Abstract
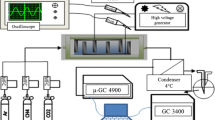
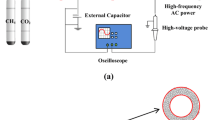
Low temperature conversion of CH4 and CO2 was investigated in a coaxial dielectric barrier discharge reactor at ambient pressure. Main parameters, including the input power, the residence time, the discharge gap, the molar ratio of the feed gases and the multi-stage ionization design were evaluated to understand the ways to improve the conversion of greenhouse gases and reduce the output of by-products. At certain input power, the conversion of CH4 and CO2 can reach 0.797 and 0.527, respectively, when the molar ratio of CH4/CO2 is one. When this ratio was low to 1:5, the conversion of CH4 was promoted to 0.843 and the selectivity to CO and H2 was almost 100%. The multi-stage ionization favored the conversion of CO2, which would also be an efficient design to promote the selectivity to the main products such as CO and H2 and suppress the selectivity to the by-products.










Similar content being viewed by others
References:
Halmann MM, Steinberg M (1999) Greenhouse gas carbon dioxide mitigation: science and technology. CRC press, Boca Raton
Haugan PM, Thorkildsen F, Alendal G (1995) Energy Convers Manag 36:461–466
Seifritz W (1990) Nature 345:486
Martin MH (1993) Chemical fixation of carbon dioxide: methods for recycling CO2 into useful products. CRC press, Boca Raton
Xu XD, Moulijn JA (1996) Energy Fuel 10:305–325
Rojey A, Jaffret C, Cornot-Gandolphe S, Durand B, Jullian S, Valais M (1997) Natural gas: production, processing, transport. Editions TECHNIP, Paris, pp 20–23
Wender I (1996) Fuel Process Technol 48:189–297
Barrai F, Jackson T, Whitmore N, Castaldi MJ (2007) Catal Today 129:391–396
Pechimuthu NA, Pant KK, Dhingra SC (2007) Ind Eng Chem Res 46:1731–1736
Edwards JH, Maitra AM (1995) Fuel Process Technol 42:269–289
Ashcroft AT, Cheetham AK, Green MLH, Vernon PDF (1991) Nature 352:225–226
Eliasson B, Kogelschatz U (1991) IEEE T Plasma Sci 19:1063–1077
Bo Z, Yan J, Li X, Chi Y, Cen K (2008) Int J Hydrog Energy 33:5545–5553
Rueangjitt N, Akarawitoo C, Sreethawong T, Chavadej S (2007) Plasma Chem Plasma Process 27:559–576
Rueangjitt N, Sreethawong T, Chavadej S (2008) Plasma Chem Plasma Process 28:49–67
Ghorbanzadeh AM, Modarresi H (2007) J Appl Phys 101:1233303
Savinov SY, Lee H, Song HK, Na BK (1999) Ind Eng Chem Res 38:2540–2547
Tsai CH, Hsieh TH (2004) Ind Eng Chem Res 43:4043–4047
Zhang JQ, Zhang JS, Yang YJ, Liu Q (2003) Energy Fuel 17:54–59
Yang Y (2002) Ind Eng Chem Res 41:5918–5926
Malik MA, Jiang XZ (1999) Plasma Chem Plasma Process 19:505–512
Huang AM, Xia GG, Wang JY, Suib SL, Hayashi Y, Matsumoto H (2000) J Catal 189:349–359
Zhou ML, Xue B, Kogelschatz U, Eliasson B (1998) Energy Fuel 12:1191–1199
Gesser HD, Hunter NR, Probawono D (1998) Plasma Chem Plasma Process 18:241–245
Liu CJ, Xue BZ, Eliasson B, He F, Li Y, Xu GH (2001) Plasma Chem Plasma Process 21:301–310
Kraus M, Eliasson B, Kogelschatz U, Wokaun A (2001) Phys Chem Chem Phys 3:294–300
Song HK, Lee H, Choi J, Na B (2004) Plasma Chem Plasma Process 24:57–72
Kogelschatz U, Eliasson B, Egli W (1997) J Phys IV 7:47–66
Kogelschatz U (2000) IEEE conference record—abstracts 27th IEEE international conference on plasma science (Cat. No.00CH37087):81
Manley TC (1943) Trans Electrochem Soc 84:83–96
Kraus M, Egli W, Haffner K, Eliasson B, Kogelschatz U, Wokaun A (2002) Phys Chem Chem Phys 4:668–675
Weast RC (1968) Handbook of chemistry and physics, 49th edn. Chemical Rubber Company
Barton MJ, Engel AV (1970) Phys Lett A 32:173–174
Fridman AA, Resanov VD (1994) Pure Appl Chem 66:1267–1278
Acknowledgments
Financial support from CNPC Innovation Foundation is acknowledged. The authors would also like to thank the continuous support from PetroChina.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Q., Yan, BH., Jin, Y. et al. Investigation of Dry Reforming of Methane in a Dielectric Barrier Discharge Reactor. Plasma Chem Plasma Process 29, 217–228 (2009). https://doi.org/10.1007/s11090-009-9173-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-009-9173-3