Abstract
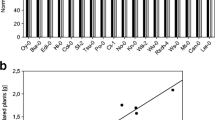
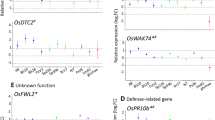
Our previous work has demonstrated that Arabidopsis thaliana can actively recruit beneficial rhizobacteria Bacillus subtilis strain FB17 (hereafter FB17) through an unknown shoot-to-root long-distance signaling pathway post a foliar bacterial pathogen attack. However, it is still not well understood which genetic targets FB17 affects in plants. Microarray analysis of A. thaliana roots treated with FB17 post 24 h of treatment showed 168 and 129 genes that were up- and down-regulated, respectively, compared with the untreated control roots. Those up-regulated include auxin-regulated genes as well as genes involved in metabolism, stress response, and plant defense. In addition, other defense-related genes, as well as cell-wall modification genes were also down-regulated with FB17 colonization. Expression patterns of 20 selected genes were analyzed by semi-quantitative RT-PCR, validating the microarray results. A. thaliana insertion mutants were used against FB17 to further study the functional response of the differentially expressed genes. Five mutants for the up-regulated genes were tested for FB17 colonization, three (at3g28360, at3g20190 and at1g21240) mutants showed decreased FB17 colonization on the roots while increased FB17 titers was seen with three mutants of the down-regulated genes (at3g27980, at4g19690 and at5g56320). Further, these mutants for up-regulated genes and down-regulated genes were foliar infected with Pseudomonas syringae pv. tomato (hereafter PstDC3000) and analyzed for Aluminum activated malate transporter (ALMT1) expression which showed that ALMT1 may be the key regulator for root FB17 colonization. Our microarray showed that under natural condition, FB17 triggers plant responses in a manner similar to known plant growth-promoting rhizobacteria and to some extent also suppresses defense-related genes expression in roots, enabling stable colonization. The possible implication of this study opens up a new dialogin terms of how beneficial microbes regulate plant genetic response for mutualistic associations.





Similar content being viewed by others
References
Ahemad M, Khan MS (2011) Functional aspects of plant growth promoting rhizobacteria: recent advancements. Insight Microbiol 1:39–54
Arkhipova TN, Veselov SU, Melentiev AI, Martynenko EV, Kudoyarova GR (2005) Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil 272:201–209
Bais HP, Fall R, Vivanco JM (2004) Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol 134:307–319
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Ann Rev Plant Biol 57:233–266
Bakker P, Ran LX, Pieterse CMJ, van Loon LC (2003) Understanding the involvement of rhizobacteria-mediated induction of systemic resistance in biocontrol of plant diseases. Canad J Plant Pathol 25:5–9
Beauregard PB, Chai Y, Vlamakis H, Losick R, Kolter R (2013) Bacillus subtilis biofilm induction by plant polysaccharides. Proc Natl Acad Sci USA 110:E1621–E1630
Bhattacharyya PN, Jha DK (2012) Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol 28:1327–1350
Brader G, Mikkelsen MD, Halkier BA, Palva ET (2006) Altering glucosinolate profiles modulates diseases resistance in plants. Plant J 46:758–767
Branda SS, Chu F, Kearns DB, Losick R, Kolter R (2006) A major protein component of the Bacillus subtilis biofilm matrix. Mol Microbiol 59:1229–1238
Brechenmacher L, Kim MY, Benitez M, Li M, Joshi T et al (2008) Transcription profiling of soybean nodulation by Bradyrhizobium japonicum. Mol Plant-Microbe Inter 21:631–645
Carpita N, McCann M (2000) The cell wall. In: Buchanan BB, Gruissem W, Jones RL (eds) Biochemistry and molecular biology of plants. American Society of Plant Physiologists, Rockville, pp 52–108
Chen S, Glawischnig E, Jorgensen K, Naur P, Jorgensen B et al (2003) CYP79F1 and CYP79F2 have distinct functions in the biosynthesis of aliphatic glucosinolates in Arabidopsis. Plant J 33:923–937
Chen Y, Cao S, Chai Y, Clardy J, Kolter R et al (2012) A Bacillus subtilis sensor kinase involved in triggering biofilm formation on the roots of tomato plants. Mol Microbiol 85:418–430
Chen Y, Yan F, Chai Y, Liu H, Kolter R et al (2013) Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environ Microbiol 15:848–864
Choudhary D, Johri BN (2009) Interactionsof Bacillus spp. and plants—with special reference to induced systemic resistance (ISR). Microbiol Res 164:493–513
Conrath U, Pieterse CM, Mauch-Mani B (2002) Priming in plant pathogen interactions. Trends Plant Sci 7:210–216
Dodd C, Zinovkina NY, Safronova VI, Belimov AA (2010) Rhizobacterial mediation of plant hormone status. Ann Appl Biol 157:361–379
El Yahyaoui F, Kuster H, Ben Amor B, Hohnjec N, Puhler A et al (2004) Expression profiling in Medicago truncatula identifies more than 750 genes differentially expressed during nodulation, including many potential regulators of the symbiotic program. Plant Physiol 136:3159–3176
Fall R, Kinsinger RF, Wheeler KA (2004) A simple method to isolate biofilm-forming Bacillus subtilis and related species from plant roots. Syst Appl Microbiol 27:372–379
Hayat R, Ali S, Amara U, Khalid R, Ahmed I (2010) Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol 60:579–598
Hein JW, Wolfe GV, Blee KA (2008) Comparison of rhizosphere bacterial communities in Arabidopsis thaliana mutants for systemic acquired resistance. Microbial Ecol 55:333–343
Hontzeas N, Saleh SS, Glick BR (2004) Changes in gene expression in canola roots induced by ACC-deaminase-containing plant-growth-promoting bacteria. Mol Plant-Microbe Interact 17:865–871
Hückelhoven R (2007) Cell wall associated mechanisms of disease resistance and susceptibility. Ann Rev Phytopathol 45:101–127
Kleijn RJ, Buescher JM, Le Chat L, Jules M, Aymerich S et al (2010) Metabolic fluxes during strong carbon catabolite repression by malate in Bacillus subtilis. J Biol Chem 285:1587–1596
Kloepper JW, Ryu CM, Zhang S (2004) Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathol 94:1259–1266
Kobayashi Y, Hoekenga OA, Itoh H, Nakashima M, Saito S et al (2007) Characterization of AtALMT1 expression in aluminum-inducible malate release and its role for rhizotoxic stress tolerance in Arabidopsis. Plant Physiol 145:843–852
Kristensen C, Morant M, Olsen CE, Ekstrøm CT, Galbraith DW et al (2005) Metabolic engineering of dhurrin in transgenic Arabidopsis plants with marginal inadvertent effects on the metabolome and transcriptome. Proc Natl Acad Sci USA 102:1779–1784
Lakshmanan V, Kitto SL, Caplan JL, Hsueh Y-H, Kearns DB et al (2012) Microbe-Associated Molecular Patterns (MAMPs)-triggered root responses mediate beneficial rhizobacterial recruitment in Arabidopsis. Plant Physiol 160:1642–1662
Lin W, Okon Y, Hardy RWF (1983) Enhanced mineral uptake by Zea mays and Sorghum bicolor roots inoculated with Azospirillum brasilense. Appl Environ Microbiol 45:1775–1779
Lugtenberg B, Kamilova F (2009) Plant growth-promoting rhizobacteria. Ann Rev Microbiol 63:541–556
Maleck K, Levine A, Eulgem T, Morgan A, Schmid J et al (2000) The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nat Genet 26:403–410
Marschner P, Neumann G, Kania A, Weisskopf L, Lieberei R (2002) Spatial and temporal dynamics of the microbial community structure in the rhizosphere of cluster roots of white lupin (Lupinus albus L.). Plant Soil 246:167–174
Millet YA, Danna CH, Clay NK, Songnuan W, Simon MD et al (2010) Innate immune responses activated in Arabidopsis roots by microbe-associated molecular patterns. Plant Cell 22:973–990
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–479
Neumann G, Martinoia E (2002) Cluster roots—an underground adaptation for survival in extreme environments. Trends Plant Sci 7:162–167
Niu DD, Liu HX, Jiang CH, Wang YP, Wang QY et al (2011) The plant growth-promoting rhizobacterium Bacillus cereus AR156 induces systemic resistance in Arabidopsis thaliana by simultaneously activating salicylate- and Jasmonate/Ethylene-dependent signaling pathways. Mol Plant-Microbe Interact 24:533–542
Ongena M, Thonart P (2006) Resistance induced in plants by nonpathogenic microorganisms: Elicitation and defense responses. In: Jaime A, Da Silva T (eds) Floriculture, ornamental and plant biotechnology: advances and topical issues, 1st edn. Global Science Books, London, pp 447–463
Pérez-García A, Romero D, de Vicente A (2011) Plant protection and growth stimulation by microorganisms: biotechnological applications of Bacilli in agriculture. Curr Opin Biotechnol 22:187–193
Pineda A, Zhengg S-J, van Loon JJA, Pieterse CMJ, Dicke M (2010) Helping plants to deal with insects: the role of beneficial soil-borne microbes. Trends Plant Sci 15:507–514
Priest F (1993) Systematics and ecology of Bacillus. In: Hoch JA, Losick R (eds) Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology Press, Washington, pp 3–16
Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, Moënne-Loccoz Y (2008) The rhizosphere: a playground and battle field for soil borne pathogens and beneficial microorganisms. Plant Soil 321:341–361
Rudrappa T, Czymmek KJ, Paré PW, Bais HP (2008) Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiol 148:1547–1556
Rudrappa T, Biedrzycki ML, Kunjeti SG, Donofrio NM, Czymmek KJ et al (2010) The rhizobacterial elicitor acetoin induces systemic resistance in Arabidopsis thaliana. Commun Integr Biol 3:130–138
Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T et al (2000) Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc Natl Acad Sci USA 97:11655–11660
Schwachtje J, Karojet S, Thormählen I, Bernholz C, Kunz S et al (2011) A naturally associated rhizobacterium of Arabidopsis thaliana induces a starvation-like transcriptional response while promoting growth. PLoS ONE 6:e29382
Trosvik P, Rudi K, Naes T, Kohler A, Chan K-S et al (2008) Characterizing mixed microbial population dynamics using time-series analysis. ISME J 2:707–715
van Loon LC, Bakker PAHM (2005) Induced systemic resistance as a mechanism of disease suppression by rhizobacteria. In: Siddiqui ZA (ed) PGPR: biocontrol and biofertilization. Springer, Dordrecht, pp 39–66
Verhagen BW, Glazebrook J, Zhu T, Chang HS, van Loon LC et al (2004) The transcriptome of rhizobacteria-induced systemic resistance in Arabidopsis. Mol Plant-Microbe Interact 17:895–908
Wang YQ, Ohara Y, Nakayashiki H, Tosa Y, Mayama S (2005) Microarray analysis of the gene expression profile induced by the endophytic plant growth-promoting rhizobacteria, Pseudomonas fluorescens FPT9601-T5 in Arabidopsis. Mol Plant-Microbe Interact 18:385–396
Welbaum G, Sturz AV, Dong Z, Nowak J (2004) Fertilizing soil microorganisms to improve productivity of agroecosystems. Crit Rev Plant Sci 23:175–193
Zamioudis C, Pieterse CMJ (2012) Modulation of host immunity by beneficial microbes. Mol Plant Microbe Interact 25:139–150
Zhang H, Kim MS, Krishnamachari V, Payton P, Sun Y et al (2007) Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta 226:839–851
Zhang M, Murzello C, Sun Y, Kim M-S, Xie X et al (2010) Choline and osmotic-stress tolerance induced in Arabidopsis by the soil microbe Bacillus subtilis (GB03). Mol Plant-Microbe Interact 23:1097–1104
Acknowledgments
All the authors are thankful to Sandra M. Mathioni, Plant and Soil Science Department, University of Delaware for helping in data analysis. H.P.B. acknowledges the support from University of Delaware Research Foundation (UDRF) and NSF Award IOS-0814477. We also thank both anonymous reviewers for insightful comments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lakshmanan, V., Castaneda, R., Rudrappa, T. et al. Root transcriptome analysis of Arabidopsis thaliana exposed to beneficial Bacillus subtilis FB17 rhizobacteria revealed genes for bacterial recruitment and plant defense independent of malate efflux. Planta 238, 657–668 (2013). https://doi.org/10.1007/s00425-013-1920-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-013-1920-2