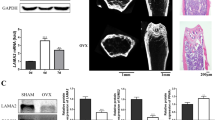
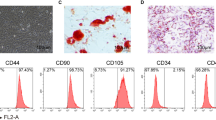
Abstract
Cbfa1/Runx2 is a bone transcription factor homologous to the Drosophila protein, Runt. Runx2 is a master gene that encodes for a protein involved in the osteogenic differentiation process from mesenchymal precursors. It is known that in Cbfa1 deficient mice (Cbfa1−/−) the lack of mature osteoblasts is associated to incomplete bone mineralization. An important aim of modern biology is the development of new molecular tools for identification of therapeutic approaches. Recent discoveries in cell and molecular biology enabled researchers in the bone tissue-engineering field to develop new strategies for gene and cell-based therapies. This review summarizes the process of osteogenic differentiation from mesenchymal stem cells and the importance of bone regeneration is discussed. In particular, given the increasing interest in the study of the transcription factor Runx2, this review highlights the role of this target gene and addresses recent strategies using Runx2 for bone regeneration.

Similar content being viewed by others
References
Lemaire, V., Tobin, F. L., Greller, L. D., et al. (2004). Modeling the interactions between osteoblast and osteoclast activities in bone remodelling. Journal of Theoretical Biology, 229, 293–309.
Neve, A., Corrado, F., & Cantatore, P. (2011). Osteoblast physiology in normal and pathological conditions. Cell and Tissue Research, 343, 289–302.
Jilka, R. L., O’Brien, C. A., Bartell, S. M., et al. (2010). Continuous elevation of PTH increases the number of osteoblasts via both osteoclast-dependent and -independent mechanisms. Journal of Bone and Mineral Research, 25, 2427–2437.
Dominici, M., Le Blanc, K., Mueller, I., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8, 315–317.
Abdallah, B. M., & Kassem, M. (2008). Human mesenchymal stem cells: From basic biology to clinical applications. Gene Therapy, 15, 109–116.
Dezawa, M., Kanno, H., Hoshino, M., et al. (2004). Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. The Journal of Clinical Investigation, 113, 1701–1710.
Luk, J. M., Wang, P. P., Lee, C. K., et al. (2005). Hepatic potential of bone marrow stromal cells: Development of in vitro co-culture and intra-portal transplantation models. Journal of Immunological Methods, 305, 39–47.
Himburg, H. A., Muramoto, G. G., Daher, P., et al. (2010). Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nature Medicine, 16, 475–482.
Janicki, P., Boeuf, S., Steck, E., et al. (2011). Prediction of in vivo bone forming potency of bone marrow-derived human mesenchymal stem cells. European Cells & Materials, 20, 488–507.
Charbord, P. (2010). Bone marrow mesenchymal stem cells. Historical overview and concepts. Human Gene Therapy, 21, 1045–1056.
Luria, E. A., Panasyuk, A. F., & Friedenstein, A. Y. (1971). Fibroblast colony formation from monolayer cultures of blood cells. Transfusion, 11, 345–349.
Foster, L. J., Zeemann, P. A., Li, C., et al. (2005). Differential expression profiling of membrane proteins by quantitative proteomics in a human mesenchymal stem cell line undergoing osteoblast differentiation. Stem Cells, 23, 1367–1377.
Valenti, M. T., Dalle Carbonare, L., Donatelli, L., et al. (2008). Gene expression analysis in osteoblastic differentiation from peripheral blood mesenchymal stem cells. Bone, 43, 1084–1092.
Rosada, C., Justesen, J., Melsvik, D., et al. (2003). The human umbilical cord blood: A potential source for osteoblast progenitor cells. Calcified Tissue International, 72, 135–142.
De Bari, C., Dell’Accio, F., Tylzanowski, P., & Luyten, F. P. (2001). Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis and Rheumatism, 44, 1928–1942.
Miura, M., Gronthos, S., Zhao, M., et al. (2003). Stem cells from human exfoliated deciduous teeth. Proceedings of the National Academy of Sciences of the United States of America, 100, 5807–5812.
De Coppi, P., Bartsch, G., Jr., Siddiqui, M. M., et al. (2007). Isolation of amniotic stem cell lines with potential for therapy. Nature Biotechnology, 25, 100–106.
Wagner, W., Wein, F., Seckinger, A., et al. (2005). Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Experimental Hematology, 33, 1402–1416.
Karsenty, G. (1999). The genetic transformation of bone biology. Genes & Development, 13, 3037–3051.
Jensen, E. D., Gopalakrishnan, R., & Westendorf, J. J. (2010). Regulation of gene expression in osteoblasts. Biofactors, 36, 25–32.
Olsen, B. R., Reginato, A. M., & Wang, W. (2000). Bone development. Annual Review of Cell and Developmental Biology, 16, 191–220.
Franceschi, R. T., Ge, C., & Xiao, G. (2007). Transcriptional regulation of osteoblasts. Annals of the New York Academy of Sciences, 1116, 196–207.
Deng, Z. L., Sharff, K. A., Tang, N., et al. (2008). Regulation of osteogenic differentiation during skeletal development. Frontiers in Bioscience, 13, 2001–2021.
Lian, J. B., Javed, A., Zaidi, S. K., et al. (2004). Regulatory controls for osteoblast growth and differentiation: Role of Runx/Cbfa/AML factors. Critical Reviews in Eukaryotic Gene Expression, 14, 1–41.
Cohen, M. M., Jr. (2009). Perspectives on RUNX genes: An update. American Journal of Medical Genetics, Part A, 149A, 2629–2646.
Yang, X., Matsuda, K., Bialek, P., et al. (2004). ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry syndrome. Cell, 117, 387–398.
Xiao, G., Wang, D., Benson, M. D., et al. (1998). Role of the alpha2-integrin in osteoblast-specific gene expression and activation of the Osf2 transcription factor. Journal of Biological Chemistry, 273, 32988–32994.
Roca, H., Phimphilai, M., Gopalakrishnan, R., et al. (2005). Cooperative interactions between RUNX2 and homeodomain protein-binding sites are critical for the osteoblast-specific expression of the bone sialoprotein gene. Journal of Biological Chemistry, 35, 30845–30855.
Imai, Y., Kurokawa, M., Yamaguchi, Y., et al. (2004). The corepressor mSin3A regulates phosphorylation-induced activation, intranuclear location, and stability of AML1. Molecular and Cellular Biology, 24, 1033–1043.
Shen, Q., & Christakos, S. (2005). The vitamin D receptor, Runx2, and the Notch signaling pathway cooperate in the transcriptional regulation of osteopontin. Journal of Biological Chemistry, 280(49), 40589–40598.
Ylönen, R., Kyrönlahti, T., Sund, M., et al. (2005). Type XIII collagen strongly affects bone formation in transgenic mice. Journal of Bone and Mineral Research, 20, 1381–1393.
Otto, F., Lubbert, M., & Stock, M. (2003). Upstream and downstream targets of RUNX proteins. Journal of Cellular Biochemistry, 89, 9–18.
Quarto, N., Behr, B., & Longaker, M. T. (2010). Opposite spectrum of activity of canonical Wnt signaling in the osteogenic context of undifferentiated and differentiated mesenchymal cells: Implications for tissue engineering. Tissue Engineering, Part A, 16, 3185–3197.
Shui, C., Spelsberg, T. C., Riggs, B. L., & Khosla, S. (2003). Changes in Runx2/Cbfa1 expression and activity during osteoblastic differentiation of human bone marrow stromal cells. Journal of Bone and Mineral Research, 18, 213–221.
Hu, R., Liu, W., Li, H., et al. (2011). A RUNX2/MIR-3960/MIR-2861 regulatory feedback loop during mouse osteoblast differentiation. Journal of Biological Chemistry, 286, 12328–12339.
Jensen, E. D., Schroeder, T. M., Bailey, J., et al. (2008). Histone deacetylase 7 associates with Runx2 and represses its activity during osteoblast maturation in a deacetylation-independent manner. Journal of Bone and Mineral Research, 23, 361–372.
Howard, T. D., Paznekas, W. A., Green, E. D., et al. (1997). Mutations in TWIST, a basic helix-loop-helix transcription factor, in Saethre-Chotzen syndrome. Nature Genetics, 15, 36–41.
Bialek, P., Kern, B., Yang, X., et al. (2004). A twist code determines the onset of osteoblast differentiation. Developmental Cell, 6, 423–435.
Xiao, G., Jiang, D., Thomas, P., et al. (2000). MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. Journal of Biological Chemistry, 11, 4453–4459.
Ge, C., Xiao, G., Jiang, D., et al. (2009). Identification and functional characterization of ERK/MAPK phosphorylation sites in the Runx2 transcription factor. Journal of Biological Chemistry, 20, 32533–32543.
Ogawa, E., Maruyama, M., Kagoshima, H., et al. (1993). PEBP2/PEA2 represents a family of transcription factors homologous to the products of the Drosophila runt gene and the human AML1 gene. Proceedings of the National Academy of Sciences of the United States of America, 90(14), 6859–6863.
Jeong, J. H., Jin, J. S., Kim, H. N., et al. (2008). Expression of Runx2 transcription factor in non-skeletal tissues, sperm and brain. Journal of Cellular Physiology, 217(2), 511–517.
Harada, H., Tagashira, S., Fujiwara, M., et al. (1999). Cbfa1 isoforms exert functional differences in osteoblast differentiation. Journal of Biological Chemistry, 274, 6972–6978.
Geoffroy, V., Corral, D. A., Zhou, L., et al. (1998). Genomic organization, expression of the human CBFA1 gene, and evidence for an alternative splicing event affecting protein function. Mammalian Genome, 9, 54–57.
Caetano-Lopes, J., Canhão, H., & Fonseca, J. E. (2007). Osteoblasts and bone formation. Acta Reumatológica Portuguesa, 32, 103–110.
Marie, P., Debiais, F., Cohen-Solal, M., & de Vernejoul, M. C. (2000). New factors controlling bone remodeling. Joint, Bone, Spine, 67, 150–156.
Enomoto, H., Enomoto-Iwamoto, M., Iwamoto, M., et al. (2000). Cbfa1 is a positive regulatory factor in chondrocyte maturation. Journal of Biological Chemistry, 275, 8695–8702.
Park, M. H., Shin, H. I., Choi, J. Y., et al. (2001). Differential expression patterns of Runx2 isoforms in cranial suture morphogenesis. Journal of Bone and Mineral Research, 16, 885–892.
Banerjee, C., Javed, A., Choi, J. Y., et al. (2001). Differential regulation of the two principal Runx2/Cbfa1 n-terminal isoforms in response to bone morphogenetic protein-2 during development of the osteoblast phenotype. Endocrinology, 142, 4026–4039.
Prince, M., Banerjee, C., Javed, A., et al. (2001). Expression and regulation of Runx2/Cbfa1 and osteoblast phenotypic markers during the growth and differentiation of human osteoblasts. Journal of Cellular Biochemistry, 80, 424–440.
Sudhakar, S., Li, Y., Katz, M. S., & Elango, N. (2001). Translational regulation is a control point in RUNX2/Cbfa1 gene expression. Biochemical and Biophysical Research Communications, 289, 616–622.
Drissi, H., Pouliot, A., Koolloos, C., et al. (2002). 1,25-(OH)2-vitamin D3 suppresses the bone-related Runx2/Cbfa1 gene promoter. Experimental Cell Research, 274, 323–333.
Tou, L., Quibria, N., & Alexander, J. M. (2001). Regulation of human cbfa1 gene transcription in osteoblasts by selective estrogen receptor modulators (SERMs). Molecular and Cellular Endocrinology, 183, 71–79.
Nojiri, C., Okano, T., Koyanagi, H., et al. (1992). In vivo protein adsorption on polymers: Visualization of adsorbed proteins on vascular implants in dogs. Journal of Biomaterials Science, Polymer Edition, 4, 75–88.
Tsaryk, R., Kalbacova, M., Hempel, U., et al. (2007). Response of human endothelial cells to oxidative stress on Ti6Al4V alloy. Biomaterials, 28, 806–813.
LeGeros, R. Z. (1993). Biodegradation and bioresorption of calcium phosphate ceramics. Clinical Materials, 14, 65–88.
Meyer, U., Büchter, A., Wiesmann, H. P., et al. (2005). Basic reactions of osteoblasts on structured material surfaces. European Cells & Materials, 9, 39–49.
Lucas, N., Bienaime, C., Belloy, C., et al. (2008). Polymer biodegradation: Mechanisms and estimation techniques. Chemosphere, 73, 429–442.
Vallés, G., González-Melendi, P., González-Carrasco, J. L., et al. (2006). Differential inflammatory macrophage response to rutile and titanium particles. Biomaterials, 27, 5199–5211.
Sreejalekshmi, K. G., & Nair, P. D. (2011). Biomimeticity in tissue engineering scaffolds through synthetic peptide modifications-altering chemistry for enhanced biological response. Journal of Biomedical Materials Research, Part A, 96, 477–491.
Søballe, K., Hansen, E. S., B-Rasmussen, H., et al. (1992). Tissue ingrowth into titanium and hydroxyapatite-coated implants during stable and unstable mechanical conditions. Journal of Orthopaedic Research, 10, 285–299.
Janatova, J. (2000). Activation and control of complement, inflammation, and infection associated with the use of biomedical polymers. ASAIO Journal, 46, S53–S62.
Laurencin, C. T., Ambrosio, A. M., Borden, M. D., & Cooper, J. A., Jr. (1999). Tissue engineering: Orthopedic applications. Annual Review of Biomedical Engineering, 1, 19–46.
Fang, J., Zhu, Y. Y., Smiley, E., et al. (1996). Stimulation of new bone formation by direct transfer of osteogenic plasmid genes. Proceedings of the National Academy of Sciences of the United States of America, 93, 5753–5758.
Franceschi, R. T. (2005). Biological approaches to bone regeneration by gene therapy. Journal of Dental Research, 12, 1093–1103.
Ishihara, A., Zekas, L. J., Weisbrode, S. E., & Bertone, A. L. (2010). Comparative efficacy of dermal fibroblast-mediated and direct adenoviral bone morphogenetic protein-2 gene therapy for bone regeneration in an equine rib model. Gene Therapy, 17, 733–744.
Ishaug-Riley, S. L., Crane-Kruger, G. M., Yaszemski, M. J., & Mikos, A. G. (1998). Three-dimensional culture of rat calvarial osteoblasts in porous biodegradable polymers. Biomaterials, 19, 1405–1412.
Cartmell, S. H., Porter, B. D., García, A. J., & Guldberg, R. E. (2003). Effects of medium perfusion rate on cell seeded three dimensional bone constructs in vitro. Tissue Engineering, 9, 1197–1203.
Buckley, C. T., & O’Kelly, K. U. (2010). Fabrication and characterization of a porous multidomain hydroxyapatite scaffold for bone tissue engineering investigations. Journal of Biomedical Materials Research, Part B, Applied Biomaterials, 2, 459–467.
Kretlow, J. D., & Mikos, A. G. (2008). From material to tissue: Biomaterial development, scaffold fabrication, and tissue engineering. AIChE Journal, 54, 3048–3067.
Cai, X., Tong, H., Shen, X., et al. (2009). Preparation and characterization of homogeneous chitosan–polylactic acid/hydroxyapatite nanocomposite for bone tissue engineering and evaluation of its mechanical properties. Acta Biomaterialia, 5, 2693–2703.
Jose, M. V., Thomas, V., Johnson, K. T., et al. (2009). Aligned PLGA/HA nanofibrous nanocomposite scaffolds for bone tissue engineering. Acta Biomaterialia, 5, 305–315.
Zhao, M., Qiao, M., Harris, S. E., et al. (2004). Smurf1 inhibits osteoblast differentiation and bone formation in vitro and in vivo. Journal of Biological Chemistry, 279, 12854–12859.
Bae, S. & Ryoo, H. (2008). Method to enhance the bone formation activity of Bmp by Runx2 acetylation. U.S. Patent 20080219962.
Jeon, E. J., Lee, K. Y., Choi, N. S., et al. (2006). Bone morphogenetic protein-2 stimulates Runx2 acetylation. Journal of Biological Chemistry, 281(24), 16502–16511.
Byers, B. A., Guldberg, R. E., & García, A. J. (2004). Synergy between genetic and tissue engineering: Runx2 overexpression and in vitro construct development enhance in vivo mineralization. Tissue Engineering, 10, 1757–1766.
Doll, B., Fu, H., Hollinger, J., & Sfeir, C. (2003). Compositions and devices comprising or encoding the Runx2 protein and method of use. U.S. Patent 20030235564.
Ogawa, T. & Jewett, A. (2009). Osteogenic enhancer composit. U.S. Patent 20090324669.
Gillissen, M., Jaworska, M., Orth, M., et al. (1997). Nacystelyn, a novel lysine salt of N-acetylcysteine, to augment cellular antioxidant defence in vitro. Respiratory Medicine, 91, 159–168.
Strong Donna, D., Linkhart Thomas, A., & Dean David, A. (2006). U.S. Patent 7741113.
Acknowledgment
The authors acknowledge Miss Lucy Percival for revising the English language.
Statement of Interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dalle Carbonare, L., Innamorati, G. & Valenti, M.T. Transcription Factor Runx2 and its Application to Bone Tissue Engineering. Stem Cell Rev and Rep 8, 891–897 (2012). https://doi.org/10.1007/s12015-011-9337-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-011-9337-4