the study location in Alison Baird Reserve, but, importantly, the results from the other two locations were consistent with what we found in Alison Baird Reserve (Figs. S5, S6, S7).
Photosynthetic rates and photosynthetic P-use efficiency (PPUE)
Photosynthetic rates on both a leaf area basis (Fig. 5a) and a leaf DW basis (Fig. 5b) for A. cygnorum plants growing next to B. attenuata were similar to those of plants growing > 10 m away from other large Proteaceae in Alison Baird Reserve. Leaf mass per area (LMA) was the same at both sites (Fig. 5c). Moreover, the photosynthetic P-use efficiency (PPUE) was similar for A. cygnorum at the two sites in Alison Baird Reserve (Fig. 5d). The average PPUE across the two sites was 0.65 mmol CO2 g− 1 P s− 1 (average photosynthetic rate = 84 nmol CO2 g− 1 DW s− 1; average leaf [P] = 0.13 mg g− 1 DW).
a Net photosynthetic rates on a leaf area basis and b on a leaf mass basis, c leaf mass per unit area (LMA) and d photosynthetic phosphorus-use efficiency (PPUE) of youngest fully-expanded leaves of Adenanthos cygnorum growing more than 10 m away from the nearest Proteaceae and within 1 m of Banksia attenuata. The bars represent means \(\pm\) SE (n = 6). Different letters indicate significant differences at p < 0.05
Leaf anatomy of Adenanthos cygnorum
There were no clear structural or anatomical differences in A. cygnorum leaves between sites at Alison Baird Reserve. Chloroplasts were mainly localised to two layers of palisade mesophyll cells beneath the upper epidermis (Fig. 6). There were abundant lignified sclereids with thick cell walls embedded in the palisade cell layers. A single layer of spongy mesophyll cells, which contained many calcium crystals, was present interior to the palisade mesophyll cells (Fig. 6). There were no stomatal crypts in the leaves.
Carboxylates and phosphatases released from Adenanthos cygnorum roots
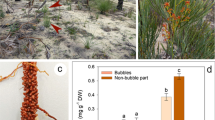
Adenanthos cygnorum plants had tiny cluster roots that lived for no more than seven days in the present hydroponic experiment, in contrast with most Proteaceae species, including Hakea prostrata (Proteaceae), which produce larger cluster roots that live for up to three weeks (Fig. 7a). Citrate, malate, oxalate and iso-citrate were detected among the exudates released by whole root systems and cluster roots of A. cygnorum (Table 1). Total carboxylate exudation rates in whole root systems were significantly faster than those in cluster roots. The major exuded carboxylates collected from whole root systems were citrate and oxalate, which accounted for 45% and 41% of the total exuded carboxylates, respectively. For cluster roots, the major exuded carboxylate was oxalate, which accounted for 68% of the total exuded carboxylates, whereas citrate accounted for only 20%.
Carboxylate-exudation rates in both whole root systems and cluster roots of A. cygnorum were slow compared with those of other Proteaceae (Table 1). In A. cygnorum, the rate of citrate exudation from cluster roots was only 0.05 nmol g− 1 root FW s− 1, which was much slower than that in other Proteaceae (Table 1).
Acid phosphatase activity in the rhizosheath of seedlings of A. cygnorum (Fig. 7b) from both the field and the glasshouse were similar at about 25 nkat g− 1 soil, which was significantly higher than the activity in the surrounding bulk soil (Fig. 8). Acid phosphatase activity in the rhizosphere soil for seedlings from the field varied more widely than that of glasshouse-grown seedlings.
Discussion
The present results provide compelling evidence that in severely P-depleted soils, P acquisition by A. cygnorum was facilitated by the P-mobilising species B. attenuata. Based on an analysis of leaf [Mn], as outlined in Zhou et al. (2020), we conclude that P uptake in A. cygnorum was enhanced by P mobilisation by the root exudates released by B. attenuata (Shi et al. 2020). In contrast with the leaf [Mn] in A. cygnorum, the leaf [P] was not affected by facilitation. This is because when P is a severely limiting resource, plants use all the Pi that is taken up for growth, without increasing their leaf [P] (de Groot et al. 2003; Shane et al. 2003). Adenanthos cygnorum did not produce large and long-lived cluster roots that released significant amounts of carboxylates into soil as do most Proteaceae. However, this species did release phosphatases into soil which hydrolyse Po. Thus, we partly accept our first hypothesis that A. cygnorum does not produce functional cluster roots and accept our second hypothesis that A. cygnorum releases phosphatases that hydrolyse Po. The concentration of Po in old soils such as those in Alison Baird Reserve, the main location for the present work, and the two other sampling locations (Fig. S6) was higher at the bottom of the dune, away from banksias than that on top of the dune, near banksias. The chemical nature of organic P in these old soils is such that it is not tightly bound (Zhong et al. 2021). Therefore, released phosphatases would provide access to Po, even without involvement of carboxylates which supports our third and fourth hypotheses. Also, A. cygnorum used P efficiently, as do most Proteaceae in P-impoverished habitats (Hayes et al. 2018) which is consistent with our fifth hypothesis.
Functional diversity of cluster roots and root exudation among species
Adenanthos cygnorum did not produce cluster roots that function like those in most Proteaceae (Shane and Lambers 2005); its short-lived and tiny cluster roots did not release substantial amounts of carboxylates into the rhizosphere to mobilise P and Mn. Instead, acid phosphatase activity in its rhizosheath was significantly higher than that in bulk soil. When grown in a low-P nutrient solution, the total exudation rates of di- and tri-carboxylates by A. cygnorum in both whole root systems and cluster roots were slow compared with those of other Proteaceae (Table 1). Hundreds of lateral roots in a single cluster root release carboxylates in an exudative burst into soil, and thus solubilise large amounts of P sorbed onto soil particles (Shane and Lambers 2005). Since cluster roots of A. cygnorum are tiny and short-lived, their ability to solubilise sorbed P will be low, despite relatively fast citrate exudation in whole root systems. The importance of carboxylate release by cluster roots relative to release by the whole root system was shown in a field experiment with three high-carboxylate-exuding Lupinus (Fabaceae) species. The two species with cluster roots (L. albus and L. luteus) produced more biomass in P-impoverished soil than the one without cluster roots (L. angustifolius) (Bolland et al. 2000). No real-soil experiment was done to fill the gap between the field and hydroponic experiment in this study. However, the low leaf [Mn] in A. cygnorum growing without a P facilitator nearby confirmed that its exudates were ineffective. In summary, exuded carboxylates are more effective when released from cluster roots over a short time in a confined volume, rather than steadily from the entire root system into a larger volume.
Depending on species, the dominant carboxylates released by roots are malate or citrate, including in most Proteaceae (Table 1) (Gardner and Boundy 1983; Lambers et al. 2006). In H. prostrata, mature cluster roots released citrate and malate at 0.7 and 1.1 nmol g− 1 root FW s− 1, respectively (Shane et al. 2004b; Roelofs et al. 2001) (Table 1). Many plants also release oxalate, especially under aluminium (Al) stress (Ryan et al. 1995). Tartrate is a dominant exudate in some species such as Medicago sativa (Fabaceae) (He et al. 2020). In contrast to most Proteaceae, oxalate was a dominant exudate from cluster roots of both A. cygnorum, and a phylogenetically distantly-related Proteaceae, Euplassa cantareirea (de Britto Costa et al. 2016).
Roots not only release carboxylates, but also phenolics, such as catechin (Weir et al. 2006), which may inhibit growth of neighbours because it triggers the production of toxic reactive oxygen species and may result in cell death in roots of neighbouring plants (Bais et al. 2003). However, oxalate reduces the negative effect of phenolics. For example, Lupinus sericeus and Gaillardia grandiflora (Asteraceae) release large amounts of oxalate in response to catechin exposure, and thus block the generation of reactive oxygen species and reduce oxidative damage caused by catechin (Weir et al. 2006). Cluster roots of B. attenuata also exude phenolics (Beeck 2017), which might lead to negative effects on A. cygnorum. Therefore, the release of oxalate by A. cygnorum may act as an antioxidant defence (Kayashima and Katayama 2002) to prevent oxidative damage caused by the phenolics released by Banksia cluster roots. This response would enhance the ability of A. cygnorum to be facilitated by B. attenuata to acquire P and provide a potential explanation of why oxalate was released from the entire root system, rather than predominantly from cluster roots.
Accessing phosphorus without carboxylates
In P-impoverished soil, it appears that acid phosphatases released by A. cygnorum can access P by hydrolysing forms of Po, such as phospholipids and nucleic acids, that are not tightly bound to soil particles, similar to the strategy used by X. occidentale to access soil Po (Zhong et al. 2021). However, different from X. occidentale, which does not show any evidence of facilitation because its leaf [Mn] is invariably low (Zhong et al. 2021), the habitat range of A. cygnorum was extended by being facilitated by neighbouring P-mobilising Banksia species to acquire P on soils with extremely low resin P and Po concentrations, which will be explained below. Like A. cygnorum, other plants exude acid phosphatases into soil to hydrolyse Po that is not tightly bound to soil particles. This is true for some Fabaceae in south-western Australia (Png et al. 2017). Generally, root phosphatase activity of legumes is higher than that of other species (Houlton et al. 2008; Olde Venterink 2011). Nicotiana tabacum (Solanaceae) also exudes enzymes that hydrolyse Po. In this case, the plants also exude citrate that helps desorb phytate from soil particles, making it more available for hydrolysis by released phytases, enhancing their effectiveness. The hydrolysis of phytate enhances shoot P accumulation and soil Po depletion (Giles et al. 2017). This carboxylate-dependent strategy was not exhibited in A. cygnorum.
The mature leaf [Mn] of Banksia species varies from 126 to 439 µg Mn g− 1 DW (Denton et al. 2007), much greater than the concentration considered adequate for crop growth (50 µg Mn g− 1 DW) (Epstein and Bloom 2005). By comparison, in the present study, leaf [Mn] of B. attenuata was 220 µg Mn g− 1 DW, and A. cygnorum growing next to it had a leaf [Mn] of 108 µg Mn g− 1 DW, significantly higher than that of A. cygnorum growing away from other Proteaceae (50 µg Mn g− 1 DW) (Fig. 2a). This difference in leaf [Mn] in A. cygnorum was despite there being no difference in soil [Mn] between the sites, indicating that differences in leaf [Mn] were due to differences other than soil [Mn]. The higher leaf [Mn] in A. cygnorum growing next to B. attenuata than in individuals growing > 10 m away from other large Proteaceae at all three sampling locations indicates facilitation of nutrient uptake, especially of P and Mn, promoted by carboxylates released by neighbouring B. attenuata. Plants cannot access sorbed P from soil directly, but both P and Mn are mobilised by carboxylates, which are released in large amounts by cluster roots (Lambers et al. 2015). In this study, soil resin [P] at the bottom of the dune at Alison Baird Reserve was significantly higher than that on top of the dune, whereas leaf [P] of plants growing on top of the dune was the same as that of plants growing at the bottom of the dune. Therefore, rather than accumulating P, the plants used all the absorbed P to support their growth, as has been shown before for other species when grown at very low P availability (de Groot et al. 2003; Shane et al. 2003). Low leaf [Mn] in A. cygnorum growing away from other large Proteaceae at all three of the locations we tested indicates that its roots did not release a large amount of carboxylates into the soil, or that the released carboxylates were rapidly metabolised by microbes in the rhizosheath soil (D’Angioli et al. 2017).
Nutrient-mobilisation-based facilitation by cluster roots involves the following processes. First, the release of nutrient-mobilising carboxylates or molecules with a similar effect into the rhizosphere (Lambers et al. 2018). Second, the ability of the roots of the facilitated plants or mycorrhizal hyphae to sense the activity of cluster roots and then intermingle with the cluster roots (Teste et al. 2020). As P is immobile in soil, root intermingling likely also happened between A. cygnorum and B. attenuata to allow facilitation of P uptake. Facilitation of Mn uptake by neighbouring plants is well documented (Gardner and Boundy 1983; Lambers et al. 2015). In steppe vegetation in Inner Mongolia, P-mobilising species also enhance leaf [Mn] in some of their non-P-mobilising neighbours (Yu et al. 2020). In a natural habitat on the Swan Coastal Plain of south-western Australia, leaf [Mn] in Hibbertia hypericoides (Dilleniaceae) is relatively high when growing next to Banksia species compared with the negative reference (Zhong et al. 2021), and the roots of Hibbertia species are found intermingled with cluster roots of adjacent Proteaceae (Lambers et al. 2022). In summary, P-mobilising species enhance Mn acquisition and leaf [Mn] in some of their neighbours, but leaf [P] may not be enhanced.
Leaf [P] of Banksia species in their natural P-impoverished habitats are extremely low, in the range of 0.14 to 0.32 mg P g− 1 DW (Denton et al. 2007), compared with concentrations of typically 2 mg P g− 1 DW that are required for crop species (Epstein and Bloom 2005). The average leaf [P] of 2548 species from 175 natural sites across the world is 0.99 mg P g− 1 DW (Wright et al. 2004). Leaf [P] of plants sampled from low-P habitats in low-rainfall environments are also low (0.38 to 1.1 mg P g− 1 DW) (Wright and Westoby 2003), but higher than those of Banksia species in south-western Australia (Denton et al. 2007). However, in the present study, irrespective of the location, leaf [P] in A. cygnorum mature leaves, at 0.13 mg P g− 1 DW, was even lower than that in Banksia species. In contrast, leaf [P] in young expanding A. cygnorum leaves collected in the field was more than 10-fold higher than that in mature leaves of many Proteaceae species. This high [P] is associated with its strategy of delayed greening, which is a P-saving strategy in plants in severely P-impoverished habitats (Sulpice et al. 2014; Kuppusamy et al. 2021). Thus, A. cygnorum took up P that was available in the soil and accumulated it in young expanding leaves, to be used to support leaf maturation.
South-western Australia has some of the most P-impoverished soils in the world; 50% of the soils in south-western Australia have total [P] less than 100 mg P kg− 1 dry soil, with 6.5% having no more than 20 mg P kg− 1 dry soil (Kooyman et al. 2017). In this study, soil total [P] was very low at all sampled sites, in the lowest or extremely P-poor category of no more than 20 mg P kg− 1 dry soil. Plant-available [P] (approximated here as resin [P]) in soil under A. cygnorum was also very low. However, soil resin [P] under A. cygnorum > 10 m away from other Proteaceae species was significantly greater than that when A. cygnorum was < 1 m away from B. attenuata or on the low dune at Alison Baird Reserve, where A. cygnorum is absent. The low resin [P], combined with the low [Po], may explain why A. cygnorum does not grow on the low dune. With no facilitating tall Banksia trees on the low dune and very low plant-available [P] and Po in soil, the soil P availability is simply too low to sustain its growth.
Phosphorus-use efficiency in photosynthesis and growth
Adenanthos cygnorum used P very efficiently as an adaptation to extremely P-impoverished soils. Photosynthetic rates on a leaf area basis for A. cygnorum were around 12 µmol CO2 m− 2 s− 1, similar to that of Hakea species (Lambers et al. 2012). Photosynthesis rates for Banksia species are typically faster than those for Hakea species (20 µmol CO2 m− 2 s− 1 and 13 µmol CO2 m− 2 s− 1, respectively) (Lambers et al. 2012). Banksia species with thick leaves have sunken stomata located in crypts (Lambers et al. 2014), epidermal depressions whose depth is tightly correlated with leaf thickness (Hassiotou et al. 2009). Deep stomatal crypts with numerous sunken stomata contribute to a shorter path for CO2 diffusion from air to chloroplast which explains the faster photosynthesis rates in Banksia species compared with those of Hakea species with similar leaf thickness, but lacking crypts (Hassiotou et al. 2009). The pathway for water vapour diffusion is shortened to the same extent by stomatal crypts, and thus they have no significant effect on photosynthetic water-use efficiency (Roth-Nebelsick et al. 2009). We found that A. cygnorum leaves lacked stomatal crypts (Fig. 5a), similar to what has been found for Hakea species (Lambers et al. 2014). The lack of crypts likely contributed to the slow photosynthesis rate of A. cygnorum compared with that of Banksia species.
Photosynthetic P-use efficiency of A. cygnorum (0.65 mmol CO2 g− 1 P s− 1) was higher than that of Banksia species (0.19 to 0.46 mmol CO2 g− 1 P s− 1) (Denton et al. 2007), other Proteaceae (mean value 0.2 mmol CO2 g− 1 P s− 1) (Guilherme Pereira et al. 2019) or global average values determined under field conditions (0.10 mmol CO2 g− 1 P s− 1) (Wright et al. 2004). Photosynthetic P-use efficiency of Pinus strobus (Pinaceae) growing in soil with different nutrient availability is very low, at 0.02 to 0.08 mmol CO2 g− 1 P s− 1 (Reich and Schoettle 1988). In a low-P tropical forests in Guyana, PPUE varies from 0.10 mmol CO2 g− 1 P s− 1 to 0.26 mmol CO2 g− 1 P s− 1 (Raaimakers et al. 1995), much lower than that of A. cygnorum. Despite its low photosynthetic rates, PPUE was very high in A. cygnorum, because of its extremely low leaf [P] of 0.13 mg P g− 1.
Plants can increase their P-use efficiency for growth by decreasing their demand for external P and efficiently resorbing and reallocating P from senescing leaves (Aerts 1996; Wright and Westoby 2003). Phosphorus re-mobilisation from senescing leaves is an important mechanism to maintain a high P-use efficiency for growth (Denton et al. 2007; Guilherme Pereira et al. 2019). Phosphorus resorption from senescing leaves is up to about 90% in Proteaceae growing on P-impoverished soils in south-western Australia (Wright et al. 2004; Denton et al. 2007). Phosphorus resorption from senescing leaves of A. cygnorum in this experiment was around 55%, taking into account the loss of leaf dry weight during senescence (Vergutz et al. 2012). Most Proteaceae from southern South America growing on P-richer soils than those in south-western Australia have much lower P-resorption efficiency from senescing leaves, i.e. about 30% (Diehl et al. 2003; Lambers et al. 2012), although high P-resorption efficiency was found in some species, such as Embothrium coccineum (Delgado et al. 2018). Evergreens are more proficient at resorbing P than deciduous species (Killingbeck 1996), though sustained, moderate drought can reduce the P proficiency in evergreens (Dallstream and Piper 2021). Leaf lifespan is also important for P-use efficiency, because nutrients can be used for a longer period when they have a longer residence time in leaves. The leaf life span of A. cygnorum is about 40 months (Veneklaas and Poot 2003), much longer than the average for 744 species growing in their native habitat (median 8.5 months; Wright et al. 2004) or Banksia species (24 to 38 months; Veneklaas and Poot 2003). So, P in A. cygnorum leaves has a longer residence time than that in Banksia species, contributing to efficient long-term P-utilisation efficiency.
Conclusion
Adenanthos cygnorum functions in a way that differs from what is known for most Proteaceae (Fig. 9). Rather than producing functional cluster roots, when growing in severely P-impoverished soils A. cygnorum depended on facilitation of its P acquisition by neighbouring B. attenuata to supplement its acquisition of P. When it grew in soil with higher [P], including higher [Po], A. cygnorum also occurred away from carboxylate-releasing neighbours. There, it could access sufficient Po that was relatively mobile by releasing acid phosphatases without substantial carboxylate exudation. Thus, by combining its ability to access P in Po by exuding phosphatases with its ability to be facilitated in its P acquisition by P-mobilising neighbours such as B. attenuata, A. cygnorum extended its range of habitats.
Schematic diagram showing mechanisms of root phosphorus (P) acquisition in Adenanthos cygnorum at different locations. a Adenanthos cynorum neighbouring Banksia attenuata grew on top of the taller dune that has a severely low soil P concentration. Carboxylates released by B. attenuata can mobilise soil bound P into inorganic P (Pi) and other nutrient elements such as manganese. Adenanthos cygnorum can also release phosphatases into soil to hydrolyse orgainc P (Po) releasing Pi. Banksia attenuata can release phytotoxic phenolics, while A. cygnorum can release oxalate into soil that may reduce the phytotoxic effects of phenolics. b Soil at the bottom of the taller dune contains sufficient P for A. cygnorum to utilise, including Po, and it can absorb Pi directly. For the higher Po, A. cygonrum can release phosphatases into soil to hydrolyse Po that is not tightly bound, such as phospholipids and RNA (Zhong et al. 2021), to release Pi
Compared with Banksia species that have thick leaves and sunken stomata in crypts, A. cygnorum, which lacked stomatal crypts, exhibited slower rates of photosynthesis, similar to those of Hakea species, which also lack stomatal crypts. However, because of its very low leaf [P], the PPUE of A. cygnorum was higher, despite slow photosynthetic rates, than that of Banksia species. The leaf lifespan of A. cygnorum is also longer than that of Banksia species (Veneklaas and Poot 2003), so the residence time of leaf P is longer, contributing to a higher P-use efficiency for growth.
In severely P-impoverished landscapes such as in south-western Australia, where Proteaceae are common, different species within this family evolved different non-mycorrhizal P-acquisition strategies as adaptations to their severely P-impoverished environment. These new findings contribute to our understanding of ecosystem functioning in severely P-impoverished highly megadiverse systems.
References
Abbott LK, Robson AD, de Boer G (1984) The effect of phosphorus on the formation of hyphae in soil by the vesicular-arbuscular mycorrhizal fungus, Glomus fasciculatum. New Phytol 97:437–446. https://doi.org/10.1111/j.1469-8137.1984.tb03609.x
Aerts R (1996) Nutrient resorption from senescing leaves of perennials: are there general patterns? J Ecol 84:597–608. https://doi.org/10.2307/2261481
Alejandro S, Höller S, Meier B, Peiter E (2020) Manganese in plants: from acquisition to subcellular allocation. Front Plant Sci 11:300. https://doi.org/10.3389/fpls.2020.00300
Amadou I, Faucon M-P, Houben D (2022) New insights into sorption and desorption of organic phosphorus on goethite, gibbsite, kaolinite and montmorillonite. Appl Geochem 143:105378. https://doi.org/10.1016/j.apgeochem.2022.105378
Bais HP, Vepachedu R, Gilroy S, Callaway RM, Vivanco JM (2003) Allelopathy and exotic plant invasion: from molecules and genes to species interactions. Science 301:1377–1380. https://doi.org/10.1126/science.1083245
Beeck D (2017) Cluster-root exudation of carboxylate and phenolic compounds by two species of Banksia. Honours, The University of Western Australia, Crawley
Bolland MDA, Sweetingham MW, Jarvis RJ (2000) Effect of applied phosphorus on the growth of Lupinus luteus, L. angustifolius and L. albus in acidic soils in the south-west of Western Australia. Aust J Exp Agric 40:79–92. https://doi.org/10.1071/EA99065
Brooks A, Woo KC, Wong SC (1988) Effects of phosphorus nutrition on the response of photosynthesis to CO2 and O2, activation of ribulose bisphosphate carboxylase and amounts of ribulose bisphosphate and 3-phosphoglycerate in spinach leaves. Photosynth Res 15:133–141. https://doi.org/10.1007/BF00035257
Canham CA, Froend RH, Stock WD (2009) Water stress vulnerability of four Banksia species in contrasting ecohydrological habitats on the Gnangara Mound, Western Australia. Plant Cell Environ 32:64–72. https://doi.org/10.1111/j.1365-3040.2008.01904.x
Canton GC, Bertolazi AA, Cogo AJD, Eutrópio FJ, Melo J, de Souza SB, Krohling A, Campostrini C, da Silva E, Façanha AG et al (2016) Biochemical and ecophysiological responses to manganese stress by ectomycorrhizal fungus Pisolithus tinctorius and in association with Eucalyptus grandis. Mycorrhiza 26:475–487. https://doi.org/10.1007/s00572-016-0686-3
Cawthray GR (2003) An improved reversed-phase liquid chromatographic method for the analysis of low-molecular mass organic acids in plant root exudates. J Chromatogr A 1011:233–240. https://doi.org/10.1016/S0021-9673(03)01129-4
Dallsteam C, Piper FI (2021) Drought promotes early leaf abscission regardless of leaf habit but increases litter phosphorus losses only in evergreens. Aust J Bot 69:121–130
D’Angioli AM, Viani RAG, Lambers H, Sawaya ACHF, Oliveira RS (2017) Inoculation with Azospirillum brasilense (Ab-V4, Ab-V5) increases Zea mays root carboxylate-exudation rates, dependent on soil phosphorus supply. Plant Soil 410:499–507. https://doi.org/10.1007/s11104-016-3044-5
de Britto Costa P, Abrahão A, Viani RAG, Brancalion PHS, Lambers H, Sawaya ACHF, Oliveira RS (2016) Cluster-root formation and carboxylate release in Euplassa cantareirae (Proteaceae) from a neotropical biodiversity hotspot. Plant Soil 403:267–275. https://doi.org/10.1007/s11104-015-2630-2
de Groot CC, Marcelis LFM, van den Boogaard R, Kaiser WM, Lambers H (2003) Interaction of nitrogen and phosphorus nutrition in determining growth. Plant Soil 248:257–268. https://doi.org/10.1023/A:1022323215010
Delgado M, Suriyagoda L, Zúñiga-Feest A, Borie F, Lambers H, Field K (2014) Divergent functioning of Proteaceae species: the South American Embothrium coccineum displays a combination of adaptive traits to survive in high-phosphorus soils. Funct Ecol 28:1356–1366. https://doi.org/10.1111/1365-2435.12303
Delgado M, Valle S, Reyes-Diaz M, Barra PJ, Zúñiga-Feest A (2018) Nutrient use efficiency of southern South America Proteaceae species. Are there general patterns in the Proteaceae family? Front Plant Sci 9:883. https://doi.org/10.3389/fpls.2018.00883
Denton MD, Veneklaas EJ, Freimoser FM, Lambers H (2007) Banksia species (Proteaceae) from severely phosphorus-impoverished soils exhibit extreme efficiency in the use and re-mobilization of phosphorus. Plant Cell Environ 30:1557–1565. https://doi.org/10.1111/j.1365-3040.2007.01733.x
Diehl P, Mazzarino MJ, Funes F, Fontenla S, Gobbi M, Ferrari J (2003) Nutrient conservation strategies in native Andean-Patagonian forests. J Veg Sci 14:63–70. https://doi.org/10.1111/j.1654-1103.2003.tb02128.x
Dinkelaker B, Hengeler C, Marschner H (1995) Distribution and function of proteoid roots and other root clusters. Bot Acta 108:183–200. https://doi.org/10.1111/j.1438-8677.1995.tb00850.x
Epstein E, Bloom AJ (2005) Mineral nutrition of plants: principles and perspectives. Sinauer Associates, Inc, Sunderland
Gardner WK, Boundy KA (1983) The acquisition of phosphorus by Lupinus albus L. Plant Soil 70:391–402. https://doi.org/10.1007/BF02374894
Giles CD, George TS, Brown LK et al (2017) Does the combination of citrate and phytase exudation in Nicotiana tabacum promote the acquisition of endogenous soil organic phosphorus? Plant Soil 412:43–59. https://doi.org/10.1007/s11104-016-2884-3
Guilherme Pereira C, Hayes PE, O’Sullivan OS, Weerasinghe LK, Clode PL, Atkin OK, Lambers H (2019) Trait convergence in photosynthetic nutrient-use efficiency along a 2-million year dune chronosequence in a global biodiversity hotspot. J Ecol 107:2006–2023. https://doi.org/10.1111/1365-2745.13158
Hassiotou F, Evans JR, Ludwig M, Veneklaas EJ (2009) Stomatal crypts may facilitate diffusion of CO2 to adaxial mesophyll cells in thick sclerophylls. Plant Cell Environ 32:1596–1611. https://doi.org/10.1111/j.1365-3040.2009.02024.x
Hayes PE, Clode PL, Oliveira RS, Lambers H (2018) Proteaceae from phosphorus-impoverished habitats preferentially allocate phosphorus to photosynthetic cells: an adaptation improving phosphorus-use efficiency. Plant Cell Environ 41:605–619. https://doi.org/10.1111/pce.13124
Hayes P, Turner BL, Lambers H, Laliberté E (2014) Foliar nutrient concentrations and resorption efficiency in plants of contrasting nutrient-acquisition strategies along a 2-million-year dune chronosequence. J Ecol 102:396–410. https://doi.org/10.1111/1365-2745.12196
He H, Wu M, Guo L, Fan C, Zhang Z, Su R, Peng Q, Pang J, Lambers H (2020) Release of tartrate as a major carboxylate by alfalfa (Medicago sativa L.) under phosphorus deficiency and the effect of soil nitrogen supply. Plant Soil 449:169–178. https://doi.org/10.1007/s11104-020-04481-9
Hinsinger P, Bengough AG, Vetterlein D, Young IM (2009) Rhizosphere: biophysics, biogeochemistry and ecological relevance. Plant Soil 321:117–152. https://doi.org/10.1007/s11104-008-9885-9
Hocking PJ, Jeffery S (2004) Cluster-root production and organic anion exudation in a group of old-world lupins and a new-world lupin. Plant Soil 258:135–150. https://doi.org/10.1023/B:PLSO.0000016544.18563.86
Houlton BZ, Wang YP, Vitousek PM, Field CB (2008) A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature 454:327–330. https://doi.org/10.1038/nature07028
Isbell R, National Committee on Soil and Terrain (NCST) (2016) Australian soil classification, 2nd edn. Australian Soil and Land Survey Handbook Series. National Committee on Soil and Terrain. CSIRO Publising, Melbourne
Jarosch KA, Kandeler E, Frossard E, Bünemann EK (2019) Is the enzymatic hydrolysis of soil organic phosphorus compounds limited by enzyme or substrate availability? Soil Biol Biochem 139:107628. https://doi.org/10.1016/j.soilbio.2019.107628
Kamh M, Horst WJ, Amer F, Mostafa H, Maier P (1999) Mobilization of soil and fertilizer phosphate by cover crops. Plant Soil 211:19–27. https://doi.org/10.1023/A:1004543716488
Kayashima T, Katayama T (2002) Oxalic acid is available as a natural antioxidant in some systems. Biochim Biophys Acta 1573:1–3. https://doi.org/10.1016/S0304-4165(02)00338-0
Keerthisinghe G, Hocking PJ, Ryan PR, Delhaize E (1998) Effect of phosphorus supply on the formation and function of proteoid roots of white lupin (Lupinus albus L.). Plant Cell Environ 21:467–478. https://doi.org/10.1046/j.1365-3040.1998.00300.x
Killingbeck KT (1996) Nutrients in senesced leaves: keys to the search for potential resorption and resorption proficiency. Ecology 77:1716–1727. https://doi.org/10.2307/2265777
Kothari SK, Marschner H, Römheld V (1991) Effect of a vesicular-arbuscular mycorrhizal fungus and rhizosphere micro-organisms on manganese reduction in the rhizosphere and manganese concentrations in maize (Zea mays L.). New Phytol 117:649–655
Kooyman RM, Laffan SW, Westoby M (2017) The incidence of low phosphorus soils in Australia. Plant Soil 412:143–150. https://doi.org/10.1007/s11104-016-3057-0
Kuppusamy T, Hahne D, Ranathunge K, Lambers H, Finnegan PM (2021) Delayed greening in phosphorus-efficient Hakea prostrata (Proteaceae) is a photoprotective and nutrient-saving strategy. Funct Plant Biol 48:218–230. https://doi.org/10.1071/FP19285
Lambers H (2022) Phosphorus acquisition and utilization in plants. Annu Rev Plant Biol 73:17–42. https://doi.org/10.1146/annurev-arplant-102720125738
Lambers H, Cramer MD, Shane MW, Wouterlood M, Poot P, Veneklaas EJ (2003) Introduction: structure and functioning of cluster roots and plant responses to phosphate deficiency. Plant Soil 248:ix–xix
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot 98:693–713. https://doi.org/10.1093/aob/mcl114
Lambers H, Raven JA, Shaver GR, Smith SE (2008) Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol 23:95–103. https://doi.org/10.1016/j.tree.2007.10.008
Lambers H, Brundrett MC, Raven JA, Hopper SD (2010) Plant mineral nutrition in ancient landscapes: high plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant Soil 334:11–31. https://doi.org/10.1007/s11104-010-0444-9
Lambers H, Cawthray GR, Giavalisco P, Kuo J, Laliberté E, Pearse SJ, Scheible W-R, Stitt M, Teste F, Turner BL (2012) Proteaceae from severely phosphorus-impoverished soils extensively replace phospholipids with galactolipids and sulfolipids during leaf development to achieve a high photosynthetic phosphorus-use-efficiency. New Phytol 196:1098–1108. https://doi.org/10.1111/j.1469-8137.2012.04285.x
Lambers H, Colmer T, Hassiotou F, Mitchell P, Poot P, Shane MW, Vaneklaas E (2014) Carbon and water relations. In: Lambers H (ed) Plant life on the sandplains in Southwest Australia, a global biodiversity hotspot. UWA Publising, Crawley, pp 129–146
Lambers H, Hayes PE, Laliberté E, Oliveira RS, Turner BL (2015) Leaf manganese accumulation and phosphorus-acquisition efficiency. Trends Plant Sci 20:83–90. https://doi.org/10.1016/j.tplants.2014.10.007
Lambers H, Albornoz F, Kotula L, Laliberté E, Ranathunge K, Teste FP, Zemunik G (2018) How belowground interactions contribute to the coexistence of mycorrhizal and non-mycorrhizal species in severely phosphorus-impoverished hyperdiverse ecosystems. Plant Soil 424:11–33. https://doi.org/10.1007/s11104-017-3427-2
Lambers H, Wright IJ, Guilherme Pereira C, Bellingham PJ, Bentley LP, Boonman A, Cernusak LA, Foulds W, Gleason SM, Gray EF, Hayes PE, Kooyman RM, Malhi Y, Richardson SJ, Shane MW, Staudinger C, Stock WD, Swarts ND, Turner BL, Turner J, Veneklaas EJ, Wasaki J, Westoby M, Xu Y (2021) Leaf manganese concentrations as a tool to assess belowground plant functioning in phosphorus-impoverished environments. Plant Soil 461:43–61. https://doi.org/10.1007/s11104-020-04690-2
Lambers H, de Britto Costa P, Cawthray GR, Denton MD, Finnegan PM, Hayes PE, Oliveira RS, Power SC, Ranathunge K, Shen Q et al (2022) Strategies to acquire and use phosphorus in phosphorus-impoverished and fire-prone environments. Plant Soil 476:133–160. https://doi.org/10.1007/s11104-022-05464-8
Lamont B (1982) Mechanisms for enhancing nutrient uptake in plants, with particular reference to mediterranean South Africa and Western Australia. Bot Rev 48:597–689. https://doi.org/10.1007/BF02860714
Leoplod M, Zhong H (2019) The soils of Alison Baird Reserve. In: Lambers H (ed) A jewel in the crown of a global biodiversity hotspot. Kwongan Foundation and the Western Australian Naturalists’ Club, Perth, pp 49–57
Li L, Li SM, Sun JH, Zhou LL, Bao XG, Zhang HG, Zhang FS (2007) Diversity enhances agricultural productivity via rhizosphere phosphorus facilitation on phosphorus-deficient soils. Proc Natl Acad Sci USA 104:11192–11196. https://doi.org/10.1073/pnas.0704591104
Li L, Tilman D, Lambers H, Zhang FS (2014) Plant diversity and overyielding: insights from belowground facilitation of intercropping in agriculture. New Phytol 203:63–69. https://doi.org/10.1111/nph.12778
Muler AL, Oliveira RS, Lambers H, Veneklaas EJ (2014) Does cluster-root activity benefit nutrient uptake and growth of co-existing species? Oecologia 174:23–31. https://doi.org/10.1007/s00442-013-2747-z
Neumann G, Massonneau A, Martinoia E, Römheld V (1999) Physiological adaptations to phosphorus deficiency during proteoid root development in white lupin. Planta 208:373–382. https://doi.org/10.1007/s004250050572
Nge FJ, Biffin E, Thiele KR, Waycott M (2021) Reticulate evolution, ancient chloroplast haplotypes, and rapid radiation of the Australian plant genus Adenanthos (Proteaceae). Front Ecol Evol. https://doi.org/10.3389/fevo.2020.616741
Olde Venterink H (2011) Legumes have a higher root phosphatase activity than other forbs, particularly under low inorganic P and N supply. Plant Soil 347:137–146. https://doi.org/10.1007/s11104-011-0834-7
Pang J, Bansal R, Zhao H, Bohuon E, Lambers H, Ryan MH, Ranathunge K, Siddique KHM (2018) The carboxylate-releasing phosphorus-mobilizing strategy can be proxied by foliar manganese concentration in a large set of chickpea germplasm under low phosphorus supply. New Phytol 219:518–529. https://doi.org/10.1111/nph.15200
Parfitt RL (1979) The availability of P from phosphate-goethite bridging complexes. Desorption and uptake by ryegrass. Plant Soil 53:55–65. https://doi.org/10.1007/BF02181879b01280.x
Pate JS, Dixon KW (1996) Convergence and divergence in the southwestern Australian flora in adaptations of roots to limited availability of water and nutrients, fire and heat stress. In: Hopper S, Utilisation of Water and Nutrients by Banksia, Chappill J, Harvey M, George A (eds) Gondwanan Heritage: Past Present and Future of the Western Australian Biota. Surrey Beatty and Sons, Chipping Norton, NSW, pp 249–258
Png GK, Turner BL, Albornoz FE, Hayes PE, Lambers H, Laliberté E, Cameron D (2017) Greater root phosphatase activity in nitrogen‐fixing rhizobial but not actinorhizal plants with declining phosphorus availability. J Ecol 105(5):1246–1255. https://doi.org/10.1111/1365-2745.12758
Raaimakers D, Boot RGA, Dijkstra P, Pot S, Pons T (1995) Photosynthetic rates in relation to leaf phosphorus content in pioneer versus climax tropical rainforest trees. Oecologia 102:120–125. https://doi.org/10.1007/BF00333319
Rayment GE, Higginson FR (1992) Australian laboratory handbook of soil and water chemical methods. Inkata Press, Melbourne
Reich PB, Schoettle AW (1988) Role of phosphorus and nitrogen in photosynthetic and whole plant carbon gain and nutrient use efficiency in eastern white pine. Oecologia 77:25–33. https://doi.org/10.1007/BF00380920
Roelofs RFR, Rengel Z, Cawthray GR, Dixon KW, Lambers H (2001) Exudation of carboxylates in Australian Proteaceae: chemical composition. Plant Cell Environ 24:891–904. https://doi.org/10.1046/j.1365-3040.2001.00741.x
Roth-Nebelsick A, Hassiotou F, Veneklaas EJ (2009) Stomatal crypts have small effects on transpiration: a numerical model analysis. Plant Physiol 151:2018–2027. https://doi.org/10.1104/pp.109.146969
Ryan PR, Delhaize E, Randall PJ (1995) Malate efflux from root apices and tolerance to aluminium are highly correlated in wheat. Funct Plant Biol 22:531–536. https://doi.org/10.1071/PP9950531
Saunders WMH, Williams EG (1955) Observations on the determination of total organic phosphorus in soils. J Soil Sci 6:254–267. https://doi.org/10.1111/j.1365-2389.1955.tb00849.x
Shane MW, Szota C, Lambers H (2004a) A root trait accounting for the extreme phosphorus sensitivity of Hakea prostrata (Proteaceae). Plant Cell Environ 27:991–1004. https://doi.org/10.1111/j.1365-3040.2004.01204.x
Shane MW, Cramer MD, Funayama-Noguchi S, Cawthray GR, Millar AH, Day DA, Lambers H (2004b) Developmental physiology of cluster-root carboxylate synthesis and exudation in harsh hakea. Expression of phosphoenolpyruvate carboxylase and the alternative oxidase. Plant Physiol 135:549–560. https://doi.org/10.1104/pp.103.035659
Shane MW, de Vos M, de Roock S, Cawthray GR, Lambers H (2003) Effects of external phosphorus supply on internal phosphorus concentration and the initiation, growth and exudation of cluster roots in Hakea prostrata R. Br. Plant Soil 248:209–219. https://doi.org/10.1023/A:1022320416038
Shane MW, Lambers H (2005) Cluster roots: a curiosity in context. Plant Soil 274:101–125. https://doi.org/10.1007/s11104-004-2725-7
Shi W, Zhang Y, Chen S, Polle A, Rennenberg H, Luo ZB (2019) Physiological and molecular mechanisms of heavy metal accumulation in nonmycorrhizal versus mycorrhizal plants. Plant Cell Environ 42:1087–1103. https://doi.org/10.1111/pce.13471
Shi J, Strack D, Albornoz FE, Han Z, Lambers H (2020) Differences in investment and functioning of cluster roots account for different distributions of Banksia attenuata and B. sessilis, with contrasting life history. Plant Soil 447:85–98. https://doi.org/10.1007/s11104-019-03982-6
Sulpice R, Ishihara H, Schlereth A (2014) Low levels of ribosomal RNA partly account for the very high photosynthetic phosphorus-use efficiency of Proteaceae species. Plant Cell Environ 37:1276–1298. https://doi.org/10.1111/pce.12240
Tabatabai M (1994) Soil enzymes. In: Weaver R, Angle S, Bottomley P, Bezdicel D, Smith S, Tabatabai M, Wollum A (eds) Methods of soil analysis. Part 2. Microbiological and biochemical properties. Soil Science Society of America, Madison, pp 775–833
Teste FP, Dixon KW, Lambers H, Zhou J, Veneklaas EJ (2020) The potential for phosphorus benefits through root placement in the rhizosphere of phosphorus-mobilising neighbours. Oecologia 193:843–855. https://doi.org/10.1007/s00442-020-04733-6
Treseder KK, Allen MF (2002) Direct nitrogen and phosphorus limitation of arbuscular mycorrhizal fungi: a model and field test. New Phytol 155:507–515. https://doi.org/10.1046/j.1469-8137.2002.00470.x
Turner BL (2007) Inositol phosphates in soil: amounts, forms and significance of the phosphorylated inositol stereoisomers. In: Turner BL, Richards JE, Mullaney E (eds) Inositol phosphates: linking agriculture and the environment. CAB Internantional, Wallingford, pp 186–206
Turner BL, Hayes PE, Laliberté E (2018) A climosequence of chronosequences in southwestern Australia. Eur J Soil Sci 69:69–85. https://doi.org/10.1111/ejss.12507
Turner BL, Romero TE (2009) Short-term changes in extractable inorganic nutrients during storage of tropical rain forest soils. Soil Sci Soc Am J 73:1972–1979. https://doi.org/10.2136/sssaj2008.0407
Veneklaas EJ, Poot P (2003) Seasonal patterns in water use and leaf turnover of different plant functional types in a species-rich woodland, south-western Australia. Plant Soil 257:295–304. https://doi.org/10.1023/A:1027383920150
Vergutz L, Manzoni S, Porporato A, Novais RF, Jackson RB (2012) Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol Monogr 82:205–220. https://doi.org/10.1890/11-0416.1
Weir TL, Bais HP, Stull VJ, Callaway RM, Thelen GC, Ridenour WM, Bhamidi S, Stermitz FR, Vivanco JM (2006) Oxalate contributes to the resistance of Gaillardia grandiflora and Lupinus sericeus to a phytotoxin produced by Centaurea maculosa. Planta 223:785–795. https://doi.org/10.1007/s00425-005-0192-x
Wright IJ, Reich PB, Westoby M et al (2004) The worldwide leaf economics spectrum. Nature 428:821–827. https://doi.org/10.1038/nature02403
Wright IJ, Westoby M (2003) Nutrient concentration, resorption and lifespan: leaf traits of Australian sclerophyll species. Funct Ecol 17:10–19. https://doi.org/10.1046/j.1365-2435.2003.00694.x
Yu RP, Li XX, Xiao ZH, Lambers H, Li L (2020) Phosphorus facilitation and covariation of root traits in steppe species. New Phytol 226:1285–1298. https://doi.org/10.1111/nph.16499
Zarcinas BA, Cartwright B, Spouncer LR (1987) Nitric acid digestion and multi-element analysis of plant material by inductively coupled plasma spectrometry. Commun Soil Sci Plant Anal 18:131–146. https://doi.org/10.1080/00103628709367806
Zhong H, Zhou J, Azmi A, Arruda AJ, Doolette AL, Smernik RJ, Lambers H (2021) Xylomelum occidentale (Proteaceae) accesses relatively mobile soil organic phosphorus without releasing carboxylates. J Ecol 109:246–259. https://doi.org/10.1111/1365-2745.13468
Zhou J, Zúñiga-Feest A, Lambers H (2020) In the beginning, there was only bare regolith—then some plants arrived and changed the regolith. J Plant Ecol 13:511–516. https://doi.org/10.1093/jpe/rtaa030
Acknowledgements
QS was supported by a scholarship from the China Scholarship Council (CSC) and a top-up scholarship from the Kwongan Foundation. Funding was provided by Australian Research Council Discovery Grant DP200101013 to HL and PMF. We thank Zihan Wang, Toby Bird and Shutong Liu for help with sample collection, Robert Creasy and Bill Piasini for glasshouse support, Todd Buters for the drone map, Greg Cawthray for carboxylate analyses and Michael Smirk for ICP analyses.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
QS, KR, PMF and HL designed the study. QS performed the experiment and collected the data with the contributions of KR and HZ. QS, KR, PMF and HL prepared the manuscript. KR, HZ, PMF and HL revised the manuscript. All authors read and approved the manuscript.
Corresponding author
Additional information
Responsible Editor: Amandine Erktan.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
ESM 1
(DPCX 4.50 MB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shen, Q., Ranathunge, K., Zhong, H. et al. Facilitation of phosphorus acquisition by Banksia attenuata allows Adenanthos cygnorum (Proteaceae) to extend its range into severely phosphorus-impoverished habitats. Plant Soil 496, 51–70 (2024). https://doi.org/10.1007/s11104-023-05935-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-05935-6