Abstract
Aims
South-western Australia has some of the most phosphorus (P)-impoverished soils in the world. Hakea prostrata (Proteaceae) has nitrate (NO3−)-uptake restraint, associated with its high P-use efficiency. This research explores how common this trait is in other Proteaceae and in co-occurring plant species in south-western Australia.
Methods
Banksia attenuata (Proteaceae), B. telmatiaea (Proteaceae), Melaleuca seriata (Myrtaceae) and M. rhaphiophylla (Myrtaceae) were grown with no NO3−, Medium (500 µM) NO3− or High (2500 µM) NO3− treatments with no other source of nitrogen (N). Plants were harvested after treatments. Leaf nitrate and phosphate concentrations were determined, as well as biomass, total N and total P concentrations of leaves, stems and roots separately.
Results
Banksia attenuata, B. telmatiaea and M. seriata had similar total N content when supplied with High NO3− as they did when supplied with Medium NO3−. All four species had the same low leaf NO3−-N concentration in High and Medium NO3− treatments, no more than 6% of leaf total N. All species had similar leaf NO3− concentrations as those of plants growing in their natural habitat where the available soil nitrate concentration was much lower. These results are in sharp contrast with plants that are typically studied, for example Arabidopsis thaliana.
Conclusions
All four species exhibited NO3−-uptake restraint to different degrees. Nitrate-uptake restraint appears a convergent trait common to many species that evolved in severely P-impoverished landscapes, allowing them to be more P-efficient.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
South-western Australia has some of the oldest and most phosphorus (P)-impoverished soils in the world (Hopper 2009; Lambers 2014) and is a global biodiversity hotspot (Myers et al. 2000). Phosphorus plays a pivotal role in plant productivity in south-western Australia because of low-P parent material and prolonged soil weathering (Hopper and Gioia 2004; Laliberté et al. 2012; Lambers et al. 2010). Compared with extremely low soil P-availability, the nutrient-poor soils in south-western Australia have relatively abundant available nitrogen (N) (Laliberté et al. 2012; Turner and Laliberté 2015). Soil N and P availability both decline during pedogenesis, but the soil N: P ratio steadily increases during long-term soil development (Turner and Laliberté 2015) which makes P the major nutrient limiting plant productivity in south-western Australia (Hopper and Gioia 2004; Laliberté et al. 2012). Native plants that evolved in this P-limiting environment have specialised P-acquisition and P-utilisation strategies, which make them highly P efficient (Hayes et al. 2021; Lambers et al. 2022).
Proteaceae is one of the dominant plant families in south-western Australia (Pate et al. 2001). Many critical traits have evolved in the Proteaceae that allow them to survive in severely P-impoverished landscapes (Lambers et al. 2015). For instance, Proteaceae acquire P from soil efficiently through their specialised cluster roots, which are composed of clusters of lateral rootlets that exude carboxylates into soil and mobilise P from poorly soluble P complexes (Shane and Lambers 2005). Proteaceae in this region also have specialised strategies of P utilisation, such as efficient remobilisation of P from senescing organs to growing organs (Lambers et al. 2012), delayed greening of young leaves (Kuppusamy et al. 2014; Lambers et al. 2012), and preferential allocation of P to photosynthetically-active mesophyll cells, rather than to epidermal cells (Hayes et al. 2018). Proteaceae leaves also have low protein and N concentrations (Hayes et al. 2014; Sulpice et al. 2014), despite the relatively high N availability compared with that of P in south-western Australia (Turner and Laliberté 2015). This results in a low demand for P in ribosomal RNA (rRNA) (Lambers et al. 2015), which is reflected by low levels of rRNA (Sulpice et al. 2014). All these traits allow Proteaceae to thrive in south-western Australia, especially on the poorest soils (Lambers et al. 2022).
Nitrate (NO3−) uptake restraint has been reported for Hakea prostrata (Proteaceae) (Prodhan et al. 2016), which has evolved in P-limiting environments of south-western Australia (Lambers et al. 2011, 2015). Hakea prostrata has all the strategies associated with efficient acquisition and utilisation of P presented above including low rRNA and protein concentrations, which reduce its demand of other nutrients, like N, sulfur (S), and micronutrients like zinc (Zn) and copper (Cu) (Prodhan et al. 2019). Nitrate restraint in H. prostrata was discovered in a hydroponics experiment where plant total N content and leaf NO3− concentration were the same in plants grown with a NO3− supply of either 5000 μM or 200 μM, both of which were in great excess to demand (Prodhan et al. 2016). This tight control of NO3− uptake and assimilation was interpreted as an adaptive strategy to P limitation (Prodhan et al. 2019). However, the prevalence of this tight control of NO3− uptake among other Proteaceae and in co-occurring plant families, such as the Myrtaceae, in south-western Australia is unknown.
Both soil ammonium (NH4+) and soil NO3− availability decline during pedogenesis in south-western Australia (Turner and Laliberté 2015). Despite the slightly higher availability of NH4+ than that of NO3− (Turner and Laliberté 2015), non-mycorrhizal species in a native Banksia woodland in Western Australia have been shown to exclusively take up nitrate as their N source (Pate et al. 1993). In this study, we selected two Proteaceae (Banksia telmatiaea and B. attenuata) and two Myrtaceae (Melaleuca rhaphiophylla and M. seriata) that occur naturally on P-impoverished soils in south-western Australia to determine whether they have NO3−-uptake restraint. The plants of all targeted species were grown in aerated hydroponics where NO3− was the only source of N. Since Proteaceae have many adaptations to low-P soils, while Myrtaceae are not known for such traits, we hypothesised that the two Proteaceae would exhibit NO3−-uptake restraint, while the two Myrtaceae would not.
Materials and methods
Study area and species selection
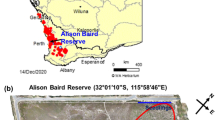
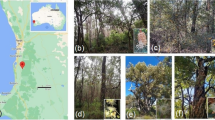
Field samples were collected in Alison Baird Reserve (32°1′19′′S 115°58′52′′E), a species-rich flora reserve southeast of Perth that has extremely impoverished soil (Leopold and Zhong 2019; Tauss et al. 2019). The reserve has a Mediterranean climate, with dry summers and wet winters (Gentilli 1972). It is located on the eastern edge of the Swan Coastal Plain, overlain by coastal dunes that are geomorphic residual outliers of Bassendean dune landforms (Tauss et al. 2019). Detailed information on coastal sand dune chronosequence systems in south-western Australia were provided in (Turner et al. 2018). The underlying sediments originated from the Darling Scarp towards the east and were deposited in the reserve over millions of years (Lane and Evans 2019). On top of a tall Bassendean dune, the deep sandy soils are moist in winter and dry in summer, while at the west of the reserve there is a flat area with lower elevation where the soils are inundated in winter and dry in summer (Tauss et al. 2019).
We selected two Proteaceae (Banksia telmatiaea and B. attenuata) and two Myrtaceae (Melaleuca rhaphiophylla and M. seriata) that occur naturally in the reserve. Banksia telmatiaea and M. rhaphiophylla both occur on the flat winter-inundated area, while B. attenuata occurs on the winter-moist top of the tall Bassendean dune (Figs. 1 and 2). Melaleuca seriata occurs almost exclusively over the entire dune, but can be found in the wet area.
Sampling sites of four targeted species in Alison Baird Reserve (ABR): Banksia attenuata and Melaleuca seriata on the Bassendean dune, Banksia telmatiaea and Melaleuca rhaphiophylla at the flat winter-inundated area. Elevation data of ABR was from Smith (2019). The image has been coloured to highlight the Bassendean dune
Sample collection from the field
Leaf samples of all species were collected for nutrient analyses, both at the winter-spring wet period (September 2019) and at the summer-autumn dry period (early April 2019 and March 2020). Most-recently fully-expanded leaves were selected from five random plants of each species in each season. Samples were snap-frozen in liquid nitrogen at harvest and stored at -80℃ to be processed later.
Plant material and growing conditions
Seeds of the two Banksia species were collected in the reserve in 2019. Seeds of the two Melaleuca species were purchased (Nindethana Seed Company, Albany, WA, Australia), after collection from natural populations in Western Australia.
Seeds of all species were germinated in Petri dishes between filter papers moistened with deionised water until the primary root and cotyledons emerged (15 °C, 12 h light, 12 h dark). Seedlings were transferred to a mixture of pasteurized sand and potting mix (1:1). After six weeks of growth, seedlings of uniform size were transferred to aerated hydroponics in cooling tanks that maintained roots at 18℃ to 20℃ (Shane et al. 2004) to start the Pre-treatment period. Before transferring to hydroponics, roots of seedlings were washed, and disinfected with 1% (v/v) sodium hypochlorite for 20 s. Disinfected seedlings were transferred to 5 L plastic pots containing 4 L nutrient solution (in µM, 2.5 PO43−, 500 NO3−, 200 Ca2+, 500 K+, 36 SO42−, 36 Mg2+, 10 Fe-EDTA, 0.24 Mn2+, 0.10 Zn2+, 0.018 Cu2+, 2.4 H3BO3, 50 SiO32− and 0.3 Mo4+. pH = 5.8) (modified from Hayes et al. (2019)) with one plant per pot. The nutrient solution was replaced twice per week. The Pre-treatment ended when the plants had produced new roots and had a healthy appearance without any sign of mineral deficiency or toxicity, or any biotic stress (four weeks for M. rhaphiophylla, five weeks for M. seriata and B. telmatiaea, seven weeks for B. attenuata).
Nutrient treatments
After the Pre-treatment, five individuals from each species were randomly selected for harvest to determine the initial nutrient status. The following treatments were then applied to five randomly assigned replicate individuals from each species. All nutrients, except N and P, were provided at established levels for plant growth as previously described (Hayes et al. 2019). Nitrate was the only source of N added. The three NO3− treatments were No NO3−, Medium (500 µM) NO3− or High (2500 µM) NO3−, added twice weekly when the entire nutrient solution was replaced. Plants of all species received sufficient P, as KH2PO4, to make sure that P was not limiting for NO3− uptake. The optimal concentration of P was determined by measuring the P concentration remaining in the nutrient solutions at each solution change. The optimal P supply was considered the concentration that was sufficient to have a small amount remaining in the solution at the next solution change, but not so high as to promote bacterial and fungal growth. The final concentrations of PO43− in the nutrient solution in each week are shown in Table 1. All NO3− treatments for each species received the same amount of P when the solution was changed twice a week. Treatments were applied until N deficiency symptoms started to appear in No NO3− treatment plants, which was after 10 weeks for the two Banksia species, nine weeks for M. seriata and five weeks for M. rhaphiophylla.
Plant harvest
Approximately 1 to 3 g fresh weight of the mature leaves were harvested, weighed, immediately frozen in liquid nitrogen and stored at -80℃ to be analysed later. All snap-frozen leaves were harvested between 9 am and 12 pm on sunny days. The remaining parts of the plants were harvested into leaves, stems and roots. After measuring fresh weights, plant material was oven dried at 65℃ for at least 72 h and weighed again to determine dry weights and dry-to-fresh weight ratios.
Snap-frozen mature leaves were freeze dried then ground to determine NO3− and inorganic phosphate (Pi) concentrations. Oven dried plant materials were also ground to determine total N and P concentrations. Total N and P content in each organ was calculated from the total N and P concentrations and biomass. The total biomass of leaves was calculated from the dry weight of the oven-dried leaves and the calculated dry weight of the snap-frozen leaves, which was calculated from the dry-to-fresh weight ratio and fresh weight of snap-frozen leaves.
Determination of total nitrogen and phosphorus
Total N concentrations were determined by combustion using an elemental analyser (Elementar Australia Pty Ltd, Sydney, Australia). Total P concentrations were determined colorimetrically using a malachite green method (Motomizu et al. 1983) after acid digestion of samples. Aliquots of 100 mg of ground freeze-dried samples were acid digested with 3 mL of concentrated HNO3 at 80℃ to 90℃, followed by digestion with 0.5 mL of HClO4 at 130℃ to 150℃, then with 1 mL of HCl at 130℃. Deionised water was added to adjust the final volume to 10 mL.
Determination of ions
Inorganic phosphate was extracted from 25 mg freeze-dried leaf samples by homogenising in ice-cold 1% (v/v) acetic acid (Precellys 24 Homogeniser, Thermo Fisher Scientific, Waltham, USA) taking care to keep the solution cold (Yan et al. 2019). The homogenate was clarified twice by centrifugation at 21,000 × g for 15 min at 4 °C. The extracts were treated with acid-washed activated charcoal to remove color interfering substances (Dayrell et al. 2021). The concentration of Pi in the final extracts was determined colorimetrically using a malachite green-based method (Motomizu et al. 1983).
Nitrate was extracted from 100 mg freeze-dried leaf samples with water while shaking at 45℃ for one hour (Cataldo et al. 1975). Interfering organic substances were removed by adding 1 mL of petroleum spirit, shaking for five minutes, separating the phases by centrifugation at 5000 × g for 5 min, removing the organic phase, and filtering the aqueous phase (25 mm diameter Polyethersulfone syringe filters; 0.45 μm; Sarstedt Australia Pty Ltd, Adelaide, Australia). The concentration of NO3− in the final extracts was determined colorimetrically using a flow injection analysis system (Lachat Instruments, Milwaukee, WI, USA).
Statistical analyses
Differences in biomass, N and P contents and concentrations, leaf N: P ratio, leaf NO3− and Pi concentrations among treatments were analysed by one-way analysis of variance (ANOVA) using Fisher's least significant difference (LSD) test at P < 0.05 (R studio, v. 3.6.0) (R Core Team. 2022).
Results
Plant biomass
Biomass was similar between Medium NO3− and High NO3− treatments for B. attenuata, B. telmatiaea and M. rhaphiophylla (Fig. 3). For M. seriata, biomass in the High NO3− treatment was lower than in the Medium NO3− treatment due to a loss of leaves caused by slight P toxicity early in the High NO3− treatment. Total biomass for all species under all NO3− treatments was significantly higher than that of the Pre-treatment, indicating that the No NO3− plants had sufficient N from the pre-treatment to sustain additional growth even when the N supply was stopped. Biomass of Banksia plants increased two-fold to three-fold during the treatment period, while biomass of Melaleuca plants increased five-fold to six-fold during the treatment period compared with the Pre-treatment plants (Fig. 3). Banksia attenuata and B. telmatiaea in High NO3− and Medium NO3− treatments all left behind more than 90% of the NO3− that was supplied in the nutrient solution. Melaleuca seriata and M. rhaphiophylla in the High NO3− treatment left behind about 87% and 69% of the total NO3− supplied, respectively, showing there was a great excess of NO3− available for plants during the treatment period. The N supply in the Medium NO3− treatment was also saturating, except for M. raphiophylla, which exhausted the NO3− in the Medium treatment.
Total biomass per plant for Banksia attenuata, B. telmatiaea, Melaleuca rhaphiophylla and M. seriata after pre-treatment (Pre) and after further growth at different supplies of nitrate. Data are means ± SE (n = 5). Significant differences among treatments were determined by ANOVA, Fisher's least significant difference (LSD) test (P < 0.05) and indicated by different letters
Total N content
In all species, plants from the No NO3− treatment had the same total N content as plants from the Pre-treatment, indicating that there was no loss of N during plant growth (Fig. 4). By contrast, the total N content of Banksia plants under Medium NO3− treatment was 65% to 130% greater than that of N-starved plants, while total N content of Melaleuca plants under Medium NO3− treatment increased even more, to at least three-fold higher than the N-starved plants (Fig. 4).
Total nitrogen (N) content per plant for Banksia attenuata, B. telmatiaea, Melaleuca rhaphiophylla and M. seriata after pre-treatment (Pre) and after further growth at different supplies of nitrate. Data are means ± SE (n = 5). Significant differences among No N, Medium N and High N treatments were determined by ANOVA, Fisher's least significant difference (LSD) test (P < 0.05) and indicated by different letters
Plants of all species except M. rhaphiophylla had the same total N content when supplied with High NO3− as they did when supplied with Medium NO3−. These three species also had the same N content in leaves, stems and roots under High NO3− treatment as they did under Medium NO3− treatment (Figs. S1, S3, S5, S7). The total N content of M. rhaphiophylla under High NO3− treatment was about 33% greater than that under Medium NO3− treatment (Fig. 4). This difference in N content between treatments for M. rhaphiophylla was due to a higher leaf N content in the High NO3− treatment than in the Medium NO3− treatment. The N content of stems and roots was the same between Medium NO3− and High NO3− treatments (Fig. S7).
Leaf nitrate concentration
The distribution of N in different organs varied among the four species (Figs. S1, S2, S3, S4, S5, S6, S7, S8). Focusing on leaf N, we found that all four species had low leaf NO3− concentrations (Fig. 5), despite the high NO3− supplies. Nitrate is an energetically inexpensive osmoticum to generate turgor compared with organic ions or sugars (Andrews et al. 2005). However, the four species examined did not use nitrate as an osmoticum, even in the high-NO3− treatment, suggesting that light energy was not a major limiting factor for growth (Andrews et al. 2005). Instead, M. seriata, M. rhaphiophylla and B. attenuata plants had the same leaf NO3− concentration when treated with Medium NO3− or High NO3− (Fig. 5). Remarkably, the leaf NO3− concentrations of all four species grown in Medium NO3− and High NO3− treatments were similar to that of plants growing in their natural habitats, and the NO3− in the leaves was only a small portion (0.18% to 5.86%) of total leaf N (Fig. 5). There was no significant difference in the proportion of NO3− in total N between Medium NO3− and High NO3− treatments for any of the four species.
Leaf nitrate and total N concentrations of Banksia attenuata, B. telmatiaea, Melaleuca rhaphiophylla and M. seriata after pre-treatment (Pre) and after further growth at different supplies of nitrate compared with plants in their natural habitats. Solid lines indicate leaf total N concentration, while dashed lines indicate leaf nitrate concentration of plants from the field site. For Pre, No N, Medium and High N treatments, n = 5 for each treatment, while for plants from the field site, n = 10 (five replicates collected in summer and five replicates collected in winter). Data are means ± SE. Statistical tests were carried out separately for total N and nitrate concentrations. Significant differences among No N, Medium N and High N treatments were determined by ANOVA, Fisher's least significant difference (LSD) test (P < 0.05) and indicated by different letters
Total P content and concentration
Within a species, all plants from all NO3− treatments received the same P supply throughout the experiment, although the P supply varied across the experiment (Table 1). Intriguingly, in the banksias, different N supplies impacted P uptake in B. attenuata, but not in B. telmatiaea (Fig. 6). The total P content in B. attenuata plants was significantly greater when plants were supplied with NO3− than in NO3−-starved plants (Fig. 6). Leaf P content in B. attenuata was greater under High NO3− than Medium NO3− treatment, whereas the P content of roots was the same, irrespective of NO3− treatment (Fig. S9). The leaf P concentration of B. attenuata was also greater under High NO3− than Medium NO3− treatment, while stem and root P concentrations were the same in both treatments (Fig. S10). In contrast to B. attenuata, neither P content nor P concentration was affected in B. telmatiaea by NO3− treatment (Figs. S11, S12). The P concentration of B. telmatiaea leaves never exceeded 0.7 mg P g−1 DW, while that in B. attenuata leaves varied from 1.0 to 3.4 mg P g−1 DW.
Total phosphorus (P) content per plant for Banksia attenuata, B. telmatiaea, Melaleuca rhaphiophylla and M. seriata after pre-treatment (Pre) and after further growth at different supplies of nitrate. Data are means ± SE (n = 5). Significant differences among No N, Medium N and High N treatments were determined by ANOVA, Fisher's least significant difference (LSD) test (P < 0.05) and indicated by different letters
Melaleuca rhaphiophylla had the same total P content in Medium NO3− and High NO3− treatments (Fig. S15). However, despite the total P content of the whole plant being the same among NO3− treatments, the NO3−-starved plants had a higher stem P content as well as stem P concentration than plants supplied with NO3− (Fig. S15). The P concentration in each organ of M. rhaphiophylla was also the same between Medium NO3− and High NO3− treatments (Fig. S16). The P content of M. seriata plants as well as leaf P content and stem P content were greater at Medium NO3− than at High NO3− (Fig. S13). The P concentrations in leaves, stems and roots of M. seriata were the same in both the High and Medium NO3− treatments (Fig. S14). The difference in leaf and stem P content between these two NO3− treatments (Fig. S13) was due to lower biomass of High NO3− plants, which was caused by transient P toxicity.
Leaf phosphate concentration
The leaf Pi concentration of B. attenuata was greater under High NO3− treatment than Medium NO3− treatment, consistent with the leaf total P concentration in these treatments (Fig. 7). In contrast, the other three species all had the same leaf Pi concentration in High NO3− and Medium NO3− treatments. In addition, the two species that occur on the Bassendean dune, B. attenuata and M. seriata, had similar leaf Pi strategies to one another: the leaf Pi concentration in both species increased when supplied with NO3−compared with that in NO3−-starved plants, and Pi was always a major component of the P pool, representing 72% to 97% of leaf total P. The other two species, B. telmatiaea and M. rhaphiophylla, which occur in the winter-wet flat area, also had similar Pi strategies to one another: leaf Pi was highest when NO3−was withheld (Fig. 7), and the proportion of Pi in total P in leaves was lower when plants were supplied with NO3−. Finally, for all species, the Pi and total P concentrations in leaves of plants grown in hydroponics were greater than those of plants growing in their natural habitat.
Total phosphorus (P) and inorganic phosphate (Pi) concentrations of Banksia attenuata, B. telmatiaea, Melaleuca rhaphiophylla and M. seriata after pre-treatment (Pre) and after further growth at different supplies of nitrate compared with plants in their natural habitats. Solid lines indicate total P concentration, while dashed lines indicate Pi concentration of plants from the field site. For Pre, No N, Medium and High N treatments n = 5, while for plants at the field site, n = 10 (five replicates collected in summer and five replicates collected in winter). Data are means ± SE. Statistical tests were carried out separately for total P and phosphate P concentrations. Significant differences among No N, Medium N and High N treatments were determined by ANOVA, Fisher's least significant difference (LSD) test (P < 0.05) and indicated by different letters
Leaf N: P ratio
There was no significant difference in leaf N : P ratio between Medium NO3− and High NO3− treatments for any of the species, indicating that N: P ratio was not dependent on NO3− supply (Fig. 8). All species, except M. rhaphiophylla, had a lower leaf N: P ratio when grown in hydroponics than plants collected in their natural habitat (Fig. 8). Interestingly, NO3−-treated B. attenuata and M. seriata plants, which naturally occur on the Bassendean dune, all had a leaf N: P ratio below 7.5. However, NO3−-treated B. telmatiaea and M. rhaphiophylla plants, which naturally occur in the flat and wetter area in Alison Baird Reserve, had a leaf N: P ratio of above 13, while the leaf N: P ratio of all four species from their natural habitats were all above 20 (Fig. 8).
Leaf N: P ratio of Banksia attenuata (B. a), B. telmatiaea (B. t), Melaleuca rhaphiophylla (M. r) and M. seriata (M. s) plants after pre-treatment and after further growth at different supplies of nitrate, and plants from their natural habitat. For Pre-, No N, Medium and High N treatments, n = 5, while for plants at the field site, n = 10 (five replicates collected in summer and five replicates collected in winter). Data are means ± SE. Significant differences were determined by ANOVA, Fisher's least significant difference (LSD) test (P < 0.05) and indicated by different letters
Discussion
Our results showed that all targeted species exhibited NO3−-uptake restraint by not taking up more NO3− than was needed to sustain growth. In contrast with our hypotheses, our results showed that NO3−-uptake restraint is not a trait unique to the Proteaceae, but may be a convergent trait common to many species that evolved in severely P-impoverished soils, allowing them to cope with extremely low P availability.
Nitrate-uptake restraint was first found in H. prostrata (Prodhan et al. 2016). The plants simply did not take up more NO3− than needed, which is fundamentally different from plants that are typically studied (Prodhan et al. 2016, 2019). All species targeted here, except M. rhaphiophylla, had the same total N content when supplied with High NO3− as they did when supplied with Medium NO3−. While total N content of M. rhaphiophylla increased when provided with more NO3−, the increase was only about 1.3-fold when the readily available NO3− in the growing medium was increased five-fold (Fig. 4). This appears to be a minimal NO3−uptake compared with the level of NO3− available. Again, it suggests a tight control of NO3−-uptake compared with that in typically studied plants, where NO3−-uptake sharply increases with an increase in NO3− availability (Greenwood and Hunt 1986; Tschoep et al. 2009).. In the case of M. rhaphiophylla, the slight increase in total N content beyond the Medium NO3− treatment may indicate that M. rhaphiophylla may need slightly more NO3− than supplied in the Medium NO3− treatment to reach its static N level in hydroponics system. While total N content was greater with higher NO3− supply in M. rhaphiophylla, even this species did not store N as NO3−, but instead converted it into organic N.
Nitrate-restraint was associated with low organ N concentration regardless of nitrate supply. The leaf and root N concentration of the two Banksia spp. remained the same (below 15 mg g−1 DW) even when NO3− supply was higher (Figs. S2, S4). This finding contrasted with what was found for a wide range of plant species that have been studied, including trees, herbs and grasses (see Prodhan et al. 2016 for summary). The stem N concentration in B. attenuata was indistinguishable between Medium and High NO3− treatments as well. Even though the stem N concentration in B. telmatiaea was 1.5-fold higher when the plants were provided with High NO3- than with Medium NO3−, it was still only about 20 mg g−1 DW. In contrast, the shoot N concentration in the model plant A. thaliana can increase to 75 mg g−1 DW (Tschoep et al. 2009), and that of other species can increase to as much as 53 mg g−1 DW (Greenwood and Hunt 1986) when supplied with more N. Melaleuca seriata also had the same leaf and stem N concentration in Medium and High NO3− treatments (Fig. S6). While the root N concentration of M. seriata was about 1.5-fold higher when supplied with High NO3− compared to Medium NO3−, it was only about 21 mg g−1 DW. In contrast, the root N concentration of many other species can reach about 57 mg g−1 DW when supplied with more N (Greenwood and Hunt 1986). For M. rhaphiophylla, leaf, root and stem N concentrations were no more than 1.3-fold higher under High NO3− treatment than those under Medium NO3− treatment. In contrast, leaf, shoot and root N concentration increased four-fold to five-fold when N supply was increased in previously studied species (Aerts 2009; Reich and Oleksyn 2004; Taylor and May 1967).
Leaf NO3− concentrations for all four target species were non-responsive to a five-fold difference in NO3− supply, as was the ratio of leaf NO3− concentration to total N concentration. This was again in sharp contrast with most plant species that have been studied, where the NO3− concentration increased up to 118-fold and the ratio of leaf NO3− concentration to total N concentration increased up to 58-fold when NO3− supply increased (Cox and Reisenauer 1973; North et al. 2009; Stewart et al. 1973). Despite leaf N concentrations in plants grown in hydroponics being higher than those of plants growing in their natural habitat (Fig. 5), leaf NO3− concentrations in hydroponics here were similar to those of these plants at the field site, where the soil N concentration was much lower than that supplied in hydroponics. However, other plants studied in their natural habitats may have up to 100-fold higher leaf NO3− concentrations when supplied with more NO3− than naturally available in field soils (Stewart et al. 1973). Thus, the low leaf NO3− concentrations seen here again demonstrates that all four targeted species restrained their NO3− uptake. Moreover, the fact that plants supplied with High NO3− had a higher leaf N concentration than plants in the field indicated that these species do indeed take up NO3− and plants grown in hydroponics were not limited by their NO3−-uptake capacity.
High NO3− treatment had a fivefold higher N: P supply ratio than the Medium NO3− treatment, yet the plants grown in these different conditions had the same leaf N: P ratio. Moreover, the NO3−-treated plants all had a much lower leaf N: P ratio than plants grown in their natural habitat. This non-responsiveness of leaf N: P ratio to high NO3− supplies again indicated that the four targeted species have NO3−-uptake restraint. The N: P ratio of less than 20 in hydroponics also indicated that P was not limiting NO3− uptake.
The four investigated species are non-mycorrhizal, and non-mycorrhizal species in a native Banksia woodland in Western Australia exclusively take up nitrate as their N source (Pate et al. 1993). However, plants take up N from soluble inorganic sources in the soil solution as well as some organic N (Lambers and Oliveira 2019). Plants growing in hypoxic soil conditions such as paddy rice take up NH4+ as the main source of N, whereas plants adapted to aerobic soils where nitrification occurs tend to prefer NO3− (Maathuis 2009; Nadelhoffer et al. 1984). In addition, nitrate is highly mobile in soil, and thus can easily be taken up by roots, while ammonia may be immobilised or nitrified by soil microorganisms (Pate et al. 1993; Shearer et al. 1974). While we do not know the source of N the targeted species prefer, the available evidence suggests that they are all likely to access nitrate in their native habitats. The plants studied here were grown in aerated hydroponics where NO3− was the only source of N. They all showed good growth and had higher total leaf N concentrations compared with plants in their natural habitat. This confirms that the plants were capable of taking up nitrate and using it as the sole source of N. They all exhibited low leaf NO3− concentrations, even when they had access to an excess of NO3−, indicating that they all exhibited NO3−-uptake restraint. Taken together, these results indicate that the actual form on N that these plants prefer, which is unknown, did not impact their NO3− uptake capacity.
Nitrate-uptake restraint is likely another P-efficiency strategy for plants that evolved in severely P-impoverished environments (Prodhan et al. 2016, 2019). Walker and Syers (1976) proposed that all terrestrial ecosystems begin with a finite amount of P in parent rock, which gradually weathers, releasing P that is eventually eroded, leached or occluded. Therefore, P limitation of plant productivity increases in ecosystems as soils age (Lambers et al. 2008; Vitousek et al. 2010). South-western Australia has some of the world’s oldest soils that are derived from parent material with low P concentrations. They have also been highly weathered and leached, making them extremely P impoverished (Hopper 2009; Lambers et al. 2010). In these extremely P-impoverished landscapes, P plays a key role in limiting plant productivity (Hopper and Gioia 2004; Laliberté et al. 2012). In contrast to many N-limited ecosystems in the world, P is in the ‘driver’s seat’ and has provided the selective pressure for plant evolution in south-western Australia (Hayes et al. 2021; Prodhan et al. 2019). Nitrate-uptake restraint would not only force plants to function at low protein concentrations that would reduce the demand for P (Lambers et al. 2015), but may also save metabolic energy by not using energy to transport unneeded NO3− across cell membranes.
Proteaceae, one of the dominant plant families in the P-impoverished ecosystems of south-western Australia, have numerous strategies to adapt to extreme P limitation. The P efficiency traits that allow Myrtaceae to be another dominant plant family in these P-limiting ecosystems are not well established. We can now add nitrate restraint as a possibly general adaptation. The highly efficient use of P decreases the requirements for N, allowing plants with NO3− restraint to economize further.
We also found that species from contrasting habitats functioned differently with respect to NO3−supplies. Banksia attenuata and M. seriata, which naturally occur in the severely P-impoverished Bassendean sand on the dune at our study site, took up more P when supplied with medium and high NO3−, compared to the NO3−-starved plants. Conversely, B. telmatiaea and M. rhaphiophylla, which occur in the winter-wet area in the reserve, where soils are somewhat richer (Gao et al. 2020; Leopold and Zhong 2019), had similar total plant P content for all three treatments. This led to relatively lower leaf N: P ratios in B attenuata and M. seriata than in B. telmatiaea and M. rhaphiophylla. Banksia attenuata and M. seriata grown in hydroponics also had higher leaf Pi concentrations than plants from their natural habitats, in contrast with B. telmatiaea and M. rhaphiophylla, which had similar leaf Pi concentrations with plants from their natural habitats. The higher Pi concentration in the two species that occur naturally on the dune of Bassendean sand suggested that they have a lower capacity to down-regulate their P-uptake. We surmise that this difference reflects the difference in P availability of their natural habitats. The plant-available P in soil on the Bassendean dune was lower than at the winter-wet areas of the reserve (Gao et al. 2020; Leopold and Zhong 2019). In a more fertile habitat, a greater capacity to down-regulate the P-uptake system would prevent P toxicity, whereas in severely P-impoverished habitats, down regulation would not be essential. Moreover, the lack of down regulation might actually be associated with a lower P-remobilisation efficiency from senescent leaves, as suggested by de Campos et al. (2013).
Based on our results, we conclude that B. attenuata, B. telmatiaea, M. rhaphiophylla and M. seriata all exhibited NO3−-uptake restraint to different degrees. None of these species stored N as NO3−, but instead converted it to organic N. However, the adaptation of these species to low P availability in their natural habitat seemed to impact on how they responded to different NO3− supplies. Thus, NO3−-uptake restraint may be a convergent trait common to many species that evolved in severely P-impoverished soils, allowing them to cope with extremely low P availability.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Aerts R (2009) Nitrogen supply effects on leaf dynamics and nutrient input into the soil of plant species in a sub-arctic tundra ecosystem. Polar Biol 32:207–214. https://doi.org/10.1007/s00300-008-0521-1
Andrews M, Maule HG, Raven JA, Mistry A (2005) Extension growth of Impatiens glandulifera at low irradiance: Importance of nitrate and potassium accumulation. Ann Bot 95:641–648. https://doi.org/10.1093/aob/mci059
Cataldo DA, Haroon M, Schrader LE, Youngs VL (1975) Rapid colorimetric determination of nitrate in plant-tissue by nitration of salicylic-acid. Commun Soil Sci Plant Anal 6:71–80. https://doi.org/10.1080/00103627509366547
Cox WJ, Reisenauer HM (1973) Growth and ion uptake by wheat supplied nitrogen as nitrate, or ammonium, or both. Plant Soil 38:363–380. https://doi.org/10.1007/Bf00779019
Dayrell RLC, Cawthray GR, Lambers H, Ranathunge K (2021) Using activated charcoal to remove substances interfering with the colorimetric assay of inorganic phosphate in plant extracts. Plant Soil. https://doi.org/10.1007/s11104-021-05195-2
de Campos MCR, Pearse SJ, Oliveira RS, Lambers H (2013) Downregulation of net phosphorus-uptake capacity is inversely related to leaf phosphorus-resorption proficiency in four species from a phosphorus-impoverished environment. Ann Bot 111:445–454. https://doi.org/10.1093/aob/mcs299
Gao JW, Wang F, Ranathunge K, Arruda AJ, Cawthray GR, Clode PL, He XH, Leopold M, Roessner U, Rupasinghe T, Zhong HT, Lambers H (2020) Edaphic niche characterization of four Proteaceae reveals unique calcicole physiology linked to hyper-endemism of Grevillea thelemanniana. New Phytol 228:869–883. https://doi.org/10.1111/nph.16833
Gentilli J (1972) Australian climate patterns. Thomas Nelson, Melbourne
Greenwood DJ, Hunt J (1986) Effect of nitrogen-fertilizer on the nitrate contents of field vegetables grown in Britain. J Sci Food Agric 37:373–383. https://doi.org/10.1002/jsfa.2740370407
Hayes P, Turner BL, Lambers H, Laliberte E (2014) Foliar nutrient concentrations and resorption efficiency in plants of contrasting nutrient-acquisition strategies along a 2-million-year dune chronosequence. J Ecol 102:396–410. https://doi.org/10.1111/1365-2745.12196
Hayes PE, Clode PL, Oliveira RS, Lambers H (2018) Proteaceae from phosphorus-impoverished habitats preferentially allocate phosphorus to photosynthetic cells: An adaptation improving phosphorus-use efficiency. Plant Cell Environ 41:605–619. https://doi.org/10.1111/pce.13124
Hayes PE, Guilherme Pereira C, Clode PL, Lambers H (2019) Calcium-enhanced phosphorus toxicity in calcifuge and soil-indifferent Proteaceae along the Jurien Bay chronosequence. New Phytol 221:764–777. https://doi.org/10.1111/nph.15447
Hayes PE, Nge FJ, Cramer MD, Finnegan PM, Fu PL, Hopper SD, Oliveira RS, Turner BL, Zemunik G, Zhong HT, Lambers H (2021) Traits related to efficient acquisition and use of phosphorus promote diversification in Proteaceae in phosphorus-impoverished landscapes. Plant Soil 462:67–88. https://doi.org/10.1007/s11104-021-04886-0
Hopper SD (2009) OCBIL theory: towards an integrated understanding of the evolution, ecology and conservation of biodiversity on old, climatically buffered, infertile landscapes. Plant Soil 322:49–86. https://doi.org/10.1007/s11104-009-0068-0
Hopper SD, Gioia P (2004) The southwest Australian floristic region: Evolution and conservation of a global hot spot of biodiversity. Annu Rev Ecol Evol Syst 35:623–650. https://doi.org/10.1146/annurev.ecolsys.35.112202.130201
Kuppusamy T, Giavalisco P, Arvidsson S, Sulpice R, Stitt M, Finnegan PM, Scheible WR, Lambers H, Jost R (2014) Lipid biosynthesis and protein concentration respond uniquely to phosphate supply during leaf development in highly phosphorus-efficient Hakea prostrata. Plant Physiol 166:1891-U1086. https://doi.org/10.1104/pp.114.248930
Laliberté E, Turner BL, Costes T, Pearse SJ, Wyrwoll KH, Zemunik G, Lambers H (2012) Experimental assessment of nutrient limitation along a 2-million-year dune chronosequence in the south-western Australia biodiversity hotspot. J Ecol 100:631–642. https://doi.org/10.1111/j.1365-2745.2012.01962.x
Lambers H (2014) Plant life on the sandplains in Southwest Australia : a global biodiversity hotspot. University of Western Australia Publishing, Crawley
Lambers H, Oliveira RS (2019) Plant physiological ecology. Springer International Publishing, Germany
Lambers H, Raven JA, Shaver GR, Smith SE (2008) Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol 23:95–103. https://doi.org/10.1016/j.tree.2007.10.008
Lambers H, Brundrett MC, Raven JA, Hopper SD (2010) Plant mineral nutrition in ancient landscapes: high plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant Soil 334:11–31. https://doi.org/10.1007/s11104-010-0444-9
Lambers H, Finnegan PM, Laliberte E, Pearse SJ, Ryan MH, Shane MW, Veneklaas EJ (2011) Phosphorus nutrition of Proteaceae in severely phosphorus-impoverished soils: Are there lessons to be learned for future crops? Plant Physiol 156:1058–1066. https://doi.org/10.1104/pp.111.174318
Lambers H, Bishop JG, Hopper SD, Laliberte E, Zuniga-Feest A (2012) Phosphorus-mobilization ecosystem engineering: the roles of cluster roots and carboxylate exudation in young P-limited ecosystems. Ann Bot 110:329–348. https://doi.org/10.1093/aob/mcs130
Lambers H, Finnegan PM, Jost R, Plaxton WC, Shane MW, Stitt M (2015) Phosphorus nutrition in Proteaceae and beyond. Nat Plants 1:15109. https://doi.org/10.1038/nplants.2015.109
Lambers H, de Britto Costa P, Cawthray GR, Denton MD, Finnegan PM, Hayes PE, Oliveira RS, Power SC, Ranathunge K, Shen Q, Wang X, Zhong H (2022) Strategies to acquire and use phosphorus in phosphorus-impoverished and fire-prone environments. Plant Soil. https://doi.org/10.1007/s11104-022-05464-8
Lane P, Evans KA (2019) Geology of the proposed Yule Brook Regional Park. In: Lambers H (ed) A Jewel in the Crown of a Global Biodiversity Hotspot. Kwongan Foundation and the Western Australian Naturalists’ Club Inc, Perth
Leopold M, Zhong H (2019) The soils of the Alison Baird Reserve. In: Lambers H (ed) A jewel in the crown of a global biodiversity hotspot. Kwongan Foundation and the Western Australian Naturalists’ Club Inc, Perth
Maathuis FJM (2009) Physiological functions of mineral macronutrients. Curr Opin Plant Biol 12:250–258. https://doi.org/10.1016/j.pbi.2009.04.003
Motomizu S, Wakimoto T, Toei K (1983) Spectrophotometric determination of phosphate in river waters with molybdate and malachite green. Analyst 108:361–367. https://doi.org/10.1039/an9830800361
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858. https://doi.org/10.1038/35002501
Nadelhoffer KJ, Aber JD, Melillo JM (1984) Seasonal patterns of ammonium and nitrate uptake in nine temperate forest ecosystems. Plant Soil 80:321–335. https://doi.org/10.1007/bf02140039
North KA, Ehlting B, Koprivova A, Rennenberg H, Kopriva S (2009) Natural variation in Arabidopsis adaptation to growth at low nitrogen conditions. Plant Physiol Biochem 47:912–918. https://doi.org/10.1016/j.plaphy.2009.06.009
Pate JS, Stewart GR, Unkovich M (1993) N-15 natural abundance of plant and soil components of a Banksia Woodland ecosystem in relation to nitrate utilization, life form, mycorrhizal status and N2-Fixing abilities of component species. Plant Cell Environ 16:365–373. https://doi.org/10.1111/j.1365-3040.1993.tb00882.x
Pate JS, Verboom WH, Galloway PD (2001) Co-occurrence of Proteaceae, laterite and related oligotrophic soils: coincidental associations or causative inter-relationships? Aust J Bot 49:529–560. https://doi.org/10.1071/Bt00086
Prodhan MA, Jost R, Watanabe M, Hoefgen R, Lambers H, Finnegan PM (2016) Tight control of nitrate acquisition in a plant species that evolved in an extremely phosphorus-impoverished environment. Plant Cell Environ 39:2754–2761. https://doi.org/10.1111/pce.12853
Prodhan MA, Finnegan PM, Lambers H (2019) How does evolution in phosphorus-impoverished landscapes impact plant nitrogen and sulfur assimilation? Trends Plant Sci 24:69–82. https://doi.org/10.1016/j.tplants.2018.10.004
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna https://www.R-project.org/
Reich PB, Oleksyn J (2004) Global patterns of plant leaf N and P in relation to temperature and latitude. P Natl Acad Sci USA 101:11001–11006. https://doi.org/10.1073/pnas.0403588101
Shane MW, Lambers H (2005) Cluster roots: A curiosity in context. Plant Soil 274:101–125. https://doi.org/10.1007/s11104-004-2725-7
Shane MW, McCully ME, Lambers H (2004) Tissue and cellular phosphorus storage during development of phosphorus toxicity in Hakea prostrata (Proteaceae). J Exp Bot 55:1033–1044. https://doi.org/10.1093/jxb/erh111
Shearer G, Duffy J, Kohl DH, Commoner B (1974) A steady-state model of isotopic fractionation accompanying nitrogen transformations in soil. Soil Sci Soc Am J 38:315–322. https://doi.org/10.2136/sssaj1974.03615995003800020030x
Smith C (2019) Factors driving the distribution and abundance of vegetation within Alison Baird Reserve. Honours thesis, the University of Western Australia, Perth
Stewart GR, Lee JA, Orebamjo TO (1973) Nitrogen-metabolism of halophytes. II. Nitrate availability and utilization. New Phytol 72:539–546. https://doi.org/10.1111/j.1469-8137.1973.tb04405.x
Sulpice R, Ishihara H, Schlereth A, Cawthray GR, Encke B, Giavalisco P, Ivakov A, Arrivault S, Jost R, Krohn N, Kuo J, Laliberte E, Pearse SJ, Raven JA, Scheible WR, Teste F, Veneklaas EJ, Stitt M, Lambers H (2014) Low levels of ribosomal RNA partly account for the very high photosynthetic phosphorus-use efficiency of Proteaceae species. Plant Cell Environ 37:1276–1298. https://doi.org/10.1111/pce.12240
Tauss C, Keighery GJ, Keighery BJ, Cloran PM, Genovese SD (2019) A new look at the flora and the vegetation patterns of the Greater Brixton Street Wetlands and Yule Brook. In: Lambers H (ed) A Jewel in the crown of a global biodiversity hotspot. Kwongan Foundation and the Western Australian Naturalists’ Club Inc, Perth
Taylor B, May L (1967) The nitrogen nutrition of the peach tree II. Storage and mobilization of nitrogen in young trees. Aust J Biol Sci 20:389–412. https://doi.org/10.1071/BI9670389
Tschoep H, Gibon Y, Carillo P, Armengaud P, Szecowka M, Nunes-Nesi A, Fernie AR, Koehl K, Stitt M (2009) Adjustment of growth and central metabolism to a mild but sustained nitrogen-limitation in Arabidopsis. Plant Cell Environ 32:300–318. https://doi.org/10.1111/j.1365-3040.2008.01921.x
Turner BL, Laliberté E (2015) Soil Development and nutrient availability along a 2 Million-Year coastal Dune Chronosequence Under Species-Rich Mediterranean Shrubland in Southwestern Australia. Ecosystems 18:287–309. https://doi.org/10.1007/s10021-014-9830-0
Turner BL, Hayes PE, Laliberté E (2018) A climosequence of chronosequences in southwestern Australia. Eur J Soil Sci 69:69–85. https://doi.org/10.1111/ejss.12507
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen-phosphorus interactions. Ecol Appl 20:5–15. https://doi.org/10.1890/08-0127.1
Walker TW, Syers JK (1976) The fate of phosphorus during pedogenesis. Geoderma 15:1–19. https://doi.org/10.1016/0016-7061(76)90066-5
Yan L, Zhang XH, Han ZM, Pang JY, Lambers H, Finnegan PM (2019) Responses of foliar phosphorus fractions to soil age are diverse along a 2 Myr dune chronosequence. New Phytol 223:1621–1633. https://doi.org/10.1111/nph.15910
Acknowledgements
We thank Toby Bird for assistance with plant growth, Toby Bird and Xue Meng Zhou for assistance with plant harvest, Chris Brouwer, Darryl Roberts and Michael Smirk for technical help, Robert Creasy and Bill Piasini for glasshouse support, and Todd Buters for drone image of Alison Baird Reserve.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This work was supported by the Australian Research Council (DP200101013) to H.L. and P.M.F. S.T.L is sponsored by a University Postgraduate Award and Australian Government Research Training Program Scholarship at The University of Western Australia.
Author information
Authors and Affiliations
Contributions
All authors designed the research; Shu Tong Liu performed the research; all authors analysed data; Shu Tong Liu wrote the manuscript; and Hans Lambers, Patrick M. Finnegan and Kosala Ranathunge provided editorial comments and suggestions to finalise the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Tim S. George.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, S.T., Ranathunge, K., Lambers, H. et al. Nitrate-uptake restraint in Banksia spp. (Proteaceae) and Melaleuca spp. (Myrtaceae) from a severely phosphorus-impoverished environment. Plant Soil 476, 63–77 (2022). https://doi.org/10.1007/s11104-022-05477-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05477-3