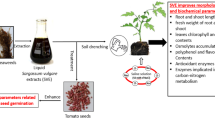
Abstract
Eggplant (Solanum melangena L.) seeds were treated with low pressure dielectric barrier air discharge plasma for the duration of \(2\), \(4\), \(6\) and 8 min in order to determine the condition of maximum seed germination rate with the plasma system used. Among the treatment conditions considered herein, 6 min treatment period provided the maximum seed germination rate of \(\sim 80\%\), where as it was \(\sim 46\%\) for control. Further, PAWs were foliar sprayed to the plants grown from the control and \(2\), \(4\), \(6\) and 8 min treated seeds. Growth parameters along with the concentrations of antioxidant activities, total phenolic content (TPC), total soluble sugar (TSS) and protein (TSP) and mineral contents were determined. The results reveal that the plant growth parameters, antioxidant activities, TPC, TSS, TSP, and mineral concentrations of \(\mathrm{Ca}\), \(\mathrm{Cu}\), \(\mathrm{Fe}\), \(\mathrm{Mn}\), and \(\mathrm{K}\) are increased, \(\mathrm{Zn}\) is only reduced with respect to control. The results obtained in this experiment may provide a way for sustainable agriculture for eggplants instead of vulnerable traditional one.








Similar content being viewed by others
References
U.S. Agricultural Research Service (2019) Eggplants. In: U.S. Department of Agriculture
Roy NC, Hasan MM, Talukder MR et al (2018) Prospective applications of low frequency glow discharge plasmas on enhanced germination, growth and yield of wheat. Plasma Chem Plasma Process 38:13–28. https://doi.org/10.1007/s11090-017-9855-1
Aktar A, Sarmin S, Irin UA et al (2021) Plasma activated water: implication as fungicide, growth and yield stimulator of potato (Solanum Tuberosum L). Plasma Med 11(1):1
Billah M, Sajib SA, Roy NC et al (2020) Effects of DBD air plasma treatment on the enhancement of black gram (Vigna mungo L.) seed germination and growth. Arch Biochem Biophys 681:1. https://doi.org/10.1016/j.abb.2020.108253
Vaka MR, Sone I, Álvarez RG et al (2019) Towards the next-generation disinfectant: Composition, storability and preservation potential of plasma activated water on baby spinach leaves. Foods. https://doi.org/10.3390/foods8120692
Chen C, Liu C, Jiang A et al (2019) The effects of cold plasma-activated water treatment on the microbial growth and antioxidant properties of fresh-cut pears. Food Bioproc Tech 12:1842–1851. https://doi.org/10.1007/s11947-019-02331-w
Ganesh GS et al (2021) Plasma-activated water from DBD as a source of nitrogen for agriculture: Specific energy and stability studies. J Appl Phys. https://doi.org/10.1063/5.0039253
Sarangapani C, Scally L, Gulan M, Cullen PJ (1947) Dissipation of pesticide residues on grapes and strawberries using plasma-activated water. Food Bioprocess Technol 13(10):1728–1741. https://doi.org/10.1007/s11947-020-02515-9/Published
Zhou R, Li J, Zhou R et al (2019) Atmospheric-pressure plasma treated water for seed germination and seedling growth of mung bean and its sterilization effect on mung bean sprouts. Innov Food Sci Emerg Technol 53:36–44. https://doi.org/10.1016/j.ifset.2018.08.006
Škarpa P, Klofáč D, Krčma F et al (2020) Effect of plasma activated water foliar application on selected growth parameters of maize (Zea mays l). Water (Switzerland) 12:1. https://doi.org/10.3390/w12123545
Shaer M el, Welily H el, Zaki A, et al (2020) Germination of wheat seeds exposed to cold atmospheric plasma in dry and wet plasma-activated water and mist
Bradu C, Kutasi K, Magureanu M et al (2020) Reactive nitrogen species in plasma-activated water: Generation, chemistry and application in agriculture. J Phys D Appl Phys 53:223001
Adhikari B, Adhikari M, Ghimire B et al (2019) Cold atmospheric plasma-activated water irrigation induces defense hormone and gene expression in tomato seedlings. Sci Rep. https://doi.org/10.1038/s41598-019-52646-z
Stoleru V, Burlica R, Mihalache G et al (2020) Plant growth promotion effect of plasma activated water on Lactuca sativa L. cultivated in two different volumes of substrate. Sci Rep 10:1. https://doi.org/10.1038/s41598-020-77355-w
Kučerová K, Henselová M, Slováková Ľ et al (2021) Effect of plasma activated water, hydrogen peroxide, and nitrates on lettuce growth and its physiological parameters. Appl Sci (Switzerland) 11:1–14. https://doi.org/10.3390/app11051985
Takaki K, Takahata J, Watanabe S, et al (2013) Improvements in plant growth rate using underwater discharge. In: Journal of physics: conference series. Institute of Physics Publishing
Rashid MM, Rashid M, Hasan MM, Talukder MR (2021) Rice plant growth and yield: foliar application of plasma activated water. Plasma Sci Technol. https://doi.org/10.1088/2058-6272/abf549
Shapira Y, Bormashenko E, Drori E (2019) Pre-germination plasma treatment of seeds does not alter cotyledon DNA structure, nor phenotype and phenology of tomato and pepper plants. Biochem Biophys Res Commun 519:512–517. https://doi.org/10.1016/j.bbrc.2019.09.034
Hossain MF, Sohan MSR, Hasan M et al (2022) Enhancement of seed germination rate and growth of Maize (Zea mays L.) through LPDBD Ar/Air plasma. J Soil Sci Plant Nutr 1:1. https://doi.org/10.1007/s42729-022-00771-6
Sajib SA, Billah M, Mahmud S et al (2020) Plasma activated water: The next generation eco-friendly stimulant for enhancing plant seed germination, vigor and increased enzyme activity, a study on black gram (Vigna mungo L.). Plasma Chem Plasma Process 40:119–143. https://doi.org/10.1007/s11090-019-10028-3
Mošovská S, Medvecká V, Halászová N et al (2018) Cold atmospheric pressure ambient air plasma inhibition of pathogenic bacteria on the surface of black pepper. Food Res Int 106:862–869. https://doi.org/10.1016/j.foodres.2018.01.066
Świecimska M, Tulik M, Šerá B et al (2020) Non-thermal plasma can be used in disinfection of scots pine (Pinus sylvestris L.) seeds infected with fusarium oxysporum. Forests 11:1. https://doi.org/10.3390/f11080837
Mitra A, Li YF, Klämpfl TG et al (2014) Inactivation of surface-borne microorganisms and increased germination of seed specimen by cold atmospheric plasma. Food Bioproc Tech 7:645–653. https://doi.org/10.1007/s11947-013-1126-4
Gao X, Zhang A, Héroux P et al (2019) Effect of dielectric barrier discharge cold plasma on pea seed growth. J Agric Food Chem 67:10813–10822. https://doi.org/10.1021/acs.jafc.9b03099
Sohan MSR, Hasan M, Hossain MF et al (2021) Improvement of seed germination rate, agronomic traits, enzymatic activity and nutritional composition of bread wheat (Triticum aestivum) using low-frequency glow discharge plasma. Plasma Chem Plasma Process 41:923–944. https://doi.org/10.1007/s11090-021-10158-7
Karmakar S, Billah M, Hasan M et al (2021) Impact of LFGD (Ar+O2) plasma on seed surface, germination, plant growth, productivity and nutritional composition of maize (Zea mays L.). Heliyon 7:e06458. https://doi.org/10.1016/j.heliyon.2021.e06458
Sivachandiran L, Khacef A (2017) Enhanced seed germination and plant growth by atmospheric pressure cold air plasma: combined effect of seed and water treatment. RSC Adv 7:1822–1832. https://doi.org/10.1039/c6ra24762h
Rashid M, Rashid MM, Reza MA, Talukder MR (2021) Combined effects of air plasma seed treatment and foliar application of plasma activated water on enhanced paddy plant growth and yield. Plasma Chem Plasma Process 41:1081–1099. https://doi.org/10.1007/s11090-021-10179-2
Liao X, Bai Y, Muhammad AI et al (2020) The application of plasma-Activated water combined with mild heat for the decontamination of Bacillus cereus spores in rice (Oryza sativa L ssp japonica). J Phys D Appl Phys 53:064003. https://doi.org/10.1088/1361-6463/ab573a
Takahata J, Takaki K, Satta N et al (2015) Improvement of growth rate of plants by bubble discharge in water. Japan Soc Appl Phys 54:1
Maniruzzaman M, Sinclair AJ, Cahill DM et al (2017) Nitrate and hydrogen peroxide generated in water by electrical discharges stimulate wheat seedling growth. Plasma Chem Plasma Process 37:1393–1404. https://doi.org/10.1007/s11090-017-9827-5
Kučerová K, Henselová M, Slováková Ľ, Hensel K (2019) Effects of plasma activated water on wheat: Germination, growth parameters, photosynthetic pigments, soluble protein content, and antioxidant enzymes activity. Plasma Processes Polym. https://doi.org/10.1002/ppap.201800131
del Río LA, Sandalio LM, Corpas FJ et al (2006) Reactive oxygen species and reactive nitrogen species in peroxisomes. Production, scavenging, and role in cell signaling. Plant Physiol 141:330–335
Sies H (2014) Role of metabolic H2O2 generation: redox signaling and oxidative stress. J Biol Chem 289:8735–8741
Rio LA, Puppo A (2009) Reactive oxygen species in plant signaling. Springer, Berlin
Foyer CH, Noctor G (2005) Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 28:1056
Im YH, Xiong Z, Elg DT, Graves DB (2019) Uptake and diffusion of plasma-generated reactive nitrogen species through keratinized membrane. J Phys D Appl Phys. https://doi.org/10.1088/1361-6463/ab0867
Duermeyer L, Khodapanahi E, Yan D et al (2018) Regulation of seed dormancy and germination by nitrate. Seed Sci Res 28:150–157. https://doi.org/10.1017/S096025851800020X
Roy NC, Hasan MM, Kabir AH et al (2018) Atmospheric pressure gliding arc discharge plasma treatments for improving germination, growth and yield of wheat. Plasma Sci Technol. https://doi.org/10.1088/2058-6272/aac647
Rashid MM, Chowdhury M, Talukder MR (2020) Textile wastewater treatment by underwater parallel-multi-tube air discharge plasma jet. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2020.104504
Lukes P, Dolezalova E, Sisrova I, Clupek M (2014) Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O 2 and HNO2. Plasma Sources Sci Technol. https://doi.org/10.1088/0963-0252/23/1/015019
ISTA (2018) International Rules for Seed Testing. International Seed Testing Association, Bassersdorf
Hara Y (1999) Calculation of population parameters using richards function and application of indices of growth and seed vigor to rice plants. Plant Prod Sci 2:129–135. https://doi.org/10.1626/pps.2.129
Su S, Zhou Y, Qin JG et al (2010) Optimization of the method for chlorophyll extraction in aquatic plants. J Freshw Ecol 25:531–538. https://doi.org/10.1080/02705060.2010.9664402
Wellburn AR (1994) The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents With Spectrophotometers of Different Resolution. J Plant Physiol 144:307–313
Giannopolitis CN, Ries SK (1977) Superoxide Dismutases
Sun M, Zigman S (1978) An improved spectrophotometric assay for superoxide dismutase based on epinephrine autoxidationl
Almeselmani M, Deshmukh PS, Sairam RK et al (2006) Protective role of antioxidant enzymes under high temperature stress. Plant Sci 171:382–388. https://doi.org/10.1016/j.plantsci.2006.04.009
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc 2:875–877. https://doi.org/10.1038/nprot.2007.102
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding
Zheng Y, Jia A, Ning T et al (2008) Potassium nitrate application alleviates sodium chloride stress in winter wheat cultivars differing in salt tolerance. J Plant Physiol 165:1455–1465. https://doi.org/10.1016/j.jplph.2008.01.001
Yusupov M, Razzokov J, Cordeiro RM, Bogaerts A (2019) Transport of reactive oxygen and nitrogen species across aquaporin: A molecular level picture. Oxid Med Cell Longev. https://doi.org/10.1155/2019/2930504
Ren B, Wang M, Chen Y et al (2015) Water absorption is affected by the nitrogen supply to rice plants. Plant Soil 396:397–410
Soriano D, Orozco-Segovia A, Marquez-Guzman J et al (2011) Seed reserve composition in 19 tree species of a tropical deciduous forest in Mexico and its relationship to seed germination and seedling growth. Ann Bot 107:939–951
Huang H, Ullah F, Zhou DX et al (2019) Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci 10:800
Zargarchi S, Saremnezhad S (2019) Gamma-aminobutyric acid, phenolics and antioxidant capacity of germinated indica paddy rice as affected by low-pressure plasma treatment. LWT 102:291–294. https://doi.org/10.1016/j.lwt.2018.12.014
Thounaojam TC, Panda P, Mazumdar P et al (2012) Excess copper induced oxidative stress and response of antioxidants in rice. Plant Physiol Biochem 53:33–39. https://doi.org/10.1016/j.plaphy.2012.01.006
Tanase C, Bujor O-C, Popa VI (2019) Phenolic natural compounds and their influence on physiological processes in plants. Elsevier, Amsterdam, pp 45–58
Li J, Zhu Z, Gerendás J (2008) Effects of nitrogen and sulfur on total phenolics and antioxidant activity in two genotypes of leaf mustard. J Plant Nutr 31:1642–1655. https://doi.org/10.1080/01904160802244860
Rossato L, Laine P, Ourry A (2001) Nitrogen storage and remobilization in Brassica napus L. during the growth cycle: nitrogen fluxes within the plant and changes in soluble protein patterns. J Exp Bot 52:1655–1663
Marschner P (2012) Mineral nutrition of higher plants, 3rd edn. Elsevier
Sugimoto T, Watanabe K, Yoshida S et al (2010) Field application of calcium to reduce phytophthora stem rot of soybean, and calcium distribution in plants. Plant Dis 94:812–819. https://doi.org/10.1094/PDIS-94-7-0812
Sánchez E, García-Bañuelos ML, Sida-Arreola JP (2012) Biofortification - promising approach to increasing the content of iron and zinc in staple food crops. J Elemntol. https://doi.org/10.5601/jelem.2014.19.3.708
Malhi SS, Nyborg M, Harapiak JT (1998) Effects of long-term N fertilizer-induced acidi®cation and liming on micronutrients in soil and in bromegrass hay. Soil Tillage Res 48:91–101
Izydorczyk G, Sienkiewicz-Cholewa U, Baśladyńska S et al (2020) New environmentally friendly bio-based micronutrient fertilizer by biosorption: from laboratory studies to the field. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2019.136061
Alejandro S, Höller S, Meier B, Peiter E (2020) Manganese in plants: from acquisition to subcellular allocation. Front Plant Sci 11:300
Acknowledgements
M.R. Talukder would like to thank to the honorable Vice Chancellor, University of Rajshahi, for partial financial support through allocation of special research grant (A-701/6/109 (research), 2022) to complete this research work. Mr. Talukder also thanks to Mizanur Rahman, Lab Technician, Plasma Science and Technology Lab. University of Rajshahi due to share his time in lab and research field.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rashid, M., Rashid, M.M., Alam, M.S. et al. Enhancement of Growth, Enzymes, Nutrition and Yield of Eggplant: Combined Effects of Plasma Treatments. Plasma Chem Plasma Process 43, 163–181 (2023). https://doi.org/10.1007/s11090-022-10301-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-022-10301-y