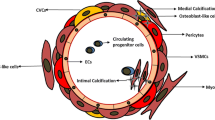
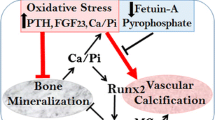
Abstract
Osteoporosis (OP) and cardiovascular diseases (CVD) are both important causes of mortality and morbidity in aging patients. There are common mechanisms underlying the regulation of bone remodeling and the development of smooth muscle calcification; a temporal relationship exists between osteoporosis and the imbalance of mineral metabolism in the vessels. Vascular calcification appears regulated by mechanisms that include both inductive and inhibitory processes. Multiple factors are implicated in both bone and vascular metabolism. Among these factors, the superfamily of tumor necrosis factor (TNF) receptors including osteoprotegerin (OPG) and its ligands has been established. OPG is a soluble decoy receptor for receptor activator of nuclear factor-kB ligand (RANKL) and TNF-related apoptosis-inducing ligand (TRAIL). OPG binds to RANKL and TRAIL, and inhibits the association with their receptors, which have been labeled as the receptor activator of NF-kB (RANK). Sustained release of OPG from vascular endothelial cells (ECs) has been demonstrated in response to inflammatory proteins and cytokines, suggesting that OPG/RANKL/RANK system plays a modulatory role in vascular injury and inflammation. For the development of potential therapeutic strategies targeting vascular calcification, critical consideration of the implications for bone metabolism must be taken into account to prevent potentially detrimental effects to bone metabolism.


Similar content being viewed by others
References
Compston JE, McClung MR, Leslie WD (2019) Osteoporosis. Lancet 393:364–376
Walsh MC, Choi Y (2014) Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol 5:511
Rochette L, Meloux A, Rigal E, Zeller M, Cottin Y, Vergely C (2018) The role of osteoprotegerin in the crosstalk between vessels and bone: its potential utility as a marker of cardiometabolic diseases. Pharmacol Ther 182:115–132
Barbu CG, Arsene AL, Florea S, Albu A, Sirbu A, Martin S, Nicolae AC, Burcea-Dragomiroiu GTA, Popa DE, Velescu BS, Dumitrescu IB, Mitrea N, Draganescu D, Lupuliasa D, Spandidos DA, Tsatsakis AM, Dragoi CM, Fica S (2017) Cardiovascular risk assessment in osteoporotic patients using osteoprotegerin as a reliable predictive biochemical marker. Mol Med Rep 16:6059–6067
Faggiano P, Dasseni N, Gaibazzi N, Rossi A, Henein M, Pressman G (2019) Cardiac calcification as a marker of subclinical atherosclerosis and predictor of cardiovascular events: a review of the evidence. Eur J Prev Cardiol. https://doi.org/10.1177/2047487319830485
Chung CP, Solus JF, Oeser A, Li C, Raggi P, Smith JR, Stein CM (2015) A variant in the osteoprotegerin gene is associated with coronary atherosclerosis in patients with rheumatoid arthritis: results from a candidate gene study. Int J Mol Sci 16:3885–3894
Feng X (2005) RANKing intracellular signaling in osteoclasts. IUBMB Life 57:389–395
Martin-Ventura JL, Munoz-Garcia B, Egido J, Blanco-Colio LM (2007) Trail and vascular injury. Front Biosci 12:3656–3667
Harper E, Forde H, Davenport C, Rochfort KD, Smith D, Cummins PM (2016) Vascular calcification in type-2 diabetes and cardiovascular disease: integrative roles for OPG, RANKL and TRAIL. Vasc Pharmacol 82:30–40
Ikeda T, Kasai M, Utsuyama M, Hirokawa K (2001) Determination of three isoforms of the receptor activator of nuclear factor-kappaB ligand and their differential expression in bone and thymus. Endocrinology 142:1419–1426
Forde H, Harper E, Davenport C, Rochfort KD, Wallace R, Murphy RP, Smith D, Cummins PM (2016) The beneficial pleiotropic effects of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) within the vasculature: a review of the evidence. Atherosclerosis 247:87–96
D’Auria F, Centurione L, Centurione MA, Angelini A, Di Pietro R (2015) Tumor necrosis factor related apoptosis inducing ligand (Trail) in endothelial response to biomechanical and biochemical stresses in arteries. J Cell Biochem 116:2427–2434
Cunha DA, Cito M, Carlsson PO, Vanderwinden JM, Molkentin JD, Bugliani M, Marchetti P, Eizirik DL, Cnop M (2016) Thrombospondin 1 protects pancreatic beta-cells from lipotoxicity via the PERK-NRF2 pathway. Cell Death Differ 23:1995–2006
Milanova V, Ivanovska N, Dimitrova P (2014) TLR2 elicits IL-17-mediated RANKL expression, IL-17, and OPG production in neutrophils from arthritic mice. Mediators Inflamm 2014:643406
Kim JY, Park YJ, Kim KJ, Choi JJ, Kim WU, Cho CS (2013) Osteoprotegerin causes apoptosis of endothelial progenitor cells by induction of oxidative stress. Arthritis Rheum 65:2172–2182
Lee J, Lee S, Lee CY, Seo HH, Shin S, Choi JW, Kim SW, Park JC, Lim S, Hwang KC (2017) Adipose-derived stem cell-released osteoprotegerin protects cardiomyocytes from reactive oxygen species-induced cell death. Stem Cell Res Ther 8:195
Eelen G, de Zeeuw P, Simons M, Carmeliet P (2015) Endothelial cell metabolism in normal and diseased vasculature. Circ Res 116:1231–1244
Rochette L, Lorin J, Zeller M, Guilland JC, Lorgis L, Cottin Y, Vergely C (2013) Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: possible therapeutic targets? Pharmacol Ther 140:239–257
Culic O, Gruwel ML, Schrader J (1997) Energy turnover of vascular endothelial cells. Am J Physiol 273:C205–C213
Schoors S, Bruning U, Missiaen R, Queiroz KC, Borgers G, Elia I, Zecchin A, Cantelmo AR, Christen S, Goveia J, Heggermont W, Godde L, Vinckier S, Van Veldhoven PP, Eelen G, Schoonjans L, Gerhardt H, Dewerchin M, Baes M, De Bock K, Ghesquiere B, Lunt SY, Fendt SM, Carmeliet P (2015) Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature 520:192–197
Iso T, Maeda K, Hanaoka H, Suga T, Goto K, Syamsunarno MR, Hishiki T, Nagahata Y, Matsui H, Arai M, Yamaguchi A, Abumrad NA, Sano M, Suematsu M, Endo K, Hotamisligil GS, Kurabayashi M (2013) Capillary endothelial fatty acid binding proteins 4 and 5 play a critical role in fatty acid uptake in heart and skeletal muscle. Arterioscler Thromb Vasc Biol 33:2549–2557
Hagberg CE, Falkevall A, Wang X, Larsson E, Huusko J, Nilsson I, van Meeteren LA, Samen E, Lu L, Vanwildemeersch M, Klar J, Genove G, Pietras K, Stone-Elander S, Claesson-Welsh L, Yla-Herttuala S, Lindahl P, Eriksson U (2010) Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature 464:917–921
Ingwall JS (2009) Energy metabolism in heart failure and remodelling. Cardiovasc Res 81:412–419
Ashrafian H, Frenneaux MP, Opie LH (2007) Metabolic mechanisms in heart failure. Circulation 116:434–448
Kobayashi-Sakamoto M, Hirose K, Isogai E, Chiba I (2004) NF-kappaB-dependent induction of osteoprotegerin by Porphyromonas gingivalis in endothelial cells. Biochem Biophys Res Commun 315:107–112
Kobayashi-Sakamoto M, Isogai E, Hirose K, Chiba I (2008) Role of alphav integrin in osteoprotegerin-induced endothelial cell migration and proliferation. Microvasc Res 76:139–144
Lommi JI, Kovanen PT, Jauhiainen M, Lee-Rueckert M, Kupari M, Helske S (2011) High-density lipoproteins (HDL) are present in stenotic aortic valves and may interfere with the mechanisms of valvular calcification. Atherosclerosis 219:538–544
Rochette L, Zeller M, Cottin Y, Vergely C (2014) Diabetes, oxidative stress and therapeutic strategies. Biochim Biophys Acta 1840:2709–2729
Zhang J, Fu M, Myles D, Zhu X, Du J, Cao X, Chen YE (2002) PDGF induces osteoprotegerin expression in vascular smooth muscle cells by multiple signal pathways. FEBS Lett 521:180–184
Kleemann R, Bureeva S, Perlina A, Kaput J, Verschuren L, Wielinga PY, Hurt-Camejo E, Nikolsky Y, van Ommen B, Kooistra T (2011) A systems biology strategy for predicting similarities and differences of drug effects: evidence for drug-specific modulation of inflammation in atherosclerosis. BMC Syst Biol 5:125
Stangl K, Stangl V (2010) The ubiquitin-proteasome pathway and endothelial (dys)function. Cardiovasc Res 85:281–290
Laina A, Stellos K, Stamatelopoulos K (2017) Vascular ageing: Underlying mechanisms and clinical implications. Exp Gerontol 109:16–30
Depre C, Wang Q, Yan L, Hedhli N, Peter P, Chen L, Hong C, Hittinger L, Ghaleh B, Sadoshima J, Vatner DE, Vatner SF, Madura K (2006) Activation of the cardiac proteasome during pressure overload promotes ventricular hypertrophy. Circulation 114:1821–1828
Ueland T, Yndestad A, Oie E, Florholmen G, Halvorsen B, Froland SS, Simonsen S, Christensen G, Gullestad L, Aukrust P (2005) Dysregulated osteoprotegerin/RANK ligand/RANK axis in clinical and experimental heart failure. Circulation 111:2461–2468
di Giuseppe R, Biemann R, Wirth J, Menzel J, Isermann B, Stangl GI, Fritsche A, Boeing H, Schulze MB, Weikert C (2017) Plasma osteoprotegerin, its correlates, and risk of heart failure: a prospective cohort study. Eur J Epidemiol 32:113–123
Min JK, Kim YM, Kim YM, Kim EC, Gho YS, Kang IJ, Lee SY, Kong YY, Kwon YG (2003) Vascular endothelial growth factor up-regulates expression of receptor activator of NF-kappa B (RANK) in endothelial cells. Concomitant increase of angiogenic responses to RANK ligand. J Biol Chem 278:39548–39557
Potente M, Carmeliet P (2017) The link between angiogenesis and endothelial metabolism. Annu Rev Physiol 79:43–66
Kobayashi-Sakamoto M, Isogai E, Holen I (2010) Osteoprotegerin induces cytoskeletal reorganization and activates FAK, Src, and ERK signaling in endothelial cells. Eur J Haematol 85:26–35
Dougall WC (2012) Molecular pathways: osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin Cancer Res 18:326–335
Hwang HJ, Jung SH, Lee HC, Han NK, Bae IH, Lee M, Han YH, Kang YS, Lee SJ, Park HJ, Ko YG, Lee JS (2016) Identification of novel therapeutic targets in the secretome of ionizing radiation induced senescent tumor cells. Oncol Rep 35:841–850
Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL (2014) Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344:630–634
Rochette L, Zeller M, Cottin Y, Vergely C (2015) Growth and differentiation factor 11 (GDF11): functions in the regulation of erythropoiesis and cardiac regeneration. Pharmacol Ther 156:26–33
Liu W, Zhou L, Zhou C, Zhang S, Jing J, Xie L, Sun N, Duan X, Jing W, Liang X, Zhao H, Ye L, Chen Q, Yuan Q (2016) GDF11 decreases bone mass by stimulating osteoclastogenesis and inhibiting osteoblast differentiation. Nat Commun 7:12794
Luo J, Yang Z, Ma Y, Yue Z, Lin H, Qu G, Huang J, Dai W, Li C, Zheng C, Xu L, Chen H, Wang J, Li D, Siwko S, Penninger JM, Ning G, Xiao J, Liu M (2016) LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat Med 22:539–546
Weitzmann MN, Ofotokun I (2016) Physiological and pathophysiological bone turnover—role of the immune system. Nat Rev 12:518–532
Goltzman D, Mannstadt M, Marcocci C (2018) Physiology of the calcium-parathyroid hormone-vitamin D axis. Front Horm Res 50:1–13
Akbari S, Rasouli-Ghahroudi AA (2018) Vitamin K and bone metabolism: a review of the latest evidence in preclinical studies. Biomed Res Int 2018:4629383
Schwalfenberg GK (2017) Vitamins K1 and K2: the emerging group of vitamins required for human health. J Nutr Metab 2017:6254836
Roumeliotis S, Dounousi E, Eleftheriadis T, Liakopoulos V (2019) Association of the inactive circulating matrix Gla protein with vitamin K intake, calcification, mortality, and cardiovascular disease: a review. Int J Mol Scie 20:628
Lok ZSY, Lyle AN (2019) Osteopontin in Vascular Disease. Arterioscler Thromb Vasc Biol:ATVBAHA118311577
Back M, Aranyi T, Cancela ML, Carracedo M, Conceicao N, Leftheriotis G, Macrae V, Martin L, Nitschke Y, Pasch A, Quaglino D, Rutsch F, Shanahan C, Sorribas V, Szeri F, Valdivielso P, Vanakker O, Kempf H (2018) Endogenous calcification inhibitors in the prevention of vascular calcification: a consensus statement from the COST action EuroSoftCalcNet. Front Cardiovasc Med 5:196
Yiu AJ, Callaghan D, Sultana R, Bandyopadhyay BC (2015) Vascular calcification and stone disease: a new look towards the mechanism. J Cardiovasc Dev Dis 2:141–164
Davaine JM, Quillard T, Brion R, Laperine O, Guyomarch B, Merlini T, Chatelais M, Guilbaud F, Brennan MA, Charrier C, Heymann D, Goueffic Y, Heymann MF (2014) Osteoprotegerin, pericytes and bone-like vascular calcification are associated with carotid plaque stability. PLoS ONE 9:e107642
Navarro R, Compte M, Alvarez-Vallina L, Sanz L (2016) Immune regulation by pericytes: modulating innate and adaptive immunity. Front Immunol 7:480
Hung CF, Mittelsteadt KL, Brauer R, McKinney BL, Hallstrand TS, Parks WC, Chen P, Schnapp LM, Liles WC, Duffield JS, Altemeier WA (2017) Lung pericyte-like cells are functional interstitial immune sentinel cells. Am J Physiol 312:L556–L567
Wu M, Rementer C, Giachelli CM (2013) Vascular calcification: an update on mechanisms and challenges in treatment. Calcif Tissue Int 93:365–373
Schneeweis LA, Willard D, Milla ME (2005) Functional dissection of osteoprotegerin and its interaction with receptor activator of NF-kappaB ligand. J Biol Chem 280:41155–41164
Garcia-Sanchez C, Posadas-Romero C, Posadas-Sanchez R, Carreon-Torres E, Rodriguez-Perez JM, Juarez-Rojas JG, Martinez-Sanchez C, Fragoso JM, Gonzalez-Pacheco H, Vargas-Alarcon G, Perez-Mendez O (2015) Low concentrations of phospholipids and plasma HDL cholesterol subclasses in asymptomatic subjects with high coronary calcium scores. Atherosclerosis 238:250–255
Wang JC, Bennett M (2012) Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res 111:245–259
Tziakas DN, Chalikias G, Pavlaki M, Kareli D, Gogiraju R, Hubert A, Bohm E, Stamoulis P, Drosos I, Kikas P, Mikroulis D, Giatromanolaki A, Georgiadis GS, Konstantinou F, Argyriou C, Munzel T, Konstantinides SV, Schafer K (2019) Lysed erythrocyte membranes promote vascular calcification: possible role of erythrocyte-derived nitric oxide. Circulation 139:2032–2048
Tesauro M, Mauriello A, Rovella V, Annicchiarico-Petruzzelli M, Cardillo C, Melino G, Di Daniele N (2017) Arterial ageing: from endothelial dysfunction to vascular calcification. J Intern Med 281:471–482
Saliques S, Teyssier JR, Vergely C, Lorgis L, Lorin J, Donzel A, Sicard P, Berchoud J, Ragot S, Touzery C, Cottin Y, Rochette L, Zeller M (2011) Smoking and FOS expression from blood leukocyte transcripts in patients with coronary artery disease. Atherosclerosis 219:931–936
Jilka RL, O’Brien CA (2016) The role of osteocytes in age-related bone loss. Curr Osteoporos Rep 14:16–25
Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL (2007) Disruption of large-scale brain systems in advanced aging. Neuron 56:924–935
Weiskopf D, Weinberger B, Grubeck-Loebenstein B (2009) The aging of the immune system. Transpl Int 22:1041–1050
Hanada R, Leibbrandt A, Hanada T, Kitaoka S, Furuyashiki T, Fujihara H, Trichereau J, Paolino M, Qadri F, Plehm R, Klaere S, Komnenovic V, Mimata H, Yoshimatsu H, Takahashi N, von Haeseler A, Bader M, Kilic SS, Ueta Y, Pifl C, Narumiya S, Penninger JM (2009) Central control of fever and female body temperature by RANKL/RANK. Nature 462:505–509
Shimamura M, Nakagami H, Osako MK, Kurinami H, Koriyama H, Zhengda P, Tomioka H, Tenma A, Wakayama K, Morishita R (2014) OPG/RANKL/RANK axis is a critical inflammatory signaling system in ischemic brain in mice. Proc Natl Acad Sci USA 111:8191–8196
Baron R, Kneissel M (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19:179–192
Yamada S, Giachelli CM (2017) Vascular calcification in CKD-MBD: roles for phosphate, FGF23, and Klotho. Bone 100:87–93
Diab DL, Watts NB (2014) Denosumab in osteoporosis. Expert Opin Drug Saf 13:247–253
Riggs MM, Cremers S (2019) Pharmacometrics and systems pharmacology for metabolic bone diseases. Br J Clin Pharmacol 85(6):1136–1146
Pietrzyk B, Smertka M, Chudek J (2017) Sclerostin: intracellular mechanisms of action and its role in the pathogenesis of skeletal and vascular disorders. Adv Clin Exp Med 26:1283–1291
Alique M, Ramirez-Carracedo R, Bodega G, Carracedo J, Ramirez R (2018) Senescent microvesicles a novel advance in molecular mechanisms of atherosclerotic calcification. Int J Mol Sci 19(7):2003
Shanahan CM (2013) Mechanisms of vascular calcification in CKD-evidence for premature ageing? Nat Rev Nephrol 9:661–670
Kranenburg G, Bartstra JW, Weijmans M, de Jong PA, Mali WP, Verhaar HJ, Visseren FLJ, Spiering W (2016) Bisphosphonates for cardiovascular risk reduction: a systematic review and meta-analysis. Atherosclerosis 252:106–115
Wu MY, Li CJ, Yiang GT, Cheng YL, Tsai AP, Hou YT, Ho YC, Hou MF, Chu PY (2018) Molecular regulation of bone metastasis pathogenesis. Cell Physiol Biochem 46:1423–1438
Lu X, Mu E, Wei Y, Riethdorf S, Yang Q, Yuan M, Yan J, Hua Y, Tiede BJ, Lu X, Haffty BG, Pantel K, Massague J, Kang Y (2011) VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer Cell 20:701–714
Weitzmann MN (2017) Bone and the immune system. Toxicol Pathol 45:911–924
Kondegowda NG, Fenutria R, Pollack IR, Orthofer M, Garcia-Ocana A, Penninger JM, Vasavada RC (2015) Osteoprotegerin and denosumab stimulate human beta cell proliferation through inhibition of the receptor activator of NF-kappaB ligand pathway. Cell Metab 22:77–85
Shirakawa J, Togashi Y, Sakamoto E, Kaji M, Tajima K, Orime K, Inoue H, Kubota N, Kadowaki T, Terauchi Y (2013) Glucokinase activation ameliorates ER stress-induced apoptosis in pancreatic beta-cells. Diabetes 62:3448–3458
Terauchi Y, Takamoto I, Kubota N, Matsui J, Suzuki R, Komeda K, Hara A, Toyoda Y, Miwa I, Aizawa S, Tsutsumi S, Tsubamoto Y, Hashimoto S, Eto K, Nakamura A, Noda M, Tobe K, Aburatani H, Nagai R, Kadowaki T (2007) Glucokinase and IRS-2 are required for compensatory beta cell hyperplasia in response to high-fat diet-induced insulin resistance. J Clin Invest 117:246–257
Panizo S, Cardus A, Encinas M, Parisi E, Valcheva P, Lopez-Ongil S, Coll B, Fernandez E, Valdivielso JM (2009) RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway. Circ Res 104:1041–1048
de Groot AF, Appelman-Dijkstra NM, van der Burg SH, Kroep JR (2018) The anti-tumor effect of RANKL inhibition in malignant solid tumors—a systematic review. Cancer Treat Rev 62:18–28
Murakami K, Kobayashi Y, Uehara S, Suzuki T, Koide M, Yamashita T, Nakamura M, Takahashi N, Kato H, Udagawa N, Nakamura Y (2017) A Jak1/2 inhibitor, baricitinib, inhibits osteoclastogenesis by suppressing RANKL expression in osteoblasts in vitro. PLoS ONE 12:e0181126
Evans BA, Elford C, Pexa A, Francis K, Hughes AC, Deussen A, Ham J (2006) Human osteoblast precursors produce extracellular adenosine, which modulates their secretion of IL-6 and osteoprotegerin. J Bone Miner Res 21:228–236
St Hilaire C, Ziegler SG, Markello TC, Brusco A, Groden C, Gill F, Carlson-Donohoe H, Lederman RJ, Chen MY, Yang D, Siegenthaler MP, Arduino C, Mancini C, Freudenthal B, Stanescu HC, Zdebik AA, Chaganti RK, Nussbaum RL, Kleta R, Gahl WA, Boehm M (2011) NT5E mutations and arterial calcifications. N Engl J Med 364:432–442
Burnstock G (2017) Purinergic signalling: therapeutic developments. Front Pharmacol 8:661
Patel JJ, Zhu D, Opdebeeck B, D’Haese P, Millan JL, Bourne LE, Wheeler-Jones CPD, Arnett TR, MacRae VE, Orriss IR (2018) Inhibition of arterial medial calcification and bone mineralization by extracellular nucleotides: the same functional effect mediated by different cellular mechanisms. J Cell Physiol 233:3230–3243
Abdel-Magid AF (2017) Inhibitors of CD73 may provide a treatment for cancer and autoimmune diseases. ACS Med Chem Lett 8:781–782
Acknowledgements
The authors wish to thank Philip Bastable for English assistance.
Funding
This work was supported by grants from French Ministry of Research, INSERM (Institut national de la santé et de la recherche médicale) and from the Regional Council of Burgundy (Conseil Régional de Bourgogne), FEDER, and Association de Cardiologie de Bourgogne. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Luc Rochette, Alexandre Meloux, Eve Rigal, Marianne Zeller, Gabriel Malka, Yves Cottin, and Catherine Vergely declare that they did not receive funding from any sources and had no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rochette, L., Meloux, A., Rigal, E. et al. The Role of Osteoprotegerin in Vascular Calcification and Bone Metabolism: The Basis for Developing New Therapeutics. Calcif Tissue Int 105, 239–251 (2019). https://doi.org/10.1007/s00223-019-00573-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-019-00573-6