Abstract
Background and aims
Plant growth and photosynthetic ability have been frequently demonstrated to increase with nitrogen (N) supply. N can also promote changes in root water absorption and shoot water status via mechanisms that remain poorly understood. This study aims at investigating the effects of N supply on water absorption.
Methods
A hydroponic experiment with two independent rice varieties (cv. ‘Shangyou63’ hybrid indica and cv. ‘Yangdao6’ conventional indica, China) supplied by three distinct N levels was performed in a greenhouse. Physiological characteristics were analyzed after a few weeks.
Results
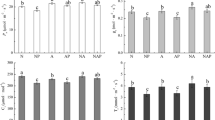
Compared to low N supply (20 mg·L−1), exposure to high N supply (100 mg·L−1) increased the light-saturated photosynthetic rate (A) and water use efficiency (WUE) by 17 % and 22 %, respectively, in Shanyou63 and by 43 % and 26 %, respectively, in Yangdao6. The leaf water potential was significantly decreased in Shanyou63 but not in Yangdao6. There were increases in the rate of water uptake and the root hydraulic conductance (L r ) under high N supply in both rice cultivars; these changes were accompanied by increased transcription levels of aquaporins (AQPs), decreased aerenchyma formation and root porosity, and decreased root lignin content. Under high N supply, Yangdao6 also exhibited much higher AQP activity, lower aerenchyma and root porosity compared with those of Shanyou63, indicating that Yangdao6 had an increased ability to absorb water compared with that of Shanyou63.
Conclusions
The enhanced expression of AQPs and decreased root aerenchyma and lignin contributed to increased water absorption ability under high N supply. In addition, the responses of each of the two rice cultivars (hybrid and conventional) to N supply is related to their water uptake ability, resulting from root porosity and an increase in AQP activity.








Similar content being viewed by others
References
Augé R, Stodola A (1990) An apparent increase in symplastic water contributes to greater turgor in mycorrhizal roots of droughted rosa plants. HortSci 25:1096–1096
Barrowclough DE, Peterson CA, Steudle E (2000) Radial hydraulic conductivity along developing onion roots. J Exp Bot 51:547–557
Beaudette PC, Chlup M, Yee J, Emery RJN (2007) Relationships of root conductivity and aquaporin gene expression in Pisum sativum: diurnal patterns and the response to HgCl2 and ABA. J Exp Bot 58:1291–1300
Bennett JM, Mutti LSM, Rao PSC, Jones JW (1989) Interactive effects of nitrogen and water stresses on biomass accumulation, nitrogen uptake, and seed yield of maize. Field Crop Res 19:297–311
Bouranis DL, Chorianopoulou SN, Siyiannis VF, Protonotarios VE, Hawkesford MJ (2003) Aerenchyma formation in roots of maize during sulphate starvation. Planta 217:382–391
Bramley H, Tyerman SD (2010) Root water transport under waterlogged conditions and the roles of aquaporins. In: Mancuso S, Shabala S (eds) Waterlogging signalling and tolerance in plants. Springer, Berlin, pp 151–180
Bramley H, Turner NC, Turner DW, Tyerman SD (2009) Roles of morphology, anatomy, and aquaporins in determining contrasting hydraulic behavior of roots. Plant Physiol 150:348–364
Bramley H, Turner NC, Turner DW, Tyerman SD (2010) The contrasting influence of short-term hypoxia on the hydraulic properties of cells and roots of wheat and lupin. Funct Plant Biol 37:183–193
Briones AM Jr, Okabe S, Umemiya Y, Ramsing NB, Reichardt W, Okuyama H (2003) Ammonia-oxidizing bacteria on root biofilms and their possible contribution to N use efficiency of different rice cultivars. Plant Soil 250:335–348
Carvajal M, Cooke D, Clarkson D (1996) Responses of wheat plants to nutrient deprivation may involve the regulation of water-channel function. Planta 199:372–381
Clarkson DT, Carvajal M, Henzler T, Waterhouse RN, Smyth AJ, Cooke DT, Steudle E (2000) Root hydraulic conductance: diurnal aquaporin expression and the effects of nutrient stress. J Exp Bot 51:61–70
Colmer T (2003) Long‐distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ 26:17–36
Cui M, Nobel PS (1992) Nutrient status, water uptake and gas exchange for three desert succulents infected with mycorrhizal fungi. New Phytol 122:643–649
Dordas CA, Sioulas C (2008) Safflower yield, chlorophyll content, photosynthesis, and water use efficiency response to nitrogen fertilization under rainfed conditions. Ind Crop Prod 27:75–85
Drew MC, He CJ, Morgan PW (1989) Decreased ethylene biosynthesis, and induction of aerenchyma, by nitrogen- or phosphate-starvation in adventitious roots of Zea mays L. Plant Physiol 91:266–271
Else MA, Coupland D, Dutton L, Jackson MB (2001) Decreased root hydraulic conductivity reduces leaf water potential, initiates stomatal closure and slows leaf expansion in flooded plants of castor oil (Ricinus communis) despite diminished delivery of ABA from the roots to shoots in xylem sap. Physiol Plantarum 111:46–54
Entry JA, Runion GB, Prior SA, Mitchell RJ, Rogers HH (1998) Influence of CO2 enrichment and nitrogen fertilization on tissue chemistry and carbon allocation in longleaf pine seedlings. In: Box J (ed) Root demographics and their efficiencies in sustainable agriculture, grasslands and forest ecosystems. Springer, Netherlands, pp 3–18
Evans JR (1983) Nitrogen and photosynthesis in the flag leaf of wheat (Triticum aestivum L.). Plant Physiol 72:297–302
Fan M, Zhu J, Richards C, Brown KM, Lynch JP (2003) Physiological roles for aerenchyma in phosphorus-stressed roots. Funct Plant Biol 30:493–506
Fan M, Bai R, Zhao X, Zhang J (2007) Aerenchyma formed under phosphorus deficiency contributes to the reduced root hydraulic conductivity in maize roots. J Integr Plant Biol 49:598–604
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Ann Rev Plant Physiol 33:317–345
Flexas J, Ribas-Carbó M, Diaz-Espejo A, Galmes J, Medrano H (2008) Mesophyll conductance to CO2: current knowledge and future prospects. Plant Cell Environ 31:602–621
Fritz C, Palacios-Rojas N, Feil R, Stitt M (2006) Regulation of secondary metabolism by the carbon-nitrogen status in tobacco: nitrate inhibits large sectors of phenylpropanoid metabolism. Plant J 46:533–548
Gloser V, Zwieniecki MA, Orians CM, Holbrook NM (2007) Dynamic changes in root hydraulic properties in response to nitrate availability. J Exp Bot 58:2409–2415
Gorska A, Ye Q, Holbrook NM, Zwieniecki MA (2008a) Nitrate control of root hydraulic properties in plants: translating local information to whole plant response. Plant Physiol 148:1159–1167
Gorska A, Zwieniecka A, Holbrook NM, Zwieniecki MA (2008b) Nitrate induction of root hydraulic conductivity in maize is not correlated with aquaporin expression. Planta 228:989–998
Green T, Mitchell R (1992) Effects of nitrogen on the response of loblolly pine to water stress I. Photosynthesis and stomatal conductance. New Phytol 122:627–633
Guehl J, Fort C, Ferhi A (1995) Differential response of leaf conductance, carbon isotope discrimination and water-use efficiency to nitrogen deficiency in maritime pine and pedunculate oak plants. New Phytol 131:149–157
Guo S, Brück H, Sattelmacher B (2002) Effects of supplied nitrogen form on growth and water uptake of French bean (Phaseolus vulgaris L.) plants. Plant Soil 239:267–275
Hacke UG, Plavcová L, Almeida-Rodriguez A, King-Jones S, Zhou W, Cooke JEK (2010) Influence of nitrogen fertilization on xylem traits and aquaporin expression in stems of hybrid poplar. Tree Physiol 30:1016–1025
Han Y, Wang Y, Jiang H, Wang M, Korpelainen H, Li C (2013) Reciprocal grafting separates the roles of the root and shoot in sex-related drought responses in Populus cathayana males and females. Plant Cell Environ 36:356–364
Hatfield R, Fukushima RS (2005) Can lignin be accurately measured? This paper was originally presented at the Lignin and Forage Digestibility Symposium, 2003 CSSA Annual Meeting, Denver, CO. Crop Sci 45:832–839
Hobbie EA, Colpaert JV (2004) Nitrogen availability and mycorrhizal colonization influence water use efficiency and carbon isotope patterns in Pinus sylvestris. New Phytol 164:515–525
Hose E, Clarkson DT, Steudle E, Schreiber L, Hartung W (2001) The exodermis: a variable apoplastic barrier. J Exp Bot 52:2245–2264
Hubbard R, Ryan M, Stiller V, Sperry J (2001) Stomatal conductance and photosynthesis vary linearly with plant hydraulic conductance in ponderosa pine. Plant Cell Environ 24:113–121
Iiyama K, Wallis AFA (1990) Determination of lignin in herbaceous plants by an improved acetyl bromide procedure. J Sci Food Agr 51:145–161
Ishikawa-Sakurai J, Hayashi H, Murai-Hatano M (2014) Nitrogen availability affects hydraulic conductivity of rice roots, possibly through changes in aquaporin gene expression. Plant Soil 379:289–300
Javot H, Maurel C (2002) The role of aquaporins in root water uptake. Ann Bot 90:301–313
Kaldenhoff R, Fischer M (2006) Functional aquaporin diversity in plants. BBA Biomembranes 1758:1134–1141
Kirk GJD, Kronzucker HJ (2005) The potential for nitrification and nitrate uptake in the rhizosphere of wetland plants: a modelling study. Ann Bot 96:639–646
Lajtha K, Whitford W (1989) The effect of water and nitrogen amendments on photosynthesis, leaf demography, and resource-use efficiency in Larrea tridentata, a desert evergreen shrub. Oecologia 80:341–348
Lawlor DW (2002) Carbon and nitrogen assimilation in relation to yield: mechanisms are the key to understanding production systems. J Exp Bot 53:773–787
Li Y, Gao Y, Xu X, Shen Q, Guo S (2009) Light-saturated photosynthetic rate in high-nitrogen rice (Oryza sativa L.) leaves is related to chloroplastic CO2 concentration. J Exp Bot 60:2351–2360
Li Y, Ren B, Ding L, Shen Q, Peng S, Guo S (2013) Does chloroplast size influence photosynthetic nitrogen use efficiency? PloS one 8(4):e62036. doi:10.1371/journal.pone.0062036
Linkohr BI, Williamson LC, Fitter AH, Leyser H (2002) Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. Plant J 29:751–760
Livingston N, Guy R, Sun Z, Ethier G (1999) The effects of nitrogen stress on the stable carbon isotope composition, productivity and water use efficiency of white spruce (Picea glauca (Moench) Voss) seedlings. Plant Cell Environ 22:281–289
Long SP, Bernacchi CJ (2003) Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J Exp Bot 54:2393–2401
Long SP, Farage PK, Garcia RL (1996) Measurement of leaf and canopy photosynthetic CO2 exchange in the field. J Exp Bot 47:1629–1642
Lu Z, Neumann PM (1999) Water stress inhibits hydraulic conductance and leaf growth in rice seedlings but not the transport of water via mercury-sensitive water channels in the root. Plant Physiol 120:143–152
Lux A, Morita S, Abe J, Ito K (2005) An improved method for clearing and staining free-hand sections and whole-mount samples. Ann Bot 96:989–996
Maggio A, Joly RJ (1995) Effects of mercuric chloride on the hydraulic conductivity of tomato root systems (evidence for a channel-mediated water pathway). Plant Physiol 109:331–335
Martre P, North GB, Nobel PS (2001) Hydraulic conductance and mercury-sensitive water transport for roots of Opuntia acanthocarpa in relation to soil drying and rewetting. Plant Physiol 126:352–362
Maurel C, Verdoucq L, Luu DT, Santoni V (2008) Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol 59:595–624
Morgan JA (1986) The effects of N nutrition on the water relations and gas exchange characteristics of wheat (Triticum aestivum L.). Plant Physiol 80:52–58
Nayyar H (2003) Accumulation of osmolytes and osmotic adjustment in water-stressed wheat (Triticum aestivum) and maize (Zea mays) as affected by calcium and its antagonists. Environ Exp Bot 50:253–264
North GB, Nobel PS (1995) Hydraulic conductivity of concentric root tissues of Agave deserti Engelm. under wet and drying conditions. New Phytol 130:47–57
North GB, Nobel PS (2000) Heterogeneity in water availability alters cellular development and hydraulic conductivity along roots of a desert succulent. Ann Bot 85:247–255
Oddo E, Inzerillo S, La Bella F, Grisafi F, Salleo S, Nardini A (2011) Short-term effects of potassium fertilization on the hydraulic conductance of Laurus nobilis L. Tree Physiol 31:131–138
Parent B, Hachez C, Redondo E, Simonneau T, Chaumont F, Tardieu F (2009) Drought and abscisic acid effects on aquaporin content translate into changes in hydraulic conductivity and leaf growth rate: a trans-scale approach. Plant Physiol 149:2000–2012
Passioura J (2007) The drought environment: physical, biological and agricultural perspectives. J Exp Bot 58:113–117
Pitre F, Cooke JK, Mackay J (2007) Short-term effects of nitrogen availability on wood formation and fibre properties in hybrid poplar. Trees 21:249–259
Postma JA, Lynch JP (2011) Root cortical aerenchyma enhances the growth of maize on soils with suboptimal availability of nitrogen, phosphorus, and potassium. Plant Physiol 156:1190–1201
Radin JW (1983) Control of plant growth by nitrogen: differences between cereals and broadleaf species. Plant Cell Environ 6:65–68
Radin JW, Boyer JS (1982) Control of leaf expansion by nitrogen nutrition in sunflower plants role of hydraulic conductivity and turgor. Plant Physiol 69:771–775
Saliendra NZ, Sperry JS, Comstock JP (1995) Influence of leaf water status on stomatal response to humidity, hydraulic conductance, and soil drought in Betula occidentalis. Planta 196:357–366
Shangguan ZP, Shao MA, Dyckmans J (2000) Nitrogen nutrition and water stress effects on leaf photosynthetic gas exchange and water use efficiency in winter wheat. Environ Exp Bot 44:141–149
Steudle E (2000a) Water uptake by plant roots: an integration of views. Plant Soil 226:45–56
Steudle E (2000b) Water uptake by roots: effects of water deficit. J Exp Bot 51:1531–1542
Steudle E (2001) The cohesion-tension mechanism and the acquisition of water by plant roots. Annu Rev Plant Biol 52:847–875
Steudle E, Peterson CA (1998) How does water get through roots? J Exp Bot 49:775–788
Tanguilig V, Yambao E, O’toole J, De Datta S (1987) Water stress effects on leaf elongation, leaf water potential, transpiration, and nutrient uptake of rice, maize, and soybean. Plant Soil 103:155–168
Tezara W, Mitchell VJ, Driscoll SD, Lawlor DW (1999) Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 401:914–917
Tian Q, Chen F, Zhang F, Mi G (2005) Possible involvement of cytokinin in nitrate-mediated root growth in maize. Plant Soil 277:185–196
Vandeleur RK, Niemietz C, Tilbrook J, Tyerman SD (2005) Roles of aquaporins in root responses to irrigation. Plant Soil 274:141–161
Vandeleur RK, Mayo G, Shelden MC, Gilliham M, Kaiser BN, Tyerman SD (2009) The role of plasma membrane intrinsic protein aquaporins in water transport through roots: diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol 149:445–460
Vander P, Vårum KM, Domard A, Eddine El Gueddari N, Moerschbacher BM (1998) Comparison of the ability of partially N-acetylated chitosans and chitooligosaccharides to elicit resistance reactions in wheat leaves. Plant Physiol 118:1353–1359
Visser EJW, Colmer TD, Blom CWPM, Voesenek LACJ (2000) Changes in growth, porosity, and radial oxygen loss from adventitious roots of selected mono- and dicotyledonous wetland species with contrasting types of aerenchyma. Plant Cell Environ 23:1237–1245
Wan X, Zwiazek JJ (1999) Mercuric chloride effects on root water transport in aspen seedlings. Plant Physiol 121:939–946
Wan X, Steudle E, Hartung W (2004) Gating of water channels (aquaporins) in cortical cells of young corn roots by mechanical stimuli (pressure pulses): effects of ABA and of HgCl2. J Exp Bot 55:411–422
Wang JR, Hawkins CDB, Letchford T (1998) Photosynthesis, water and nitrogen use efficiencies of four paper birch (Betula papyrifera) populations grown under different soil moisture and nutrient regimes. Forest Ecol Manag 112:233–244
Warren CR, Adams MA (2006) Internal conductance does not scale with photosynthetic capacity: implications for carbon isotope discrimination and the economics of water and nitrogen use in photosynthesis. Plant Cell Environ 29:192–201
Welander NT, Ottosson B (2000) The influence of low light, drought and fertilization on transpiration and growth in young seedlings of Quercus robur L. Forest Ecol Manag 127:139–151
Yang X, Li Y, Ren B, Ding L, Gao C, Shen Q, Guo S (2012) Drought-induced root aerenchyma formation restricts water uptake in rice seedlings supplied with nitrate. Plant Cell Physiol 53:495–504
Acknowledgments
This work was supported by the National Basic Research Program of China (2013CB127403), the National Natural Science Foundation of China (31172020 and 31272236), Jiangsu Postdoctoral Science Foundation (1402148C) and China Postdoctoral Science Foundation (2015M571768).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ad C. Borstlap.
Rights and permissions
About this article
Cite this article
Ren, B., Wang, M., Chen, Y. et al. Water absorption is affected by the nitrogen supply to rice plants. Plant Soil 396, 397–410 (2015). https://doi.org/10.1007/s11104-015-2603-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2603-5