Abstract
Background & aims
Small organic N compounds could contribute to N nutrition, but an alternative view is that root uptake may serve to recapture compounds that efflux out of roots. However, it is unclear if plants can recapture leaked organic N compounds because no studies have examined quantitative relationships between efflux and uptake at sub-micromolar concentrations.
Methods
This study examines efflux and uptake of a broad suite of small organic N compounds by wheat (Triticum aestivum L.). 15N-labeling and capillary electrophoresis-mass spectrometry were used to estimate efflux and uptake.
Results
One hundred and ten organic N compounds were detected in exudates. Amino acids were abundant but accounted for less than half of organic N. Other abundant compound classes were amines and polyamines, quaternary ammonium compounds, nucleobases and nucleosides. Uptake occurred simultaneously with efflux for all 45 compounds for which rates of efflux could be reliably determined, even though concentrations were 0.01 to 0.5 μM.
Conclusions
These findings indicate that wheat is highly proficient at recapturing much of the diverse array of organic N compounds in root exudates. The ability to salvage effluxed compounds present at very low concentrations means that wheat might also be able to take up organic N compounds from the soil solution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several strands of evidence suggest that small organic N compounds could contribute to N nutrition of plants (Näsholm et al. 2009). First, the pool and/or diffusive fluxes of small organic N compounds in the soil solution can be as large as those of inorganic N (Inselsbacher and Näsholm 2012; Warren 2014a). Second, it has been known for at least 100 years that plants can grow with, or take up from hydroponic solution, different forms of organic N (e.g. see review by: Hutchinson and Miller 1912). More recent studies have identified in diverse taxa the transporters for organic N compounds including amino acids (Wipf et al. 2002; Rentsch et al. 2007; Näsholm et al. 2009), quaternary ammonium compounds (Breitkreuz et al. 1999) and amines + polyamines (Igarashi and Kashiwagi 2010). Finally, injecting soil with organic N compounds in which C and/or N are isotope labeled (e.g. 13C and 15N, or 14C) has shown plants can take up organic N compounds including amino acids (Schmidt and Stewart 1999; Persson and Nasholm 2001; Warren 2006; Näsholm et al. 2009), amino sugars (Kerley and Read 1995; Roberts and Jones 2012), quaternary ammonium compounds (Warren 2013c), and peptides (Hill et al. 2011). Despite these various lines of evidence, the quantitative significance of organic N to plant nutrition remains unclear (Jones et al. 2005; Näsholm et al. 2009; Warren 2012). An alternative view of organic N uptake is that that root uptake mechanisms may primarily be involved in retrieval of compounds that have “leaked” out of root cells (Jones et al. 2005). The view that uptake of organic N is primarily a mechanism to recapture effluxed compounds is based on the premise that the efflux of small organic N compounds from roots into soil would represent a costly loss of N to the plant, and thus there is a strong incentive to recapture leaked N.
Whether or not plants can recapture leaked organic N compounds depends on quantitative relationships between efflux and uptake, but there is little consensus among the few studies to have compared efflux with uptake. In Medicago sativa, Medicago truncatula, Zea mays, Lolium multiflorum and Triticum aestivum the rates of uptake of 16 amino acids supplied at 2.5 μM exceeded efflux rates by 5–545 % (Phillips et al. 2004, 2006). In contrast Lesuffleur et al. (2007) working with amino acids supplied at 1, 10 and 20 μM found that efflux of glycine was faster than uptake in six crop species, while efflux of serine was faster than uptake in Medicago sativa, Trifolium repens, and Brassica napus. These contrasting results might reflect that different methods were used to disentangle efflux from uptake. In the study of Phillips et al. (and also: Jones and Darrah 1993) efflux was estimated indirectly by blocking uptake with an inhibitor of active transport (carbonyl cyanide m-chlorophenylhydrazone, CCCP), whereas Lesuffluer et al. used an isotope labelling approach to enable the direct and independent measurement of efflux and uptake. There are pros and cons to both approaches, but a recent study with metabolic inhibitors suggested that efflux of at least some compounds may be actively controlled (Lesuffleur and Cliquet 2010), and thus the CCCP pre-treatment used to estimate efflux (Jones and Darrah 1993; Phillips et al. 2004, 2006). May in itself alter rates of efflux.
Designing realistic experiments to determine if plants can re-capture effluxed organic N compounds is not straightforward. In the context of re-capture of effluxed compounds, use of realistically low compound concentrations is vitally important given that uptake of organic N compounds is concentration dependent (e.g. amino acids: Schobert and Komor 1987; Jämtgård et al. 2008). In this context it is worth noting that past studies showing simultaneous efflux and re-capture of amino acids supplied amino acids at arbitrary concentrations of 1 10 & 20 μM (Lesuffleur et al. 2007; Lesuffleur and Cliquet 2010) or 2.5 μM (Phillips et al. 2004, 2006) or 100 μM (Jones and Darrah 1994). In general these concentrations are larger and bear little semblance to concentrations that occur in exudates. Hence, it remains unclear if the ability to take up organic N compounds extends to the re-capture of the highly dilute organic N compounds effluxed from roots. An additional challenge for understanding the role of efflux in organic N uptake is that we only have data on re-capture of amino acids, yet a broader suite of organic N compounds probably efflux from plants roots and could be re-captured. For example, root exudates have been reported to contain a wide variety of organic N compounds including amino acids, purines, nucleosides, peptides and quaternary ammonium compounds (Fan et al. 2001; Dakora and Phillips 2002; Walker et al. 2003; Badri and Vivanco 2009; Strehmel et al. 2014), although with the excpetion of amino acids we do not yet have good quantitative data for these compound classes. Clearly, more experimentation is needed to resolve the issue of which organic N compounds efflux from roots and if they can be re-captured at the low concentrations that occur in exudates.
This study examines efflux and uptake of a broad suite of small organic N compounds by wheat (Triticum aestivum L.). The aims were first to describe what small organic N compounds are in root exudates. Capillary electrophoresis-mass spectrometry (CE-MSn) was used for separation, ID and quantification of small (<1 kDa) organic N compounds in root exudates (Warren 2013b). The second aim of this study was to use a 15N-labeling methodology (Fig. 1) to obtain direct estimates of efflux and uptake of a broad suite of organic N compounds at field-relevant concentrations. Previous studies using 15N-labeling to determine efflux and uptake have supplied plants with a modest number of amino acids at arbitrarily chosen concentrations (Lesuffleur et al. 2007; Lesuffleur and Cliquet 2010). The approach used in the present study was instead to harvest the natural complement of exudate compounds from plants grown with N at natural abundance. These natural abundance exudates were then presented to plants that were fully 15N-labelled. Efflux was determined from the appearance of 15N isotopologues in the hydroponic solution, while uptake was determined from disappearance of 14N isotopologues from the hydroponic solution. The final aim of this study was to examine the hypothesis that the profile of organic N compounds in exudates reflects the profile of organic N compounds in root extracts.
Methods
Plant material and growth conditions
Several experiments were performed over a period of 6 months. In each case the plant material was grown identically. Seeds of Triticum aestivum L. were surface sterilized, germinated and then grown in sterile hydroponic solution. The hydroponic solution was a modified Hoagland solution that contained 6 mM N (as KNO3 and (NH4)2SO4), 0.125 mM P, 3 mM K, 2 mM Ca, 1 mM Mg, 1 mM S, and micronutrients. To avoid adding unlabeled N, iron was supplied as FeSO4 rather than the more usual EDTA chelate. The pH of the hydroponic solution during growth and experimentation was between 6.5 and 6.8. Plants grown with N at natural abundance and 15N were grown identically expect that for 15N plants the N was supplied as 99 atom% 15KNO3 and (15NH4)2SO4, (Isotec inc., Miamisburg, USA). The roots of plants were sealed into individual, sterile 250-mL polypropylene containers that contained an aerated hydroponic solution. Preliminary experiments established that the aquarium pump and tubing used for aeration led to contamination of the hydroponic solution with plasticisers and other exogenous compounds that affected subsequent mass spectrometry analyses. To minimise contamination, hydroponic solutions were aerated by passing the air from the aquarium pump through a hydrocarbon trap (BHT-4, Agilent Technologies, Santa Clara, USA) to remove organics, with the cleaned air being connected to sterilized miniature aquarium stones via short lengths of PTFE tubing. These precautions ensured that hydroponic solutions did not contain detectable amounts of plasticisers. Light was excluded from the hydroponic solution and roots by tightly wrapping containers with aluminium foil. The hydroponic solution was exchanged every 2 to 6 days depending on the size of plants. Plants were grown at 22 °C with a 12-h photoperiod of 250–400 μmol PAR m−2 s−1 at plant height. Plants were grown for 4 weeks prior to experimentation.
Determining the time-course and steady-state concentrations of exudates
One experiment was carried out to determine the time-course of exudation, whether there was a steady-state concentration of exudates, and provide samples for identification of the N-containing organic compounds in exudates. Three 4-week-old natural abundance and three 15N-labelled wheat plants were removed from the hydroponic solutions that they had been grown in. To remove residual hydroponic solution and old exudates adhering to roots, roots were gently sprayed with N-free hydroponic solution. Plants were placed into individual 250-mL polypropylene containers filled with aerated 1/10 strength N-free hydroponic solution. Experiments were carried out in 1/10-strength hydroponic solution because the large salt load in full-strength solution interfered with mass spectrometry analyses. Preliminary experiments in which exudates were analysed by GC-MS as methoximated-trimethylsilyl derivatives (Warren et al. 2012) established that steady-state exudate concentrations did not vary between plants in full-strength hydroponic solution and plants in 1/10-strength N-free hydroponic solution. Light was excluded from the hydroponic solution and roots by tightly wrapping containers with aluminium foil. Experiments were carried out during daylight hours with plants exposed to the same light intensity and temperatures as used for growth (i.e. 22 °C; 250–400 μmol PAR m−2 s−1 at plant height). For natural abundance plants samples of hydroponic solution were collected immediately before plants were added (T0), immediately after plants had been added (~1 min), then 1 h, 3 h, 5 h, 7 h after plants were added. For 15N plants samples were collected at T0 and after 7 h only. On each occasion the hydroponic solution was gently swirled to ensure adequate mixing, then two duplicate 1.5 mL samples were withdrawn and transferred to pre-labeled polypropylene tubes that contained 0.5 mL of ice-cold methanol. Samples were next filtered with a 0.22 μm pore size syringe filter, and then frozen at −80 °C for no more than 2 weeks until they were analysed.
Efflux of CCCP pre-treated plants
One way of visualising efflux is by blocking uptake via pre-treatment of plants with carbonyl cyanide m-chlorophenylhydrazone (CCCP) (Jones and Darrah 1993). Three 4-week-old natural abundance wheat plants were pre-treated for 30 min in a 1/10 strength hydroponic solution that contained 50 μM CCCP. After pre-treatment, roots were rinsed and then efflux was measured by transferring plants into 250-mL polypropylene containers filled with aerated 1/10 strength N-free hydroponic solution and then samples of hydroponic solution were collected after 1 min, 1 h, 2 h, 4 h.
Extraction of roots to determine organic N compounds
Two types of aqueous extracts were obtained from roots. Extraction of roots with water was used to obtain a simple aqueous extract that could represent compounds leaking from roots into water, whereas the aqueous phase of a methanol:chloroform:water extract was expected to contain increased representation of lipophilic compounds. To obtain root extracts, four 4-week-old plants grown with N at natural abundance were harvested. Root material was ground to a fine powder with the aid of liquid N and then extracted with either ultra-pure water (10 mL g−1 FW), or methanol:chloroform:water (2:1:2). Extracts were centrifuged with the supernatant of the H2O extract being retained for analysis and the upper polar phase of the MCW extract being retained for analysis. For both extract types the ratio of plant material to (polar) extractant was identical.
15N-labeling to determine gross efflux and uptake
To obtain independent, direct estimates of efflux and uptake a 15N-labeling methodology was used (Fig. 1). In the first step, exudates were harvested from eight 4-week-old plants grown with N at natural abundance. To collect exudates, plants were removed from their hydroponic solution, gently rinsed, and then placed into 250-mL polypropylene containers filled with aerated 1/10 strength N-free hydroponic solution. Preliminary experiments established that under these conditions 4 h was sufficient time to allow exudates to reach at least 75 % of the “steady state” concentrations observed after 7 h (see also Fig. 3). After 4 h had elapsed the plants were removed and the collected exudate solutions were filtered to 0.22 μm and then refrigerated at 1–3 °C for no more than 24 h before subsequent experimentation. Next, wheat plants that had been grown with 15N for 4 weeks were rinsed then transferred to the natural abundance exudate solution, which had previously been brought to room temperature (22 °C). Samples of hydroponic solution were collected immediately before plants were added (T0), then 1 min, 1, 2 and 4 h after plants were added. An additional experiment involving “interrupted uptake” (Jämtgård et al. 2008) was performed to verify that the changes in compound concentrations were due to plants rather than exoenzymes or microbial contamination. The experiment was carried out as described above, except that at 1 h plants were removed. Samples of hydroponic solution were collected as per usual from T0 to 4 h.
Sterility assessment
Sterility of hydroponic solutions was assessed in two ways. First, the presence of culturable microorganisms was determined at the conclusion of experiments by incubating an aliquot of hydroponic solution on nutrient agar. Second, microbial contamination was assessed in every sample analysed by checking for the presence of specific metabolites that occur in microbes but not axenic wheat. The main microbial marker metabolites used were ectoine, hercynine and aminobutylhomospermidine. These three compounds were highly sensitive indicators of contamination because they do not occur in axenic wheat, were three of the most abundant compounds detected in hydroponic solutions that were intentionally contaminated, and have detection limits better than most of the compounds in root exudates. Exudate samples were also checked for the presence of muramic acid and diaminopimelic acid, two markers of bacterial cell walls. Experiments known to be contaminated either with cultureable microorganisms, or microbe-specific metabolites, were discarded.
Analysis by capillary electrophoresis-mass spectrometry
Samples of hydroponic solution and root extracts were defrosted, concentrated and analysed in a random order in batches of six. Prior to analysis evaporation under reduced pressure (Vacufuge, Eppendorf) was used to concentrate samples of hydroponic solution 36-fold and root extracts 2-fold. Blanks of 1/10 hydroponic solution that had not been used for plant growth or experimentation were also concentrated 36-fold and analysed. Significant background contamination was not detected in any blanks. Samples were subsequently made up in 100 mM ammonium formate (pH 9) in 25 % (v/v) acetonitrile that contained an internal standard (0.4 μg mL−1 methionine sulfone). Capillary electrophoresis-mass spectrometry (CE-MS) was used for untargeted profiling of small organic N compounds, essentially as described previously (Warren 2013b, 2014b). All samples were analyzed at least twice, once with the mass spectrometer set to scan a range of 50–255 Da to obtain optimal detection limits for small compounds, and then again with a wider scan range of 100–1000 Da for larger compounds. Samples were injected by pressure (3 psi for 30 s) and separated with an electrolyte of 2 M formic acid with 20 % (v/v) methanol under 30 kV positive polarity. For the purposes of compound identification CE-MSn analyses were carried out using natural abundance and 15N exudate samples collected at 7 h. Samples were re-analysed to obtain MS2 spectra, and for some compounds MS3 spectra. MS2 and MS3 spectra were obtained using data-dependent (auto MSn) data acquisition with three MS/MS precursors per MS, active exclusion after three spectra, include after 0.15 min, isolation width of 1.0 m/z, fragmentation amplitude of 0.6 V modulated by SmartFrag from 30 to 200 %. MS3 spectra were automatically triggered and obtained for MS2 spectra dominated by neutral losses of water or ammonia. Around 20 low abundance compounds were not fragmented by the auto-MSn routine, so to obtain MS2 and MS3 spectra for these compounds it was necessary to perform additional CE-MSn analyses in which specific precursors were targeted for fragmentation (based on m/z and time window).
Identification and quantification of compounds
Identification of compounds in exudate samples collected at 7 h was an iterative multi-step process involving integration of all peaks, searching of CE-MS and mass spectral databases, de novo mass spectral interpretation, and comparisons with migration times and mass spectra of standards (for full details see Notes S1). Quantification was based on area of the [M+H]+ peak of the 14N isotopologue or the [M+H]+ peak of the fully 15N-labelled isotopologue. 15N isotopologues were assumed to have the same response as their 14N isotopologues. Corrections were made for the contribution of naturally occurring isotopes to the signal for the 15N isotopologue based on calculations made with the software package IsotopePattern (Bruker).
Calculation of gross efflux and uptake
When 15N-grown plants were placed in natural abundance exudates, gross efflux was quantified from the rate at which 15N isotopologues appeared while uptake was determined from the rate the 14N isotopologues disappeared. To calculate uptake and efflux the initial plan was to fit linear regressions to isotopologue concentrations as a function of time from 1 min to 4 h. However, this approach was abandoned because for samples collected at ≥ 2 h the pool of exudates in the hydroponic solution contained a significant amount of 15N isotopologues from contemporaneous efflux. To minimize this problem efflux and uptake were estimated from the change in isotopologue concentrations between 1 min and 1 h. The 1 min (rather than T0) sample was used as the first data point to account for the dilution of the hydroponic solution that occurred when the plant was added, and also for passive desorption of compounds from the root surface and apoplastic space.
Statistics
Principal Components Analysis (PCA) was performed with SIMCA P+ 12.01 (Umetrics, Umeå, Sweden). Owing to the large differences in absolute concentrations between extract types, PCA was performed with normalized (i.e. relative) compound concentrations. Normalised compound concentrations were calculated by dividing the individual compound concentration by the summed concentration of all compounds in the sample. Data were further pre-processed using typical procedures for mass-spectrometry based metabolite data (Wiklund et al. 2008), namely, Pareto scaling and log transformation.
Results
Net efflux of organic N compounds in control & CCCP-treated plants
The number of compounds detectable in exudates increased with the duration of exudate collection. In control plants, around 60 compounds could be detected after 1 min while after 7 h a total of 110 N-containing organic compounds were detected. Of the 110 compounds detected after 7 h, 74 were positively identified by comparison of migration time and mass spectra with standards run on the same instrument, 29 compounds were identified by de novo mass spectral interpretation and/or matches of migration times and/or mass spectra with data reported in published papers or collated in online databases (Table S1). For six of the identified compounds it was not possible to identify the exact isomer. Only seven compounds could not be identified and none of the seven unidentified compounds were abundant (assuming the unknowns had a mass spectral response equivalent to compounds of similar molecular weight and CE migration time).
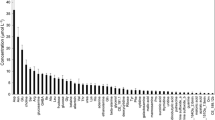
It was possible to determine reliably the concentrations of the approximately 50 most abundant compounds in exudates, but not those compounds present at smaller concentrations that were close to the detection limits of the method. Concentrations of the 50 most abundant compounds had a mean relative standard deviation (standard deviation/mean) of 14 %, indicating good precision of the analytical method and modest biological variation despite concentrations as small as 0.02 μM. The concentrations of the 50 most abundant organic N compounds measured at 7 h ranged from almost 2 μM down to 0.02 μM (Fig. 2). The compounds quantified represented ten compound classes plus one group of unknowns (Table 1). Protein amino acids accounted for the largest number of compounds and were the single most abundant compound class on a molar and N basis, but accounted for only 38 % of concentration on a molar basis and 28 % on an N basis. The other quantitatively significant compound classes were non-protein amino acids, amines + polyamines, quaternary ammonium compounds, nucleobases + nucleosides. Compound classes that were consistently present and quantifiable but made a minor contribution to organic N in exudates included the azetine class which was represented by a single compound 2′-deoxymugineic acid, and hydroxy-cinnamic acid amides, peptides and sugar amines all of which were represented by one compound in top 50.
The concentration of compounds in root exudates of wheat (Triticum aestivum) measured after roots had been placed in a dilute hydroponic solution for 7 h. The right y-axis indicates the average rate of net efflux per unit root dry mass for the 7-h period. Amino acids are indicated with standard 3-letter abbreviations. HCA = hydroxycinnamic acid amide. 164 and 237 = unknowns with nominal protonated masses of 164 and 237 Da, respectively. Data are means of three replicate plants; error bars are one standard error
The total concentration of compounds measured in exudates increased dramatically in the first minute and, to a lesser extent, in the first hour after plants were transferred to the trapping solution (Fig. 3a). Concentrations increased more slowly over the ensuing 7 h. Between 5 and 7 h there was only a modest increase in total exudate concentration (from 12.9 to 13.4 μM). Rates of net efflux calculated from the differences in compound concentrations between different time points (Fig. 3b) confirmed that efflux was very rapid in the first minute and, to a lesser extent, in the first hour. Rates of net efflux decreased to almost zero between 5 and 7 h. In plants that had been pre-treated with CCCP, exudate concentrations and rates of net efflux were around three times greater than control plants (Fig. 3a and b). Moreover, in CCCP-treated plants concentrations of exudate compounds increased quasi-linearly between 2 and 6 h and showed little evidence that a steady-state concentration was being reached or that net efflux was approaching zero (Fig. 3b). Note that efflux experiments with CCCP-treated plants did not extend beyond 4 h because at 4 h the concentrations of many compounds had reached the upper end of the linear calibration range of the CE-MS method.
The time course of efflux of organic N from wheat (Triticum aestivum). Data are a the total concentration of the 50 most abundant organic N compounds in root exudates, and b the rate of net efflux per unit dry mass of roots. Exudates were collected from wheat placed in a dilute hydroponic solution. Prior to measuring efflux plants were pre-treated for 30 min in a dilute hydroponic solution (control), or for 30 min in dilute hydroponic solution that contained 50 μM carbonyl cyanide m-chlorophenylhydrazone (CCCP). Rates of net efflux were calculated from the change in concentration between samples collected at different time points. Data are means of three replicate plants; error bars are one standard error
Simultaneous estimates of gross efflux and uptake
When a plant grown with 15N was placed into a solution containing exudates at natural isotopic abundance, efflux led to the appearance of 15N isotopologues within the solution while uptake led to the decrease in concentration of 14N isotopologues (see Fig. 4 for an example).
The concentration of arginine isotopologues in a a natural abundance hydroponic solution at time zero (before plant added), and b 1 h after adding a 15N-labelled wheat (Triticum aestivum) plant. The natural abundance exudate solution was generated by collecting exudates from a plant grown with N at natural isotopic abundance. Mass spectra are shown for the < 0.1 min separation window corresponding to arginine. Mass spectra were collected over a broad mass range, but for clarity only a restricted mass range is shown here
Rates of gross efflux and uptake could not be determined reliably for many compounds. Rates could not be estimated for the more than 50 compounds present at low concentrations because ≥ 2 h was required for them to reach the lower limit of quantification by which time re-uptake of effluxed compounds was a large problem. There were also five compounds for which specific problems precluded determination of uptake and efflux. Rates of uptake and efflux could not be determined for Gln, Asn and (oxidized)glutathione because they were unstable in hydroponic solution. This was confirmed with spike and recovery tests with sterile hydroponic solution filtered to <0.2 μm suggesting instability could have been due to exuded extracellular enzymes and/or non-enzymatic de-amidation and oxidation. Data are not reported for 2′-deoxymugineic acid and nicotianamine because loss of either compound from solution could be due to uptake and/or formation of complexes with metal ions.
Rates of gross efflux were determined for 45 out of the 50 most abundant compounds. For all 45 of these compounds rates of uptake were also significant. There was a tendency for uptake/efflux to be small for compounds present at low concentrations, with uptake being more than twice as slow as efflux in spermidine, acetylcholine, aminoadipic acid, and proline (Fig. 5). For 41 out of 45 compounds rates of uptake were at least half as fast as the rate of efflux, while rates of uptake were faster than efflux for 21 out of 45 compounds. Uptake/efflux did not vary systematically among compound classes or physico-chemical groupings of compounds (e.g. anionic, cationic, neutral). Instead, in all of the major compound classes (protein amino acids, non-protein amino acids, amines + polyamines, quaternary ammonium compounds, nucleobase + nucleosides, and peptides) there were compounds in which uptake was as fast or faster than rates of efflux.
The relationship between concentration of 45 organic N exudate compounds and the rate of uptake divided by the rate of efflux (uptake/efflux). To determine efflux and uptake exudates were first collected for 4 h from plants grown with N at natural isotopic abundance. Concentrations at T0 are the concentration of the compound in the natural abundance exudate solution before the plant was added. Note that these concentrations are somewhat lower than reported in Fig. 2 because data in Fig. 2 are for exudates collected after 7 h. Wheat plants that had been grown with 15N were placed in the natural abundance exudate solution with efflux being estimated from the rate at which 15N isotopologues appeared between samples collected at 1 min and 1 h, while uptake was determined from the rate at which 14N isotopologues disappeared between samples collected at 1 min and 1 h. Data are means of four replicate plants; error bars are one standard error
Control experiments suggested that the measured change in compound concentrations were due to plants rather than microbial contamination or exoenzymes. When efflux/uptake were interrupted by removing the plant after 1 h, there was no subsequent uptake or efflux of organic N compounds following removal of the plant (linear regression of concentrations at 1, 2 and 4 h, P > 0.05). The exceptions were Gln, Asn and (oxidized)glutathione which were lost from the hydroponic solution (see above).
Is the profile of root exudates similar to root extracts?
Principal components analysis (PCA) provided an overview of multivariate relationships among normalised concentrations of organic N monomers (Fig. 6). Differences in multivariate relationships among exudate concentrations at 4 h, root H2O extracts and root MCW extracts were large relative to variability among replicates, as indicated by the tight grouping of the different sample types in principal components analysis (Fig. 6a). One reason the multivariate pattern of exudates concentrations at 4 h might diverge strongly from root extracts is that exudate concentration is a function of not only efflux but also uptake. However, estimates of gross efflux, while different to exudate concentrations, were also clearly different to both types of root extracts (Fig. 6b).
The multivariate relationships among H2O extracts of roots, the aqueous phase of a methanol:chloroform:water extract of roots and the concentration of exudates measured at 4 h (a) and also the gross rate of efflux calculated between 1 min and 1 h (b). Data are the first two principal components of a principal components analysis performed with normalized (i.e. relative) compound concentrations or efflux rates. Normalised compound concentrations and efflux rates were calculated by dividing the individual compound concentration or efflux rate by the summed concentration or efflux rate of all compounds. Data were Pareto scaled and log transformed and then PCA was performed with SIMCA-P
Univariate comparisons also illustrated widespread differences between concentrations of organic N compounds in gross efflux and organic N compounds in root extracts. Of the 45 organic N compounds for which efflux could be reliably estimated, normalized concentrations of 19 compounds differed between gross efflux and H2O extracts (Fig S1), while normalized concentrations of 22 out of 45 organic N monomers differed between gross efflux and MCW extracts (Fig S2). There were some compounds whose normalized concentrations differed between H2O extracts and efflux but not between MCW extracts and efflux, and vice versa. One consistent difference was that efflux was characterized by proportionally larger concentrations of betaine, several nucleosides (e.g. adenosine and deoxyadenosine) and amines + polyamines (e.g. putrescine, homospermidine, spermidine) than roots extracted with H2O or MCW. The correlation of normalized concentrations in root extracts with gross efflux was weak (Fig. 7). Standout features of these correlations were amines + polyamines, nucleobases and nucleosides that were present at or near detection limits in root extracts but were abundant in gross efflux from roots (ellipses in Fig. 7a and b). Other outliers that were abundant in root extracts but not in efflux were Asp in H2O extracts and a hydroxycinnamic acid amide in MCW extracts (marked in Fig. 7 as Asp and HCA, respectively).
Relationships between rates of gross efflux (calculated between 1 min and 1 h) and the concentration of compounds in a H2O extract (a), and the concentration of compounds in the aqueous phase of a root extracted with methanol:chloroform:water (MCW) (b). Data are shown for the 45 compounds for which uptake and efflux could be estimated reliably. Efflux and concentrations in extracts were normalized based on total efflux of all compounds or total concentration of all compounds. Data are means of four replicate plants; error bars are one standard error. Two outliers, aspartic acid (Asp) and hydroxy-cinnamic acid amide (HCA), are indicated on the figure.
Discussion
Detection of organic N compounds that efflux from wheat roots
A variety of compounds and compound classes have been previously reported from root exudates (Dakora and Phillips 2002; Walker et al. 2003; Badri and Vivanco 2009; Chaparro et al. 2013; Strehmel et al. 2014), and the present study adds to this literature by uncovering a large diversity of organic N compounds in root exudates of wheat. A total of 110 organic N compounds were detected of which 74 were positively identified, 29 were putatively identified and 7 could not be identified (Table S1). No single study has previously reported such a large number and diversity of organic N compounds in root exudates, instead individual studies have reported the presence of a modest number of organic N compounds (typically < 20). Nevertheless most of the compound classes and many of the individual compounds have been previously reported from root exudates. For example, compounds and classes that were present in root exudates of wheat and have been reported previously from wheat and other species include the polyamine putrescine (Kuiper et al. 2001), quaternary ammonium compounds such as betaine and choline (Ikegami et al. 1982; de Rudder et al. 1999; Fan et al. 2001), nucleobases and nucleosides (Dakora and Phillips 2002; Strehmel et al. 2014), the phytosiderophore 2′-deoxymugineic acid (Fan et al. 2001; Oburger et al. 2014) and small peptides (Strehmel et al. 2014).
The idea that exudates contain a broad suite of organic N compounds is not new, but much of the focus has been placed on amino acids because few studies have established the quantitative significance of the different compound classes. Indeed, many studies on organic N compounds have focussed solely on amino acids (Jones and Darrah 1993; Phillips et al. 2006; Lesuffleur et al. 2007; Jämtgård et al. 2008; Lesuffleur and Cliquet 2010), while reviews and summaries often imply that amino acids are the main organic N compound in root exudates. For example, the chemical composition of root exudates has been summarised as: sugars (50–70 % of exudate), carboxylic acids (20–30 % of exudate) and amino acids (10–20 % of exudate) (Kraffczyk et al. 1984; Jones 1998; Hutsch et al. 2002; Farrar et al. 2003). Broad characterisation of the pool of organic N revealed that while amino acids were abundant, they accounted for less than half of the organic N in root exudates of wheat (Table 1) and only five of the ten most abundant compounds were amino acids (Fig. 2). Clearly, future studies of organic N compounds in root exudates ought to consider not only amino acids but also other quantitatively significant compound classes including amines and polyamines, quaternary ammonium compounds, nucleobases and nucleosides.
The large number and diversity of organic N compounds reported here was because the analytical procedure permitted taking a broad view of organic N compounds, rather than because there was anything unusual about the plant material or how exudates were collected. Concentrations of amino acids at steady-state and rates of efflux were, in fact, broadly similar to those reported in previous studies focusing solely on amino acids (Jones and Darrah 1993; Phillips et al. 2006; Lesuffleur et al. 2007; Jämtgård et al. 2008; Lesuffleur and Cliquet 2010). Hence, the major point of difference with previous studies is that the CE-MS procedure used here permitted taking a broad view of multiple classes of organic N (Warren 2013a, b). In contrast, the most commonly used methods for small organic N compounds in root exudates have involved pre-column derivatization (e.g. LC of o-phthalaldehyde derivatives; GC of t-butyldimethylsilyl or trimethylsilyl derivatives) and are highly selective for amino acids and effectively blind to most other classes of organic N (Knapp 1979; Toyo’oka 1999). Hence, the diversity of compounds seen here, and their absence from many previous studies, is most probably because in previous studies the compounds were present but not detected.
What drives efflux?
The efflux of small organic compounds from plant roots is generally regarded as passive leakage (e.g. Jones and Darrah 1993). If exudation were passive leakage across a non-selective membrane the compounds detected in exudates would reflect those detected in root extracts, but this is clearly not the case for root exudates of wheat. Principal components analysis (Fig. 6) and univariate analyses (Figs. 7, S1 and S2) showed that there were large and consistent differences in relative compound concentrations between exudates and extracts. A more realistic view of “leakage” is that it is determined also by the compound-specific resistance to diffusion across the plasma membrane such that leakage should be faster for compounds that are uncharged and hydrophobic than compounds that are charged and hydrophilic (Chakrabarti and Deamer 1992). However, this cannot offer a general explanation for the differences between exudates and extracts because the compound classes that were more abundant in exudates than root extracts were those that should leak across membranes slowly, viz., the neutral and hydrophilic quaternary ammonium compounds, nucleobases and nucleosides, and the strongly cationic and hydrophilic amines + polyamines (Fig. 7a and b). Hence, neither the “steady-state” concentrations of exudate compounds nor rates of gross efflux are consistent with passive leakage from root cells into solution. These findings are consistent with previous studies suggesting that efflux of amino acids could be selective (Lesuffleur et al. 2007; Lesuffleur and Cliquet 2010).
If root exudates do not strictly reflect passive leakage from root cells, what is it that drives efflux? Studies have identified transporters involved in the active secretion of some root exudates (Badri et al. 2012; Fourcroy et al. 2014) and thus for at least some compounds there could be an active component to efflux. An alternative or perhaps complementary explanation is that root extracts are poorly representative of the source of exudates. For example, there can be large amounts of polyamines in xylem (Friedman et al. 1986) and thus the presence of large amounts of amines + polyamines in exudates could reflect their leakage from xylem rather than root cells. Another possibility is that large amounts of polyamines exist within roots in a conjugated form as hydroxy-cinnamic acid amides (see Fig. 7b, and: Samborsk and Rohringe 1970), but efflux from roots as free polyamines. Finally, it is conceivable that some of the compounds detected in exudates are not leaking out of cells but are instead produced at the root surface or in solution by catabolism of larger compounds. For example, the large amounts of nucleosides in exudates could be explained by the enzymatic depolymerisation of high molecular weight exudates or root debris. Additional experimentation is required to distinguish among these possibilities.
Wheat can re-capture organic N compounds effluxed from roots
Wheat can take up organic N compounds present at the low concentrations found in root exudates. In addition to the quantitative estimates of efflux and uptake provided by isotope labelling (Figs. 4 and 5), two additional observations illustrate active uptake at low concentrations. First, exudate concentrations of control plants reached a quasi-steady state after several hours (Fig. 3a) and net efflux approached zero (Fig. 3b), consistent with an equilibrium being reached between rates of efflux and uptake. Second, when active transport was inhibited by pre-treating plants with CCCP, exudate concentrations were much greater and there was no evidence that a steady-state was being reached (Fig. 3a and b). These multiple strands of evidence indicate that wheat can (partially or wholly) re-capture a broad array of organic N compounds that efflux from roots and greatly builds upon earlier studies showing simultaneous uptake and efflux of amino acids (Jones and Darrah 1994; Phillips et al. 2004; Lesuffleur et al. 2007; Lesuffleur and Cliquet 2010) and inorganic N (Kronzucker et al. 1995a, b; Mata et al. 2000; Britto et al. 2001).
Quantitative relationships between efflux and uptake are key to determining whether or not plants recapture leaked organic N compounds. Uptake occurred simultaneously with efflux for all 45 compounds for which rates of efflux could be reliably determined, even though concentrations were only ~0.01–0.5 μM. Four compounds had rates of uptake that were less than 50 % of the rate of efflux, 20 compounds had rates of uptake that were 50–100 % of the rate of efflux, while in the remaining 21 compounds rates of uptake were more than 100 % of rates of efflux (Fig. 5). The relationship between uptake and efflux did not vary systematically among compound classes or physico-chemical groupings of compounds (e.g. anionic, cationic, neutral). Instead, in all of the major compound classes (protein amino acids, non-protein amino acids, amines + polyamines, quaternary ammonium compounds, nucleobase + nucleosides) there were compounds for which uptake was as fast or faster than rates of efflux. A similarly well-developed capability for uptake at low concentrations was seen in Medicago sativa, Medicago truncatula, Zea mays, Lolium multiflorum and Triticum aestivum in which rates of uptake of 16 amino acids supplied at 2.5 μM exceeded efflux rates by 5–545 % (Phillips et al. 2004, 2006). Very different results were reported by Lesuffleur et al. (2007) who found that uptake of glycine was slower than efflux in six crop species, while uptake of serine was slower than efflux in Medicago sativa, Trifolium repens, and Brassica napus. No such evidence for selective efflux of glycine and/or serine was seen in wheat, instead rates of uptake approximately balanced efflux in glycine (uptake/efflux = 0.92 ± 0.09) and in serine (1.0 ± 0.15) (Fig. 5). Differences among studies could indicate inherent species differences or the procedures used to estimate efflux and uptake. For example, Lesuffleur et al. (2007) may have over-estimated efflux by using a very short exposure time (5–10 min) during which time much of the apparent efflux could have been rapid desorption and efflux from apoplastic spaces rather than the slower efflux from the symplast (e.g. see fast rates of net efflux calculated between 0 and 1 min, Fig. 3b).
The diversity of organic N compounds taken up by wheat is not unprecedented, but providing the first evidence that uptake occurs at sub-micromolar external concentrations has ecological implications. Previous studies have shown uptake of diverse forms of organic N (Hutchinson and Miller 1912; Miller and Schmidt 1965; Audley and Tan 1968; Kerley and Read 1995; Schmidt and Stewart 1999; Persson and Nasholm 2001; Näsholm et al. 2009; Warren 2013c) but used concentrations from 10 μM to several mM rather than the (generally) sub-micromolar concentrations of root exudates (Fig. 2). The present study has shown that wheat has the capacity to re-capture a significant proportion of the organic N that effluxes from roots. Organic N compounds in the soil solution are also present at generally sub-micromolar concentrations (Warren 2014a), and thus the ability to salvage effluxed compounds means that wheat has a more general capacity to take up organic N compounds from the soil solution.
Methodological considerations
Experiments here have shown the occurrence of simultaneous efflux and uptake of a broad suite of organic N compounds, but it is unclear how readily these results can be translated to soil. Wheat was grown in a sterile hydroponic system so as to exclude complications from microbes (Kuijken et al. 2015), whereas in soil the efflux and uptake of N from roots could be modified by specific microbial metabolites (Phillips et al. 2004) and a suite of soil processes (e.g., adsorption, mass flow, diffusion, microbial competition). For example, many studies have shown that microbes out-compete plants for inorganic and organic forms of N (Hodge et al. 2000; Bardgett et al. 2003; Jones et al. 2005), and thus while plants may be able to salvage effluxed compounds in sterile hydroponic solution this capability may not be fully realized in soil.
The workflow presented here enabled estimation of uptake and efflux, but low compound concentrations presented some challenges. Slow rates of efflux and the concomitant low concentration of compounds in exudates and slow uptake, meant that a long duration was required to estimate efflux and uptake. Hence, the initial approach had been to estimate efflux and uptake from the difference in isotopologue concentrations between 1 min and 4 h. However, this approach was subsequently abandoned because for anytime ≥ 2 h there was substantial uptake of effluxed compounds and this led to erroneous estimates of efflux and uptake rates. To minimize the uptake of effluxed compounds, efflux and uptake were estimated only between 1 min and 1 h. In samples collected at 1 h there would have been some uptake of effluxed compounds, but this was reasoned to be acceptably small given that after 1 h 15N isotopologues (i.e. derived from efflux) accounted for no more than 15 % of any compound’s concentration in the hydroponic solution. The other problem associated with low compound concentrations is that it was not possible to determine efflux and uptake rates for the ~50 least abundant compounds. Use of mass spectrometry instrumentation with better detection limits and quantitative capabilities (e.g. triple quadrupole) might enable estimates of uptake and efflux to be made on less abundant compounds. Moreover, instrumentation with better detection limits would enable experiments to be carried out more quickly so as to minimize problems arising from uptake of effluxed compounds.
Conclusions
Past studies have recognized the diversity of organic N compounds in root exudates, but the quantitative significance of the different compound classes has been unclear. Broad characterisation of the pool of organic N revealed that while amino acids were abundant, they accounted for less than half of the organic N in root exudates of wheat. A workflow utilizing 15N labelling and capillary electrophoresis-mass spectrometry showed that uptake occurred simultaneously with efflux for all 45 compounds for which rates of efflux could be reliably determined, even though concentrations were only ~0.01–0.5 μM. These findings indicate that wheat can take up a broad suite of organic N compounds present at the sub-micromolar concentrations that are typical of root exudates. The ability to salvage effluxed compounds present at very low concentrations means that wheat may also be able to tap into organic N compounds that occur at similarly low concentrations in the soil solution.
References
Audley BG, Tan CH (1968) The uptake of ergothioneine from the soil into the latex of Hevea brasiliensis. Phytochemistry 7:1999–2000
Badri DV, Vivanco JM (2009) Regulation and function of root exudates. Plant Cell Environ 32:666–681
Badri DV, Chaparro JM, Manter DK, Martinoia E, Vivanco JM (2012) Influence of ATP-binding cassette transporters in root exudation of phytoalexins, signals, and in disease resistance. Front Plant Sci 3:149
Bardgett RD, Streeter TC, Bol R (2003) Soil microbes compete effectively with plants for organic-nitrogen inputs to temperate grasslands. Ecology 84:1277–1287
Breitkreuz KE, Shelp BJ, Fischer WN, Schwacke R, Rentsch D (1999) Identification and characterization of GABA, proline and quaternary ammonium compound transporters from Arabidopsis thaliana. FEBS Lett 450:280–284
Britto DT, Siddiqi MY, Glass ADM, Kronzucker HJ (2001) Futile transmembrane NH4 + cycling: a cellular hypothesis to explain ammonium toxicity in plants. Proc Natl Acad Sci U S A 98:4255–4258
Chakrabarti AC, Deamer DW (1992) Permeability of lipid bilayers to amino-acids and phosphate. Biochim Biophys Acta 1111:171–177
Chaparro JM, Badri DV, Bakker MG, Sugiyama A, Manter DK, Vivanco JM (2013) Root exudation of Phytochemicals in Arabidopsis follows specific patterns that are developmentally programmed and correlate with soil microbial functions. PLoS One 8
Dakora FD, Phillips DA (2002) Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 245:35–47
de Rudder KEE, Sohlenkamp C, Geiger O (1999) Plant-exuded choline is used for rhizobial membrane lipid biosynthesis by phosphatidylcholine synthase. J Biol Chem 274:20011–20016
Fan TWM, Lane AN, Shenker M, Bartley JP, Crowley D, Higashi RM (2001) Comprehensive chemical profiling of gramineous plant root exudates using high-resolution NMR and MS. Phytochemistry 57:209–221
Farrar J, Hawes M, Jones D, Lindow S (2003) How roots control the flux of carbon to the rhizosphere. Ecology 84:827–837
Fourcroy P, Siso-Terraza P, Sudre D, Saviron M, Reyt G, Gaymard F, Abadia A, Abadia J, Alvarez-Fernandez A, Briat JF (2014) Involvement of the ABCG37 transporter in secretion of scopoletin and derivatives by Arabidopsis roots in response to iron deficiency. New Phytol 201:155–167
Friedman R, Levin N, Altman A (1986) Presence and identification of polyamines in xylem and phloem exudates of plants. Plant Physiol 82:1154–1157
Hill PW, Quilliam RS, DeLuca TH, Farrar J, Farrell M, Roberts P, Newsham KK, Hopkins DW, Bardgett RD, Jones DL (2011) Acquisition and assimilation of nitrogen as peptide-bound and D-enantiomers of amino acids by wheat. PLoS One 6
Hodge A, Robinson D, Fitter A (2000) Are microorganisms more effective than plants at competing for nitrogen? Trends Plant Sci 5:304–308
Hutchinson HB, Miller NHJ (1912) The direct assimilation of inorganic and organic forms of nitrogen by higher plants. J Agric Sci 4:282–U284
Hutsch BW, Augustin J, Merbach W (2002) Plant rhizodeposition - an important source for carbon turnover in soils. J Plant Nutr Soil Sci-Z Pflanzenernahr Bodenkd 165:397–407
Igarashi K, Kashiwagi K (2010) Characteristics of cellular polyamine transport in prokaryotes and eukaryotes. Plant Physiol Biochem 48:506–512
Ikegami F, Kuo YH, Lambein F (1982) Release of amino-acids, trigonelline and related-compounds from imbibing Lathyrus odoratus Seeds. Arch Int Physiol Biochim Biophys 90:B35–B36
Inselsbacher E, Näsholm T (2012) The below-ground perspective of forest plants: soil provides mainly organic nitrogen for plants and mycorrhizal fungi. New Phytol 195:329–334
Jämtgård S, Näsholm T, Huss-Danell K (2008) Characteristics of amino acid uptake in barley. Plant Soil 302:221–231
Jones DL (1998) Organic acids in the rhizosphere—a critical review. Plant Soil 205:25–44
Jones DL, Darrah PR (1993) Influx and efflux of amino-acids from Zea mays L roots and their implications for N-nutrition and the rhizosphere. Plant Soil 156:87–90
Jones DL, Darrah PR (1994) Amino-acid influx at the soil-root interface of Zea mays L and its implications in the rhizosphere. Plant Soil 163:1–12
Jones DL, Healey JR, Willett VB, Farrar JF, Hodge A (2005) Dissolved organic nitrogen uptake by plants - an important N uptake pathway? Soil Biol Biochem 37:413–423
Kerley SJ, Read DJ (1995) The biology of mycorrhiza in the Ericaceae.18. Chitin degradation by Hymenoscyphus ericae and transfer of chitin-nitrogen to the host-plant. New Phytol 131:369–375
Knapp DR (1979) Handbook of analytical derivatization reactions. Wiley, New York, p 768
Kraffczyk I, Trolldenier G, Beringer H (1984) Soluble root exudates of maize - influence of potassium supply and rhizosphere microorganisms. Soil Biol Biochem 16:315–322
Kronzucker HJ, Siddiqi MY, Glass ADM (1995a) Compartmentation and flux characteristics of ammonium in spruce. Planta 196:691–698
Kronzucker HJ, Siddiqi MY, Glass ADM (1995b) Compartmentation and flux characteristics of nitrate in spruce. Planta 196:674–682
Kuijken RCP, Snel JFH, Heddes MM, Bouwmeester HJ, Marcelis LFM (2015) The importance of a sterile rhizosphere when phenotyping for root exudation. Plant Soil 387:131–142
Kuiper I, Bloemberg GV, Noreen S, Thomas-Oates JE, Lugtenberg BJJ (2001) Increased uptake of putrescine in the rhizosphere inhibits competitive root colonization by Pseudomonas fluorescens strain WCS365. Mol Plant-Microbe Interact 14:1096–1104
Lesuffleur F, Cliquet JB (2010) Characterisation of root amino acid exudation in white clover (Trifolium repens L.). Plant Soil 333:191–201
Lesuffleur F, Paynel F, Bataille MP, Le Deunff E, Cliquet JB (2007) Root amino acid exudation: measurement of high efflux rates of glycine and serine from six different plant species. Plant Soil 294:235–246
Mata C, van Vemde N, Clarkson DT, Martins-Loucao MA, Lambers H (2000) Influx, efflux and net uptake of nitrate in Quercus suber seedlings. Plant Soil 221:25–32
Miller RH, Schmidt EL (1965) Uptake and assimilation of amino acids supplied to the sterile soil: root environment of the bean plant (Phaseolus vulgaris). Soil Sci 100:323–330
Näsholm T, Kielland K, Ganeteg U (2009) Uptake of organic nitrogen by plants. New Phytol 182:31–48
Oburger E, Gruber B, Schindlegger Y, Schenkeveld WDC, Hann S, Kraemer SM, Wenzel WW, Puschenreiter M (2014) Root exudation of phytosiderophores from soil-grown wheat. New Phytol 203:1161–1174
Persson J, Nasholm T (2001) Amino acid uptake: a widespread ability among boreal forest plants. Ecol Lett 4:434–438
Phillips DA, Fox TC, King MD, Bhuvaneswari TV, Teuber LR (2004) Microbial products trigger amino acid exudation from plant roots. Plant Physiol 136:2887–2894
Phillips DA, Fox TC, Six J (2006) Root exudation (net efflux of amino acids) may increase rhizodeposition under elevated CO2. Glob Chang Biol 12:561–567
Rentsch D, Schmidt S, Tegeder M (2007) Transporters for uptake and allocation of organic nitrogen compounds in plants. FEBS Lett 581:2281–2289
Roberts P, Jones DL (2012) Microbial and plant uptake of free amino sugars in grassland soils. Soil Biol Biochem 49:139–149
Samborsk DJ, Rohringe R (1970) Abnormal metabolites of wheat - occurrence, isolation and biogenesis of 2-Hydroxyputrescine amides. Phytochemistry 9:1939–1945
Schmidt S, Stewart GR (1999) Glycine metabolism by plant roots and its occurrence in Australian plant communities. Aust J Plant Physiol 26:253–264
Schobert C, Komor E (1987) Amino acid uptake by Ricinus communis roots - characterization and physiological significance. Plant Cell Environ 10:493–500
Strehmel N, Bottcher C, Schmidt S, Scheel D (2014) Profiling of secondary metabolites in root exudates of Arabidopsis thaliana. Phytochemistry 108:35–46
Toyo’oka T (1999) Modern derivatization methods for separation sciences, England. pp. 312
Walker TS, Bais HP, Grotewold E, Vivanco JM (2003) Root exudation and rhizosphere biology. Plant Physiol 132:44–51
Warren CR (2006) Potential organic and inorganic N uptake by six Eucalyptus species. Funct Plant Biol 33:653–660
Warren CR (2012) Post-uptake metabolism affects quantification of amino acid uptake. New Phytol 193:522–531
Warren CR (2013a) Development of a capillary electrophoresis–mass spectrometry method for small peptides in the soil solution. Soil Biol Biochem 63:80–84
Warren CR (2013b) High diversity of small organic N observed in soil water. Soil Biol Biochem 57:444–450
Warren CR (2013c) Quaternary ammonium compounds can be abundant in some soils and are taken up as intact molecules by plants. New Phytol 198:476–485
Warren CR (2014a) Organic N molecules in the soil solution: what is known, what is unknown and the path forwards. Plant Soil 375:1–19
Warren CR (2014b) Response of osmolytes in soil to drying and rewetting. Soil Biol Biochem 70:22–32
Warren CR, Aranda I, Cano FJ (2012) Metabolomics demonstrates divergent responses of two Eucalyptus species to water stress. Metabolomics 8:186–200
Wiklund S, Johansson E, Sjostrom L, Mellerowicz EJ, Edlund U, Shockcor JP, Gottfries J, Moritz T, Trygg J (2008) Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal Chem 80:115–122
Wipf D, Ludewig U, Tegeder M, Rentsch D, Koch W, Frommer WB (2002) Conservation of amino acid transporters in fungi, plants and animals. Trends Biochem Sci 27:139–147
Acknowledgments
Charles Warren is supported by a Future Fellowship from the Australian Research Council. The University of Sydney is thanked for financial support via the major equipment scheme.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ad C. Borstlap.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig S1
The relative concentrations of organic N compounds in gross efflux from roots, root H2O extracts and the aqueous phase of root methanol:chloroform:water extracts. (DOCX 50 kb)
Fig S2
The relative concentrations of organic N compounds in gross efflux from roots, root H2O extracts and the aqueous phase of root methanol:chloroform:water extracts. (DOCX 50 kb)
ESM 1
Details of how organic N compounds were identified. (DOCX 26 kb)
Table S1
Compounds identified in root exudates of wheat (Triticum aestivum L.). (XLSX 25 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Warren, C.R. Wheat roots efflux a diverse array of organic N compounds and are highly proficient at their recapture. Plant Soil 397, 147–162 (2015). https://doi.org/10.1007/s11104-015-2612-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2612-4