Abstract
Background
Vascular calcification (VC) is a major predictor of cardiovascular diseases that represent the principal cause of mortality among type-2 diabetic patients. Accumulating data suggest the vital role of some microRNAs on vascular calcification as an epigenetic regulator. Thus, we assessed herein, the role of serum miR-433-3p in vascular calcification in type-2 diabetic patients.
Methods
Twenty healthy subjects (control group) and forty diabetic patients (20 without VC and 20 with VC) were involved in the study. miR-433-3p gene expression was measured. Runx2, Dickkopf-1 (DKK1), β-catenin, Receptor activator of nuclear factor kappa-B ligand (RANKL), and osteoprotegerin (OPG) levels in serum were assessed by ELISA technique.
Results
Diabetes patients had significantly lower levels of miR-433-3p expression in comparison to the control group, with the lowest levels being found in diabetic patients with VC. Furthermore, Runx2, β-catenin, and RANKL levels were significantly increased with concomitant lower DKK1 and OPG levels detected in the two diabetic groups especially those with VC.
Conclusion
Collectively, the study documented that down-regulation of miR-433-3p may contribute to the development of VC through activating WNT/β-Catenin and RANKL/RANK/OPG signaling pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vascular calcification (VC) is a major causative risk factor and a strong predictor of cardiovascular diseases (CVD), which remain the main cause of mortality in patients with type 2 diabetes [1]. It is defined as an ectopic abnormal deposit of calcium and phosphate minerals that leads to thickening and dysfunction of blood vessels, which is an important key factor contributing to CVD complications and mortality. Calcification of the vessel wall can occur in both the intimal and medial layers. However, it may occur also in heart valves [2].
Although VC occurs as a part of the normal aging process, diseases like diabetes, chronic kidney disease, and hypertension can also hasten their onset. [3]. In diabetic patients, VC commonly exists in the aortic wall, coronary arteries, and lower extremity arteries. Indeed, hyperglycemia has been reported to be one of the metabolic states promoting aortic valve fibrosis and calcification. [4].
Noteworthy, vascular calcification has complex and numerous pathogenic mechanisms. It results not only from high-calcium and phosphorous milieu but also happens as a result of oxidative stress, inflammatory cell infiltration, vascular smooth muscle cells (VSMCs) trans-differentiation endothelial dysfunction, osteogenesis, and matrix turnover [5, 6].
Interestingly, during VC, VSMCs exhibit a phenotypic switch into cells resembling osteoblasts (bone-forming cells). These cells undergo loss of smooth muscle biomarkers associated with increased expression of osteogenic biomarkers e.g. Runt-related transcription factor 2 (Runx2), osteocalcin, and osteopontin. Runx2 is a transcription factor that is essential for the differentiation of osteoblasts. VSMCs show upregulated Runx2 expression, which may be the reason for this cellular transdifferentiation [7, 8].
Runx2 is considered to be responsible for the osteogenic switch of VSMCs. Runx2 is targeted directly by an important signaling pathway known as the canonical Wnt signaling cascade, which is known to activate the gene and control the development of bone during the period of embryogenesis. It also regulates direct bone turnover as well as bone remodeling [9]. In the canonical (β-catenin dependent) Wnt signaling pathway, the ligands bind to a specific cell membrane receptor known as the Frizzled (Fz) receptor. This binding enhances nuclear translocation of β-catenin from the cytoplasm inducing the transcription of target genes. Runx2 is one of the target genes of the Wnt signaling pathway, and stimulation of Runx2 by Wnt activates osteoblast differentiation and the formation of bone [10].
Runx2 is a chief transcription factor that is critical for ectopic blood vessel calcification. While Runx2 is not normally expressed in the aortic valves and blood vessels, several studies have reported that de novo Runx2 expression is associated with the formation of cartilaginous and calcified bone-like lesions in atherosclerotic plaques, aortic valve disease, and diabetic calcifying lesions [11].
Runx2 regulates the expression of both alkaline phosphatase (ALP) and osteocalcin genes via binding to the osteoblast-specific cis-acting element, found in the promoter regions of both genes. This may clarify the necessity for Runx2 as a molecular switch for osteogenic differentiation. Vascular cells normally exhibit low expression of Runx2, which is upregulated in atherosclerotic calcified lesions. Runx2 expression is improperly regulated by diabetes mellitus illness, which worsens aortic stiffness [12].
There is strong evidence that the RANKL/RANK/OPG signaling axis is the main key to VC. Receptor activators of NF-kB ligand (RANKL), and osteoprotegerin (OPG) are the main components of this signaling system. RANKL is a homotrimeric protein that is produced by preosteoblasts, osteoblasts, osteocytes, periosteal cells, and some other cells like activated T cells [13]. When RANKL binds to RANK, it forms a homotrimer, which triggers stimulation of nuclear factor kappa-B (NF-κB) and its nuclear translocation, which in turn increases osteogenesis gene production. In contrast, osteoprotegerin (OPG) is a glycoprotein that belongs to the family of TNF receptors. It is also called TNF receptor superfamily member 11b (TNFRS11B). OPG hinders the binding of RANKL and RANK by serving as a decoy receptor for RANK [14, 15].
A substantial body of evidence revealed that epigenetic modifications are involved in VC development and progress [16]. In the epigenetic hallmarks, microRNAs (miRNAs), are a rising class of endogenous, small noncoding single-stranded RNAs (with an average of 22 nucleotides in length). miRNAs mediate epigenetic regulation through the regulation of gene expression, either by degrading mRNAs or by inhibiting their translation; thus, miRNAs may be attractive biomarkers that will be useful in clinical practice [17, 18].
Previous studies propose that microRNAs play a bio-vital role in VSMCs trans-differentiation. However, the influence of miR-433-3p is not well documented [19]. Thus, we aimed to explore the role of serum miR-433-3p in vascular calcification in type-2 diabetes mellitus.
Materials and methods
Patients and samples collection
The present research was carried out at Tanta University in the Internal Medicine Department, Endocrinology and Diabetes Unit. The study included 40 patients with type 2 diabetes mellitus who were divided into two groups: 20 diabetic patients without VC (11 females and 9 males) and 20 diabetic patients with VC (10 females and 10 males). Twenty healthy subjects taken as the control group (8 females and 12 males) were also included. The American Diabetes Association's criteria were used to diagnose type 2 diabetes mellitus [20]. Diabetic patients were under treatment by either oral hypoglycemic drugs or insulin and were included in the Endocrinology and Diabetes Outpatient Clinic at Tanta University Hospital.
Inclusion criteria
Age of 40–63 years, 40 patients with type 2 diabetes, 20 patients of them having VC detected either by multi-slice CT coronary calcium scoring, non-contrast CT images of the aorta and both lower limbs or valvular calcification detected by echocardiography, the other 20 diabetic patients and the control non-diabetic group are free of VC detected by the same methods of screening.
Exclusion criteria
The current study excluded participants who were using medications like glucocorticoids, immunosuppressive agents, or cytotoxic agents, or who had a chronic illness other than diabetes, such as congestive heart failure, end-stage renal disease, liver failure, or cancer.
Ethical statement
An informed consent was obtained prior to the start of the participation. The study protocol (which adhered to the principles of the Declaration of Helsinki II) was accepted by The Local Research Ethics Committee of Faculty of Medicine, Tanta University (Approval code: 35904/10/22).
Clinical evaluation
Patients enrolled in this study underwent a clinical history and physical examination. This involved age, family history of diabetes, duration of diabetes, and symptoms suggestive of DM. Symptoms of chest pain, dyspnea, or intermittent claudication were also inquired.
All the participants were submitted to multi-slice CT coronary calcium scoring for detection of coronary calcification, non-contrast CT images of the aorta and both lower limbs for detection of aortic and peripheral arterial calcification, and echocardiography for detection of valvular calcification.
Biochemical analysis
All subjects had overnight fasting blood samples taken under strict aseptic conditions. Samples were split into two portions, one of which was used to collect serum in plain tubes to measure fasting blood glucose (FBG), triglycerides (TG), total cholesterol (TC), and high-density lipoprotein cholesterol (HDL-c) levels were measured using colorimetric techniques (Spinreact, Spain), and the Friedewald equation [21] was used to estimate low- density lipoprotein cholesterol (LDL-c) levels.
For microRNA extraction, the remaining portion of the collected blood sample was stored in tubes that had been treated with ethylene diamine tetra acetic acid (EDTA).
Two hours- postprandial blood sample was obtained for assay of 2 h- post-prandial blood glucose (PP.BG).
Enzyme-linked immunosorbent assays (ELISAs)
It was used for the measurement of serum levels of Dickkopf-1 (MyBiosource Inc. California, USA, catalog number: MBS165224), Runx2 (MyBiosource Inc. California, USA, catalog number: MBS2512456), β-catenin (Biovision, USA, catalog number: EK3381), RANKL (Boster biological technology, USA, catalog number: EK0842), OPG (Boster biological technology, USA, catalog number: EK0480) using ELISA Reader (Stat Fax 2100, Fisher Bioblock Scientific, France).
Quantitative analysis of miR-433-3p gene expression
Using the Gene JET RNA Purification Kit (Thermo Scientific, USA), RNA was isolated according to the manufacturer's instructions from EDTA peripheral blood samples. To evaluate the relative expression of the miR-433-3p gene using the Step One Plus real-time PCR system (Applied Biosystem, USA), reverse transcription of total RNA was conducted using Revert Aid H minus Reverse Transcriptase (Thermo Scientific, USA) to obtain cDNA to be utilized as a template. The Primer 5.0 software was used to create the primers, and they had the following sequences: miR-433-3p Forward: 5′-GGAGAAGTACGGTGAGCCTGT-3′ and Reverse: 5′-GAACACCGAGGAGCCCATCAT-3′). The housekeeping gene small nuclear RNA U6 [22] with primer sequences; Forward: 5′-CGCTTCGGCAGCACATATACTAAAAT-3′ and Reverse: 5′-CGCTTCACGAATTTGCGTGTCAT-3′. The thermal cycling settings were initial denaturation (for 10 min.) at 95 °C, 40–45 cycles of amplification of DNA denaturation (for 15 s) at 95 °C, annealing (for 30 s) at 60 °C, extension (for 30 s) at 72 °C C. For melting curve analysis, the temperature was raised at the end of the last cycle from 63 to 95 °C C. Target gene and housekeeping gene cycle threshold (Ct) values were computed, Using the 2−ΔΔCt technique, the relative gene expression was assessed [23].
Statistical analysis
The computer program SPSS (Statistical Package for the Social Science; SPSS, Chicago, USA) version 21 for Microsoft Windows, USA, was used to present and analyze the current study's statistics. The expression for the variables was mean ± SD. One-way analysis of variance (ANOVA) was used to compare statistical differences between variables, and post hoc analysis was done afterward. In order to identify the association between various characteristics, the Pearson correlation was used. With related factors acting as independent variables, multiple linear regression analysis was used to consider the factors impacting miR-433-3p expression. Sensitivity and specificity were eventually used to complete the analysis, and the Receiver Operating Characteristic (ROC) curve was used to identify the appropriate cutoff point. If p < 0.05 statistical significance was considered to exist.
Results
Demographic and clinical characteristics
The demographic and clinical results of all subjects are presented in Table 1. No significant differences in age were found among all studied groups. However, a significant difference was found in the duration of diabetes mellitus between the two diabetic groups. Moreover, BMI, SBP and DBP were significantly different among groups. Furthermore, there were significant differences in FBG, Pp.BG, HbA1c, A/C ratio, lipid profile, blood urea, and serum creatinine were among the studied groups.
FBG, Pp. BG and HbA1c% levels, A/C ratio, TC, TG, and LDL-c levels were significantly increased in diabetic groups (with a significant decrease in HDL-c level) as compared with control subjects. Diabetic patients showed a significant increase in SBP and DBP in comparison with control subjects. A statistically significant difference concerning blood urea and serum creatinine levels was also detected among the studied groups.
All patients were kept on the current clinical recommendations, including diet control, lifestyle modification and medical treatment by oral hypoglycemic drugs or insulin, aspirin and statins. In diabetic patients without VC, oral hypoglycemic drugs were prescribed in 14 (70%) while 4 (20%) of patients were treated with insulin and only 2 (10%) were treated with both insulin and oral hypoglycemic drugs. In diabetic patients with VC, 10 (50%) of patients were maintained on oral hypoglycemic drugs and 7 (35%) of patients were treated with insulin but both insulin and oral hypoglycemic drugs were prescribed in 3(15%). Statins were used in 15 (75%) of diabetic patients without VC and in 18 (90%) of those with VC. In addition, 19 (95%) of diabetic patients without VC was maintained on aspirin while all case in the VC group used aspirin.
Biochemical results
In comparison to the control group, the mean values of Runx2, β-catenin, and RANKL were significantly greater in the two diabetes groups, with significantly higher levels found in diabetic patients with VC. However, there were significant differences between the examined groups regarding relative miR-433-3p expression, DKK1, and OPG levels, with significantly lower values reported in the diabetic groups compared to the control group as presented in (Table 2 and Fig. 1).
Correlations between the studied parameters
A significant negative correlation was detected between miR-433-3p relative expression and Runx2, β-catenin, RANKL, FBG, and Pp.BG, TC, TG, blood urea and serum creatinine, HbA1c, and A/C ratio. While miR-433-3p expression level showed a significant positive correlation with DKK1 and OPG levels. (Table 3).
Multiple linear regression analysis of miR-433-3p -related factors
HbA1c was considered as the dependent variable while the other studied parameters were considered as independent variables. miR-433-3p relative expression was revealed to be the independent predictor for VC (B 2.919, P value < 0.001*) (Table 4).
ROC curve of relative miR-433-3p expression as an early marker for discriminating diabetic patients with VC from healthy controls and diabetic patients without VC
ROC curve was used to measure miR-433-3p relative expression value for discriminating diabetic patients with VC from healthy controls (Fig. 2A) where the optimal cut-off point was 0.93 with a sensitivity of 95% and specificity of 92%. The area under the curve was 0.973. In (Fig. 2B) the ROC curve was applied to discriminate diabetic patients with VC from those without VC where the optimal cut-off point was 0.72 with a sensitivity of 95% and specificity of 95%. The area under the curve was 0.98.
Discussion
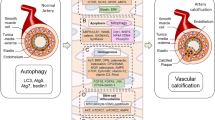
Vascular calcification (VC) is highly prevalent in patients with type-2 diabetes, chronic kidney disease, and hypertension, leading to an augmented risk of cardiovascular morbidity and mortality [24]. Numerous molecular processes and signaling pathways, including aberrant calcium or phosphate deposition, osteogenic trans-differentiation of vascular smooth muscle cells (VSMCs), oxidative stress, inflammation, and apoptosis, have been identified as contributing to its pathogenesis. [25].
At present, researchers pay attention to the imminent role of microRNAs (miRNAs) as newly revealed key factors involved in the pathogenesis of a wide variety of diseases. miRNAs are classes of non-coding small RNAs that can regulate numerous target genes post-transcriptionally. Consequently, miRNA-based regulation has been recognized as a prospective possibility for researching disease genesis and treating several disorders at the epigenetic level [26].
According to Goettsch et al., miRNAs are highly stable and found in blood (plasma, platelets, erythrocytes, and nucleated blood cells) due to their encapsulation in extracellular vesicles, association with a protein complex containing the RNA-binding protein Argonaute 2, or inclusion in lipoprotein complexes. These associations protect the miRNAs from degrading while they are in circulation [27].
In CVD patients, altered miRNA expression has been detected, and certain miRNAs have been documented to be involved in multiple cardiovascular diseases, such as atherosclerosis, VC, arrhythmias, and myocardial infarction [28]. A variety of miRNAs have been involved in the pathogenesis of vascular calcification, whereas others may have a protective role. Therefore, miRNA might be effective targets for preventing VC and its detrimental consequences [29].
In our study herein, we reported the role of miR-433-3p in VC for the first time. We identified miR-433-3p as a negative regulator of VSMC calcification. However, other research studies its role in the trans-differentiation of VSMCs and atherosclerosis [30, 31].
Our data revealed that a statistically significant down-regulation of miR-433-3p was noted among both diabetic groups with and without VC compared to each other and the control group, with profound downregulation in those with VC. The ROC curve analysis displayed that the miR-433-3p relative expression could discriminate diabetic patients with VC from healthy control with high levels of accuracy (AUC = 0.973). Also, it has a significant ability to discriminate diabetic patients with VC from those without VC patients (AUC = 0.98).
Likewise, Mir et al. [32] found that miR-433-3p was down-regulated in patients with obesity and metabolic syndrome and negatively correlated with HbA1c, suggesting its pathophysiologic role in various metabolic diseases like diabetes. Similarly, miR-433-3p was overexpressed in healthy individuals compared to diabetic nephropathy patients, ensuring miR-433-3p’s protective role in diabetic conditions [33]
The miR-433-3p level was decreased in women after gestational diabetes mellitus diagnosis, according to Sørensen et al. [34]. The miR-433-3p was down-regulated in insulin-secreting MIN6 cells in a high glucose environment in vitro; however, the β-cells were protected by restoring miR-433-3p levels using miR-mimics. In an effort to maintain glucose homeostasis, it is conceivable that miR-433-3p could contribute to the compensatory effects [34].
It was also reported that low levels of miR-433-3p were associated with a higher risk of type 2 diabetes seen in PCOS women since they are thought to protect pancreatic β-cells from glucose toxicity [35].
Likewise, previous studies demonstrated that miR-433-3p directly targets cyclooxygenase 2 to protect mouse pancreatic cells from the high glucose-induced decrease of cell viability. Its expression was shown to be down-regulated in high glucose, and miR-433 mimic transfection significantly increased its expression, which significantly increased cell viability and proliferation in high glucose conditions by suppressing apoptosis and accelerating cell cycle progression, suggesting its pathophysiologic role in various metabolic diseases including diabetes [36].
According to Li et al., miR-433 plays a significant role in TGF-β/Smad3-induced renal fibrosis by creating a positive feedback loop that amplifies TGF-β/Smad3 signaling. This suggests that it could be a promising therapeutic target for tissue fibrosis [37].
Moreover, miR-433-3p was found to be expressed in the osteoblast lineage but not in osteoclasts. A single nucleotide polymorphism in human osteonectin, a plentiful non-collagenous bone matrix protein that controls osteoblast development and survival, was discovered to be variably targeted by miR-433-3p [38].
Notably, miR-433-3p is progressively reduced during differentiation of osteoblasts from primary mouse bone marrow stromal cells in vitro. It was detected to target Runx2 and also to be inhibited by bone morphogenetic protein (BMP) signaling. Similarly, miR-433-3p targets R-spondin 3 (Rspo3), a leucine-rich repeat-containing G-protein coupled receptor (LGR) ligand that augments WNT signaling. Particularly, WNT canonical signaling is also suppressed by the activity of miR-433-3p [39].
In this context, it was recognized that miR-433-3p was shown to be reduced during BMP2-induced osteoblastic differentiation of C3H10T1/2 cells and to target the Runx2 3′ UTR [40].
Runx2, also known as AML3 or Cbfa1, is an important transcription factor that is necessary for skeletal formation and remodeling. It is the chief regulator of bone formation. It is up-regulated within calcifying VSMCs and may be responsible for this trans-development; it is also important for osteoblast differentiation [41]. Lin et al. demonstrated that SMC-specific Runx2 has an imminent role in both osteoblastic differentiation and chondrocyte maturation during atherosclerosis-induced VC [42].
Unsurprisingly, our results supported earlier research [43, 44] and validated that the Runx2 level was significantly greater in diabetic groups with and without VC compared to the control group with a higher level demonstrated in those with VC.
Emerging evidence testifies the involvement of the canonical WNT signaling pathway in the pathogenesis of VC as WNT/β-catenin signaling is essential for the osteogenic differentiation of pluripotent mesenchymal cells. The extracellular signal, membrane segment, cytoplasmic segment, and nuclear segment are the four segments that make up the Wnt/β-catenin pathway. Wnt proteins, including Wnt3a, Wnt1, and Wnt5a, play a major role in the transmission of extracellular signals. The Wnt signaling pathway, which is made up of a large part of the β-catenin protein, is crucial for cell signaling [45].
The phosphorylation of β-catenin was brought on by the lack of Wnt ligands. The fragments of the broken-down complex cannot be bonded to or spread out by the phosphorylated β-catenin. Because β-catenin cannot be degraded, it accumulates within cells and is subsequently released into the extracellular matrix. Studies on β-catenin focus mostly on its accumulation within the cell and its transcription into the nucleus [46].
Recently, a small number of instances have proven that β-catenin can be found in serum and may be related to the progression of type 2 diabetes, PTEN hamartoma tumor syndrome, early-onset ulcerative colitis, hepatitis C, and hepatitis B. These results revealed that serum disease-associated indicators may be identified by β-catenin [47].
Runx2 is induced as a result of pro-osteogenic stimuli activating the WNT/ β -catenin signaling pathway. Runx2 then controls the production of many bone-related proteins, including sclerostin, osteocalcin, and osterix, and also can control crucial processes necessary for osteoblast development and phenotypic characterization [8, 44].
Atherosclerotic injury is the reason that triggered the heightened plasma RUNX2 level. Therefore, early arterial lesions and calcification appear to be more caused by elevated levels of RUNX2. RUNX2 is crucial for the calcification of vascular smooth muscle cells brought on by oxidative stress. This indicates that arterial injury is more affected by elevated plasma RUNX2 levels. Therefore, it appears that early artery lesions are more likely to be caused by elevated levels of RUNX2 [48].
Indeed, a notable soluble dominant antagonist of the canonical WNT protein is Dickkopf1 (DKK1). It was the first and most characteristic molecule to bind to the single-pass trans-membrane receptor proteins Kremen 1 and Kremen 2, as well as to the plasma membrane-located WNT co-receptors LRP5 and LRP6 to suppress canonical WNT signaling [49].
In a previous study, DKK1 concentration was inversely co-related to aortic calcification and coronary artery disease [50]. Likewise, our results revealed that the two diabetes groups, particularly diabetic patients with VC, showed a considerable rise in β-catenin levels together with a concurrent decline in DDK1. In agreement with our finding, Tang et al. proved the vital role of miR-433-3p in the DKK1/WNT/β-catenin signaling pathway by reducing DKK1 expression and enhancing osteoblasts differentiation [51].
In accordance with our results, RANKL/RANK/OPG axis dysregulation appears to be involved in the progression of VC [52]. It appears to be the final effector of many osteotropic factors. It consists of the transmembrane protein RANK, its ligand (RANKL), and the soluble receptor OPG that binds to the RANKL, thereby interfering with the RANK-RANKL interaction [5, 53].
Bucay et al. reported that OPG knock-out (OPG−/−) mice developed osteoporosis and massive arterial calcification [54]. Additionally, RANKL expression was up-regulated in calcified arteries [55]. RANKL directly promotes the calcification of vascular smooth muscle cells by binding to RANK and promoting BMP4 production via the alternative NF-B pathway. RANKL also acts indirectly by raising macrophage paracrine pro-calcific activity by promoting the release of tumor necrosis factor-alpha and interleukin-6 [56].
Nie et al. showed that the WNT/ β-catenin pathway activation significantly promoted the calcification and up-regulated RANKL gene expression. Furthermore, WNT/β-catenin pathway activity had a positive relation with the degree of arterial or cell calcification. An active WNT/-catenin pathway during calcification increased the degree of calcification, especially in the early stage, which was likely induced by increased expression of the proteins RANKL, WNT3a, and WNT7a [57].
In agreement with this premise, Motovska et al. showed a connection between the RANKL/RANK/OPG axis, WNT/DKK-1 signaling, and the onset of atherosclerosis. These findings suggest that these factors may be involved in the regulation of VC in calcified aortic stenosis [5].
Conclusion
Our study validated, for the first time the protective role of miR-433-3p against the development of VC, offering an innovative guard against the common mechanisms of VC pathogenesis via targeting of the WNT/β-Catenin and RANKL/RANK/OPG signaling pathways. The data presented here also suggest that miR-433-3p could be considered a biomarker useful to support the diagnosis and prediction of VC.
Limitation
The current study has some limitations. First, a small number of patients in this study is an important limitation, so a large number of patients is required to validate our findings. Also, this study is based on a single center, which does not reflect the whole population. Thus, multicenter studies are required to validate these results.
For better data validation, silence/overexpress miR-433-3p in VSCMs and its effect on osteogenic differentiation genes and VC should be studied in the upcoming research.
Data availability
It is available upon reasonable request.
Abbreviations
- ALP :
-
Alkaline phosphatase
- ANOVA :
-
One-way analysis of variance
- BMI :
-
Body mass index
- BMP :
-
Bone morphogenetic protein
- CVD :
-
Cardiovascular diseases
- DKK1 :
-
Dickkopf-1
- DM :
-
Diabetes mellitus
- ELISA :
-
Enzyme-linked immunosorbent assay
- FBG :
-
Fasting blood glucose
- HDL-c :
-
High- density lipoprotein cholesterol
- LDL-c :
-
Low- density lipoprotein cholesterol
- miR-433-3p :
-
MicroRNA- 433-3p
- NF-κB :
-
Nuclear factor kappa-B
- OPG :
-
Osteoprotegerin
- RANKL :
-
Receptor activator of nuclear factor kappa-B ligand
- ROC :
-
Receiver Operating Characteristic
- Runx2 :
-
Runt-related transcription factor 2
- TC :
-
Total cholesterol
- TG :
-
Triglycerides
- TNF :
-
Tumor necrosis factor
- UACR :
-
Urinary albumin/creatinine ratio
- VC :
-
Vascular calcification
- VSMCs :
-
Vascular smooth muscle cells
References
Ghosh S, Luo D, He W, Chen J, Su X, Huang H (2020) Diabetes and calcification: the potential role of anti-diabetic drugs on vascular calcification regression. Pharmacol Res 158:104861. https://doi.org/10.1016/j.phrs.2020.104861
Wang SS, Wang C, Chen H (2020) MicroRNAs are critical in regulating smooth muscle cell mineralization and apoptosis during vascular calcification. J Cell Mol Med 24(23):13564–13572. https://doi.org/10.1111/jcmm.16005
Lee SJ, Lee IK, Jae-Han J (2020) Vascular calcification—new insights into its mechanism. Int J Mol Sci 21(8):2685. https://doi.org/10.3390/ijms21082685
Kopytek M, Mazur P, Ząbczyk M, Undas A, Natorska J (2021) Diabetes concomitant to aortic stenosis is associated with increased expression of NF-κB and more pronounced valve calcification. Diabetologia 64:2562–2574
Lee SJ, Lee IK, Jeon JH (2020) Vascular Calcification-new insights into its mechanism. Int J Mol Sci 21(8):2685. https://doi.org/10.3390/ijms21082685
Zununi Vahed S, Mostafavi S, Hosseiniyan Khatibi SM, Shoja MM, Ardalan M (2020) Vascular calcification: an important understanding in nephrology. Vasc Health Risk Manag 12(16):167–180. https://doi.org/10.2147/VHRM.S242685
Bundy K, Boone J, Simpson CL (2021) Wnt signaling in vascular calcification. Front Cardiovasc Med 8:708470. https://doi.org/10.3389/fcvm.2021.708470
Cai T, Sun D, Duan Y, Wen P, Dai C, Yang J, He W (2016) WNT/β-catenin signaling promotes VSMCs to osteogenic transdifferentiation and calcification through directly modulating Runx2 gene expression. Exp Cell Res 345(2):206–217. https://doi.org/10.1016/j.yexcr.2016.06.007
Reinhold S, Blankesteijn WM, Foulquier S (2020) The interplay of WNT and PPARγ signaling in vascular calcification. Cells 9(12):2658. https://doi.org/10.3390/cells9122658
Cobb AM, Yusoff S, Hayward R, Ahmad S, Sun M, Verhulst A et al (2020) Runx2 (Runt-Related Transcription Factor 2) links the DNA damage response to osteogenic reprogramming and apoptosis of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 2:1339–1357. https://doi.org/10.1161/ATVBAHA.120.315206
Nguyen N, Naik V, Speer MY (2013) Diabetes mellitus accelerates cartilaginous metaplasia and calcification in atherosclerotic vessels of LDLr mutant mice. Cardiovasc Pathol 22:167–175. https://doi.org/10.1016/j.carpath.2012.06.007
Raaz U et al (2015) Transcription factor Runx2 promotes aortic fibrosis and stiffness in type 2 diabetes mellitus. Circ Res 117:513–524
Tobeiha M, Moghadasian MH, Amin N, Jafarnejad S (2020) RANKL/RANK/OPG pathway: a mechanism involved in exercise-induced bone remodeling. Biomed Res Int 2020:6910312. https://doi.org/10.1155/2020/6910312
Dalle Carbonare L, Innamorati G, Valenti MT (2012) Transcription factor Runx2 and its application to bone tissue engineering. Stem Cell Rev Rep 8(3):891–897. https://doi.org/10.1007/s12015-011-9337-4
Ndip A, Williams A, Jude EB, Serracino-Inglott F, Richardson S, Smyth JV, Boulton AJ, Alexander MY (2011) The RANKL/RANK/OPG signaling pathway mediates medial arterial calcification in diabetic Charcot neuroarthropathy. Diabetes 60(8):2187–2196. https://doi.org/10.2337/db10-1220
Papadopouli AE, Klonaris CN, Theocharis SE (2008) Role of OPG/RANKL/RANK axis on the vasculature. Histol Histopathol 23(4):497–506. https://doi.org/10.14670/HH-23.497
Dekker M, Waissi F, Silvis MJM, Bennekom JV, Schoneveld AH, de Winter RJ, Isgum I, Lessmann N, Velthuis BK, Pasterkamp G, Mosterd A, Timmers L, de Kleijn DPV (2021) High levels of osteoprotegerin are associated with coronary artery calcification in patients suspected of a chronic coronary syndrome. Sci Rep 11(1):18946. https://doi.org/10.1038/s41598-021-98177-4
Hou YC, Lu CL, Yuan TH, Liao MT, Chao CT, Lu KC (2020) The epigenetic landscape of vascular calcification: an integrative perspective. Int J Mol Sci 21(3):980. https://doi.org/10.3390/ijms21030980
Infante T, Forte E, Punzo B, Cademartiri F, Cavaliere C, Soricelli A, Salvatore M, Napoli C (2019) Correlation of circulating miR-765, miR-93-5p, and miR-433-3p to obstructive coronary heart disease evaluated by cardiac computed tomography. Am J Cardiol 124(2):176–182. https://doi.org/10.1016/j.amjcard.2019.04.016
American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021 Jan;44(Suppl 1):S15-S33. doi: https://doi.org/10.2337/dc21-S002. Erratum in: Diabetes Care. 2021 Sep;44(9): 2182.
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18(6):499–502
Tang X, Lin J, Wang G, Lu J (2017) MicroRNA-433–3p promotes osteoblast differentiation through targeting DKK1 expression. PloS one 12(6):e0179860. https://doi.org/10.1371/journal.pone.0179860
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Wu W, Shang YQ, Dai SL, Yi F, Wang XC (2017) MiR-26a regulates vascular smooth muscle cell calcification in vitro through targeting CTGF. Bratisl Lek Listy 118(8):499–503. https://doi.org/10.4149/BLL_2017_096
Liu J, Xiao X, Shen Y, Chen L, Xu C, Zhao H, Wu Y, Zhang Q, Zhong J, Tang Z, Liu C, Zhao Q, Zheng Y, Cao R, Zu X (2017) MicroRNA-32 promotes calcification in vascular smooth muscle cells: implications as a novel marker for coronary artery calcification. PloS one 12(3):e0174138. https://doi.org/10.1371/journal.pone.0174138
Saiyed AN, Vasavada AR, Johar SRK (2022) Recent trends in miRNA therapeutics and the application of plant miRNA for prevention and treatment of human diseases. Futur J Pharm Sci 8(1):24. https://doi.org/10.1186/s43094-022-00413-9
Goettsch C, Hutcheson JD, Aikawa E (2013) MicroRNA in cardiovascular calcification: focus on targets and extracellular vesicle delivery mechanisms. Circ Res 112(7):1073–1084. https://doi.org/10.1161/CIRCRESAHA.113.300937.Erratum.In:CircRes.2013Nov8;113(11):e106.PMID:23538277;PMCID:PMC3668680
Liu H, Wang H, Yang S, Qian D (2019) Downregulation of miR-542-3p promotes osteogenic transition of vascular smooth muscle cells in the aging rat by targeting BMP7. Hum Genomics 13(1):67. https://doi.org/10.1186/s40246-019-0245-z
Alkagiet S, Tziomalos K (2017) Vascular calcification: the role of microRNAs. Biomol Concepts 8(2):119–123. https://doi.org/10.1515/bmc-2017-0001
Feinberg MW, Moore KJ (2016) MicroRNA regulation of atherosclerosis. Circ Res 118(4):703–720
Tang X, Lin J, Wang G, Lu J (2017) MicroRNA-433–3p promotes osteoblast differentiation through targeting DKK1 expression. PloS one 12(6):e0179860. https://doi.org/10.1371/journal.pone
Mir FA, Mall R, Iskandarani A et al (2022) Characteristic MicroRNAs linked to dysregulated metabolic pathways in Qatari adult Subjects with obesity and metabolic syndrome. Front Endocrinol 13:937089. https://doi.org/10.3389/fendo.2022.937089
Asadi G, Rezaei Varmaziar F, Karimi M, Rajabinejad M, Ranjbar S, Gorgin Karaji A, Salari F, Afshar Hezarkhani L, Rezaiemanesh A (2021) Determination of the transcriptional level of long non-coding RNA NEAT-1, downstream target microRNAs, and genes targeted by microRNAs in diabetic neuropathy patients. Immunol Lett 232:20–26. https://doi.org/10.1016/j.imlet.2021.01.007
Sørensen AE, van Poppel MNM, Desoye G, Simmons D, Damm P, Jensen DM, Dalgaard LT, The Dali Core Investigator Group (2022) The temporal profile of circulating miRNAs during Gestation in overweight and obese women with or without gestational diabetes mellitus. Biomedicines 10(2):482. https://doi.org/10.3390/biomedicines10020482
Wang M (2017) miR 433 protects pancreatic β cell growth in high glucose conditions. Mol Med Rep 16(3):2604–2610. https://doi.org/10.3892/mmr.2017.6925
Dole NS, Kapinas K, Kessler CB, Yee SP, Adams DJ, Pereira RC, Delany AM (2015) A single nucleotide polymorphism in osteonectin 3’ untranslated region regulates bone volume and is targeted by miR-433. J Bone Miner Res 30(4):723–732. https://doi.org/10.1002/jbmr.2378
Li R, Chung AC, Dong Y, Yang W, Zhong X, Lan HY (2013) The microRNA miR-433 promotes renal fibrosis by amplifying the TGF-β/Smad3-Azin1 pathway. Kidney Int 84(6):1129–1144. https://doi.org/10.1038/ki.2013.272
Garcia J, Smith SS, Karki S, Drissi H, Hrdlicka HH, Youngstrom DW, Delany AM (2021) miR-433-3p suppresses bone formation and mRNAs critical for osteoblast function in mice. J Bone Miner Res 36(9):1808–1822. https://doi.org/10.1002/jbmr.4339
Smith SS, Dole NS, Franceschetti T, Hrdlicka HC, Delany AM (2016) MicroRNA-433 dampens glucocorticoid receptor signaling, impacting circadian rhythm and osteoblastic gene expression. J Biol Chem 291(41):21717–21728. https://doi.org/10.1074/jbc.M116.737890
Lian JB, Stein GS (2003) Runx2/Cbfa1: a multifunctional regulator of bone formation. Curr Pharm Des 9(32):2677–2685. https://doi.org/10.2174/1381612033453659
Lin ME, Chen TM, Wallingford MC, Nguyen NB, Yamada S, Sawangmake C, Zhang J, Speer MY, Giachelli CM (2016) Runx2 deletion in smooth muscle cells inhibits vascular osteochondrogenesis and calcification but not atherosclerotic lesion formation. Cardiovasc Res 112(2):606–616. https://doi.org/10.1093/cvr/cvw205
Sun Y, Byon CH, Yuan K, Chen J, Mao X, Heath JM, Javed A, Zhang K, Anderson PG, Chen Y (2012) Smooth muscle cell-specific runx2 deficiency inhibits vascular calcification. Circ Res 111(5):543–552. https://doi.org/10.1161/CIRCRESAHA.112.267237
Bundy K, Boone J, Simpson CL (2021) Wnt signaling in vascular calcification. Front Cardiovasc Med 8:708470. https://doi.org/10.3389/fcvm.2021.708470
Li S, Huang M, Liu Q, Wang D, Wu R, Zhang X, Chen W, Duan L (2019) Serum expression of β-Catenin is a potential detection marker in patients with colorectal cancer. Dis Markers 24(2019):5070524. https://doi.org/10.1155/2019/5070524
Rahmani F, Avan A, Hashemy SI, Hassanian SM (2018) Role of Wnt/β-catenin signaling regulatory microRNAs in the pathogenesis of colorectal cancer. J Cell Physiol 233(2):811–817. https://doi.org/10.1002/jcp.25897
Galatola M, Paparo L, Duraturo F et al (2012) Beta catenin and cytokine pathway dysregulation in patients with manifestations of the “PTEN hamartoma tumor syndrome.” BMC Med Genet 13(1):1–10. https://doi.org/10.1186/1471-2350-13-28
Yuan X, Wang Z, Wang L, Zhao Q, Gong S, Sun Y, Liu Q, Yuan P (2020) Increased levels of runt-related transcription factor 2 are associated with poor survival of patients with idiopathic pulmonary arterial hypertension. Am J Mens Health 14(4):1557988320945458. https://doi.org/10.1177/1557988320945458
Niederhoffer N, Lartaud-Idjouadiene I, Giummelly P, Duvivier C, Peslin R, Atkinson J (1997) Calcification of medial elastic fibers and aortic elasticity. Hypertension 29(4):999–1006. https://doi.org/10.1161/01.HYP.29.4.999
Li X, Liu XL, Li X, Zhao YC, Wang QQ, Zhong HY, Liu DD, Yuan C, Zheng TF, Zhang M (2022) Dickkopf1 (Dkk1) alleviates vascular calcification by regulating the degradation of phospholipase D1 (PLD1). J Cardiovasc Transl Res. https://doi.org/10.1007/s12265-022-10251-y
Register TC, Hruska KA, Divers J, Bowden DW, Palmer ND, Carr JJ, Wagenknecht LE, Hightower RC, Xu J, Smith SC, Dietzen DJ, Langefeld CD, Freedman BI (2013) Plasma Dickkopf1 (DKK1) concentrations negatively associate with atherosclerotic calcified plaque in African-Americans with type 2 diabetes. J Clin Endocrinol Metab 98(1):E60–E65. https://doi.org/10.1210/jc.2012-3038
Tang X, Lin J, Wang G, Lu J (2017) MicroRNA-433–3p promotes osteoblast differentiation through targeting DKK1 expression. PloS one 12(6):e0179860. https://doi.org/10.1371/journal.pone.0179860
Rochette L, Meloux A, Rigal E, Zeller M, Malka G, Cottin Y, Vergely C (2019) The role of osteoprotegerin in vascular calcification and bone metabolism: the basis for developing new therapeutics. Calcif Tissue Int 105(3):239–251. https://doi.org/10.1007/s00223-019-00573-6
Motovska Z, Vichova T, Doktorova M, Labos M, Maly M, Widimsky P (2015) Serum Dickkopf-1 signaling and calcium deposition in aortic valve are significantly related to the presence of concomitant coronary atherosclerosis in patients with symptomatic calcified aortic stenosis. J Transl Med 15(13):63. https://doi.org/10.1186/s12967-015-0423-2
Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS (1998) osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev 12(9):1260–1268. https://doi.org/10.1101/gad.12.9.1260
Kawakami R, Nakagami H, Noma T, Ohmori K, Kohno M, Morishita R (2016) RANKL system in vascular and valve calcification with aging. Inflamm Regen 1(36):10. https://doi.org/10.1186/s41232-016-0016-3
Wu M, Rementer C, Giachelli CM (2013) Vascular calcification: an update on mechanisms and challenges in treatment. Calcif Tissue Int 93(4):365–373. https://doi.org/10.1007/s00223-013-9712-z
Nie B, Zhang SY, Guan SM, Zhou SQ, Fang X (2019) Role of Wnt/β-Catenin pathway in the arterial medial calcification and its effect on the OPG/RANKL system. Curr Med Sci 39(1):28–36. https://doi.org/10.1007/s11596-019-1996-4
Acknowledgements
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No organizations provided funds for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elshamy, A.M., Hafez, Y.M., Safa, M.A.E. et al. The role of miR-433-3p in vascular calcification in type 2 diabetic patients: targeting WNT/β-Catenin and RANKL/RANK/OPG signaling pathways. Mol Biol Rep 50, 9073–9083 (2023). https://doi.org/10.1007/s11033-023-08792-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08792-9