Abstract
This paper is a contribution to the development of microwave plasma-based technology for hydrogen (H2) production from a so-called synthetic biogas, considered as a mixture of methane (CH4) and carbon dioxide (CO2). The efficiency of hydrogen production via reforming of CH4 in the synthetic biogas flowing through a waveguide-supplied metal cylinder-based microwave plasma source (MPS) operating at atmospheric pressure was tested experimentally. The MPS operated at a frequency of 915 MHz. The working plasma-forming gas was a mixture of CH4:CO2 of volume ratios from 0 to 1. Its flow rate was varied from 3 to 12 m3/h. The microwave power absorbed by the plasma was up to 7.5 kW. The experiment showed that using this kind of MPS, the plasma processing of the synthetic biogas can be run stably at high flow rates (up to 12 m3/h). The optimal CH4:CO2 ratio in terms of high energy efficiency of the microwave plasma reforming of CH4 was found to be 40:60. The maximum achieved hydrogen production rate was 156 g(H2)/h at a microwave absorbed power of 7.5 kW (at a flow rate of 6 m3/h) with the energy efficiency of hydrogen production of 21 g(H2)/kWh. The maximum energy yield of hydrogen production of 24 g(H2)/kWh was achieved at 4.5 kW of the microwave absorbed power (the hydrogen production rate was 108 g(H2)/h in this condition). The maximum methane conversion degree and maximum hydrogen selectivity were 86.5% and 73.3%, respectively at an absorbed microwave power of 6.5 kW (at 3 m3/h).
Similar content being viewed by others
Introduction
Effective energy sources alternative to fossil-based fuels become the main area of research for sustainable energy development. The declining reserves of fossil fuels and the thread of global warming impose that the newly developed energy source must simultaneously meet the requirements of being renewable and ecological. In this aspect hydrogen (H2) is one of the most promising renewable and environmentally clean energy sources. The technologies related to H2 production from fossil fuels and other resources have been described in many review papers, e.g. [1,2,3,4].
Although H2 has a high combustion heat (142 kJ/g) [1], it is envisaged to be used in the future as an energy carrier for activating the fuel cells [5,6,7,8] rather than a fuel for heat production through the combustion [9]. The applicability of hydrogen for fuel cells boosts an interest in new sources and production methods of hydrogen. Biogas is regarded as a new ecological and renewable hydrogen source. It becomes an alternative for methane (CH4), which has so far been a common hydrogen source [2].
Biogas is a gas formed during the breakdown of organic matter in the absence of oxygen. It can be produced from raw materials such as green waste, household waste, agricultural waste, municipal waste, sewage etc. [10]. Biogas is a hydrogen carrier similar to fossil natural gas. Methane (CH4), carbon dioxide (CO2), oxygen (O2)., nitrogen (N2), ammonia (NH3), volatile organic compounds (VOCs), including organic silicon compounds, halogenated compounds and sulphur compounds (mainly hydrogen sulphide (H2S)) are components of biogas from landfills, waste water treatment plants (WWTP) sludge digesters and biogas plants processing different materials [11, 12]. The content of CH4 in the biogas from landfills, WWTPs and biogas plants usually ranges from 50 to 70%, CO2 content—from 35 to 45% and N2 content—from 1 to 3%. O2 content is less than 1%. Biogas can also contain water vapour (H2O), carbon oxide (CO) and particulate matter. Thus, the main components of typical biogas are: CH4 and CO2. It should be pointed out that the origin of CO2 in biogas produced from plants is ecologically neutral because it is absorbed by the plants from the atmosphere. The production-and-use cycle of biogas is continuous, and it does not generate any net CO2. These attributes make biogas a renewable and ecological hydrogen source [11, 13].
For many of the applications biogas has to be cleaned from some components and upgraded in terms of higher concentration of CH4 in it [1, 14]. For example, the typical concentration of corrosive H2S in raw biogas is enough to destroy the biogas installations and devices [15]. To have the same standards as fossil natural gas, biogas has be cleaned and upgraded to natural gas standards to become bio-methane, which consisting of 98% of CH4 is capable of being used in local natural gas networks. In particular, both cleaning and upgrading have most likely to be applied, if high-purity H2 for activating the fuel cells is expected to obtained from biogas.
Since the major component of biogas is CH4, several conventional CH4 reforming processes can be adopted for producing hydrogen from biogas. They are pyrolysis, steam reforming, dry reforming, partial oxidation, auto-thermal reforming:
The conventional methods (1)–(5a and 5b) for producing hydrogen from biogas have been broadly discussed in many papers (e.g. [11,12,13, 16]). It is acknowledged that some of the conventional reforming technologies listed above are already or will be soon commercialized (steam reforming, partial oxidation reforming and autho-thermal reforming). In almost all conventional reforming technologies using biogas as raw material, high activity catalysts have to be employed to increase the efficiency of the reforming process and useful lifetime of the reforming devices. The main problems encounter in the biogas conventional reforming are related to coke formation on the catalyst surface and its poisoning by substances containing sulphur, which lead to deactivation of the catalyst and reduction of H2 production.
In the recent two decades an alternative technology has been proposed and developed for hydrogen production. This technology uses thermal and non-thermal plasma for reforming gaseous and liquid compounds containing hydrogen (e.g. review papers: [17,18,19,20,21,22,23,24,25,26,27,28]). These compounds can originate from fossil fuels and biofuels.
Different types of plasma have been investigated for reforming biogas-like mixtures (containing CH4 and CO2) into syngas (H2 and CO): corona discharges [29, 30], glow discharges [31], dielectric barrier discharges (DBDs) [15, 32,33,34], arc discharges [35,36,37,38] and microwave discharges [39,40,41,42].
In this paper, we present a microwave plasma-based technology for hydrogen production from methane-containing gases, in particular from biogas. Microwave plasma-based technology appears to create the most promising plasma environment for such thermal and chemical processing as coal/biomass/waste pyrolysis/gasification, waste gas treatment, plasma reforming and CO2 conversion. Microwave plasma is a gas ionized by means of a high frequency electromagnetic field (0.3–300 GHz). The electric field energizes the electrons which become capable of exciting and ionizing the neutral atoms and molecules, creating the plasma and sustaining the so-called microwave discharge. The technologically important advantage of microwave discharge over other discharges is that it does not require internal electrodes, which need regular maintenance or replacement due to erosion caused by contaminants. Microwave discharges can operate over a wide pressure range (from a fraction of mbar to several bars). Furthermore, the energy efficiency transfer from microwaves to plasma efficiency is high (up to 90%). The microwave plasma parameters (mainly the electron concentration and energy) result in high concentrations of active species enhancing the chemical reactions in the working gas forming the plasma. These properties of microwave plasma make it attractive for many applications, including reforming the biogas for hydrogen production.
The microwave plasma-based technology for hydrogen production presented in this paper concerns the efficiency of hydrogen production via reforming of the so-called synthetic biogas flowing through a waveguide-supplied metal cylinder-based microwave plasma source (MPS). The synthetic biogas is a mixture of CH4 and CO2, which are the main constituents of a raw biogas. In the experiment carried out in this work, the ratio of CH4:CO2 in the synthetic gas was varied from 0 to 1. We will call the processes occurring in the MPS the microwave plasma dry (CO2) reforming of CH4.
Experimental
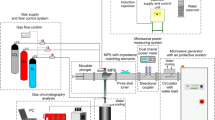
The experimental setup used for hydrogen production via synthetic biogas reforming in atmospheric-pressure microwave plasma is presented in Fig. 1. The main parts of microwave system were: microwave (915 MHz, 20 kW) generator with an isolator, power measuring system (directional coupler: Mega Industries, power meter: Agilent N1914A, power heads: Agilent E9301A), microwave plasma source (MPS) impedance maching elements (three stub tuner + movable plunger) and gas supply and flow control system (Bronkhorst EL-FLOW). The error of the measured gas flow rate and microwave power did not exceed ± 3%.
The microwave power PA absorbed in the plasma was determined as the difference between incident (PI) and reflected (PR) powers, measured by the power measuring system. This is a reasonable approximation despite of the fact that in addition to the microwave absorption by the plasma other microwave energy losses (e.g. losses in the tapered section, microwave leakage) exist in the microwave duct around the microwave plasma. As a consequence of such an approximation, the microwave power PA calculated as the difference (PI–PR) is overestimated. This results in the underestimation of energy efficiency of the biogas reforming presented in this work. The additional losses seem to be small, however, an estimation of these losses is difficult. The errors of measuring the incident and reflected microwave powers were ± 1%.
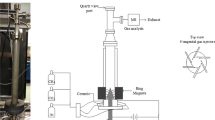
The microwave duct was formed by a standard rectangular waveguide WR 975, which also was a mount base for the elements of microwave system. The plasma was generated by the waveguide-supplied metal-cylinder-based type MPS with a section of reduced-height, preceded by tapered section (Fig. 2). The microwave plasma flame was generated inside a quartz cylindrical tube passing through the waveguide as shown in Fig. 2. A cylindrical brass shield surrounding the quartz tube had a 5 mm slit for plasma observation and monitoring. The working gas was introduced to the MPS by four gas inlets which formed a swirl flow inside the quartz tube. The matching of the microwave generator with the discharge section was set at a level of 75%, using three stub tuner and movable plunger (see Fig. 1). This means that 75% of the incident microwave power was absorbed by the plasma. The MPS was described in detail in [43].
The experimental tests were performed with the working gas simulating biogas (a mixture of CH4 and CO2). Its flow rate through the MPS was varied from 3 m3/h up to 12 m3/h. The absorbed microwave power PA ranged from 4.5 kW up to 7.5 kW. The CH4:CO2 ratio was varied from 0 (pure CO2) to 1 (CH4–50 Vol.% and CO2–50 Vol.%).
The working gas composition after its processing by the MPS was measured using a gas chromatographs: Shimadzu GC-2014 and SRI 8610C. The presence of following components: H2, O2, N2, CO, CO2, CH4, C2H2, C2H4 and C2H6 in the outlet gas was detected and their concentrations were measured. The average of 3 measuring trial runs was taken to determine the concentrations of each component in every gas sample. The error of measured concentration of each component did not exceed ± 5%.
The volumetric flow of the working gas changed when processed by the plasma due to reactions between its components. This influenced the individual volumetric flows of plasma processing components. To determine the individual component volumetric flows, an additional stream of N2 with a flow rate of 0.6 m3/h was introduced as a flow marker into the outlet gas, as shown in (Figs. 1 and 2). Analyzing the concentrations of gas components in a gas sample taken at the MPS outlet (Fig. 1) enabled determining the individual volumetric flows of plasma processing components.
To determine the effectiveness of hydrogen production by the MPS the hydrogen production rate and the energy yield of hydrogen production were measured in this work. The hydrogen production rate in g(H2)/h shows how many grams of hydrogen is produced per unit of time. The energy yield of hydrogen production in g(H2)/kWh determines the amount of grams of hydrogen produced using 1 kWh of microwave energy. The other parameters determined in this work were: the methane conversion degree and the hydrogen selectivity. The methane conversion degree is a fraction (or percent) of the methane introduced into the microwave plasma, which was converted into plasma non-methane products. It is given by a ratio:
where [CH4]inlet is the number of CH4 mol at the MPS inlet, and [CH4]outlet is the number of CH4 mol at the MPS outlet. The hydrogen selectivity is defined as:
where [H2]outlet is the number of moles of produced H2 and ([CH4]inlet - [CH4]outlet) is the number of moles of CH4 converted in the plasma. This parameter describes a fraction of the methane introduced into plasma converted finally into hydrogen.
Results and Discussion
Figure 3 shows images of plasma flame generated in the waveguide-supplied metal-cylinder-based type MPS at different CH4:CO2 ratio at a working plasma gas flow rate of 6 m3/h and an microwave absorbed power of 6.5 kW. The images of plasma were taken through the slit along the outer cylindrical shield. The plasma flame length was about 20 cm measured from the bottom wall of the waveguide for pure CO2 plasma (CH4:CO2 ratio = 0) and decreased with increasing CH4:CO2 ratio, i.e. with increasing CH4 content in the working gas. As it can be seen from the images, the microwave plasma changed significantly when a CH4:CO2 ratio was higher than 40:60 (about 0.7). It changed not only colour but became clearly shorter and brighter (note that the camera exposure time at CH4:CO2 = 50:50 had to be shortened to avoid overexposure). For a CH4:CO2 ratio of 50:50 the microwave discharge became unstable and intense soot production could be observed. Further increasing CH4:CO2 ratio (above 1) resulted in extinguishing the discharge. The soot deposited on the quartz tube inner surface and in the plasma zone, similarly as we observed earlier in the decomposition of ethanol in nitrogen plasma [44]. The images of CH4:CO2 plasma shown in Fig. 3 suggest that the parameters of the plasma change substantially with increasing content of CH4. This undoubtly influences the plasmo-chemical processes in the plasma, and, eventually, the hydrogen production efficiency.
Figure 4 presents the hydrogen production rate and energy yield of hydrogen production as a function of CH4:CO2 ratio in the MPS. As it could be expexted the hydrogen production rate incrased with the increasing CH4 content in the working gas. The MPS operated very stably up to a CH4:CO2 ratio of 40:60 (about 0.7). The hydrogen production parameters, the hydrogen production rate and the energy yield were highest at CH4:CO2 = 40:60. As mentioned above, the plasma started to change significantly at CH4:CO2 > 40, and the discharge became unstable even for anabsorbed microwave power higher than 7.5 kW.
Such a behaviour of the microwave discharge can be explained by changes in the chemical processes caused by the higher content of CH4 in the CH4:CO2 micture. For the CH4:CO2 of 40:60, an overall reaction occurring in such a plasma can be formulated as follows:
According to reaction (8) there should not been any solid carbon (soot) in the MPS. This was confirmed in our experiment (manifested by no soot in the MPS and stable operation of the microwave discharged).
On the other hand, for the CH4:CO2 ratio higher than 50:50 (or 1) the chemical processes in the MPS can be described by an overall reaction:
The production of solid carbon predicted in reaction (9) resulted in soot presence and deposition in the MPS, which caused instability of the microwave discharge.
For the CH4:CO2 ratio equal to about 50:50 (or 1), the so-called dry (or CO2) reforming of CH4, descibed by reaction (3) should occur in the MPS, and soot should not been produced there. This is true in the thermodynamic equilibrium conditions. The experimental results showed, however, that soot was produced at the CH4:CO2 ratio equal to about 50:50 (or 1) and it choked the microwave discharge (Figs. 4 and 5). The above discrepancy can be explained by axial and radial temperature inhomogeneity inside the working gas stream, in which hot plasma regions and colder swirl flow regions exist. It means that the working gas stream is far from being in the thermodynamical equilibrium. In the colder plasma regions the temperature cannot be high enough to run the dry reforming (3) but sufficient to run pyrolysis of CH4 instead. Pyrolysis of CH4 (reaction (1)) results in soot presence in the MPS.
Figure 5a shows that the methane conversion degree decreased with increasing CH4:CO2 ratio. This means that the high ability of microwave CH4:CO2 plasma for converting CH4 weakens when the content of CH4 in the CH4:CO2 increases. In contrast, the hydrogen selectivity increased with increasing CH4:CO2 ratio (Fig. 5b). This can be attributed to the decreasing presence of oxygen in the microwave CH4:CO2 plasma when the content of CH4 in the CH4:CO2 increases (see Eqs. (8), (9), and (10)). At lower contents of CH4, a significant amounts of the produced H2 as well as the introduced CH4 can be combusted due to the presence of oxygen.
The hydrogen production rate increased with increasing microwave absorbed power (see Fig. 6a). The maximum achieved hydrogen production rate was 156 g(H2)/h at a microwave absorbed power of 7.5 kW and CH4:CO2 ratio of 40:60 with an energy yield of hydrogen production of 21 g(h2)/kWh. On the other hand, the maximum energy yield of hydrogen production [24 g(H2)/kWh] was achieved at an microwave absorbed power of 4.5 kW (Fig. 6b) with a hydrogen production rate of 108 g(H2)/h. Thus the highest hydrogen production rate can be achieved at a loss of the energy yield (also see Table 1).
Figure 7a, b show the hydrogen production rate and the energy yield do not depend on working plasma gas flow rate in a range from 3 m3/h to 12 m3/h. This proves that the MPS can operate with at high gas-flow output, which can be attractive for various plasmo-chemical processing purposes and technologies. However, in the case of the biogas simulator having different CH4:CO2 ratios, the optimal working gas flow rate is about 3 m3/h or a bit less (a lower flow rate can result in overheating the MPS). As it is seen in Fig. 8a, b, the methane conversion degree and the hydrogen selectivity are highest when the working gas flow rate is about 3 m3/h, and they decrease significantly with increasing working gas flow rate. This means that operating at flow rates higher than 3 m3/h would not give any positive effects in term of the hydrogen production efficiency. On the contrary, the microwave processing of the biogas simulator at the flow rates higher than 3 m3/h would lead to a loss of the unprocessed biogas (a significant fraction of the biogas would just pass the MPS unprocessed).
The major components of outlet gas are listed in Table 1. They are H2, CO2, CO, CH4, C2H2, C2H4, and C2H6. At the optimal conditions, H2 content in the outlet gas was 22.4% and 30.3% for 4.5 kW and 7.5 kW of the microwave absorbed power, respectively. The content of the other components were: CO2 (34.3% and 24.7%, respectively), CH4 (22.9% and 15%, respectively), CO (19% and 28%, respectively), C2H2 (1.1% and 1.4%, respectively), C2H4 (0.1% and 0.16%, respectively) and C2H6 (0.06%).
Conclusions
The experiment showed that the microwave plasma reforming of synthetic biogas (a mixture of CH4:CO2) can be run stably at high gas flow rates (up to 12 m3/h) in the waveguide-supplied metal cylinder-based microwave plasma source (MPS) operating at atmospheric pressure. This makes the MPS attractive for different plasmo-chemical processing purposes and technologies. The CH4:CO2 ratio optimal in terms of hydrogen production from the synthetic biogas was found to be 40:60. The working gas flow rate optimal from the view point of hydrogen production efficiency was found to be about 3 m3/h. The highest hydrogen production rate of 156 g(H2)/h [1.8 m3(H2)/h] was obtained at a microwave absorbed power of 7.5 kW. The highest energy yield of hydrogen production of 24 g(H2)/kWh [0.3 m3(H2)/kWh] was achieved at 4.5 kW of a microwave absorbed power.
Table 2 summarizes to some extend the plasma technologies, along with their energy efficiencies, tested for hydrogen production from (CH4 and CO2) mixtures, which are the main components of typical biogases. As a reference, parameters of the large-scale conventional steam reforming of methane is also given in Table 2. The large-scale conventional steam reforming of methane is an established industrial process, which exhibits an energy yield of hydrogen production at 60 g(H2)/kWh. This value is recognized by the U.S. Department of Energy (DOE) as a 2020 target for large-scale emerging technologies to be able to compete with the well-established technologies for hydrogen production [9, 24, 25]. An accepTable 2020 target for small-scale plasma technologies intended for the distributed hydrogen production is expected to be lower than 60 g(H2)/kWh.
In the plasma processing of (CH4 and CO2) mixtures shown in Table 2 several conventional CH4 reforming processes were adopted: pyrolysis (Eq. 1), steam reforming (Eq. 2), dry reforming (Eq. 3), and auto-thermal reforming (Eqs. 5a an 5b). From the thermodynamic point of view (considering the standard reaction enthalpy and the thermodynamic limit of hydrogen production energy yield) the most effective processing is the auto-thermal reforming, then the pyrolysis, the steam reforming, and finally the dry reforming [25]. As seen from Table 2, regardless of the reforming processing adopted, only the arc and microwave plasmas have a potential for hydrogen production with a high energy yield, closed to the DOE’s 2020 target. A very high energy yield of hydrogen production was actually achieved in the auto-thermal reforming of (CH4 and CO2) mixture by an AC-pulse arc plasma [35]. However, this result has to be taken with cautiousness because the energy from the CH4 combustion has not been included in the energy consumed for the hydrogen production. Applying the classic pyrolysis of methane for hydrogen production from (CH4 and CO2) mixtures requires costly removal of CO2 from them prior to the processing. As shown in [40], the microwave plasma pyrolysis of methane is characterized by a relatively high hydrogen production rate and energy yield, however, severe soot production makes the microwave plasma malfunction, limiting the hydrogen production rate and energy yield. To avoid or at least reduce the soot production, the microwave plasma steam reforming can be used. However, in this case first CO2 has to be removed from (CH4 and CO2) mixtures and then H2O vapour has to be introduced to form stable and efficient (CH4 and H2O) microwave plasma. To avoid costly removal of CO2, the so-called combined dry and stream reforming (CH4 + CO2 + H2O) for microwave plasma processing of (CH4 and CO2) mixtures has been tested in [41]. As found, the combined dry and steam reforming allowed stable plasma processing of (CH4 + CO2) mixtures of a higher content of methane without the soot problems, whereby the hydrogen production rate and energy yield higher than in the classic dry reforming could be achieved. Although the thermodynamic limit of hydrogen production energy yield for the classic dry reforming is lower than that of the DOE’s 2020 target of hydrogen production yield [25], the classic dry reforming processing has been tested employing the rotating gliding arc plasma [36] and the microwave plasmas ([40, 42] and the present work). This is justified because the dry reforming is attractive from the technological point of view. It offers almost direct introducing the biogas (considered as a CH4 and CO2 mixture) into the plasma source, without its costly modification, when, for example adding H2O vapour is considered. As the present work showed, using the classic dry reforming in the 915 MHz microwave system resulted in a relatively high (156 [g(H2)/h]) hydrogen production rate compared to the other plasma methods (at the similar value of the energy yield). The promising results achieved when using the 915 MHz microwave system may suggest that attractive results can be obtained if such a system is employed for processing (CH4 and CO2) mixtures according to the combined dry and steam reforming and auto-thermal reforming schemes. Such tests are planned in the near future. We believe that they will give results which may meet the DOE’s 2020 requirements, taking into account that the reforming of renewable and ecological biogas is considered.
References
Holladay JD, Hu J, King DL, Wang Y (2009) An overview of hydrogen production technologies. Catal Today 139:244–260
Abbas HF, Wan Daud WMA (2010) Hydrogen production by methane decomposition: a review. Int J Hydrogen Energy 35:1160–1190
Dincer I, Acar C (2015) Review and evaluation of hydrogen production methods for better sustainability. Int J Hydrogen Energy 40:11094–11111
Nikolaidis P, Poullikkas A (2017) A comparative overview of hydrogen production processes. Renew Sustain Energy Rev 67:597–611
Sengodan S, Lan R, Humphreys J, Du D, Xu W, Wang H, ShanwenTao S (2018) Advances in reforming and partial oxidation of hydrocarbons for hydrogen production and fuel cell applications. Renew Sustain Energy Rev 82:761–780
Ono Y, Haneda T, IkegamiT Akisawa A (2017) Potential of producing hydrogen for fuel-cell vehicles by residential fuel cell co-generation utilizing idle capacity. J Jpn Inst Energy 96(10):478–486
Kirillov VA, Shigarov AB (2016) Biofuels as a promising source of hydrogen for fuel cell power plants. Theor Found Chem Eng 50(4):351–365
Ahmed S, Krumpelt M (2001) Hydrogen from hydrocarbon fuels for fuel cells. Int J Hydrogen Energy 26:291–301
Randolph K (2013) Hydrogen production. In: Annual Merit Review and Peer Evaluation Meeting. U.S. http://www.hydrogen.energy.gov/pdfs/review13/pd000_randolph_2013_o.pdf. Accessed 16 May 2013
Divya D, Gopinath LR, Christy PM (2015) A review on current aspects and diverse prospects for enhancing biogas production in sustainable means. Renew Sustain Energy Rev 42:690–699
Balata M, Balata H (2009) Biogas as a renewable energy source—a review. Energy Source Part A 31:1280–1293
Alves HJ, Junior CB, Niklevicz RR, Frigo EP, Frigo MS, Coimbra-Araujo CH (2013) Overview of hydrogen production technologies from biogas and the applications in fuel cells. Int J Hydrogen Energy 38:5215–5225
Mengistua MG, Simanea B, Esheteb G, Worknehc TS (2015) A review on biogas technology and its contributions to sustainable rural livelihood in Ethiopia. Renew Sustain Energy Reviews 48:306–316
Woolcock PJ, Brown RC (2013) A review of cleaning technologies for biomass-derived syngas. Biomass Bioenergy 52:54–84
Dors M, Izdebski T, Berendt A, Mizeraczyk J (2012) Hydrogen production via biomethane reforming in DBD reactor. Int J Plasma Environ Sci Technol 6(2):93–97
Pakhare D, Spivey J (2014) Review paper: a review of dry (CO2) reforming of methane over noble metal catalysts. Chem Soc Rev 43:7813–7837
Cormier JM, Rusu I (2001) Syngas production via methane steam reforming with oxygen: plasma reactors versus chemical reactors. J Phys D Appl Phys 34:2798–2803
Deminsky M, Jivotov V, Potapkin B, Rusanov V (2002) Plasma-assisted production of hydrogen from hydrocarbons. Pure Appl Chem 74:413–418
Chang JS, Urashima K (2007) Plasma fuel reforming: a critical review, Trans. of the Institute of Fluid-flow. Machinery 119:17–28
Rusu I (2007) Development trends of cold plasma reactors in the global context of carbon emission reduction. Environ Eng Manag J 6(3):211–217
Petitpas G, Rollier JD, Darmon A, Gonzales-Aguilar J, Metkemeijer R, Fulcheri L (2007) A comparative study of non-thermal plasma assisted reforming technologies. Int J Hydrogen Energy 32:2848–2867
Chen HL, Lee HM, Chen SH, Chao Y, Chang MB (2008) Review of plasma catalysis on hydrocarbon reforming for hydrogen production—interaction, integration, and prospects. Appl Catal B: Environ 85:1–9
Tao X, Bai M, Li X, Long H, Shang S, Yin Y, Dai X (2011) CH4-CO2 reforming by plasma—challenges and opportunities. Prog Energy Combust Sci 37:113–124
Mizeraczyk J, Urashima K, Jasinski M, Dors M (2014) Hydrogen production from gaseous fuels by plasmas—a review. Int J Plasma Environ Sci Technol 8(2):89–97
Mizeraczyk J, Jasinski M (2016) Plasma processing methods for hydrogen production. Eur Phys J Appl Phys 75:24702
Snoeckx R, Bogaerts A (2017) Plasma technology—a novel solution for CO2 conversion? Chem Soc Rev 46(19):5805–5863
de la Fuente JF, Kiss AA, Radoiu MT, Stefanidis GD (2017) Microwave plasma emerging technologies for chemical processes. J Chem Technol Biotechnol 92:2495–2505
Nahar G, Mote D, Dupont V (2017) Hydrogen production from reforming of biogas: review of technological advances and an Indian perspective. Renew Sustain Energy Rev 76:1032–1052
Li M, Xu G, Tian Y, Chen L, Fu H (2004) Carbon dioxide reforming of methane using DC corona discharge plasma reaction. J Phys Chem A 108:1687–1693
Nguyen HH, Nasonova A, Nah IW, Kim K-S (2015) Analysis on CO2 reforming of CH4 by corona discharge process for various process variables. J Ind Eng Chem 32:58–62
Ghorbanzadeh AM, Lotfalipour R, Rezaei S (2009) Carbon dioxide reforming of methane at near room temperature in low energy pulsed plasma. Int J Hydrogen Energy 34:293–298
Wang Q, Yan B, Jin Y, Cheng Y (2009) Investigation of dry reforming of methane in a dielectric barrier discharge reactor. Plasma Chem Plasma Process 29:217–228
Goujard V, Tatiboue J-M, Batiot-Dupeyrat C (2011) Carbon dioxide reforming of methane using a dielectric barrier discharge reactor: effect of helium dilution and kinetic model. Plasma Chem Plasma Process 31:315–325
Gallon HJ, Tu X, Whitehead JC (2012) Effects of reactor packing materials on H2 production by CO2 reforming of CH4 in a dielectric barrier discharge. Plasma Process Polym 9:90–97
Chung W-J, Park H-W, Park D-W (2017) Effects of arc discharge mode on the efficiency of biogas reforming in an AC-pulsed arc plasma system. Plasma Chem Plasma Process 37:383–399
Zhu F, Zhang H, Yan X, Yan J, Ni M, Li X, Xin TuX (2017) Plasma-catalytic reforming of CO2-rich biogas over Ni/c-Al2O3 catalysts in a rotating gliding arc reactor. Fuel 199:430–437
Tan Z, Ai P (2017) CO2 reforming of biogas to obtain synthesis gas using non-thermal plasma. J Energy Inst 90(2017):864–874
Mao S, Tan Z, Zhang L, Huang Q (2018) Plasma-assisted biogas reforming to syngas at room temperature condition. J Energy Inst 91:172–183
Zhang J, Zhang J, Yang Y, Liu Q (2003) Oxidative coupling and reforming of methane with carbon dioxide using a pulsed microwave plasma under atmospheric pressure. Energy Fuels 17:54–59
Jasiński M, Czylkowski D, Hrycak B, Dors M, Mizeraczyk J (2013) Atmospheric pressure microwave plasma source for hydrogen production. Int J Hydrogen Energy 38:11473–11483
Czylkowski D, Hrycak B, Jasiński M, Dors M, Mizeraczyk J (2016) Microwave plasma-based method of hydrogen production via combined steam reforming of methane. Energy 113:653–661
Chun SM, Hong YCH, Choi DH (2017) Reforming of methane to syngas in a microwave plasma torch at atmospheric pressure. J CO2 Util 19:221–229
Miotk R, Jasinski M, Mizeraczyk J (2014) Optical emission spectroscopy of microwave (915 MHz) plasma in atmospheric pressure nitrogen with addition of ethanol vapour. Acta Phys Pol, A 125:1329–1331
Hrycak B, Czylkowski D, Miotk R, Dors M, Jasinski M, Mizeraczyk J (2015) Hydrogen production from ethanol in nitrogen microwave plasma at atmospheric pressure. Open Chem 13:317–324
Acknowledgements
This research was supported by Institute of Fluid Flow Machinery, under the programme No. FBW 30.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hrycak, B., Czylkowski, D., Jasiński, M. et al. Hydrogen Production via Synthetic Biogas Reforming in Atmospheric-Pressure Microwave (915 MHz) Plasma at High Gas-Flow Output. Plasma Chem Plasma Process 39, 695–711 (2019). https://doi.org/10.1007/s11090-019-09962-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-019-09962-z