Abstract
Background and aims
A fast and reliable phenotyping system forms a major bottleneck in root exudation research. Our goal was to develop such a system to quantify genetic and environmental effects on root exudation. Another aim was characterizing non-sterile phenotyping.
Methods
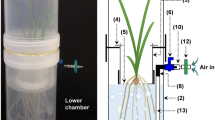
We developed a system in which plants can be grown with non-sterile shoot environments and sterile rhizospheres. These sterile systems were compared with non-sterile controls.
Results
In non-sterile rhizospheres exogenous carbon disappeared quickly with a half-life of 2 to 3 h and root exudate concentrations remained below detection limit. In sterile rhizospheres exogenous carbon levels were relatively stable or depleted slower than in non-sterile rhizospheres and organic acid build-up occurred. Tomato (Solanum lycopersicum) could be grown with sterile roots for several months.
Conclusions
The differences in carbon depletion in sterile and non-sterile rhizospheres was most likely due to the absence of microbial catabolism in sterile rhizospheres. These results prove that using a sterile phenotyping system is essential to study the quantity and composition of root exudates. The sterile system described in this paper eliminates the obscuring effect caused by microbes on exudation levels. It offers a stable, reliable and easy phenotyping method and can be used to investigate genetic and environmental effects on exudation.





Similar content being viewed by others
References
Aulakh MS, Wassmann R, Bueno C, Kreuzwieser J, Rennenberg H (2001) Characterization of root exudates at different growth stages of ten rice (Oryza sativa L.) cultivars. Plant Biol 3:139–148
Barazani O, Friedman J (1999) Is IAA the major root growth factor secreted from plant-growth-mediating bacteria? J Chem Ecol 25:2397–2406. doi:10.1023/a:1020890311499
Bengough AG, McKenzie BM, Hallett PD, Valentine TA (2011) Root elongation, water stress, and mechanical impedance: a review of limiting stresses and beneficial root tip traits. J Exp Bot 62:59–68. doi:10.1093/jxb/erq350
Dakora FD, Phillips DA (2002) Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 245:35–47
Dechassa N, Schenk MK (2004) Exudation of organic anions by roots of cabbage, carrot, and potato as influenced by environmental factors and plant age. J Plant Nutr Soil Sci-Z Pflanzenphysiol Bodenkd 167:623–629. doi:10.1002/jpln.200420424
Dessureault-Rompré J, Nowack B, Schulin R, Luster J (2006) Modified micro suction cup/rhizobox approach for the in-situ detection of organic acids in rhizosphere soil solution. Plant Soil 286:99–107
Dinkelaker B, Romheld V, Marschner H (1989) Citric-acid excretion and precipitation of calcium citrate in the rhizosphere of white lupin (Lupinus-albus). Plant Cell Environ 12:285–292
Egle K, Romer W, Keller H (2003) Exudation of low molecular weight organic acids by Lupinus albus L., Lupinus angustifolius L. and Lupinus luteus L. as affected by phosphorus supply. Agronomie 23:511–518. doi:10.1051/agro:2003025
Farrar J, Hawes M, Jones D, Lindow S (2003) How roots control the flux of carbon to the rhizosphere. Ecology 84:827–837. doi:10.1890/0012-9658(2003)084[0827:hrctfo]2.0.co;2
Gaume A, Machler F, Frossard E (2001) Aluminum resistance in two cultivars of Zea mays L: root exudation of organic acids and influence of phosphorus nutrition. Plant Soil 234:73–81
Gent MPN, Parrish ZD, White JC (2005) Nutrient uptake among subspecies of Cucurbita pepo L. is related to exudation of citric acid. J Am Soc Hortic Sci 130:782–788
Gherardi MJ, Rengel Z (2004) The effect of manganese supply on exudation of carboxylates by roots of lucerne (Medicago sativa). Plant Soil 260:271–282
Haase S, Neumann G, Kania A, Kuzyakov Y, Romheld V, Kandeler E (2007) Elevation of atmospheric CO2 and N-nutritional status modify nodulation, nodule-carbon supply, and root exudation of Phaseolus vulgaris L. Soil Biol Biochem 39:2208–2221. doi:10.1016/j.soilbio.2007.03.014
Hodge A, Berta G, Doussan C, Merchan F, Crespi M (2009) Plant root growth, architecture and function. Plant Soil 321:153–187. doi:10.1007/s11104-009-9929-9
Hoekenga OA, Maron LG, Pineros MA, Cancado GMA, Shaff J, Kobayashi Y, Ryan PR, Dong B, Delhaize E, Sasaki T, Matsumoto H, Yamamoto Y, Koyama H, Kochian LV (2006) AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc Natl Acad Sci U S A 103:9738–9743. doi:10.1073/pnas.0602868103
Hoffland E, Vandenboogaard R, Nelemans J, Findenegg G (1992) Biosynthesis and root exudation of citric and malic-acids in phosphate-starved rape plants. New Phytol 122:675–680
Hoffland E, Wei CZ, Wissuwa M (2006) Organic anion exudation by lowland rice (Oryza sativa L.) at zinc and phosphorus deficiency. Plant Soil 283:155–162. doi:10.1007/s11104-005-3937-1
Imas P, BarYosef B, Kafkafi U, GanmoreNeumann R (1997) Release of carboxylic anions and protons by tomato roots in response to ammonium nitrate ratio and pH in nutrient solution. Plant Soil 191:27–34
Johnson JF, Allan DL, Vance CP, Weiblen G (1996) Root carbon dioxide fixation by phosphorus-deficient lupinus albus - contribution to organic acid exudation by proteoid roots. Plant Physiol 112:19–30
Jones DL, Darrah PR (1994) Role of root derived organic-acids in the mobilization of nutrients from the rhizosphere. Plant Soil 166:247–257
Jones DL, Darrah PR (1995) Influx and efflux of organic-acids across the soil-root interface of Zea-mays L and its implications in rhizosphere C flow. Plant Soil 173:103–109
Jones DL, Edwards AC (1998) Influence of sorption on the biological utilization of two simple carbon substrates. Soil Biol Biochem 30:1895–1902
Jones DL, Hodge A, Kuzyakov Y (2004) Plant and mycorrhizal regulation of rhizodeposition. New Phytol 163:459–480
Kaku N, Yonezawa N, Kodama Y, Watanabe K (2008) Plant/microbe cooperation for electricity generation in a rice paddy field. Appl Microbiol Biotechnol 79:43–49. doi:10.1007/s00253-008-1410-9
Kamh M, Horst WJ, Amer F, Mostafa H, Maier P (1999) Mobilization of soil and fertilizer phosphate by cover crops. Plant Soil 211:19–27. doi:10.1023/a:1004543716488
Kamilova F, Kravchenko LV, Shaposhnikov AI, Azarova T, Makarova N, Lugtenberg B (2006a) Organic acids, sugars, and l-tryptophane in exudates of vegetables growing on stonewool and their effects on activities of rhizosphere bacteria. Mol Plant-Microbe Interact 19:250–256. doi:10.1094/MPMI-19-0250
Kamilova F, Kravchenko LV, Shaposhnikov AI, Makarova N, Lugtenberg B (2006b) Effects of the tomato pathogen Fusarium oxysporum f. sp radicis-lycopersici and of the biocontrol bacterium pseudomonas fluorescens WCS365 on the composition of organic acids and sugars in tomato root exudate. Mol Plant-Microbe Interact 19:1121–1126. doi:10.1094/mpmi-19-1121
Kirk GJD, Santos EE, Santos MB (1999) Phosphate solubilization by organic anion excretion from rice growing in aerobic soil: rates of excretion and decomposition, effects on rhizosphere pH and effects on phosphate solubility and uptake. New Phytol 142:185–200
Kochian LV, Pineros MA, Hoekenga OA (2005) The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil 274:175–195. doi:10.1007/s11104-004-1158-7
Kuzyakov Y, Domanski G (2000) Carbon input by plants into the soil review. J Plant Nutr Soil Sci-Z Pflanzenerna hr Bodenkd 163:421–431
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot 98:693–713. doi:10.1093/aob/mcl114
Leifert C, Cassells AC (2001) Microbial hazards in plant tissue and cell cultures. In Vitro Cell Dev Biol-Plant 37:133–138
Liang CY, Pineros MA, Tian J, Yao ZF, Sun LL, Liu JP, Shaff J, Coluccio A, Kochian LV, Liao H (2013) Low pH, aluminum, and phosphorus coordinately regulate malate exudation through GmALMT1 to improve soybean adaptation to acid soils. Plant Physiol 161:1347–1361. doi:10.1104/pp. 112.208934
Ligaba A, Maron L, Shaff J, Kochian L, Pineros M (2012) Maize ZmALMT2 is a root anion transporter that mediates constitutive root malate efflux. Plant Cell Environ 35:1185–1200. doi:10.1111/j.1365-3040.2011.02479.x
Lynch JM, Whipps JM (1990) Substrate flow in the rhizosphere. Plant Soil 129:1–10
Ma JF (2000) Role of organic acids in detoxification of aluminum in higher plants. Plant Cell Physiol 41:383–390
Marschner P, Fu QL, Rengel Z (2003) Manganese availability and microbial populations in the rhizosphere of wheat genotypes differing in tolerance to Mn deficiency. J Plant Nutr Soil Sci-Z Pflanzenerna hr Bodenkd 166:712–718. doi:10.1002/jpln.200320333
Meharg AA, Killham K (1991) A novel method of quantifying root exudation in the presence of soil microflora. Plant Soil 133:111–116
Meharg AA, Killham K (1995) Loss of exudates from the roots of perennial ryegrass inoculated with a range of microorganisms. Plant Soil 170:345–349
Mimmo T, Hann S, Jaitz L, Cesco S, Gessa CE, Puschenreiter M (2011) Time and substrate dependent exudation of carboxylates by Lupinus albus L. and Brassica napus L. Plant Physiol Biochem 49:1272–1278. doi:10.1016/j.plaphy.2011.08.012
Nakano Y, Nakano A, Watanabe S, Okano K, Tatsumi J (2003) External and internal root structures of tomato plants grown hydroponically in a humid atmosphere or in a nutrient solution. J Jpn Soc Hortic Sci 72:148–155
Neumann G, Romheld V (1999) Root excretion of carboxylic acids and protons in phosphorus-deficient plants. Plant Soil 211:121–130
Neumann G, Massonneau A, Martinoia E, Romheld V (1999) Physiological adaptations to phosphorus deficiency during proteoid root development in white lupin. Planta 208:373–382
Oburger E, Jones DL, Wenzel WW (2011) Phosphorus saturation and pH differentially regulate the efficiency of organic acid anion-mediated P solubilization mechanisms in soil. Plant Soil 341:363–382. doi:10.1007/s11104-010-0650-5
Paterson E, Thornton B, Midwood AJ, Sim A (2005) Defoliation alters the relative contributions of recent and non-recent assimilate to root exudation from Festuca rubra. Plant Cell Environ 28:1525–1533
Paterson E, Sim A, Standing D, Dorward M, McDonald AJS (2006) Root exudation from Hordeum vulgare in response to localized nitrate supply. J Exp Bot 57:2413–2420. doi:10.1093/jxb/erj214
Pearse SJ, Veneklaas EJ, Cawthray GR, Bolland MDA, Lambers H (2006) Carboxylate release of wheat, canola and 11 grain legume species as affected by phosphorus status. Plant Soil 288:127–139. doi:10.1007/s11104-006-9099-y
Pearse SJ, Veneklaas EJ, Cawthray G, Bolland MDA, Lambers H (2007) Carboxylate composition of root exudates does not relate consistently to a crop species’ ability to use phosphorus from aluminium, iron or calcium phosphate sources. New Phytol 173:181–190. doi:10.1111/j.1469-8137.2006.01897.x
Petersen W, Bottger M (1991) Contribution of organic-acids to the acidification of the rhizoshpere of maize seedlings. Plant Soil 132:159–163
Radersma S, Grierson PF (2004) Phosphorus mobilization in agroforestry: organic anions, phosphatase activity and phosphorus fractions in the rhizosphere. Plant Soil 259:209–219
Ragsdale SW, Pierce E (2008) Acetogenesis and the wood-ljungdahl pathway of CO2 fixation. Biochim Biophys Acta Protein Proteomics 1784:1873–1898. doi:10.1016/j.bbapap.2008.08.012
Rasmussen J (2011) Why we need to restrict the use of “rhizodeposition” and the Janzen and Bruinsma equation. Soil Biol Biochem 43:2213–2214. doi:10.1016/j.soilbio.2011.05.023
Ryan PR, Raman H, Gupta S, Horst WJ, Delhaize E (2009) A second mechanism for aluminum resistance in wheat relies on the constitutive efflux of citrate from roots. Plant Physiol 149:340–351. doi:10.1104/pp. 108.129155
Sandnes A, Eldhuset TD, Wollebaek G (2005) Organic acids in root exudates and soil solution of Norway spruce and silver birch. Soil Biol Biochem 37:259–269. doi:10.1016/j.soilbio.2004.07.036
Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H (2004) A wheat gene encoding an aluminum-activated malate transporter. Plant J 37:645–653. doi:10.1111/j.1365-313X.2003.01991.x
Shane MW, Lambers H, Cawthray GR, Kuhn AJ, Schurr U (2008) Impact of phosphorus mineral source (Al-P or Fe-P) and pH on cluster-root formation and carboxylate exudation in Lupinus albus L. Plant Soil 304:169–178. doi:10.1007/s11104-007-9535-7
Strader LC, Beisner ER, Bartel B (2009) Silver ions increase auxin efflux independently of effects on ethylene response. Plant Cell 21:3585–3590. doi:10.1105/tpc.108.065185
Strik D, Timmers RA, Helder M, Steinbusch KJJ, Hamelers HVM, Buisman CJN (2011) Microbial solar cells: applying photosynthetic and electrochemically active organisms. Trends Biotechnol 29:41–49. doi:10.1016/j.tibtech.2010.10.001
Tracy SR, Black CR, Roberts JA, Sturrock C, Mairhofer S, Craigon J, Mooney SJ (2012) Quantifying the impact of soil compaction on root system architecture in tomato (Solanum lycopersicum) by X-ray micro-computed tomography. Ann Bot 110:511–519. doi:10.1093/aob/mcs031
Veneklaas EJ, Stevens J, Cawthray GR, Turner S, Grigg AM, Lambers H (2003) Chickpea and white lupin rhizosphere carboxylates vary with soil properties and enhance phosphorus uptake. Plant Soil 248:187–197
Wang B, Shen JB, Tang CX, Rengel Z (2008) Root morphology, proton release, and carboxylate exudation in lupin in response to phosphorus deficiency. J Plant Nutr 31:557–570. doi:10.1080/01904160801895084
Wichern F, Eberhardt E, Mayer J, Joergensen RG, Muller T (2008) Nitrogen rhizodeposition in agricultural crops: methods, estimates and future prospects. Soil Biol Biochem 40:30–48. doi:10.1016/j.soilbio.2007.08.010
Wright GD (2010) Antibiotic resistance in the environment: a link to the clinic? Curr Opin Microbiol 13:589–594. doi:10.1016/j.mib.2010.08.005
Zhao FJ, Hamon RE, McLaughlin MJ (2001) Root exudates of the hyperaccumulator Thlaspi caerulescens do not enhance metal mobilization. New Phytol 151:613–620
Acknowledgments
This research was supported by the EU Seventh Framework program Future and emerging technologies under grant number 226532. We thank Diaan Jamar, Marije Hoogendoorn and Esther Meinen for technical assistance, Monsanto Holland B.V. for providing tomato seeds and Jacques Withagen for statistical support. We thank Dr. Anja Dieleman for her constructive comments on this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Peter J. Gregory .
Rights and permissions
About this article
Cite this article
Kuijken, R.C.P., Snel, J.F.H., Heddes, M.M. et al. The importance of a sterile rhizosphere when phenotyping for root exudation. Plant Soil 387, 131–142 (2015). https://doi.org/10.1007/s11104-014-2283-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2283-6