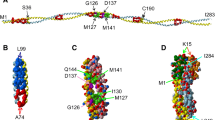
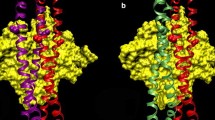
Abstract
Tropomyosin, best known for its role in the steric regulation of muscle contraction, polymerizes head-to-tail to form cables localized along the length of both muscle and non-muscle actin-based thin filaments. In skeletal and cardiac muscles, tropomyosin, under the control of troponin and myosin, moves in a cooperative manner between blocked, closed and open positions on filaments, thereby masking and exposing actin-binding sites necessary for myosin crossbridge head interactions. While the coiled-coil signature of tropomyosin appears to be simple, closer inspection reveals surprising structural complexity required to perform its role in steric regulation. For example, component α-helices of coiled coils are typically zippered together along a continuous core hydrophobic stripe. Tropomyosin, however, contains a number of anomalous, functionally controversial, core amino acid residues. We argue that the atypical residues at this interface, including clusters of alanines and a charged aspartate, are required for preshaping tropomyosin to readily fit to the surface of the actin filament, but do so without compromising tropomyosin rigidity once the filament is assembled. Indeed, persistence length measurements of tropomyosin are characteristic of a semi-rigid cable, in this case conducive to cooperative movement on thin filaments. In addition, we also maintain that tropomyosin displays largely unrecognized and residue-specific torsional variance, which is involved in optimizing contacts between actin and tropomyosin on the assembled thin filament. Corresponding twist-induced stiffness may also enhance cooperative translocation of tropomyosin across actin filaments. We conclude that anomalous core residues of tropomyosin facilitate thin filament regulatory behavior in a multifaceted way.




Similar content being viewed by others
References
Bai F, Wang L, Kawai M (2013) A study of tropomyosin’s role in cardiac function and disease using thin-filament reconstituted myocardium. J Muscle Res Cell Motil 34:295–310
Bailey K (1948) Tropomyosin: a new asymmetric protein component of the muscle fibril. Biochem J 43:271–273
Barua B, Winkelmann DA, White HD, Hitchcock-DeGregori SE (2012) Regulation of actin-myosin interaction by conserved periodic sites of tropomyosin. Proc Natl Acad Sci USA 109:18425–18430
Barua B, Fagnant PM, Winkelmann DA, Trybus KM, Hitchcock-Degregori SE (2013) A periodic pattern of evolutionarily-conserved basic and acidic residues constitutes the binding interface of actin- tropomyosin. J Biol Chem 288:9602–9609
Behrmann E, Müller M, Penczek PA, Mannherz HG, Manstein DJ, Raunser S (2012) Structure of the rigor actin-tropomyosin-myosin complex. Cell 150:327–338
Brown JH, Cohen C (2005) Regulation of muscle contraction by tropomyosin and troponin: how structure illuminates function. Adv Protein Chem 71:121–159
Brown JH, Cohen C, Parry DA (1996) Heptad breaks in alpha-helical coiled coils: stutters and stammers. Proteins 26:134–145
Brown JH, Kim KH, Jun G, Greenfield NJ, Dominguez R, Volkmann N, Hitchcock-Degregori SE, Cohen C (2001) Deciphering the design of the tropomyosin molecule. Proc Natl Acad Sci USA 98:8496–8501
Brown JH, Zhou Z, Reshetnikova L, Robinson H, Yammani RD, Tobacman LS, Cohen C (2005) Structure of the mid-region of tropomyosin: bending and binding sites for actin. Proc Natl Acad Sci USA 102:18878–18883
Cohen C, Holmes KC (1963) X-ray diffraction evidence for the alpha-helical coiled-coils in native muscle. J Mol Biol 6:423–432
Conway JF, Parry DAD (1990) Structural features in the heptad substructure and longer range repeats of two-stranded α-fibrous proteins. Int J Biol Macromol 12:328–334
Corsi A, Perry SV (1958) Some observations on the localization of myosin, actin and tropomyosin in the rabbit myofibril. Biochem J 68:12–17
Crick FHC (1953) The packing of α-helices: Simple coiled-coils. Acta Cryst 6:689–697
Das KM, Bajpai M (2008) Tropomyosins in human diseases: ulcerative colitis. Adv Exp Med Biol 644:158–167
Desai R, Geeves MA, Kad NM (2015) Using fluorescent myosin to directly visualize cooperative activation of thin filaments. J Biol Chem 290:1915–1925
Dominguez R (2011) Tropomyosin: the gatekeeper’s view of the actin filament revealed. Biophys J 100:797–798
Ebashi S (1963) Third component participating in the superprecipitation of “Natural Actomyosin”. Nature 200:1010–1010
Finer JT, Simmons RM, Spudich JA (1994) Single myosin molecule mechanics: piconewton forces and naometre steps. Nature 368:113–119
Flicker PF, Phillips GN Jr, Cohen C (1982) Troponin and its interactions with tropomyosin. An electron microscope study. J Mol Biol 162:495–501
Geeves MA (2012) Thin filament regulation. In: Egelman EH, Goldman YE, Ostap EM (eds) Comprehensive biophysics. Molecular motors and motility. vol 4. Academic Press, Oxford, pp 251–267
Geeves MA, Lehrer SS (2002) Modeling thin filament cooperativity. Biophys J 82:1677–1681
Ghosh A, Janco M, Böcking T, Gunning PW, Lehman W, Rynkiewicz MJ (2019) Structure of the Tpm3.1 N-terminus: a new target for anti-cancer treatment. Biophys J 116
Gunning PW, Hardeman EC (2017) Tropomyosins. Curr Biol 27:R1–R18
Gunning PW, Ghoshdastider U, Whitaker S, Popp D, Robinson RC (2015a) The evolution of compositionally and functionally distinct actin filaments. J Cell Sci 128:2009–2019
Gunning PW, Hardeman EC, Lappalainen P, Mulvihill DP (2015b) Tropomyosin—master regulator of actin filament function in the cytoskeleton. J Cell Sci 128:2965–2971
Hagemann UB, Mason JM, Müller KM, Arndt KM (2008) Selectional and mutational scope of peptides sequestering the Jun-Fos coiled-coil domain. J Mol Biol 381:73–88
Hanson J, Huxley HE (1953) Structural basis of cross-striations in muscle. Nature 172:530–532
Hanson J, Huxley HE (1955) The structural basis of contraction in striated muscle. Symp Soc Exp Biol 9:228–264
Hanson J, Lowy J (1964) The structure of actin filaments and the origin of the axial periodicity in the I-substance of vertebrate striated muscle. Proc R Soc Lond B 160:449–460
Haselgrove JC (1972) X-ray evidence for a conformational change in actin-containing filaments of vertebrate striated muscle. Cold Spring Harbor Symp Quant Biol 37:341–352
Helfman DM, Flynn P, Khan P, Saeed A (2008) Tropomyosin as a regulator of cancer cell development. Adv Exp Med Biol 644:124–131
Hill TL, Eisenberg E, Greene L (1980) Theoretical model for the cooperative equilibrium binding of myosin subfragment 1 to the actin–troponin–tropomyosin complex. Proc Natl Acad Sci USA 77:3186–3190
Hill TL, Eisenberg E, Greene L (1982) Alternate model for the cooperative equilibrium binding of myosin subfragment-1-nucleotide complex to the actin–troponin–tropomyosin. Proc Natl Acad Sci USA 80:60–64
Hitchcock-DeGregori SE (2008) Tropomyosin: function follows form. Tropomyosin and the steric mechanism of muscle regulation. Adv Exp Med Biol 644:60–67
Holmes KC (1995) The actomyosin interaction and its control by tropomyosin. Biophys J 68:2 s–7 s
Holmes KC, Lehman (2008) Gestalt-binding of tropomyosin to actin filaments. J Muscle Res Cell Motil 29:213–219
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38
Hunt CW (1912) The manufacture of manila rope: Its use for transmission and hoisting. Sci Am Suppl 74:404–407
Huxley HE (1969) The mechanism of muscle contraction. Science 164:1356–1366
Huxley HE (1970) Structural changes in muscle and muscle proteins during contraction. In: Proceedings of 8th international congress biochemistry, Interlaken, vol 4, pp 23–23
Huxley HE (1972) Structural changes in actin and myosin-containing filaments during contraction. Cold Spring Harbor Symp Quant Biol 37:361–376
Huxley HE, Brown W (1967) The low-angle X-ray diagram of vertebrate striated muscle and its behavior during contraction and rigor. J Mol Biol 30:383–434
Huxley HE, Hanson J (1954) Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature 173:973–976
Huxley AF, Niedergerke R (1954) Structural changes in muscle during contraction; interference microscopy of living muscle fibres. Nature 173:971–973
Huxley HE, Brown W, Holmes KC (1965) Constancy of axial spacings in frog Sartorius muscle during contraction. Nature 206:1358–1358
Kee AJ, Hardeman EC (2008) Tropomyosins in skeletal muscle diseases. Adv Exp Med Biol 644:143–157
Kee AJ, Yang L, Lucas CA, Greenberg MJ, Martel N, Leong GM, Hughes WE, Cooney GJ, James DE, Ostap EM, Han W, Gunning PW, Hardeman EC (2015) An actin filament population defined by tropomyosinTpm3.1 regulates glucose uptake. Traffic 16:691–711
Kee AJ, Chagan J, Chan JY, Bryce NS, Lucas CA, Zeng J, Hook J, Treutlein H, Laybutt DR, Stehn JR, Gunning PW, Hardeman EC (2018) On-target action of anti-tropomyosin drugs regulates glucose metabolism. Sci Rep 8:4604
Kiani FA, Lehman W, Fischer S, Rynkiewicz MJ (2019) Spontaneous transitions of actin-bound tropomyosin toward blocked and closed states. J Gen Physiol 151:4–8
Kwok SC, Hodges RS (2004) Stabilizing and destabilizing clusters in the hydrophobic core of long two-stranded α-helical coiled-coils. J Biol Chem 279:21576–21588
Laki K, Maruyama K, Kominz DR (1962) Evidence for the interaction between tropomyosin and actin. Arch Biochem Biophys 98:323–330
Lehman W (2016) Thin filament structure and the steric blocking model. Comp Physiol 6:1043–1069
Lehman W (2017) Switching muscles on and off in steps: the McKillop–Geeves three-state model of muscle regulation. Biophys J 112:2459–2466
Lehman W, Craig R, Vibert P (1994) Ca2+-induced tropomyosin movement in Limulus thin filaments revealed by three-dimensional reconstruction. Nature 368:65–67
Lehman W, Hatch V, Korman V, Rosol M, Thomas L, Maytum R, Geeves MA, Van Eyk JE, Tobacman LS, Craig R (2000) Tropomyosin and actin isoforms modulate the localization of tropomyosin strands on actin filaments. J Mol Biol 302:593–606
Lehman W, Li X, Kiani FA, Moore JR, Campbell SG, Fischer S, Rynkiewicz MJ (2018) Precise binding of tropomyosin on actin involves sequence-dependent variance in coiled-coil twisting. Biophys J 115:1082–1092
Lehrer SS (1994) The regulatory switch of the muscle thin filament: Ca2+ or myosin heads? J Muscle Res Cell Motil 15:232–236
Lehrer SS (2011) The 3-state model of muscle regulation revisited: is a fourth state involved? J Muscle Res Cell Motil 32:203–208
Lehrer SS, Geeves MA (1998) The muscle thin filament as a classical cooperative/allosteric regulatory system. J Mol Biol 277:1081–1089
Lehrer SS, Geeves MA (2014) The myosin-activated thin filament regulatory state, M–-open: a link to hypertrophic cardiomyopathy (HCM). J Muscle Res Cell Motil 35:153–160
Li XE, Holmes KC, Lehman W, Jung H-S, Fischer S (2010) The shape and flexibility of tropomyosin coiled-coils: implications for actin filament assembly and regulation. J Mol Biol 395:327–399
Li X, Tobacman LS, Mun JY, Craig R, Fischer S, Lehman W (2011) Tropomyosin position on F-actin revealed by EM reconstruction and computational chemistry. Biophys J 100:1005–1013
Loong CK, Badr MA, Chase PB (2012) Tropomyosin flexural rigidity and single Ca2+ regulatory unit dynamics: implications for cooperative regulation of cardiac muscle contraction and cardiomyocyte hypertrophy. Front Physiol 3:1–10 (article 80)
Lorenz M, Poole KJV, Popp D, Rosenbaum G, Holmes KC (1995) An atomic model of the unregulated thin filament obtained by X-ray fiber diffraction on oriented actin–tropomyosin gels. J Mol Biol 246:108–119
Marston S, Gautel M (2013) Introducing a special edition of the Journal of Muscle Research and Cell Motility on tropomyosin form and function. J Muscle Res Cell Motil 34:151–153
Marston SB, Taylor EW (1980) Comparison of the myosin and actomyosin ATPase mechanisms of the four types of vertebrate muscles. J Mol Biol 139:573–600
Marston S, Memo M, Messer A, Papadaki M, Nowak K, McNamara E, Ong R, El-Mezgueldi M, Li X, Lehman W (2013) Mutations in repeating structural motifs of tropomyosin cause gain of function in skeletal muscle myopathy patients. Hum Mol Genet 22:4978–4987
Matyushenko AM, Artemova NV, Shchepkin DV, Kopylova GV, Bershitsky SY, Tsaturyan AK, Sluchanko NN, Levitsky DI (2014) Structural and functional effects of two stabilizing substitutions, D137L and G126R, in the middle part of α-tropomyosin molecule. FEBS J 281:2004–2016
Matyushenko AM, Shchepkin DV, Kopylova GV, Bershitsky SY, Koubassova NA, Tsaturyan AK, Levitsky DI (2018) Functional role of the core gap in the middle part of tropomyosin. FEBS J 285:871–886
Maytum R, Lehrer SS, Geeves MA (1999) Cooperativity and switching within the three-state model of muscle regulation. Biochemistry 38:1102–1110
McConnell M, Tal Grinspan L, Williams MR, Lynn ML, Schwartz BA, Fass OZ, Schwartz SD, Tardiff JC (2017) Clinically divergent mutation effects on the structure and function of the human cardiac tropomyosin overlap. Biochemistry 56:3403–3413
McKillop DF, Geeves MA (1993) Regulation of the interaction between actin and myosin subfragment-1: evidence for three states of the thin filament. Biophys J 65:693–701
Memo M, Marston S (2013) Skeletal muscle myopathy mutations at the actin tropomyosin interface that cause gain- or loss-of-function. J Muscle Res Cell Motil 34:165–169
Mijailovich SM, Kayser-Herold O, Li X, Griffiths H, Geeves MA (2012) Cooperative regulation of myosin-S1 binding to actin filaments by a continuous flexible Tm-Tn chain. Eur Biophys J 41:1015–1032
Minakata S, Maéda K, Oda N, Wakabayashi K, Nitanai Y, Maéda Y (2008) Two-crystal structures of tropomyosin C-terminal fragment 176–273: exposure of the hydrophobic core to the solvent destabilizes the tropomyosin molecule. Biophys J 95:710–719
Moore JR, Li X, Nirody J, Fischer S, Lehman W (2011) Structural implications of conserved aspartate residues located in tropomyosin’s coiled-coil core. Bioarchitecture 1:250–255
Moore JR, Campbell SG, Lehman W (2016) Structural determinants of muscle thin filament cooperativity. Arch Biochem Biophys 594:8–17
Nesser H (2006) Borkmann’s point: an Inspector Van Veeteren mystery. Translated from Swedish by L. Thompson. Knopf-Doubleday Publishing Group, New York (Vintage Crime/Black Lizard Edition)
Nevzorov IA, Levitsky DI (2011) Tropomyosin: double helix from the protein world. Biochemistry 76:1507–1527
Orzechowski M, Li XE, Fischer S (2014a) An atomic model of the tropomyosin cable on F-actin. Biophys J 107:694–699
Orzechowski M, Fischer S, Moore JR, Lehman W, Farman GP (2014b) Energy landscapes reveal the myopathic effects of tropomyosin mutations. Arch Biochem Biophys 564:89–99
Parry DAD, Squire JM (1973) Structural role of tropomyosin in muscle regulation: analysis of the X-ray diffraction patterns from relaxed and contracting muscles. J Mol Biol 75:33–55
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612
Phillips GN Jr, Fillers JP, Cohen C (1986) Tropomyosin crystal structure and muscle regulation. J Mol Biol 192:111–131
Phillips GN Jr, Chacko S (1996) Mechanical properties of tropomyosin and implications for muscle regulation. Biopolymers 38:89–95
Poole KJ, Lorenz M, Evans G, Rosenbaum G, Pirani A, Tobacman LS, Lehman W, Holmes KC (2006) A comparison of muscle thin filament models obtained from electron microscopy reconstructions and low-angle X-ray fibre diagrams from non-overlap muscle. J Struct Biol 155:273–284
Redwood C, Robinson P (2013) Alpha-tropomyosin mutations in inherited cardiomyopathies. J Muscle Res Cell Motil 34:285–294
Regnier M, Rivera AJ, Wang CK, Bates MA, Chase PB, Gordon AM (2002) Thin filament near-neighbour regulatory unit interactions affect rabbit skeletal muscle steady-state force-Ca2+ relations. J Physiol 540:485–497
Rynkiewicz MJ, Schott V, Orzechowski M, Lehman W, Fischer S (2015) Electrostatic interaction map reveals a new binding position for tropomyosin on F-actin. J Muscle Res Cell Motil 36:525–533
Savill SA, Leitch HF, Harvey JN, Thomas TH (2016) Inflammatory adipokines decrease expression of two high molecular weight isoforms of tropomyosin similar to the change in type 2 diabetes patients. PLoS One 11:e0162908
Scellini B, Piroddi N, Matyushenko AM, Levitsky DI, Poggesi C, Lehrer SS, Tesi C (2017) The relaxation properties of myofibrils are compromised by amino acids that stabilize α-tropomyosin. Biophys J 112:376–387
Schmidt WM, Lehman W, Moore JR (2015) Direct observation of tropomyosin binding to actin filaments. Cytoskeleton 72:292–303
Shchepkin DV, Matyushenko AM, Kopylova GV, Artemova NV, Bershitsky SY, Tsaturyan AK, Levitsky DI (2013) Stabilization of the central part of tropomyosin molecule alters the Ca2+-sensitivity of actin-myosin interaction. Acta Nat 5:126–129
Siemankowski RF, White HD (1984) Kinetics of the interaction between actin, ADP and cardiac myosin-S1. J Biol Chem 259:5015–5053
Singh A, Hitchcock-Degregori SE (2003) Local destabilization of the tropomyosin coiled coil gives the molecular flexibility required for actin binding. Biochemistry 42:14114–14121
Singh A, Hitchcock-DeGregori SE (2006) Dual requirement for flexibility and specificity for binding of the coiled-coil tropomyosin to its target, actin. Structure 14:43–50
Smith DA, Geeves MA (2003) Cooperative regulation of myosin-actin interactions by a continuous flexible chain II: actin-tropomyosin-troponin and regulation by calcium. Biophys J 84:3168–3180
Smith DA, Maytum R, Geeves MA (2003) Cooperative regulation of myosin-actin interactions by a continuous flexible chain I: actin-tropomyosin systems. Biophys J 84:3155–3167
Sousa D, Cammarato A, Jang K, Graceffa P, Tobacman LS, Li XE, Lehman W (2010) Electron microscopy and persistence length analysis of semi-rigid smooth muscle tropomyosin strands. Biophys J 99:1–7
Stehn JR, Haass NK, Bonello T, Desouza M, Kottyan G, Treutlein H, Zeng J, Nascimento PR, Sequeira VB, Butler TL, Allanson M, Fath T, Hill TA, McCluskey A, Schevzov G, Palmer SJ, Hardeman EC, Winlaw D, Reeve VE, Dixon I, Weninger W, Cripe TP, Gunning PW (2013) A novel class of anticancer compounds targets the actin cytoskeleton in tumor cells. Cancer Res 73:5169–5182
Strelkov S, Burkhard P (2002) Analysis of α-helical coiled coils with the program TWISTER reveals a structural mechanism for stutter compensation. J Struct Biol 137:54–64
Sumida JP, Wu E, Lehrer (2008) Conserved Asp-137 imparts flexibility to tropomyosin and affects function. J Biol Chem 283:6728–6734
Szent-Györgyi AG, Cohen C (1957) Role of prolines in polypeptide chain configuration of proteins. Science 126:697–698
Tardiff JC (2005) Sarcomeric proteins and familial hypertrophic cardiomyopathy: linking mutations in structural proteins to complex cardiovascular phenotypes. Heart Fail Rev 10:237–248
Tardiff JC (2011) Thin filament mutations: developing an integrative approach to a complex disorder. Circ Res 108:765–782
Vibert P, Craig R, Lehman W (1997) Steric-model for activation of muscle thin filaments. J Mol Biol 266:8–14
Viswanathan MC, Schmidt W, Rynkiewicz MJ, Agarwal K, Gao J, Katz J, Lehman W, Cammarato A (2017) Distortion of the actin A-triad results in contractile disinhibition and cardiomyopathy. Cell Rep 20:2612–2625
von der Ecken J, Müller M, Lehman W, Manstein DJ, Penczek PA, Raunser S (2015) Structure of the F-actin-tropomyosin complex. Nature 519:114–117
Walcott S, Warshaw DM, Debold EP (2012) Mechanical coupling between myosin molecules cuases differences between ensemble and single-molecule measurements. Biophys J 103:501–510
Williams MR, Tardiff JC, Schwartz SD (2018) Mechanism of cardiac tropomyosin transitions on filamentous actin as revealed by all-atom steered molecular dynamics simulations. J Phys Chem Lett 5:3301–3306
Yar S, Chowdhury SA, Davis RT 3rd, Kobayashi Monasky MM, Rajan S, Wolska BM, Gaponenko V, Kobayashi T, Wieczorek DF, Solaro RJ (2013) Conserved Asp-137 is important for both structure and regulatory functions of cardiac α-tropomyosin (α-TM) in a novel transgenic mouse model expressing α-TM-D137L. J Biol Chem 288:16235–16246
Zheng W, Barua B, Hitchcock-DeGregori SE (2013) Probing the flexibility of tropomyosin and its binding to filamentous actin using molecular dynamics simulations. Biophys J 105:1882–1892
Zheng W, Hitchcock-DeGregori SE, Barua B (2016) Investigating the effects of tropomyosin mutations on its flexibility and interactions with filamentous actin using molecular dynamics simulation. J Muscle Res Cell Motil 37:131–147
Acknowledgements
These studies were supported by NIH Grants R01HL036153 (to W.L.) and R01HL123774 (to J.R.M. and W.L.). The Massachusetts Green High Performance Computing Center provided considerable computational resources to analyze the data presented. We thank Dr. Anita Ghosh for her analysis of crystal structure B factors. The current paper can be regarded as a sequel to Holmes and Lehman (2008), also published in the Journal of Muscle Research and Cell Motility, and is a tribute to Kenneth C. Holmes, who first conceptualized the notion of tropomyosin’s ‘Gestalt-Binding’ to actin filaments, as well as to Brown and Cohen who laid out tropomyosin’s building blocks and to Lehrer and Geeves who clarified thin filament cooperativity for us.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lehman, W., Rynkiewicz, M.J. & Moore, J.R. A new twist on tropomyosin binding to actin filaments: perspectives on thin filament function, assembly and biomechanics. J Muscle Res Cell Motil 41, 23–38 (2020). https://doi.org/10.1007/s10974-019-09501-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-019-09501-5