Abstract
This paper is a contribution to the development of microwave plasma-based technology aimed at efficient hydrogen (H2) production from a so-called synthetic biogas, considered a mixture of methane (CH4) and carbon dioxide (CO2), which can contain up to 70% CH4. In this work, we tested the performance of a waveguide-supplied metal cylinder-based microwave plasma source (MPS) operating at 915 MHz at atmospheric pressure as a tool for the efficient production of H2 in the steam reforming of the synthetic biogas. The test showed that the steam reforming of the synthetic biogas could be carried out under a wide range of working parameters without soot formation and extinction of the microwave discharge. We found that there is a minimal H2Osteam consumption rate for a given CH4 input volume content, which ensures stable operation of the MPS (no soot). The experiments did not show that increasing the amount of H2Osteam rate above the minimal value for a given CH4 input volume content results in an increase in the H2 production rate, energy yield, CH4 conversion degree, and H2 output concentration. To describe the MPS performance, which also takes into account a factor of the utilization of the CH4 feedstock, we introduced a new parameter, called an energy–CH4 feedstock consumption yield. The best results in terms of the H2 production rate, the energy yield, and the CH4 conversion degree were 239 g[H2]/h 36.8 g[H2]/kWh, and 74.3%, respectively. This shows that the application of the steam reforming, instead of the dry reforming, resulted in a 1.5-fold increase of the H2 production rate and the corresponding energy yield.
Similar content being viewed by others
Introduction
The thread of global warming and the declining resources of fossil fuels force the newly developed energy source to meet the requirements of renewable energy and environmental friendliness at the same time. One of the most promising renewable and ecologically clean energy sources seems to be hydrogen (H2). Many reviews described technologies related to the production of H2 from various resources1,2,3,4.
In the future hydrogen is predicted to be used not as a fuel to produce heat by combustion5 but rather as an energy carrier to activate fuel cells6,7,8,9. This increases the interest in new sources and methods of hydrogen production. As an alternative to methane (CH4), which so far has been a common source of hydrogen2, biogas is considered to be renewable and ecological.
A wide variety of raw materials such as green waste, agricultural waste, municipal waste, household waste, sewage etc. can be used to produce biogas10. The components of biogas from landfills, waste water treatment plants (WWTP), sludge digesters and biogas plants processing various materials are methane (CH4), carbon dioxide (CO2), oxygen (O2), nitrogen (N2), ammonia (NH3), volatile organic compounds (VOCs) including organic silicon compounds, halogenated compounds and sulphur compounds (mainly hydrogen sulphide (H2S))11,12. CH4 and CO2 are the main compounds of a typical biogas. The content of CH4 and CO2 in biogas from landfills, WWTPs and biogas plants usually ranges from 50 to 70% and from 35 to 45%, respectively. Biogas contains also: N2 (1–3%) and O2 (less than 1%). Other biogas components can be: water vapour (H2O), carbon monoxide (CO) and solid particles.
The CO2 contained in biogas produced from plants is absorbed by plants from the atmosphere. Therefore its origin is ecologically neutral. The continuous production-and-use cycle of biogas does not generate any net CO2. This cause that biogas can be consider as renewable and ecological H2 source11,13.
Many applications require that some components must be removed from biogas and a higher concentration of CH4 in the biogas have to be ensured1,14. For example, the typical concentration of corrosive H2S in the raw biogas is sufficient to destroy biogas installations and devices15. In order to have the same standards as fossil natural gas, the biogas needs to be cleaned and the concentration of CH4 needs to be raised up to natural gas standards to become bio-methane (98% CH4 content). Such an upgraded biogas is capable of being used in local natural gas networks. In particular, both cleaning and upgrading are most likely required if high purity H2 for fuel cell activation is expected to be obtained from the biogas.
Several conventional CH4 reforming processes (pyrolysis, steam reforming, dry reforming, partial oxidation, auto-thermal reforming) can be adopted to produce H2 from biogas, since the major component of biogas is CH4:
All conventional methods (1)–(5a, 5b) of H2 production have been widely discussed in many publications11,12,13,16. Some of these conventional reforming technologies mentioned above are already or will be soon commercialized (steam reforming, partial oxidation reforming and auto-thermal reforming).
Over the last two decades an alternative technology to produce H2 has been proposed and developed. This technology is based on thermal and non-thermal plasmas generated by electrical discharges. Such plasmas are used to reform gaseous and liquid hydrogen carriers to produce H217,18,19,20,21,22,23,24,25,26,27,28,29. These discharges are: glow discharges30, corona discharges31,32, arc discharges33,34,35,36, dielectric barrier discharges (DBD)15,37,38,39 and microwave discharges40,41,42,43,44.
The microwave plasma is an ionized gas sustained by an electromagnetic field of high frequency (0.3–300 GHz). A technologically important advantage of a microwave discharge over other discharges is the lack of internal electrodes, which require regular maintenance or/and replacement due to erosion. Microwave discharges can be generated over a wide pressure range (from a mbar to a few bar) of the working gas. Moreover, the efficiency of energy transfer from the electromagnetic field to the plasma is very high (up to 90%). Microwave plasma is characterized by a relatively high concentration and energy of electrons. This cause a high concentration of active ingredients that enhance the chemical reactions in the working gas. The microwave discharges generate one of the most promising plasma environments for the production of H2 from CH4-containing gases, in particular biogas.
Our research to date showed that microwave plasma-based technologies at 2.45 GHz for the steam reforming of synthetic biogas42 and at 915 MHz used for the dry reforming of synthetic biogas44 revealed the limitations of both microwave plasma technologies in terms of the H2 production efficiency, determined by the H2 production rate and energy yield. Firstly, the use of classical dry reforming of synthetic biogas in a 915 MHz microwave system resulted in a relatively high (156 [g(H2)/h]) rate of hydrogen production and energy yield (about 21 g(H2)/kWh)44 compared to other plasma methods. However, a further increase of these parameters was inhibited by soot formation on the quartz tube inner surface and in the plasma zone which led to choking and extinguishing the microwave discharge when the CH4 input volume content increased above 40%. This suggests that the limitation of the CH4 input volume content to 40% in the microwave plasma dry reforming of biogas in a 915 MHz microwave system is the major obstacle to approaching the energy yield of H2 production of 60 g(H2)/kWh, which is recognized by the U.S. Department of Energy (DOE) as an adopted target for small-scale technologies intended for industrial distributed hydrogen production5. Following the conclusions of our previous investigation in the steam reforming of biogas-like mixtures in a 2.45 GHz microwave system42, this steam reforming could be an effective way to overcome the CH4 input volume content limitation encountered in 915 MHz microwave dry plasma reforming. Secondly, our previous research in the steam reforming of biogas-like mixtures in a 2.45 GHz microwave system with an input CH4 volume content up to 70% resulted in a hydrogen production rate of 192 g(H2)/h and a hydrogen energy yield of 43 g(H2)/kWh, both with 4.5 kW of absorbed microwave power (Pabs). This was an important advance in the development of microwave plasma technology for efficient H2 production from biogas-like mixtures, but the resulting hydrogen energy yield of 43 g(H2)/kWh was still below the cost-effective DOE’s limit of 60 g(H2)/kWh. A further possible increase in the hydrogen energy yield in the 2.45 GHz system by increasing the absorbed microwave power above 4.5 kW is limited due to a thread of electrical breakdowns occurring in the typical 2.45 GHz systems at higher input microwave powers. This power limitation can be overcome by using a 915 MHz microwave system in place of a 2.45 GHz microwave plasma system. The 915 MHz microwave system safely provides more power to the microwave discharge than the 2.45 GHz one.
In this work, we present a technology based on microwave plasma for H2 production by steam reforming, the so-called synthetic biogas flowing through a waveguide-supplied, metal-cylinder microwave plasma source (MPS) working at 915 MHz. The used in this work synthetic biogas was a mixture of CH4 and CO2 which are the main components of a typical raw biogas.
Experimental procedure and setup
The experimental procedure and main parts of the experimental setup used for studying the combined reforming of synthetic biogas in atmospheric-pressure microwave plasma were described in detail in our previous investigations in the dry reforming of synthetic biogas44. Only a general description of the experimental part of this work is provided below to assist in following up the experimental results.
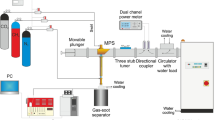
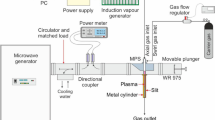
The experimental setup consisted of a microwave generator (915 MHz) with a protective isolator, microwave power measuring system, impedance matching elements (three stub tuner + movable plunger), microwave plasma source (MPS), gas supply system with a flow control unit, supplemented with a water vapour supply system and a gas-soot separator unit (Fig. 1). The drawing detailing the MPS setup (with dimensions) and a schematic drawing of the swirl gas inlets are shown in Fig. 2. The MPS used in this experiment (Figs. 2 and 3) was described in detail in45. The experiments were performed with a synthetic gas simulated by a mixture of CH4 and CO2. Before entering the MPS, a mixture of CH4 and CO2 in various proportions was mixed with water vapour (H2Osteam), together forming the working gas. The working gas in the quartz discharge tube was supplied by four tangential inlets, enabling the formation of a gas swirl flow through the tube. The mixture of CH4 and CO2 was introduced via three inlets while water vapour was introduced to one of the remaining inlets. The input CH4 volume content in the CH4:CO2 mixture was varied from 40 to 100%. The flow rate of the CH4:CO2 mixture through the MPS was varied from 3000 NL/h to 12,000 NL/h. In this system the H2Osteam with a temperature of 250–350 °C, was produced using an induction vaporizer, which was fed with water from a reservoir by a peristaltic pump. To avoid steam condensation the induction vaporizer outlet was placed as close as possible to the one MPS inlet which additionally was isolated. The H2Osteam consumption rate was varied from 0.5 to 3 kg/h. The microwave power absorbed by the microwave discharge changed from 4.5 to 6.5 kW, i.e. 2 kW more than in the previous dry reforming using a 2.45 GHz microwave system44.Under these conditions, the MPS 915 MHz worked stably at high flow rates (up to 12,000 NL/h) and high CH4 input volume content (up to 100%) in the CH4:CO2 mixture. The appropriate addition of H2Osteam to the CH4:CO2 mixture effectively prevented the soot formation and the extinction of the microwave discharge.
To determine the volume flow rates of the output gas components, an additional stream of N2 with a flow rate of 180 NL/h was introduced as a flow marker into the output gas (Figs. 1 and 2) like in44,46,47.
The concentrations of the output gas components were measured using a gas chromatography. For cleaning the output gas from the carbon soot formed during the methane conversion a lab-made centrifuge-type gas-soot separator was placed at the discharge quartz tube outlet. Its upper part was water cooling. Inside the gas-soot separator, the output gas was also mixed. The output gas was sampled after leaving the separator and the gas samples were collected into a Tedlar® bags to be analyzed using SRI 8610C and Shimadzu GC-2014 gas chromatographs.The presence of following components: H2, O2, N2, CO, CO2, CH4, C2H2, C2H4 and C2H6 in the output gas was detected and their volume flow rates were calculated.
To determine the effectiveness of the hydrogen production process the following parameters were used: hydrogen production rate, energy yield of the hydrogen production, CH4 conversion degree and H2 concentration in the output gas. The hydrogen production rate in g(H2)/h presents how many hydrogen is produced per hour. The energy yield of hydrogen production in g(H2)/kWh shows the amount of hydrogen produced consuming 1 kWh of microwave energy. The CH4 conversion degree is given by the ratio:
where [CH4]input is the number of CH4 moles at the MPS input, and [CH4]output is the number of CH4 moles at the MPS output. It is the percent of the CH4 introduced into the microwave plasma, that was converted into non-methane products. The H2 concentration in the output gas determinates the percentage of H2 by volume is in the output gas.
In this work, the energy yield of hydrogen production and the hydrogen production rate are two basic parameters taken into account when considering the practical suitability of the presented hydrogen production method. Certainly, these are not the only factors which have to be eventually considered by the industry when deciding the implementation of the method. Other factors, like raw material extraction, manufacturing and processing, transportation, usage and retail, and waste disposal may severe influence the final implementation of the method. A method with worse energy performance, expressed by the energy yield and production rate, may be competitive and worth implementation when the others factors do not prevail. It is worth mentioning that the economic landscape is changing, and new criteria for evaluating hydrogen production may appear. Extensive research has been carried out by many governmental and private organizations all over the world to assess the hydrogen production economy at present and propose a road map for the production and distribution of hydrogen, as well as fuel cells and hydrogen systems. The most advanced assessments of the present hydrogen policy needs have been made by the U.S. Department of Energy (DOE) (e.g.,5,48,49,50,51,52,53, the International Energy Agency (IEA) (e.g.,54,55), and the European Union Commission (e.g.,56,57). This policy aims to identify research pathways leading to hydrogen production technologies that produce near-zero net greenhouse gas emissions and use renewable energy sources, nuclear energy, and coal (with carbon dioxide capture, utilization, and storage—CCUS). DOE evaluates the competitiveness and industrial suitability of hydrogen production methods by relating the cost of production of a kg of hydrogen to the cost of production of a gallon of gasoline (3.79 L) in the USA. At present, the cost of production of a gallon of gasoline is about US$2 (excluding delivery, storage, and tax). A gallon of gasoline is approximately equal to a kilogram of hydrogen on an energy-containing basis. Several conventional methods of mass-scale hydrogen production from fossil-originated resources (methane, natural gas and higher hydrocarbons reforming, coal gasification reforming) are currently well developed. Their hydrogen production costs are similar to that of a gasoline gallon, i.e., about US$2/kg(H2). However, in the future, fossil-originated technologies for hydrogen production will have to be replaced by new technologies, which will reduce the dependency on fossil resources and their impact on climate and human health and contribute to the energy supplies' reliability, stability, and security. To assess the attractiveness of new hydrogen production methods for the energy market, DOE introduced a reference cost for hydrogen production. This reference cost was set at above mentioned US$2/kg(H2). Also other organizations (e.g. IEA, EU Commission) evaluate that the accepted cost of hydrogen production in the Net Zero Emission Scenario in 2050 will be about US$(1–2)/kg(H2). This means that for new environmentally friendly technologies to be accepted in the energy market in the near future, they must meet the hydrogen production cost target of at least USD 2/kg(H2) unless other economic factors prevail.
The reference hydrogen production cost introduced by DOE in energy-market oriented units (US$/kg(H2) was modified in the scientific and technical societies to a more practical energy parameter called the energy yield expressed in units of g(H2)/kWh24,25,58, also used in this work. This modification is especially useful when electric energy is used for hydrogen production. In such cases, it is convenient to express the DOE’s reference hydrogen production cost in inverse units, that is in g(H2)/kWh. Assuming that the pricing of 1 kWh of electric energy is US$ 0.12 (an averaged value over the last years in the USA), the reference hydrogen production cost of US$2/kg(H2) corresponds to the hydrogen energy yield of 60 g(H2)/kWh. Thus, this value is used in this paper as the DOE’s target for the hydrogen production cost that the newly offered hydrogen production methods must meet. When the pricing of 1 kWh of electric energy is lower due to the availability of a cheap energy source (e.g., from solar or wind farms), the DOE’s target will be more advantageous for the new methods.
Results and discussion
As it was found in44, in the dry reforming process of the CH4:CO2 mixture in the 915 MHz microwave system a CH4 input volume content above 40% causes an increase in the amount of soot formation in the area of the microwave discharge. It causes choking of the discharge, plasma instability, and accelerated degradation of the quartz discharge tube. As a result, there is a decrease in the energy parameters of H2 production. This can be seen in Fig. 4a,b (the curves labeled (green diamond) and (green square), respectively), showing a clear breakdown of the growth trend of H2 production rate, energy yield and H2 output concentration with the increase of CH4 input volume content above 40% in the CH4:CO2 mixture. Therefore, the value of 40% of the CH4 input volume content determines its upper limit for the dry reforming of biogas in 915 MHz microwave plasma. The growth trend of the H2 production rate, energy yield and H2 output concentration with the increase in the CH4 input volume content above 40% in the CH4:CO2 mixture could be sustained by removing the cause of its breakdown which is soot production. This can be done by eliminating the generation of soot in the microwave discharge with steam (H2Osteam). The addition of H2Osteam to the CH4:CO2 mixture in the microwave discharge causes oxidation of the soot produced in it, and, in consequently, quenching of the microwave discharge at high CH4 input volume contents ceases. This process is called steam reforming of the CH4:CO2 mixture.
H2 production rate, energy yield (a), CH4 conversion degree and H2 output concentration (b) as a function of the CH4 input volume content in the CH4:CO2 mixture for various H2Osteam consumption rates (Pabs—6.5 kW, total flow rate—6000 NL/h). The green measuring points (green diamond; green square) correspond to the dry reforming of the CH4:CO2 mixture [a].
Figure 5 shows the minimal amounts of H2Osteam which must be supplied to the microwave discharge in the CH4:CO2 mixture in order to eliminate the formation of soot and enable the CH4 input volume content to be increased above 40% during the microwave plasma reforming of the CH4:CO2 mixture. The diagram in Fig. 5 defines the areas of stable operation of the MPS during the steam reforming of the CH4:CO2 mixture for various CH4 input volume contents for total flow rates of CH4 input volume contents of 3000 NL/h and 6000 NL/h. It is worth explaining that the higher minimal H2Osteam consumption rate (kg/h) for the CH4 input volume content (%) of 80% at the CH4:CO2 mixture total flow rate of 6000 NL/h than that for the CH4:CO2 mixture total flow rate of 3000 NL/h is in that the absolute CH4 amount in NL/h for 6000 NL/h is twice that for 3000 NL/h. Thus, the former needs more H2Osteam to effectively suppress soot formation of from a higher number of CH4 molecules.
As it seen from Fig. 4a, at a total flow rate of the CH4:CO2 mixture of 6000 NL/h the addition of H2Osteam with a flow rate of 2 kg/h ensures stable operation of the 915 MHz MPS with a CH4 input volume content up to 60%. It is 20% higher than the CH4 input volume content limit for stable operation of the dry reforming of the CH4:CO2 mixture. The H2Osteam consumption rate of 3 kg/h ensures stable operation with a CH4 input volume content reaching 75%-80%. The increase in the CH4 input volume content from 40 to 75% almost doubled the H2 production rate and energy yield to 239 g(H2)/h and 36.8 g(H2)/kWh, respectively (Fig. 4a). These were the best results in terms of the H2 production rate and energy yield archived in this experiment (see Table 1). Figure 4a shows that above the CH4 volumetric content of 75%, the growth trend of the H2 production rate and energy yield collapses at the H2Osteam consumption rate of 3 kg/h. A possible breakdown of this trend is the insufficient amount of H2Osteam in the microwave discharges to stop soot production. However, this supposition could not be verified in this experiment due to the H2O vaporizer capacity being limited to 3 kg/h.
Figure 4b shows that in the dry reforming of the CH4:CO2 mixture, the high ability of the microwave discharge to CH4 conversion, expressed by the CH4 conversion degree (in %), weakens when the CH4 volume content in the CH4:CO2 mixture increases up to 40%. However, as can be easily calculated, despite the decreasing CH4 conversion degree, the absolute amount of CH4 (in NL/h) in the CH4:CO2 mixture increases resulting in an increase in the absolute amount of the converted CH4 (in NL/h). That is the reason that despite the decreasing CH4 conversion degree the H2 output concentration increases with the CH4 input volume content increase. For this reason, despite the decreasing degree of CH4 conversion, the output H2 concentration increases with the increasing CH4 input volume content.
Figure 4b also shows that after adding an appropriate amount of H2O vapour to the CH4:CO2 mixture, the CH4 conversion rate decreased with the increase in the CH4 input volume in the CH4:CO2 mixture above 40%. With a sufficient amount of H2Osteam the CH4 conversion degree is about 50% for the CH4 input volume content in the range of 40% up to 80%. Adding an appropriate amount of H2Osteam to the CH4:CO2 mixture for CH4 input volume contents higher than 40% causes the H2 output concentration to continue its increasing as the CH4 input volume content increases. With a CH4 volume content of 80%, the total flow rate of the CH4:CO2 mixture of 6000 NL/h, and an H2Osteam consumption rate of 3 kg/h, the H2 output concentration is about 40%.
It follows from the above text that the addition of H2Osteam to the microwave discharge in the CH4:CO2 mixture plays a crucial role in stabilizing the microwave discharge at higher contents of CH4. The minimal H2Osteam consumption rates that ensures the stable operation of the MPS during the steam reforming of the CH4:CO2 mixture at various CH4 input volume contents are presented in Fig. 5. As seen from Fig. 6a,b, adding H2Osteam to the microwave discharge in the CH4:CO2 mixture above the minimal amount that ensures the stable operation of the MPS for a given CH4 content, almost does not change the H2 production rate, energy yield, CH4 conversion degree, and H2 output concentration. Also, the percentages of the major components of the output gas does not change with the increasing amount of H2Osteam (Table 2 for a CH4 input volume content of 50% as a typical example). In other words, the surplus of amount of H2Osteam over the minimal value in the microwave discharge in the CH4:CO2 mixture does not directly improve the hydrogen production parameters. On the other hand, the H2Osteam was found to be beneficial for hydrogen production by suppressing soot production and ensuring the introduction of more CH4 into the microwave discharge. In consequence, more CH4 in the CH4:CO2 mixture results in better hydrogen production parameters (Fig. 4a,b).
Thus, the results presented in Fig. 6a,b show that introducing more H2Osteam than the minimal amount for a given CH4 input volume content is useless in terms of the meaningful improvement of the H2 production rate, and energy yield, CH4 conversion degree, and H2 output concentration.
For example, a minimal H2Osteam consumption rate of 1 kg/h is sufficient to provide the stable operation of the MPS with the best values of the H2 production parameters in the case of 60% of the CH4 input volume content at 6.5 kW of the absorbed power, and 6000 NL/h of the total flow rate of the CH4:CO2 mixture.
Within the measurement error (± 5%), the CH4 conversion degree and H2 output concentration were constans with the increasing H2Osteam consumption rate at a constant Pabs, CH4 input volume content and CH4:CO2 mixture total flow rate (Fig. 6b). It suggests that the H2 produced in the steam reforming of the CH4:CO2 mixture comes mainly from the conversion of CH4, not from the conversion of H2Osteam.
Figure 7a shows that, within the experimental error, the H2 production rate and energy yield remain constant with an increasing total flow rate of the CH4:CO2 mixture in the range of 3000–12,000 NL/h for the case of 6.5 kW of Pabs, 50% of the input volume CH4 and 2 kg/h of the consumption rate of H2Osteam. Under the same conditions, the CH4 conversion degree and H2 output concentration decrease considerably with the increasing total flow rate of the CH4:CO2 mixture (Fig. 7b). It is worth noting that although the H2 production rate and energy yield remain constant with an increasing total flow rate of the CH4:CO2 mixture in the range of 3000–12,000 NL/h, the percentage of unconverted CH4 (in other words unutilized feedstock) increases from 25% at 3000 NL/h to 57% at 12,000 NL/h. This means that the best performance of the MPS in terms of the H2 production rate, energy yield, CH4 conversion degree, and H2 output concentration was obtained for low CH4:CO2 mixture flow rates. This was probably due to the sufficiently long residence time of the CH4:CO2 mixture with a low flow rate in the microwave discharge, the absorbed microwave power Pabs of which was limited to 6.5 kW. CH4:CO2 mixtures with higher flow rates that remain for a shorter time in the microwave discharge would require a more powerful discharge to achieve a high CH4 conversion rate. However, if the flow rates of the CH4:CO2 mixture were too low, the cooling swirl flow inside the quartz tube could not be created and the MPS could not function properly, mainly due to overheating which could also seriously damage the MPS.
We found out that for an absorbed microwave power of 6.5 kW the minimal total flow of the CH4:CO2 mixture ensuring safe and stable operation of the MPS without overheating and damage was 3000 NL/h. As seen from Fig. 8a, for a constant CH4 input volume content (in %) an increase in the total flow rate of the CH4:CO2 mixture from 3000 NL/h to 6000 NL/h did change the H2 production rate and energy yield. Note, however, that at a given CH4 input volume content, the absolute CH4 amount in NL/h for 3000 NL/h is half that for 6000 NL/h. This means that when working with the total flow rate of the CH4:CO2 mixture of 3000 NL/h, the same H2 production rate and energy yield as those with a total flow rate of 6000 NL/h are obtained with the absolute CH4 input amount in NL/h two times lower. This is advantageous with regard to the final H2 production cost that eventually also includes the cost of the CH4 feedstock.
H2 production rate, energy yield (a), CH4 conversion degree and H2 output concentration (b) as a function of the CH4 input volume content (%) in the CH4:CO2 mixture for a recommended total flow rate of 3000 NL/h and, for comparison, for 6000 NL/h (Pabs—6.5 kW). The applied H2Osteam consumption rates ensure stable operation of the MPS at a given CH4 input volume content.
As seen from Fig. 8a, in both cases (3000 NL/h and 6000 NL/h), the H2 production rate and energy yield increase with the increasing CH4 input volume content (with sufficient H2Osteam for each CH4 input volume content).
In the dry reforming of the CH4:CO2 mixture (at the CH4 input volume content of 40%)—Fig. 8b, the CH4 conversion degree for the total flow rate of the CH4:CO2 mixture of 3000 NL/h is higher (about 90%) than that for 6000 NL/h (about 55%). It is possibly because of the shorter residence time of the CH4:CO2 mixture in the microwave discharge at 6000 NL/h and lower specific energy input. The CH4 conversion degrees for 3000 NL/h and 6000 NL/h decrease as the CH4 input volume content (with sufficient H2Osteam) increases above 40%. Then, they remain constant for the CH4 input volume contents higher than 50%. In this range, the CH4 conversion degree for the total flow rate of the CH4:CO2 mixture of 3000 NL/h is higher (about 70%) than that for 6000 NL/h (about 50%). This means that the amounts of unconverted CH4 are about 30% and 50% for 3000 NL/h and 6000 NL/h, respectively. The high amount (50%) of unconverted CH4 at 6000 NL/h shows the poor performance of the MPS at this total flow rate in terms of the utilization of the CH4 feedstock.
Although the CH4 conversion degree (in %) is approximately constant as the CH4 input volume content increases (from 50 to 100% with sufficient H2Osteam) for the total flow rates of 3000 NL/h and 6000 NL/h, it can be calculated using Fig. 8b that in both cases the absolute amount of converted CH4 (in NL/h) increases with the increasing CH4 input volume content. This results in an increased H2 production rate and energy yield at higher CH4 input volume contents (Fig. 8a).
For both total flow rates, the H2 output concentration increases with the increasing CH4 input volume content. In the case of the total flow rate of 3000 NL/h, the H2 output concentration reaches about 60%, while for 6000 NL/h it is always about 16% lower. It is worth remembering that the total flow rates of the CH4:CO2 mixtures of 3000 NL/h and 6000 NL/h refer to the MPS input, while the H2 output concentrations are measured at the MPS output, where the output gas volume flow rates differ from those at the MPS input. For example, in the case of the CH4 input volume content of 60% and the H2Osteam consumption rate of 2 kg/h, the output gas volume flow rates are 4860 NL/h and 7020 NL/h for the total flow rates of the CH4:CO2 mixtures of 3000 NL/h and 6000 NL/h, respectively. The greater increase in the volume of the output gas for the CH4:CO2 mixture with 3000 NL/h can be explained by the residence time in the microwave discharge and specific energy input, twice as long and twice as that of the mixture with 6000 NL/m, respectively. It results in greater heating and an increase in the volume of the output gas due to the higher decomposition of the CH4:CO2 mixture.
Figures 7b and 8b showed the high amount of unutilized CH4 feedstock (from 25% at 3000 NL/h to 57% at 12,000 NL/h) during the steam reforming of the CH4:CO2 mixture in the presented MPS. This has a negative impact on the overall assessment of the performance of the MPS. However, the customary energy parameters (H2 production rate and energy yield) describing the performance of the MPS do not provide information on the performance of the MPS in terms of the utilization of the CH4 feedstock. Poor utilization of the CH4 feedstock causes an additional the cost of H2 production.
Therefore, we propose a new parameter, called the energy–CH4 feedstock consumption yield, to describe the MPS performance, which also takes into account a factor of the utilization of the CH4 feedstock. The energy–CH4 feedstock consumption yield shows the amount of hydrogen produced in a unit time (1 h) with the consumption of 1 kWh of microwave energy and 1 kNL/h of CH4 feedstock (or the ratio of the energy yield to the amount of the CH4 feedstock consumed in a unit time (1 h)), expressed in g(H2)/h/[kNL(CH4)/h × kWh]. For example, the energy–CH4 feedstock consumption yields for various total flow rates of the CH4:CO2 mixture under the conditions: Pabs—4.5 kW, H2Osteam consumption—2 kg/m, CH4 input volume content—50%, Fig. 7a) are shown in Table 3. As can be seen from this Table, under the same conditions, a fourfold increase in the total flow rate of the CH4:CO2 mixture (from 3000 to 12,000 NL/h) results in an almost fourfold reduction in the energy–CH4 feedstock consumption yield. In other words, when increasing 4 times the total flow rate, almost 4 times less H2 is produced from a unit of the CH4 feedstock. This confirms the poor performance of the MPS at higher total flow rates of the CH4:CO2 mixtures with respect to the utilization of the CH4 feedstock, already reported above when discussing the results shown in Fig. 7a,b. As shown, the energy–CH4 feedstock consumption yield can be a useful parameter to evaluate the performance of the MPS.
Figure 9 shows the H2 production parameters as a function of the absorbed microwave power Pabs for the total flow rate—6000 NL/h, CH4 input volume content—50%, and H2Osteam consumption rate—2 kg/h. As it can be seen, the H2 production rate, CH4 conversion degree and H2 output concentration increase with the increasing Pabs. However, the energy yield remains constant despite the increase in Pabs. This all means that operating with Pabs higher than 6.5 kW may result in higher H2 production (about 300 g(H2)/h at 10 kW, assuming a linear increase in the H2 production as shown in Fig. 9). However, no increase in the energy yield above 30 g(H2)/kWh should be expected, which is clearly lower than that assumed by the DOE.
Summary and conclusions
This work is devoted to the development of microwave plasma-based technology aimed at the efficient production of hydrogen from biogas, which can contain up to 70% CH4. In the so-called upgraded biogas or bio-methane, the CH4 content is up to 98%. Our research to date on the suitability of microwave-based technology to produce H2 from the so-called synthetic biogas (a mixture of CH4 and CO2 in various proportions)42,44, showed that real progress in the efficiency of H2 production could be made if a microwave plasma system of a relatively high microwave power with a relatively high CH4 input volume content (at least 70%) is used. In this work, we tested the performance of a microwave plasma system operating at a frequency of 915 MHz as a tool for efficient production of H2 in the steam reforming of synthetic biogas. The results of these tests, widely described above can be concluded as follows.
-
1.
By using a 915 MHz microwave plasma system we could test steam reforming of a CH4:CO2 mixture with an absorbed microwave power Pabs up to 6.5 kW, i.e. 2 kW more than in our previous 2.45 GHz microwave system42. In this work, we found that the H2 production rate increased as the absorbed microwave power increased to 6.5 kW.
-
2.
The appropriate addition of H2Osteam to the synthetic biogas effectively prevented soot formation and the extinction of the microwave discharge, which previously in the dry reforming of synthetic biogas44 limited operation of the MPS to the CH4 contents of not higher than 40% (in contrary to 100% at present work).
-
3.
We found that there is a minimal H2Osteam consumption rate for a given CH4 input volume content, which ensures stable operation of the MPS (no soot).
-
4.
The experiments did not show that increasing the amount of H2Osteam rate above the minimal value for a given CH4 input volume content results in an increase in the H2 production rate, energy yield, CH4 conversion degree, and H2 output concentration. However, the H2Osteam suppresses soot production and ensures the introduction of more CH4 into the microwave discharge. In consequence, more CH4 in the CH4:CO2 mixture results in better hydrogen production parameters.
-
5.
Within the scope of the above working conditions, the best results obtained in terms of the H2 production rate, energy yield, and CH4 conversion degree were 239 g[H2]/h, 36.8 g[H2]/kWh, and 74.3%, respectively (see Table 1). This means that the application of the steam reforming, instead of the dry reforming, resulted in a 1.5-fold increase of the H2 production rate and the corresponding energy yield.
-
6.
Within the limits of the absorbed microwave power of 6.5 kW and H2Osteam consumption rate of 3 kg/h in the 915 MHz MPS used in this experiment, the best performance of the MPS in terms of the H2 production rate, energy yield, CH4 conversion degree, and H2 output concentration was obtained for low CH4:CO2 mixture flow rates.
-
7.
The results showed a high amount of unutilized CH4 feedstock (from 25% at 3000 NL/h to 57% at 12,000 NL/h of the total flow rate) during the steam reforming of the CH4:CO2 mixture in the presented MPS. Therefore, we introduced a new parameter, called the energy–CH4 feedstock consumption yield, to describe the MPS performance, which also takes into account a factor of the utilization of the CH4 feedstock. Our calculations showed that under the same conditions, a fourfold increase in the total flow rate of the CH4:CO2 mixture (from 3000 NL/h to 12,000 NL/h) results in an almost fourfold reduction in the energy–CH4 feedstock consumption yield. This confirmed the poor performance of the MPS at higher total flow rates of the CH4:CO2 mixtures with respect to the utilization of the CH4 feedstock. The energy–CH4 feedstock consumption yield can be a useful parameter to evaluate the performance of an MPS.
-
8.
Despite the progress obtained in this work (see Table 4), the energy yield of H2 production by the microwave plasma-based technologies (about 40 g[H2]/kWh) has to be improved to meet the Department of Energy’s (USA) requirement (the target of 60 g(H2)/kWh).
-
9.
Better results than those obtained in this work can be expected for a higher absorbed microwave power Pabs (around 10 kW), higher total flow rates of the CH4:CO2 mixture with an optimal CH4 input volume content, and correspondingly higher H2Osteam consumption rates (higher than 3 kg/h).
In summarizing, taking into account the above, one could consider microwave (915 MHz) atmospheric pressure plasma-based technology for hydrogen (H2) production from the so-called synthetic biogas as one of the promising techniques of hydrogen production for further investigations.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Holladay, J. D., Hu, J., King, D. L. & Wang, Y. An overview of hydrogen production technologies. Catal. Today 139, 244–260 (2009).
Abbas, H.F. & Wan Daud W.M.A. Hydrogen production by methane decomposition: A review. Int. J. Hydrogen Energy 35, 1160–1190 (2010).
Dincer, I. & Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrogen Energy 40, 11094–11111 (2015).
Nikolaidis, P. & Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 67, 597–611 (2017).
Randolph, K. Hydrogen production. In: Annual Merit Review and Peer Evaluation Meeting. U.S. DOE; May 16, 2013. http://www.hydrogen.energy.gov/pdfs/review13/pd000_randolph_2013_o.pdf.
Sengodan, S. et al. Advances in reforming and partial oxidation of hydrocarbons for hydrogen production and fuel cell applications. Renew. Sustain. Energy Rev. 82, 761–780 (2018).
Ono, Y., Haneda, T., Ikegami, T. & Akisawa, A. Potential of producing hydrogen for fuel-cell vehicles by residential fuel cell co-generation utilizing idle capacity. J. Japan Inst. Energy 96(10), 478–486 (2017).
Kirillov, V. A. & Shigarov, A. B. Biofuels as a promising source of hydrogen for fuel cell power plants. Theor. Found. Chem. Eng. 50(4), 351–365 (2016).
Ahmed, S. & Krumpelt, M. Hydrogen from hydrocarbon fuels for fuel cells. Int. J. Hydrogen Energy 26, 291–301 (2001).
Divya, D., Gopinath, L. R. & Christy, P. M. A review on current aspects and diverse prospects for enhancing biogas production in sustainable means. Renew. Sustain. Energy Rev. 42, 690–699 (2015).
Balata, M. & Balata, H. Biogas as a renewable energy source—a review. Energy Source Part A 31, 1280–1293 (2009).
Alves, H. J. et al. Overview of hydrogen production technologies from biogas and the applications in fuel cells. Int. J. Hydrogen Energy 38, 5215–5225 (2013).
Mengistua, M. G., Simanea, B., Esheteb, G. & Worknehc, T. S. A review on biogas technology and its contributions to sustainable rural livelihood in Ethiopia. Renew. Sustain. Energy Rev. 48, 306–316 (2015).
Woolcock, P. J. & Brown, R. C. A review of cleaning technologies for biomass-derived syngas. Biomass Bioenergy 52, 54–84 (2013).
Dors, M., Izdebski, T., Berendt, A. & Mizeraczyk, J. Hydrogen production via biomethane reforming in DBD reactor. Int. J. Plasma Environ. Sci. Technol. 6(2), 93–97 (2012).
Pakhare, D. & Spivey, J. Review paper: A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 43, 7813–7837 (2014).
Cormier, J. M. & Rusu, I. Syngas production via methane steam reforming with oxygen: Plasma reactors versus chemical reactors. J. Phys. D: Appl. Phys. 34, 2798–2803 (2001).
Deminsky, M., Jivotov, V., Potapkin, B. & Rusanov, V. Plasma-assisted production of hydrogen from hydrocarbons. Pure Appl. Chem. 74, 413–418 (2002).
Chang, J.S. & Urashima, K. Plasma fuel reforming: a critical review. Trans. Inst. Fluid-Flow Mach. 119, 17–28 (2007).
Rusu, I. Development trends of cold plasma reactors in the global context of carbon emission reduction. Environ. Eng. Manag. J. 6(3), 211–217 (2007).
Petitpas, G. et al. A comparative study of non-thermal plasma assisted reforming technologies. Int. J. Hydrogen Energy 32, 2848–2867 (2007).
Chen, H. L., Lee, H. M., Chen, S. H., Chao, Y. & Chang, M. B. Review of plasma catalysis on hydrocarbon reforming for hydrogen production—interaction, integration, and prospects. Appl. Catal. B: Environ. 85, 1–9 (2008).
Tao, X. et al. CH4-CO2 reforming by plasma—challenges and opportunities. Prog. Energy Combust. Sci. 37, 113–124 (2011).
Mizeraczyk, J., Urashima, K., Jasinski, M. & Dors, M. Hydrogen production from gaseous fuels by plasmas—a review. Int. J. Plasma Environ. Sci. Technol. 8(2), 89–97 (2014).
Mizeraczyk, J. & Jasiński, M. Plasma processing methods for hydrogen production. Eur. Phys. J. Appl. Phys. 75, 24702 (2016).
Snoeckx, R. & Bogaerts, A. Plasma technology—a novel solution for CO2 conversion?. Chem. Soc. Rev. 46(19), 5805–5863 (2017).
de la Fuente, J. F., Kiss, A. A., Radoiu, M. T. & Stefanidis, G. D. Microwave plasma emerging technologies for chemical processes. J. Chem. Technol. Biotechnol. 92, 2495–2505 (2017).
Nahar, G., Mote, D. & Dupont, V. Hydrogen production from reforming of biogas: Review of technological advances and an Indian perspective. Renew. Sustain Energy Rev. 76, 1032–1052 (2017).
Chen, G., Tu, X., Homm, G. & Weidenkaff, A. Plasma pyrolysis for a sustainable hydrogen economy. Nat. Rev. Mater 7, 333–334 (2022).
Ghorbanzadeh, A. M., Lotfalipour, R. & Rezaei, S. Carbon dioxide reforming of methane at near room temperature in low energy pulsed plasma. Int. J Hydrogen Energy 34, 293–298 (2009).
Li, M., Xu, G., Tian, Y., Chen, L. & Fu, H. Carbon dioxide reforming of methane using DC corona discharge plasma reaction. J Phys. Chem. A 108, 1687–1693 (2004).
Nguyen, H. H., Nasonova, A., Nah, I. W. & Kim, K.-S. Analysis on CO2 reforming of CH4 by corona discharge process for various process variables. J. Ind. Eng. Chem. 32, 58–62 (2015).
Chung, W.-J., Park, H.-W. & Park, D.-W. Effects of arc discharge mode on the efficiency of biogas reforming in an AC-pulsed arc plasma system. Plasma Chem. Plasma Process. 37, 383–399 (2017).
Zhu, F. et al. Plasma-catalytic reforming of CO2-rich biogas over Ni/c-Al2O3 catalysts in a rotating gliding arc reactor. Fuel 199, 430–437 (2017).
Tan, Z. & Ai, P. CO2 reforming of biogas to obtain synthesis gas using non-thermal plasma. J Energy Inst. 90, 864–874 (2017).
Mao, S., Tan, Z., Zhang, L. & Huang, Q. Plasma-assisted biogas reforming to syngas at room temperature condition. J Energy Inst. 91, 172–183 (2018).
Wang, Q., Yan, B., Jin, Y. & Cheng, Y. Investigation of dry reforming of methane in a dielectric barrier discharge reactor. Plasma Chem. Plasma Process. 29, 217–228 (2009).
Goujard, V., Tatiboue, J.-M. & Batiot-Dupeyrat, C. Carbon dioxide reforming of methane using a dielectric barrier discharge reactor: Effect of helium dilution and kinetic model. Plasma Chem. Plasma Process. 31, 315–325 (2011).
Gallon, H. J., Tu, X. & Whitehead, J. C. Effects of reactor packing materials on H2 production by CO2 reforming of CH4 in a dielectric barrier discharge. Plasma Processes Polym. 9, 90–97 (2012).
Zhang, J.-Q., Zhang, J.-S., Yang, Y.-J. & Liu, Q. Oxidative coupling and reforming of methane with carbon dioxide using a pulsed microwave plasma under atmospheric pressure. Energy Fuels 17, 54–59 (2003).
Jasiński, M., Czylkowski, D., Hrycak, B., Dors, M. & Mizeraczyk, J. Atmospheric pressure microwave plasma source for hydrogen production. Int. J. Hydrogen Energy 38, 11473–11483 (2013).
Czylkowski, D., Hrycak, B., Jasiński, M., Dors, M. & Mizeraczyk, J. Microwave plasma-based method of hydrogen production via combined steam reforming of methane. Energy 113, 653–661 (2016).
Chun S.M., Hong Y.Ch. & Choi D.H. Reforming of methane to syngas in a microwave plasma torch at atmospheric pressure. J CO2 Util. 19, 221–229, (2017).
Hrycak, B., Czylkowski, D., Mizeraczyk, J., Jasiński, M. & Dors, M. Hydrogen production via synthetic biogas reforming in atmospheric-pressure microwave (915 MHz) plasma at high gas-flow output. Plasma Chem. Plasma Process. 39, 695–711 (2019).
Miotk, R., Jasiński, M. & Mizeraczyk, J. Optical emission spectroscopy of microwave (915 MHz) plasma in atmospheric pressure nitrogen with addition of ethanol vapour. Acta Phys. Pol. A 125, 1329–1331 (2014).
Li, X.-S., Zhu, B., Shi, Ch., Xu, Y. & Zhu, A.-M. Carbon dioxide reforming of methane in Kilohertz spark-discharge plasma at atmospheric pressure. AIChE J. 57, 2854–2860 (2011).
Lian, H.-Y., Li, X.-S., Liu, J.-L., Zhu, X. & Zhu, A.-M. Oxidative pyrolysis reforming of methanol in warm plasma for an on-board hydrogen production. Int. J. Hydrogen Energy 42, 13617–13624 (2017).
U.S. Department of Energy, Hydrogen and Fuel Cells Program, DOE/EE-0651, September 2011, www.hydrogen.energy.gov.
U.S. Department of Energy, Hydrogen and Fuel Cells Program Record #12024: Hydrogen Production Cost Using Low-Cost Natural Gas, 2012, http://www.hydrogen.energy.gov/pdfs/12024_h2_production_cost_natural_gas.pdf.
U.S. Department of Energy, Hydrogen and Fuel Cells Program #12001, 2012, http://www.hydrogen.energy.gov/pdfs/12001_h2_pd_cost_apportionment.pdf.
U.S. DRIVE Partnership, Hydrogen Production Technical Team Roadmap, November 2017, https://www.energy.gov/sites/prod/files/2017/11/f46/HPTT%20Roadmap%20FY17%20Final_Nov%202017.pdf.
U.S. Department of Energy, Hydrogen 2021 Annual Merit Review and Peer Evaluation (AMR) Report: Hydrogen Technologies, https://hydrogen.energy.gov/pdfs/review21/2021-amr-04-hydrogen-technologies.pdf.
DOE National Clean Hydrogen Strategy and Road Map, https://hydrogen.energy.gov/roadmaps_vision.html.
IEA (International Energy Agency), The Future of Hydrogen, Seizing today’s opportunities, France, June 2019, www.iea.org.
IEA (International Energy Agency), The Global Hydrogen Review, October 2021, www.iea.org.
Communication from The Commission to The European Parliament, The Council, The European Economic And Social Committee and The Committee of The Regions, A hydrogen strategy for a climate-neutral Europe, Brussels, 08. 07. 2020, https://op.europa.eu/en/publication-detail/-/publication/5602f358-c136-11ea-b3a4-01aa75ed71a1/language-en.
Fuel Cells and Hydrogen 2 Joint Undertaking, Hydrogen roadmap Europe: a sustainable pathway for the European energy transition, Publications Office, 2019, fch.europa.eu, 2019. https://doi.org/10.2843/341510
Bespalko, S. & Mizeraczyk, J. Overview of the hydrogen production by plasma-driven solution electrolysis. Energies 15, 7508 (2022).
Acknowledgements
The authors thank Dr. Sergii Bespalko for the fruitful discussion on the hydrogen production rate and energy yield. The project was financed within the program of the Ministry of Science and Higher Education called “Regionalna Inicjatywa Doskonałości” in the years 2019–2022; the project number was 006/RID/2018/19 and the sum of financing was PLN 11,870,000. Moreover, this work was supported by Institute of Fluid Flow Machinery, Polish Academy of Sciences under programme IMP PAN O3Z1T1.
Author information
Authors and Affiliations
Contributions
All the authors made substantial contributions to the experimental investigations as well as to the preparing of the submitted manuscript. All of them have approved the submitted version of the manuscript. The particular contribution of the authors was as follows: B.H.—corresponding author, preparation of the experimental setup, conduction of the plasma reforming experiments, GC analysis, analysis and interpretation of the experimental data, preparation of the manuscript tables and figures, writing the main text of the manuscript. J.M.—funding acquisition, project administration, conceptualization and methodology of the research, interpretation of the experimental data, preparation of the manuscript tables, writing the main text of the manuscript. D.C.—preparation of the experimental setup, conduction of the plasma reforming experiments, writing the part of the manuscript. M.D.—analysis and interpretation of the experimental data, supervision of the GC analysis, writing the part of the manuscript. M.B.—preparation of the experimental setup, conduction of the plasma reforming experiments. M.J.—funding acquisition, conceptualization and methodology of the research, supervision all the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hrycak, B., Mizeraczyk, J., Czylkowski, D. et al. Hydrogen production by the steam reforming of synthetic biogas in atmospheric-pressure microwave (915 MHz) plasma. Sci Rep 13, 2204 (2023). https://doi.org/10.1038/s41598-023-29433-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-29433-y
- Springer Nature Limited