Abstract
Plants and microorganisms establish beneficial associations that can improve their development and growth. Recently, it has been demonstrated that bacteria isolated from the skin of amphibians can contribute to plant growth and defense. However, the molecular mechanisms involved in the beneficial effect for the host are still unclear. In this work, we explored whether bacteria isolated from three tropical frogs species can contribute to plant growth. After a wide screening, we identified three bacterial strains with high biostimulant potential, capable of modifying the root structure of Arabidopsis thaliana plants. In addition, applying individual bacterial cultures to Solanum lycopersicum plants induced an increase in their growth. To understand the effect that these microorganisms have over the host plant, we analysed the transcriptomic profile of A. thaliana during the interaction with the C32I bacterium, demonstrating that the presence of the bacteria elicits a transcriptional response associated to plant hormone biosynthesis. Our results show that amphibian skin bacteria can function as biostimulants to improve agricultural crops growth and development by modifying the plant transcriptomic responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
Three species of bacteria isolated from amphibian skin exhibited an effect on plant growth in A. thaliana and tomato plants. Transcriptomic analysis revealed a transcriptional regulation of hormonal pathways involved in the growth of the plant A. thaliana.
Introduction
For years, chemical fertilizers have been used to change the physical, biological, and chemical properties of soils, which favors the nutritional status of plants, providing the necessary components to improve their development and growth (Neina 2019; Sharma and Chetani 2017). However, excessive application of these chemical agents leads to water, air, and soil pollution, as well as biological imbalances and reduced biodiversity (Kumar et al. 2019; O’Donnell et al. 2001). The use of microorganisms present in the environment has been proposed as an ecological alternative to help promoting plant growth and development, tolerance to biotic and abiotic stress, as well as helping in the assimilation of nutrients stored in the soil or rhizosphere (Ferreira et al. 2019; O’Brien 2017; Rouphael and Colla 2020).
Bacteria that promote plant growth are known as Plant-Growth Promoting Bacteria [PGPB] or Bacteria Plant Biostimulants (De Zelicourt et al. 2013). However, the mechanisms by which PGPBs act on plant biology are still not fully understood in terms of ecology and molecular function (Vandenkoornhuyse et al. 2015). It is proposed that biostimulants function through direct and indirect mechanisms. Direct mechanisms include biological nitrogen fixation [BNF], solubilization of nutrients, such as phosphate, zinc, and potassium (Morales-Cedeño et al. 2021), and secretion of plant growth-promoting substances, including several hormones such as auxins [indole-3-acetic acid or IAA], cytokinins [CK], gibberellins (Basu et al. 2021; Cano 2011; Rehman et al. 2020; Santner et al. 2009; Vega-Celedón et al. 2016), brassinosteroids [BR] (Hussain et al. 2020), and ethylene [ET] (Iqbal et al. 2017). While indirect mechanisms include the production of siderophores that help to solubilize iron [Fe3+], and the production of microbial volatile organic compounds [mVOCs] that trigger the induced or acquired systemic defense responses, to combat pathogenic microorganisms (del Rosario Cappellari et al. 2019; Singh 2018).
Biostimulants possess several of the above-mentioned characteristics, which in many cases will depend on changes in the environment where the bacterium develops (Morales-Cedeño et al. 2021). Bacteria of the genus Azospirrillum (de-Bashan et al. 2012), Bacillus (Sansinenea 2019), Pseudomonas (Santoyo et al. 2012), Burkholderia (Suárez-Moreno et al. 2012) and Enterobacter (Jha et al. 2011), have been reported as plant growth promoters, and/or biocontrol agents [BCAs]. However, many of these microorganisms are often pathogenic to humans, thus posing an ecological and human health risk, which must be addressed before commercial production; furthermore, these PGPBs may also exhibit resistance to plant pathogens (Ramakrishna et al. 2019). Therefore, the identification of new biostimulants and description of the molecular mechanisms behind their beneficial/protective effect are important.
Biostimulant bacteria have been identified from several sources in environment. One promising source is the amphibian skin microbiota since it has been shown that some of their members are able to protect amphibians against fungal diseases such as chytridiomycosis, caused by the chytrid fungus Batrachochytrium dendrobatidis [Bd] (Harris et al. 2009; Rebollar et al. 2019). For instance, Susilawati et al. (2021) found that bacteria present on the skin of wild frogs have the potential to control diseases caused by pathogenic fungi in plants. They identified 106 bacteria isolated from three different frogs species [Hyla japonica, Pelophylax porosus porosus and Buergeria burger], three of which significantly inhibited the growth of the fungal pathogen Colletotrichum orbiculare, [anthracnose], and produced changes in the root structure of cucumber [Cucumus sativus] plants. The molecular mechanisms used by these bacteria to inhibit the plant pathogen and influence plant growth, and development are still undescribed. Moreover, we have recently identified that, bacteria from the genus Acinetobacter isolated from the skin of a tropical frog species, reduced the pathogenic activity against Bd, and the plant fungal pathogen Botrytis cinerea [Bc] (Cevallos et al. 2022). However, their phytostimulant activity in plants is still undescribed.
In order to find bacterial candidates with biostimulant potential, we analysed the growth promoting effect of three bacterial strains isolated from the frog Craugastor fitzingeri that inhibit the growth of the fungal pathogens Bd and Bc (Cevallos et al. 2022; Rebollar et al. 2019; Rebollar and Harris 2019). We determined that growth of Arabidopsis thaliana and Solanum lycopersicum was improved by the exogenous application of these bacteria. Additionally, to understand the biostimulants molecular effect of one strain [Acinetobacter sp. C32I], we studied the transcriptomic changes induced by its application on A. thaliana, observing modifications in the expression levels for genes related to hormones. These results demonstrate that bacteria from amphibian skin are a good source of bacteria that can have plant biostimulant effects.
Materials and methods
Bacteria strain
The bacteria strains used in this study were previously isolated from the skin of the frog Craugastor fitzigeri and described in Rebollar et al. (2019). Two of the bacteria were recently identified as members of the Acinetobacter genus [C26G and C32I] (Cevallos et al. 2022), and one was only identified at the family level as Enterobacteraceae [C23F] using 16S rRNA sequencing (Rebollar et al. 2019). All of them were able to inhibit the growth of the amphibian fungal pathogen Bd (Cevallos et al. 2022; Rebollar et al. 2019). To analyze their effect in the plant, bacteria were cultured on Luria–Bertani medium [LB] and incubated at 30 °C for 24 h until an optical density of 0.6 [O.D. 600 nm] was reached.
Plant-bacteria interaction in vitro
Arabidopsis thaliana ecotype Col-0 seeds were surface sterilized with ethanol 70% three times for five minutes followed by absolute ethanol for ten minutes, the ethanol was decanted and the seeds were placed in square plates containing 0.2× MS agar medium pH 5.7, supplemented with sucrose [0.5% w/v] and 1.5% [w/v] agar (Murashige and Skoog 1962). The plates were placed at 4 °C for 48 h for vernalization and were incubated at 22 ± 2 °C in a plant growth chamber with 16 h/ 8 h light/dark cycles. After four days of germination, 30 µl of each bacterial suspension normalized to an optical density of 0.6 [O.D. 600 nm] was placed on the opposite side of the plants in the plates. Plants were monitored for fifteen days to evaluate primary root growth. At the end of the experiment, root and rosette fresh weight were evaluated. The number of root hairs was analysed under the optical microscope [Zeiss Axioskop 2, 10×] as previously described (Napsucialy-Mendivil and Dubrovsky 2018). The experiments were repeated with at least three biological replicates, each with three technical replicates [5 plants per treatment].
Histochemical analysis of A. thaliana DR5::GUS reporter line inoculated with Acinetobacter sp. C32I
Sterile seeds of the A. thaliana DR5::GUS reporter line were germinated on plates containing 0.2× MS medium pH 5.7. After four days, plates were inoculated with Acinetobacter sp. C32I bacteria cell suspension. Seven days-post-inoculation [dpi], the seedlings were subjected to GUS histochemical staining during 12 h of incubation at 37 °C in a GUS reaction buffer [0.5 mg/ml of 5-bromo-4-chloro-3-indolyl-β-D-glucuronide in 100 mM sodium phosphate, pH 7] (Jefferson et al. 1987). Tissue clarification was carried out with a solution of methanol: acetone [3:1] during 2 h. For analysis, 20 plantlets [10 plantlets per plate] were analysed at 10 × magnification under a Zeiss Axioskop 2 microscope. Imagens are representative of the experiment. All experiments were carried in triplicate.
Root hair analyses of the A. thaliana rdh6 mutant in interaction with Acinetobacter spp. C32I
For the study, seeds of the A. thaliana rdh6 mutant, which shows reduced number of root hairs in absence of auxins, were used. Sterile seeds were germinated on plates with MS medium 0.2× pH 5.7. Four-day-old seedlings were inoculated with 30 µl of a suspension of Acinetobacter sp. C32I cells. After fifteen days of interaction, the seedlings were placed in a 50% sterile glycerol solution for microscopic observation. The number of root hairs was counted as previously described (Napsucialy-Mendivil and Dubrovsky 2018). As control, we used uninoculated seedlings. Ten seedlings were measured for each treatment [n = 10]. All experiments were performed at least three times.
RNA extraction and leaves transcriptomic analysis of A. thaliana leaves
For RNA-seq analysis, Arabidopsis thaliana Col-0 plants were root-inoculated with Acinetobacter sp. C32I under greenhouse conditions. Ten leaves per rosette were collected after four weeks post interaction and under uninoculated conditions. Total RNA for RNA-seq was isolated from two different biological replicates for each treatment using the TRizol method according to the manufacturer’s instructions [Invitrogen]. Total RNA concentration and purity were measured with a NanoDrop spectrophotometer [Implen NP80, Thermo Fisher Scientific, USA]. Library construction and sequencing were performed by Beijing Genomics Institute [BGI] Americas2 using DNBSeq TM technology. Differentially expressed genes [DEGs] were identified using the software DESeq2 in the Integrated Differential Expression Analysis MultiEXperiment [IDEAMEX] (Jiménez-Jacinto et al. 2019), with a FoldChange ≥ 2, and adjusted p-value ≤ 0.05. DEGs were functionally annotated with Gene Ontology terms by PANTHER [v17.0]. GO Term Enrichment for plant analysis was performed by using the web tool TAIR [The Arabidopsis Information Resource [TAIR], https://www.arabidopsis.org/tools/go_term_enrichment.jsp, on www.arabidopsis.org, Feb,24, 2022] employing Fisher’s exact test and correction with an FDR. Non-redundant enriched terms were obtained by using REVIGO software (Supek et al. 2011). Plots were created with the ggplot2 library using R [v4.2.1] libraries ggplot2 and heatmap. KEGG pathways maps were generated by using KEGG Mapper – Color [https://www.genome.jp/kegg/mapper/color.html, Feb, 24, 2022].
Bacteria- Solanum lycopersicum interactions
Solanum lycopersicum seeds were placed in 50 mL Falcon tubes and washed three times with 3% sodium hypochlorite and a final wash with absolute ethanol. Seeds were germinated in hydrated vermiculite. Seven-day-old plants were placed in plastic containers containing a mixture of soil and vermiculite [3:1] and maintained under greenhouse conditions with 2 weekly irrigations of 200 mL of tap water. For five weeks, a suspension of bacteria, grown in 0.2× MS medium pH 5.7, was added to the soil every third day. After this time, root and stem length, fresh, dry were measured. We performed two biological replicates, each with 8 plants per treatment. The experiment was replicated to assess both production and fresh weight.
Statistical analysis
All results are reported as mean values [± SEM]. A one-way ANOVA for non-parametric data and Tukey´s analysis [p < 0.05] was carried out to determine statistically significant differences between each experiment. The software GraphPad Prism version 9.2.0 was used [GraphPad Software, San Diego, CA, USA, 2019]. All the data analysed were obtained from three independent experiments.
Results
Frog skin microbiota modify the root structure of A. thaliana
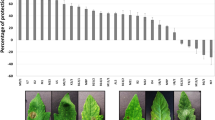
To investigate the effect of bacteria on the growth of A. thaliana Col-0 plants, seeds were germinated on MS 0.2× for four days under in vitro conditions, then inoculated with bacterial isolates C23F, C26G, and C32I, and compared to the uninoculated control. After fifteen days post interaction [dpi], we evaluated the primary root growth, root and stem fresh weight, and the number of root hairs for each treatment (Fig. 1). The results obtained showed that there was no significant difference in the growth of the primary root of A. thaliana between the control and the treatments (Fig. 1B). However, when evaluating the fresh weight of both root and stem, we observed that the plants in the presence of each bacterium individually presented a significantly higher weight compared to the uninoculated control (Fig. 1C, D). Additionally, it was observed that all bacteria modified the root structure of A. thaliana by promoting the growth of secondary roots and the development of root hairs (Fig. 1E). Our analysis showed that plants inoculated with C23F promoted root hair formation approximately of 40 roots hair/mm, followed by C26G and C32I with 38 and 34 roots hair/mm respectively, compared to the control which showed only five roots hair per mm (Fig. 1F). These data correlate with the increase in root and stem mass, which could confer a benefit on the plant for water and macronutrient and micronutrient uptake.
Frog skin bacterial isolates modify the root structure of A. thaliana. Four-day-old Arabidopsis thaliana Col-0 seedlings were exposed, individually, to 30 µl of cell suspensions containing C23F, C26G, or C32I bacteria. Plant growth was observed over a 15 dpi. We conducted an analysis to evaluate the influence of each bacterial strain on root hair production in Arabidopsis roots. (A) Representative images of the interaction of each bacterium with the plant, with uninoculated plants using as the control. (B) Root length was monitored throughout the experiment from the apical zone to the stem collar. (C) Fresh weight of rosettes and (D) fresh weight of roots in A. thaliana plants were determined. (E) Frog skin bacteria were found to modify the root structure, leading to an increase in the number of root hairs in the apical zone. (F) Quantification was performed per millimeter of both inoculated and non-inoculated plant roots. Letters indicate a statistically significant difference, according to one-way analysis of variance [ANOVA] [p ≤ 0.05] followed by a Tukey test. Scale bar 100 µm
Presence of frog skin C32I bacteria induces transcriptional changes in the A. thaliana plant
To elucidate the molecular effect of frog skin bacteria on growth of A. thaliana, we selected the C32I bacteria because it generated the greatest change in plant growth under in-vitro conditions (Fig. 1). After four weeks we collected rosette-leaves samples from uninoculated and inoculated plants and analysed the expression levels by RNAseq (Supplementary Data 1, Fig. 2). We identified that the number of differentially expressed genes [DEGs] in plants inoculated with C32I were 543 versus the control treatment without bacteria. Two hundred forty-six genes were up-regulated and 297 were down-regulated respectively (Fig. 2A).
Transcriptome analysis of A. thaliana leaves in response to Acinetobacter spp. C32I. Plants of A. thaliana Col-0 were inoculated with the cellular suspension of Acinetobacter sp. C32I at the root level. Leaves-collected from each plant for sequencing [RNA-seq] of total RNA. (A) Venn diagrams show the number of up-regulated and down-regulated DEGs identified in the treatment versus the control [Log2FC ≥ 2 or ≤ -2]. (B) Distribution of the different biological functions of Gene Ontology [GO] for the up-regulated genes [p < 0.05]. The circle size represents the number of genes biological process associated. The graph represents the enrichment of “Biological process” GO-terms [FDR < 0.01]
To further identify the plant biological processes that changed in response to the presence of C32I, a comparison analysis between uninoculated and inoculated plants was performed using REVIGO software. Our results showed that 543 genes were significantly classified into 20 biological processes. From the up-regulated genes, we identified GO categories for: sulfur compound metabolic process, secondary metabolic processes, response to wounding, response to stress, response to stimulus, response to bacterium, response to biotic stimulus, regeneration, macromolecule metabolic process and biological processes involved in interspecies interaction between organisms, related to the biological activity of the plant (Supplementary Data 2, Fig. 2B). The analysis suggests that plants in the presence of C32I bacteria, triggers the expression of various functional groups of genes that are usually associated with the interaction between plants and bacteria (Kudoyarova et al. 2019), promoting plant growth and defence mechanisms. These results help to explain the observed traits of the plants previously observed in the in-vitro analysis.
Acinetobacter sp. C32I induces transcriptional changes on plant hormonal signal transduction pathways
Since we found a growth promoting effect of frog skin bacteria on the plant, we investigated the hormone biosynthetic pathways that could induced by the presence of C32I. We found 33 genes from Auxin [AUX], Cytokinin [CK], Brassinosteroids [BR] and Salicylic Acid [SA] biosynthesis pathways using the Kyoto Encyclopedia of Genes and Genomes [KEGG] analysis (Supplementary Fig. 1). With respect to the AUX pathway, 7 genes related to Indole-Acetic Acid [IAA] biosynthesis were identified, of which genes IAA3, IAA27, IAA17, IAA28, IAA19 and IAA8, showed an increase in expression in inoculated plants, except for the gene IAA2 which showed a decrease its expression. Similarly, ARF1 and ARF7 were down-regulated in inoculated plants; these genes are transcription factors [TFs] which bind to auxin response elements [AREs], that carefully regulate the plant’s response, and prevent inappropriate overexpression of certain genes in response to auxin (Mallick et al. 2022). On the other hand, it was observed that the genes corresponding to the small auxin up-regulated RNA [SAUR] gene family showed an increase in expression in inoculated versus uninoculated plants, including SAUR6, SAUR3, SAUR26, SAUR31 and SAUR14 (Fig. 3, Supplementary Data 3).
KEGG pathway image of plant hormonal signal transduction DEGs related to plant growth in A. thaliana plants treated with Acinetobacter sp. C32I. Relative transcript abundance of genes of plant growth-related hormones after four weeks interaction with bacterium compared to the uninoculated control. The DEGs coding for each gene family are represented by boxes. Identified genes related to hormone transduction pathways related to Auxin [AUX], Cytokinin [CK], Brassinosteroid [BR], and Salicylic Acid [SA] synthesis. Red boxes represent up-regulated genes and blue boxes represent down-regulated genes. IAA: indole acetic acid, ARF: auxin response factor, GH3: Gretchen Hagen 3, SAUR: small auxin upregulated, BSK: brassinosteroid-signaling kinase, CYCD3: D-type cyclin, CRE1: cytokinin response 1, AHP: histidine phosphotransfer, B_ARR: Arabidopsis response regulator type B, A_ARR: Arabidopsis response regulator type A, TGA: TGACG-binding factors, PR1: protein pathogenesis-related
Based on the gene expression involved in the auxin biosynthesis, the transcriptomic levels of the genes of the cytokinin [CK] (Kakimoto 2003) and brassinosteroid [BR] (Chung and Choe 2013) pathways were evaluated. We observed that plants inoculated with Acinetobacter sp. C32I showed up-regulation of CYCD3 gene expression. This gene, involved in the CK pathway, has a crucial function in the regulation of the cell cycle, DNA synthesis, and plant development, while BSK8 was down-regulated (Fig. 3, Supplementary Data 3). Continuing with cytokinins, the highest expression levels were found in for the AHP family of genes encoding for a histidine phosphotransfer proteins such as AHP2 and AHP1. Interestingly, members of the Response Regulator Gene Family to Cytokinin [ARR gene family] which is subdivided into type A and B (Ferreira and Kieber 2005), were identified where two typo B genes ARR5 and ARR7 and three typo A genes ARR3, ARR16, and ARR9 were found to be expressed in the plant in response to the presence of the bacteria versus the control (Fig. 3, Supplementary Data 3).
The phytohormone salicylic acid [SA] is one of the best known and described molecules involved in plant biology, specifically during plant-pathogen interactions (Maruri-López et al. 2019). Based on this knowledge, we observed that the expression of TGA1, TGA2, and TGA4 were down-regulated while PR1 was up-regulated. Taken together, the transcriptomic analysis of the interaction of A. thaliana plants inoculated with Acinetobacter sp. C32I suggest that the bacterium triggers plant development through the modification of induction of phytohormones-mediated signalling pathways.
Acinetobacter sp. C32I induces auxin production and accumulation in the A. thaliana
To validate our transcriptomic analysis, we verified the accumulation and biosynthesis of auxin in A. thaliana in the presence of C32I. First, we were interested to know if this bacterium regulates and restores the WT phenotype of the rdh6 mutant, deficient in auxin-mediated root hair production (Masucci and Schiefelbein 1994). Thus, we inoculated four-day-old plants with the Acinetobacter sp. C32I, and then analysed the root structure in plants by microscopy, using uninoculated plants as controls (Fig. 4). We found was that plants inoculated with Acinetobacter sp. C32I presented a higher number of root hairs, compared to the control, where there was a deficiency in hair production as expected (Fig. 4A, B). To further investigate the role of C32I in root development, we analysed auxin accumulation and distribution in roots using the A. thaliana DR5:GUS reporter line (Ulmasov et al. 1997). For this purpose, four-day-old plants were inoculated with the isolate C32I in direct interaction, and after seven dpi the roots were analysed by microscopy. The histochemical staining pattern of inoculated plants showed a higher GUS signal in the apical zone of the root and the foliar zone of the leaf corresponding to sites of high auxin production, in contrast to the control (Fig. 4C). We conclude that auxin production and accumulation are induced in the presence of the bacteria, and this observation supports by our transcriptomic analysis.
Acinetobacter sp. C32I induces auxin production and accumulation. A. thaliana rdh6 mutant seeds deficiency in root hair production and DR5::GUS reporter line, were germinated on plates containing 0.2× MS medium in the presence of C32I bacterium. The parameters were evaluated at 7 dpi. (A) Representative images of each treatment are show. The plant treated with Acinetobacter sp. C32I exhibits an induction in the production of root hairs, in contrast to the non-inoculated control. (B) The analysis of the roots in rdh6 plants, unveiled an increased count of root hairs in plants inoculated with the bacteria in comparison to the uninoculated control. (C) The DR5::GUS reporter line shows a different signal in leaves and roots in the presence of the bacterium versus control. The experiments were repeated three times with similar results [n = 20 ± SD]. Letters indicate a statistically significant difference, according to a one-way analysis of variance [ANOVA] [p ≤ 0.05] followed by the Tukey test. Mocks represent the plants with MS medium. Scale bar for rdh6 200 µm. Scale for DR5:GUS 100 µm
Frog skin microbiota promote growth of tomato plants
Previously, we found that three bacterial isolates obtained from frog skin induced and modified the growth and root structure of A. thaliana (Fig. 1). As part of the characterization of the three isolates, we decided to determine the effect of these bacteria on the economically important crop model tomato [S. lycopersicum]. First, we monitored the growth of the stem for five weeks, and observed that plants inoculated individually with C23F, C26G, and C32I isolates, after seventeen days, significantly increased in height compared to the control without bacteria (Fig. 5B). Subsequently, the plants were removed from the greenhouse and carefully washed to remove the substrate. Roots were measured and had a greater length in roots inoculated with each bacterium compared to plants without bacteria (Fig. 5C). Biomass was measured [fresh and dry weight], where the plants treated with bacteria showed an increase of 150% compared to control plants (Fig. 5D, E). This analysis suggests that these bacteria from the amphibian skin may exert a beneficial effect on tomato plant development and thereby improve plant performance compared to uninoculated controls.
Effect of soil inoculation with frog skin bacteria on the growth of tomato plants. Tomato plants were germinated under greenhouse conditions, periodic inoculated with C32I bacterium. After 5 weeks of interaction, morphological parameters of the plants were evaluated. (A) Representative images for each treatment display a increase in plant growth when treated with individual bacteria, as compared to the non-inoculated control. (B) Stem length over time. (C) Stem length. (D) Fresh weight. (E) Dry weight. Graphs represent five plants per treatment. Letters indicate a statistically significant difference, according to a one-way analysis of variance [ANOVA] [p ≤ 0.05] followed the Tukey test. Mocks represent the plants with MS medium. Scale bar 10 cm
To determine the effect of the bacteria on the size and number of tomato fruits, the plants were maintained for four months in a greenhouse, with periodic inoculation of the bacteria cultures and constant water irrigation (Fig. 6A). The data obtained showed no differences in the number of fruits per plant of each treatment (Supplementary Fig. 2), however, during harvest treated plants with each bacterium showed a larger size of fruits compared to the control without bacteria (Fig. 6B). A measurement of the fruits of each treatment showed that the control had an average of approximately 12 g per fruit, while the inoculated tomatoes showed a biomass of approximately 23–25 g per fruit (Fig. 6C). The results suggest that although there were no differences in the number of fruits, an increase in the size of the tomatoes was observed, which suggests frog skin bacteria can influence the growth of the plant and the size of the fruits.
Effect of frog skin bacteria C32I on tomato fruit production. Tomato plants treated with C32I bacterium, were cultivated until that produced mature fruits [four months post inoculation]. (A) Representative image of the control treatment. (B) In the treatments with each bacterium individually, a larger size is evident compared to the non-inoculated control, where the fruit exhibited a smaller size. Mock indicate inoculation with MS 0.2× medium as a control. (C) Quantification of fresh weight of tomato fruits. The graphs represent two experiments that were done [n = 20 ± SD]. Letters indicate a statistically significant difference, according to a one-way analysis of variance [ANOVA] [p ≤ 0.05] followed the Tukey test. Scale bar 1 cm
Transcriptional reprograming induced by C32I is different than other BCAs previously reported
Very few reports have analysed the transcriptomic responses of plants stimulated by the presence of PGPBs. In order to compare our results with other biostimulant agents, we compared the differentially expressed genes [DEGs] with transcriptional reports of other bacteria such as Pseudomonas fluorescens SS101, Bacillus amyloliquefaciens FZB42, Burkholderia phytofirmans PsJN (Poupin et al. 2013; Tzipilevich et al. 2021; van de Mortel et al. 2012), and compounds released by the yeast Hanseniaspora opuntia [HoFs] (Ferreira-Saab et al. 2018) (Fig. 7, Supplementary Data 4). Interestingly, we found that there is no central core of differentially expressed genes [DEGs] among all the reported agents, indicating that each strain or compound elicits a plant-specific response. However, each bacterium does show a pattern of overexpression in functional groups related to plant growth (Supplementary Data 4). For instance, SS101 exhibited genes associated with root morphogenesis, secondary metabolism, and SA signalling (van de Mortel et al. 2012). Similarly, previous studies have reported that inoculation of A. thaliana with PsJn (Poupin et al. 2013) and FZB42 (Tzipilevich et al. 2021), respectively, led to overexpression of hormone-related pathways, particularly auxins. These findings align with the results of our study, where we observed the up-regulation of genes related to hormone biosynthesis.
Comparative transcriptomic analysis between other biocontrol agents. Transcriptomic data of A. thaliana with Acinetobacter sp. C32I, were compared with Pseudomonas fluorescens SS101, Bacillus amyloliquefaciens FZB42, Burkholderia phytofirmans PsJN and Hanseniaspora opuntia [HoFs] biocontrols previously reported. Venn diagrams representing overlapping of (A) upregulated and (B) downregulated genes
Discussion
Chemical fertilizers and other agrochemicals have been used to promote plant growth, however, it has been observed that these chemicals cause harmful effects on the environment and human health, so environmentally friendly alternatives are needed (Basu et al. 2021). In nature, plants interact with diverse microorganisms such as bacteria, fungi, nematodes and viruses (Compant et al. 2010; Khare et al. 2018). With respect to bacteria, they can establish a beneficial association with plants, favoring plant growth, enhanced defense systems, and tolerance against biotic and abiotic stresses (Custodio et al. 2022; Eichmann et al. 2021; Islam et al. 2020; Jones et al. 2019; Kang et al. 2019; Vandenkoornhuyse et al. 2015; Woodhams et al. 2023). Similarly, amphibians such as frogs and salamanders are known to possess a microbial diversity in their skin, which protects them against various pathogens such as chytrid fungus B. dendrobatids a pathogen responsible for the extinction of several amphibian species (Rebollar et al. 2018; Scheele et al. 2019; Varga et al. 2019). Whilst several microorganisms isolated from soil that have been characterized as biostimulant agents, bacteria from the amphibian skin has recently gained attention as many of them have antifungal properties and could likely promote plant growth (Rebollar et al. 2020; Woodhams et al. 2015).
On the other hand, bacteria associated with both plants and amphibians can indirectly influence each other through their biotic and abiotic interactions (Berendsen et al. 2012; Rebollar and Harris 2019). It has been proposed that changes in the diversity of bacteria present in animals and plants may be influenced by variations in precipitation, thereby facilitating the exchange of microbial communities between habitats (Bernardo-Cravo et al. 2020; Ikeda-Ohtsubo et al. 2018; Van Stan II et al. 2020). Despite the evidence of bacteria-host interactions in plants or animals, and on the antifungal mechanisms exerted on pathogenic fungi, it is not known whether bacteria isolated from amphibian skin can exert beneficial effects on plant growth. To our knowledge, there is only one report in which bacteria from three different amphibian species were characterized as biostimulant plant growth modifying agents. This study included 106 bacteria, of which three were selected to study their interaction with cucumber [Cucumus sativus] plants. These three bacteria, identified as HjD57, HjD92 [isolated from Hyla japonica], and B341 [isolated from Buergeria burgeri], did not produce changes in germination or shoot production and only B341 produced changes in root growth (Susilawati et al. 2021). Our study shows that three bacterial strains isolated from tropical frog Craugastor fitzigeri (Rebollar et al. 2019) named C23F, C26G, and C32I did produce changes in the growth of the A. thaliana model plant, mainly in the roots. In particular, those belonging to the genus Acinetobacter (Cevallos et al. 2022), are of particular interest, since bacteria from this genus had previously shown to promote plant growth and development (Molina-Romero et al. 2017).
Moreover, to characterize the molecular effects underlying A. thaliana, we selected one of the previously characterized bacteria. We identified that Acinetobacter sp. C32I regulates several biological processes related to plant growth and development. Interestingly, a previously study found that tobacco plants [Nicotiana tabacum] inoculated with the bacterium Bacillus cereus, showed significant differential expressions in categories related to biological processes, mainly in plant hormone signal transduction (Li et al. 2020). Our KEGG analysis identified genes [DEGs] that are up-regulated from the auxin, cytokinin, brassinosteroid, and salicylic acid pathways. Multiple reports have implicated the direct involved of these phytohormones in the modification of plant leaf and root structure (Spaepen 2014) (Fig. 3). Additionally, these results are similar to previous studies, where microorganisms such as Pseudomonas, Agrobacterium, Rhizobium, Bradyrhizobium and Azospirillum, can regulate the biosynthesis of hormones, specifically auxins (Costacurta and Vanderleyden 1995).
Auxins are one of the most important hormones in plant life, and it is known that soil-borne bacteria produce indole-3-acetic acid [IAA], whose main function is to contribute to plant root growth (Zhao 2010). Spaepen et al. (2014) reported that the bacterium Azospirillum brasilense contributes to the increase of lateral roots and root hairs, increasing the concentration of internal auxin in the plant. Similarly, Samaras et al. (2022) reported that inoculation of the Bacillus subtilis MBI600 in cucumber [Cucumis sativus L.] altered the expression of genes involved in phytohormone production, mainly in indole-3-acetic acid-induced genes ARG7 and auxin-responsive proteins Csa_2G011420 and Csa_3G035310. Interestingly, in our study A. thaliana showed an increase in the expression of auxin hormone-related genes after inoculation with C32I, mainly those related to structural changes in the root. Similarly, Lakshmanan et al. (2013) also described that A. thaliana inoculated with Bacillus subtilis FB17 showed significant changes in auxin-responsive genes AT1G29460 and AT1G29500. Collectively, our study indicates that the amphibian skin bacteria are involved in auxin-mediated root development reprograming. Therefore, it is likely that this microorganism acts beneficially in modifying growth and plant development supporting the proof of concept that implementation of microorganisms from non-plant systems could be implemented as new ecological alternatives that act in benefit of the plant.
On the other hand, auxin can interact with other hormones like ethylene, collectively contributing to root development by inhibiting main root elongation and promoting root hair emergence (Hu et al. 2017; Weijers et al. 2018). Furthermore, soil microbial communities can influence auxin concentrations (Compant et al. 2019). Studies show that Pseudomonas sp. SP01 affects auxin distribution (Chu et al. 2020). In another study, Méndez-Gómez et al. (2021) report that indirect contact of A. thaliana DR5:GUS plants with Azospirillum brasilense reduces the GUS signaling, while direct contact increases the signal. Our study supports these findings, demonstrating that direct inoculation of Acinetobacter sp. C32I influences auxin accumulation, particularly in the root apical zone. However, further research is needed to understand auxin biogenesis and distribution during plant morphogenesis.
To look deeper into the effect that biostimulant agents have in growth and development, mutant plants like A. thaliana rdh6 [ROOT HAIR DEFECTIVE 6] have been used. Previous studies have demonstrated that A. brasilense Sp245, induces lateral roots and root hairs in rdh6 mutants (Spaepen et al. 2014). These results are like ours, in which C32I reestablished the production of root hairs compared to the control. Our study suggests that components of auxin and ethylene [signalling and transport] play a crucial role during beneficial microbe interactions. Collectively, our findings indicates that amphibian skin bacteria can induce auxin-mediated root development reprograming, thus promoting plant growth and plant development. This supports the proof of concept that implementation of microorganisms from non-plant systems could be implemented as new ecological alternatives that act in benefit of the plant.
There are several studies that have demonstrated the influence of bacteria on crop improvement and productivity, mainly in the acquisition of nutrients, fixation and solubilization of insoluble minerals, production of siderophores, regulation and production of phytohormones and improvement of the plant defense system (Hamid et al. 2021). Indeed, there are several bacteria that have been characterized as biostimulants in crops such as the Bacillus spp. (Sansinenea 2019), Pseudomonas spp. (Widnyana and Javandira 2016), Burkholderia spp. (Coutinho et al. 2015), Acinetobacter spp. (Molina-Romero et al. 2017; Mujumdar et al. 2023), and others. Additionally, it has been proposed that the mixture of different bacterial species through a consortium can enhance plant development (Molina-Romero et al. 2021). Application of bacteria from the skin of amphibians to rice and cucumber, showed a protective effect against pathogens but did not improve their growth and development (Susilawati et al. 2021). We demonstrate the effect of amphibian skin bacteria on the growth and development of the A. thaliana, along with transcriptional changes elicited in the host after bacterial inoculation.
To determine if the effect of this bacterium could be similar in an agronomical important crop. We showed that C23F, C26G and C32I improved growth of tomato plants [Solanum lycopersicum], demonstrating the potential of amphibian skin bacteria to improve plant growth and development in several plant species. Therefore, the discovery of new biostimulant agents can be of great help to replace chemical agents that affect the environment. However, we still need studies to prove the potential of these bacteria in agricultural soils and their implication as a new ecological alternative and that are harmless to human health.
Conclusion
Plant growth-promoting bacteria have the potential to be used as biostimulants for the benefit of different crops of agricultural interest. However, the potential use of bacteria from animals such as amphibians as new and efficient agricultural inoculants has been poorly explored. We demonstrate, in this study, that three bacteria isolated from the skin of frogs, two Acinetobacter sp. C26G and C32I, and one Enterobacteraceae C23F, improve plant development in A. thaliana plants mainly in the modification of root structure, production of a greater number of root hairs and an increase in biomass. Additionally, we identified that inoculation with one of these bacteria [C32I], triggers transcriptomic changes in A. thaliana, inducing the expression of genes related to plant growth hormones, specifically auxins. Finally, we identify that the inoculation of the three bacterial isolates in a plant of agricultural interest, such as tomato, produces an increase in the length of the root and stem, and an increase in the biomass of the fruits. Based on the results, our study can contribute to the identification and characterization of potential PGPBs from animals to improve the development and growth of agronomically important plants.
Data Availability
The RNA sequencing data produced in this study have been submitted to the NCBI’s Sequence Read Archive and are accessible under the following link: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA986187
References
Basu A, Prasad P, Das SN, Kalam S, Sayyed R, Reddy M, El Enshasy H (2021) Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: recent developments, constraints, and prospects. Sustainability 13(3):1140
Berendsen RL, Pieterse CMJ, Bakker PAHM (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17(8):478–486. https://doi.org/10.1016/j.tplants.2012.04.001
Bernardo-Cravo AP, Schmeller DS, Chatzinotas A, Vredenburg VT, Loyau A (2020) Environmental factors and host microbiomes shape host-pathogen dynamics. Trends Parasitol 36(7):616–633. https://doi.org/10.1016/j.pt.2020.04.010
Cano MA (2011) Interacción de microorganismos benéficos en plantas: Micorrizas, Trichoderma spp. y Pseudomonas spp. Una revisión. Revista UDCA Actualidad & Divulgación Científica 14(2):15–31
Cevallos MA, Basanta MD, Bello-López E, Escobedo-Muñoz AS, González-Serrano FM, Nemec A, Romero-Contreras YJ, Serrano M, Rebollar EA (2022) Genomic characterization of antifungal Acinetobacter bacteria isolated from the skin of the frogs Agalychnis callidryas and Craugastor fitzingeri. FEMS Microbiol Ecol 98(12):fiac126. https://doi.org/10.1093/femsec/fiac126
Chu TN, Bui LV, Hoang MTT (2020) Pseudomonas PS01 isolated from maize rhizosphere alters root system architecture and promotes plant growth. Microorganisms 8(4):471. https://www.mdpi.com/2076-2607/8/4/471
Chung Y, Choe S (2013) The regulation of brassinosteroid biosynthesis in Arabidopsis. Crit Rev Plant Sci 32(6):396–410
Compant S, Van Der Heijden MG, Sessitsch A (2010) Climate change effects on beneficial plant–microorganism interactions. FEMS Microbiol Ecol 73(2):197–214
Compant S, Samad A, Faist H, Sessitsch A (2019) A review on the plant microbiome: ecology, functions, and emerging trends in microbial application. J Adv Res 19:29–37. https://doi.org/10.1016/j.jare.2019.03.004
Costacurta A, Vanderleyden J (1995) Synthesis of phytohormones by plant-associated bacteria. Crit Rev Microbiol 21(1):1–18
Coutinho BG, Licastro D, Mendonça-Previato L, Cámara M, Venturi V (2015) Plant-influenced gene expression in the rice endophyte Burkholderia kururiensis M130. Mol Plant Microbe Interact 28(1):10–21
Custodio V, Gonin M, Stabl G, Bakhoum N, Oliveira MM, Gutjahr C, Castrillo G (2022) Sculpting the soil microbiota. Plant J 109(3):508–522
De Zelicourt A, Al-Yousif M, Hirt H (2013) Rhizosphere microbes as essential partners for plant stress tolerance. Mol Plant 6(2):242–245
de-Bashan LE, Hernandez JP, Bashan Y (2012) The potential contribution of plant growth-promoting bacteria to reduce environmental degradation – a comprehensive evaluation. Appl Soil Ecol 61:171–189. https://doi.org/10.1016/j.apsoil.2011.09.003
del Rosario Cappellari L, Chiappero J, Banchio E (2019) Invisible signals from the underground: a practical method to investigate the effect of microbial volatile organic compounds emitted by rhizobacteria on plant growth. Biochem Mol Biol Educ 47(4):388–393
Eichmann R, Richards L, Schäfer P (2021) Hormones as go-betweens in plant microbiome assembly. Plant J 105(2):518–541
Ferreira FJ, Kieber JJ (2005) Cytokinin signaling. Curr Opin Plant Biol 8(5):518–525
Ferreira CMH, Soares HMVM, Soares EV (2019) Promising bacterial genera for agricultural practices: an insight on plant growth-promoting properties and microbial safety aspects. Sci Total Environ 682:779–799. https://doi.org/10.1016/j.scitotenv.2019.04.225
Ferreira-Saab M, Formey D, Torres M, Aragón W, Padilla EA, Tromas A, Sohlenkamp C, Schwan-Estrada KRF, Serrano M (2018) Compounds released by the biocontrol yeast Hanseniaspora opuntiae protect plants against Corynespora cassiicola and Botrytis cinerea [Original Research]. Front Microbiol 9(1596):01596. https://doi.org/10.3389/fmicb.2018.01596
Hamid B, Zaman M, Farooq S, Fatima S, Sayyed R, Baba ZA, Sheikh TA, Reddy MS, El Enshasy H, Gafur A (2021) Bacterial plant biostimulants: a sustainable way towards improving growth, productivity, and health of crops. Sustainability 13(5):2856
Harris RN, Brucker RM, Walke JB, Becker MH, Schwantes CR, Flaherty DC, Lam BA, Woodhams DC, Briggs CJ, Vredenburg VT, Minbiole KPC (2009) Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. ISME J 3(7):818–824. https://doi.org/10.1038/ismej.2009.27
Hu Y, Vandenbussche F, Van Der Straeten D (2017) Regulation of seedling growth by ethylene and the ethylene–auxin crosstalk. Planta 245(3):467–489. https://doi.org/10.1007/s00425-017-2651-6
Hussain MA, Fahad S, Sharif R, Jan MF, Mujtaba M, Ali Q, Ahmad A, Ahmad H, Amin N, Ajayo BS, Sun C, Gu L, Ahmad I, Jiang Z, Hou J (2020) Multifunctional role of brassinosteroid and its analogues in plants. Plant Growth Regul 92(2):141–156. https://doi.org/10.1007/s10725-020-00647-8
Ikeda-Ohtsubo W, Brugman S, Warden CH, Rebel JMJ, Folkerts G, Pieterse CMJ (2018) How can we define “Optimal Microbiota?”: a comparative review of structure and functions of microbiota of animals, fish, and plants in agriculture [Review]. Front Nutr 5. https://doi.org/10.3389/fnut.2018.00090
Iqbal N, Khan NA, Ferrante A, Trivellini A, Francini A, Khan MIR (2017) Ethylene role in plant growth, development and senescence: interaction with other phytohormones [Review]. Front Plant Sci 8. https://doi.org/10.3389/fpls.2017.00475
Islam W, Noman A, Naveed H, Huang Z, Chen HY (2020) Role of environmental factors in shaping the soil microbiome. Environ Sci Pollut Res 27(33):41225–41247
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. Embo J 6(13):3901–3907. https://doi.org/10.1002/j.1460-2075.1987.tb02730.x
Jha CK, Aeron A, Patel BV, Maheshwari DK, Saraf M. (2011) Enterobacter: role in plant growth promotion. In Bacteria in agrobiology: plant growth responses. Springer, pp 159–182
Jiménez-Jacinto V, Sanchez-Flores A, Vega-Alvarado L (2019) Integrative differential expression analysis for multiple experiments (IDEAMEX): a web server tool for integrated RNA-seq data analysis. Front Genet 10:279
Jones P, Garcia BJ, Furches A, Tuskan GA, Jacobson D (2019) Plant host-associated mechanisms for microbial selection. Front Plant Sci 10:862
Kakimoto T (2003) Biosynthesis of cytokinins. J Plant Res 116:233–239
Kang S-M, Hamayun M, Khan MA, Iqbal A, Lee I-J (2019) Bacillus subtilis JW1 enhances plant growth and nutrient uptake of Chinese cabbage through gibberellins secretion. J Appl Bot Food Qual 92:172–178
Khare E, Mishra J, Arora NK (2018) Multifaceted interactions between endophytes and plant: developments and prospects. Front Microbiol 9:2732
Kudoyarova G, Arkhipova T, Korshunova T, Bakaeva M, Loginov O, Dodd IC (2019) Phytohormone mediation of interactions between plants and non-symbiotic growth promoting bacteria under edaphic stresses. Front Plant Sci 10:1368
Kumar R, Kumar R, Prakash O (2019) Chapter-5 the impact of chemical fertilizers on our environment and ecosystem. Chief Ed 35:69
Lakshmanan V, Castaneda R, Rudrappa T, Bais HP (2013) Root transcriptome analysis of Arabidopsis thaliana exposed to beneficial Bacillus subtilis FB17 rhizobacteria revealed genes for bacterial recruitment and plant defense independent of malate efflux. Planta 238(4):657–668. https://doi.org/10.1007/s00425-013-1920-2
Li Y, Zhao M, Chen W, Du H, Xie X, Wang D, Dai Y, Xia Q, Wang G (2020) Comparative transcriptomic analysis reveals that multiple hormone signal transduction and carbohydrate metabolic pathways are affected by Bacillus cereus in Nicotiana tabacum. Genomics 112(6):4254–4267. https://doi.org/10.1016/j.ygeno.2020.07.022
Mallick A, Dey S, Datta S, Barman M, Samui S, Dutta G (2022) Auxin and cytokinin signaling in plant stress response. In Auxins, cytokinins and gibberellins signaling in plants. Springer, pp 213–234
Maruri-López I, Aviles-Baltazar NY, Buchala A, Serrano M (2019) Intra and extracellular journey of the phytohormone salicylic acid. Front Plant Sci 10:423
Masucci JD, Schiefelbein JW (1994) The rhd6 mutation of Arabidopsis thaliana alters root-hair initiation through an auxin- and ethylene-associated process. Plant Physiol 106(4):1335–1346. https://doi.org/10.1104/pp.106.4.1335
Méndez-Gómez M, Barrera-Ortiz S, Castro-Mercado E, López-Bucio J, García-Pineda E (2021) The nature of the interaction Azospirillum-Arabidopsis determine the molecular and morphological changes in root and plant growth promotion. Protoplasma 258(1):179–189. https://doi.org/10.1007/s00709-020-01552-7
Molina-Romero D, Baez A, Quintero-Hernández V, Castañeda-Lucio M, Fuentes-Ramírez LE, Bustillos-Cristales M, Rodríguez-Andrade O, Morales-García YE, Munive Muñoz-Rojas AJ (2017) Compatible bacterial mixture, tolerant to desiccation, improves maize plant growth. PLoS ONE 12(11):e0187913. https://doi.org/10.1371/journal.pone.0187913
Molina-Romero D, Juárez-Sánchez S, Venegas B, Ortíz-González CS, Baez A, Morales-García YE, Muñoz-Rojas J (2021) A bacterial consortium Interacts with different varieties of maize, promotes the plant growth, and reduces the application of chemical fertilizer under field conditions [original research]. Front Sustain Food Syst 4:616757. https://doi.org/10.3389/fsufs.2020.616757
Morales-Cedeño LR, Orozco-Mosqueda MDC, Loeza-Lara PD, Parra-Cota FI, de los Santos-Villalobos S, Santoyo G (2021) Plant growth-promoting bacterial endophytes as biocontrol agents of pre- and post-harvest diseases: fundamentals, methods of application and future perspectives. Microbiol Res 242:126612. https://doi.org/10.1016/j.micres.2020.126612
Mujumdar S, Bhoyar J, Akkar A, Hundekar S, Agnihotri N, Jaybhay P, Bhuyan S (2023) Chapter 15 – Acinetobacter: A versatile plant growth-promoting rhizobacteria (PGPR). In: Swapnil P, Meena M, Harish A, Marwal S, Vijayalakshmi Zehra (eds) Plant-microbe interaction – recent advances in molecular and biochemical approaches. Academic Press, pp 327–362. https://doi.org/10.1016/B978-0-323-91875-6.00009-8
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Napsucialy-Mendivil S, Dubrovsky JG (2018) Genetic and Phenotypic analysis of lateral root development in Arabidopsis thaliana. In: Ristova D, Barbez E (eds) Root development: methods and protocols. Springer, New York, pp 47–75. https://doi.org/10.1007/978-1-4939-7747-5_4
Neina D (2019) The role of soil pH in plant nutrition and soil remediation. Appl Environ Soil Sci 2019:5794869. https://doi.org/10.1155/2019/5794869
O’Brien PA (2017) Biological control of plant diseases. Aust Plant Pathol 46(4):293–304. https://doi.org/10.1007/s13313-017-0481-4
O’Donnell AG, Seasman M, Macrae A, Waite I, Davies JT (2001) Plants and fertilisers as drivers of change in microbial community structure and function in soils. Plant Soil 232(1):135–145. https://doi.org/10.1023/A:1010394221729
Poupin MJ, Timmermann T, Vega A, Zuñiga A, González B (2013) Effects of the plant growth-promoting bacterium Burkholderia phytofirmans PsJN throughout the life cycle of Arabidopsis thaliana. PLoS ONE 8(7):e69435
Ramakrishna W, Yadav R, Li K (2019) Plant growth promoting bacteria in agriculture: two sides of a coin. Appl Soil Ecol 138:10–18
Rebollar EA, Gutiérrez-Preciado A, Noecker C, Eng A, Hughey MC, Medina D, Walke JB, Borenstein E, Jensen RV, Belden LK, Harris RN (2018) The skin microbiome of the neotropical frog Craugastor fitzingeri: inferring potential bacterial-host-pathogen interactions from metagenomic data. Front Microbiol 9:466. https://doi.org/10.3389/fmicb.2018.00466
Rebollar EA, Bridges T, Hughey MC, Medina D, Belden LK, Harris RN (2019) Integrating the role of antifungal bacteria into skin symbiotic communities of three Neotropical frog species. ISME J 13(7):1763–1775. https://doi.org/10.1038/s41396-019-0388-x
Rebollar EA, Martínez-Ugalde E, Orta AH (2020) The amphibian skin microbiome and its protective role against chytridiomycosis. Herpetologica 76(2):167–177
Rebollar EA, Harris RN (2019) Ecology of amphibian-microbial symbioses. Front Microbiol 10:443471. https://doi.org/10.3389/fmicb.2019.00766
Rehman F, Kalsoom M, Adnan M, Toor M, Zulfiqar A (2020) Plant growth promoting rhizobacteria and their mechanisms involved in agricultural crop production: a review. SunText Rev Biotechnol 1(2):1–6
Rouphael Y, Colla G (2020) Toward a sustainable agriculture through plant biostimulants: from experimental data to practical applications. Agronomy 10(10):1461
Samaras A, Kamou N, Tzelepis G, Karamanoli K, Menkissoglu-Spiroudi U, Karaoglanidis GS (2022) Root transcriptional and metabolic dynamics induced by the plant growth promoting Rhizobacterium (PGPR) Bacillus subtilis Mbi600 on cucumber plants. Plants 11(9):1218. https://www.mdpi.com/2223-7747/11/9/1218
Sansinenea E (2019) Bacillus spp.: as plant growth-promoting bacteria. Secondary metabolites of plant growth promoting rhizomicroorganisms 225–237
Santner A, Calderon-Villalobos LIA, Estelle M (2009) Plant hormones are versatile chemical regulators of plant growth. Nat Chem Biol 5(5):301–307. https://doi.org/10.1038/nchembio.165
Santoyo G, Orozco-Mosqueda MDC, Govindappa M (2012) Mechanisms of biocontrol and plant growth-promoting activity in soil bacterial species of Bacillus and Pseudomonas: a review. Biocontrol Sci Tech 22(8):855–872
Scheele BC, Pasmans F, Skerratt LF, Berger L, Martel A, Beukema W, Acevedo AA, Burrowes PA, Carvalho T, Catenazzi A (2019) Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 363(6434):1459–1463
Sharma A, Chetani R (2017) A review on the effect of organic and chemical fertilizers on plants. Int J Res Appl Sci Eng Technol 5:677–680
Singh I (2018) Plant Growth Promoting Rhizobacteria (PGPR) and their various mechanisms for plant growth enhancement in stressful conditions: a review. Eur J Biol Res 8(4):191–213
Spaepen S, Bossuyt S, Engelen K, Marchal K, Vanderleyden J (2014) Phenotypical and molecular responses of Arabidopsis thaliana roots as a result of inoculation with the auxin-producing bacterium Azospirillum brasilense. New Phytol 201(3):850–861
Spaepen S (2014) Plant hormones produced by microbes. In Principles of plant-microbe interactions: microbes for sustainable agriculture. Springer, pp 247–256
Suárez-Moreno ZR, Caballero-Mellado J, Coutinho BG, Mendonça-Previato L, James EK, Venturi V (2012) Common features of environmental and potentially beneficial plant-associated Burkholderia. Microbial Ecol 63(2):249–266. https://doi.org/10.1007/s00248-011-9929-1
Supek F, Bošnjak M, Škunca N, Šmuc T (2011) REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE 6(7):e21800
Susilawati L, Iwai N, Komatsu K, Arie T (2021) Antifungal activity of bacteria isolated from Japanese frog skin against plant pathogenic fungi. Biol Contr 153:104498. https://doi.org/10.1016/j.biocontrol.2020.104498
Tzipilevich E, Russ D, Dangl JL, Benfey PN (2021) Plant immune system activation is necessary for efficient root colonization by auxin-secreting beneficial bacteria. Cell Host Microbe 29(10):1507–1520.e1504
Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9(11):1963–1971
van de Mortel JE, de Vos RC, Dekkers E, Pineda A, Guillod L, Bouwmeester K, van Loon JJ, Dicke M, Raaijmakers JM (2012) Metabolic and transcriptomic changes induced in Arabidopsis by the Rhizobacterium Pseudomonas fluorescens SS101. Plant Physiol 160(4):2173–2188
Van Stan II JT, Morris CE, Aung K, Kuzyakov Y, Magyar D, Rebollar EA, Remus-Emsermann M, Uroz S, Vandenkoornhuyse P (2020) Precipitation partitioning—hydrologic highways between microbial communities of the plant microbiome? Precipitation partitioning by vegetation: a global synthesis 229–252
Vandenkoornhuyse P, Quaiser A, Duhamel M, Le Van A, Dufresne A (2015) The importance of the microbiome of the plant holobiont. New Phytolog 206(4):1196–1206. https://doi.org/10.1111/nph.13312
Varga JF, Bui-Marinos MP, Katzenback BA (2019) Frog skin innate immune defences: sensing and surviving pathogens. Front Immunol 9:3128
Vega-Celedón P, Canchignia Martínez H, González M, Seeger M (2016) Biosíntesis de ácido indol-3-acético y promoción del crecimiento de plantas por bacterias. Cultivos Tropicales 37:33–39
Weijers D, Nemhauser J, Yang Z (2018) Auxin: small molecule, big impact. J Exp Bot 69(2):133–136. https://doi.org/10.1093/jxb/erx463
Widnyana IK, Javandira C (2016) Activities Pseudomonas spp. and Bacillus sp. to stimulate germination and seedling growth of tomato plants. Agric Agric Sci Proc 9:419–423. https://doi.org/10.1016/j.aaspro.2016.02.158
Woodhams DC, Alford RA, Antwis RE, Archer H, Becker MH, Belden LK, Bell SC, Bletz M, Daskin JH, Davis LR (2015) Antifungal isolates database of amphibian skin-associated bacteria and function against emerging fungal pathogens: ecological archives E096–059. Ecology 96(2):595–595
Woodhams DC, McCartney J, Walke JB, Whetstone R (2023) The adaptive microbiome hypothesis and immune interactions in amphibian mucus. Develop Comp Immunol 145:104690. https://doi.org/10.1016/j.dci.2023.104690
Zhao Y (2010) Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol 61:49–64
Acknowledgements
We thank Martha Torres for her technical assistance and Antony Buchala for critical reading and comments on the manuscript.
Funding
This study was supported by funds from Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica [PAPIIT]-UNAM grants IN203720 and IA200921 [to M.S. and E.A.R], respectively, Y.J.R.C and F.G-S., are doctoral students from Programa de Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de México, and he received the fellowship CVU_745733 and CVU_856429, respectively from Consejo Nacional de Humanidades Ciencias y Tecnologías [CONAHCYT].
Author information
Authors and Affiliations
Contributions
YJRC, FGS, WA, MAC, EAR, and MS conceived and designed the experiments. YJRC, FGS, EBL performed the experiments. YJRC, FGS, WA, DF, EAR and MS analysed the data and wrote and revised the paper. All authors have read and agreed to the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that the research was conducted without any commercial or financial relationships that could be perceived as a potential conflict of interest.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Romero-Contreras, Y.J., González-Serrano, F., Bello-López, E. et al. Bacteria from the skin of amphibians promote growth of Arabidopsis thaliana and Solanum lycopersicum by modifying hormone-related transcriptome response. Plant Mol Biol 114, 39 (2024). https://doi.org/10.1007/s11103-024-01444-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11103-024-01444-x