Abstract
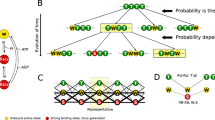
The 3-state model of muscle regulation has been useful in explaining the roles of Ca2+ and myosin heads in activation and relaxation of striated muscle contraction. However, there are some phenomena, which cannot simply be explained by the 3-state model. These include increased Ca2+-binding caused by strong-binding myosin heads and residual active force at low Ca2+ in the case of familial hypertrophic cardiomyopathy. Here, I review experimental data which provide evidence for an additional state, a myosin-induced Open state present in the absence of Ca2+ (Open−Ca2+) which like the normal Open+Ca2+ state, is an active state and can allow myosin heads to cycle and generate force. A schematic diagram is presented which shows that the formation of the Open−Ca2+ state is on a parallel path with the formation of the Open+Ca2+ state and can contribute to activation.

Similar content being viewed by others
References
Bai F, Weis A, Takeda AK, Chase PB, Kawai M (2011) Enhanced active cross-bridges during diastole: molecular pathogenesis of tropomyosin’s HCM mutations. Biophys J 100(4):1014–1023
Bremel RD, Weber A (1972) Cooperation within actin filament in vertebrate skeletal muscle. Nature New Biol 238:97–101
Chalovich JM (2002) Regulation of striated muscle contraction: a discussion. J Muscle Res Cell Motil 23(4):353–361
Chang AN, Harada K, Ackerman MJ, Potter JD (2005) Functional consequences of hypertrophic and dilated cardiomyopathy-causing mutations in alpha-tropomyosin. J Biol Chem 280(40):34343–34349
Coulton A, Lehrer SS, Geeves MA (2006) Functional homodimers and heterodimers of recombinant smooth muscle tropomyosin. Biochemistry 45(42):12853–12858
Dong WJ, Cheung HC (1996) Calcium-induced conformational change in cardiac troponin C studied by fluorescence probes attached to Cys-84. Biochim Biophys Acta 1295(2):139–146
Dong W-J, Robinson JM, Xing J, Umeda PK, Cheung HC (2000) an interdomain distance in cardiac troponin C determined by fluorescence spectroscopy. Protein Sci 9:280–289
Farah CS, Miyamoto CA, Ramos CH, da Silva AC, Quaggio RB, Fujimori K, Smillie LB, Reinach FC (1994) Structural and regulatory functions of the NH2- and COOH-terminal regions of skeletal muscle troponin I. J Biol Chem 269(7):5230–5240
Geeves MA, Lehrer SS (1994) Dynamics of the muscle thin filament regulatory switch: the size of the cooperative unit. Biophys J 67(1):273–282
Geeves MA, Chai M, Lehrer SS (2000) Inhibition of actin-myosin subfragment 1 ATPase activity by troponin I and IC: relationship to the thin filament states of muscle. Biochemistry 39(31):9345–9350
Gordon AM, Homsher E, Regnier M (2000) Regulation of contraction in striated muscle. Physiol Rev 80(2):853–924
Grabarek Z, Grabarek J, Leavis PC, Gergely J (1983) Cooperative binding to the Ca2+−specific sites of troponin C in regulated actin and actomyosin. J Biol Chem 258(23):14098–14102
Heeley DH, Belknap B, White HD (2006) Maximal activation of skeletal muscle thin filaments requires both rigor myosin S1 and calcium. J Biol Chem 281(1):668–676
Hitchcock SE (1975) Regulation of muscle contraction: bindings of troponin and its components to actin and tropomyosin. Eur J Biochem 52(2):255–263
Hoffman PA, Fuchs F (1987) Effect of length and cross-bridge attachment on Ca2+ binding to cardiac troponin C. Am J Physiol 253:C90–C96
Houmeida A, Heeley DH, Belknap B, White HD (2010) Mechanism of regulation of native cardiac muscle thin filaments by rigor cardiac myosin-S1 and calcium. J Biol Chem 285(43):32760–32769
Kobayashi T, Solaro RJ (2005) Calcium, thin filaments, and the integrative biology of cardiac contractility. Annu Rev Physiol 67:39–67
Lehrer SS (1994) The regulatory switch of the muscle thin filament: Ca2+ or myosin heads? J Muscle Res Cell Motil 15(3):232–236
Lehrer SS, Geeves MA (1998) The muscle thin filament as a classical cooperative/allosteric regulatory system. J Mol Biol 277(5):1081–1089
Lehrer SS, Morris EP (1982) Dual effects of tropomyosin and troponin–tropomyosin on actomyosin subfragment 1 ATPase. J Biol Chem 257(14):8073–8080
Lehrer SS, Golitsina NL, Geeves MA (1997) Actin-tropomyosin activation of myosin subfragment 1 ATPase and thin filament cooperativity. The role of tropomyosin flexibility and end-to-end interactions. Biochemistry 36(44):13449–13454
Maytum R, Westerdorf B, Jaquet K, Geeves MA (2003) Differential regulation of the actomyosin interaction by skeletal and cardiac troponin isoforms. J Biol Chem 278(9):6696–6701
McKillop DF, Geeves MA (1993) Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys J 65(2):693–701
Moss RL, Razumova M, Fitzsimons DP (2004) Myosin crossbridge activation of cardiac thin filaments: implications for myocardial function in health and disease. Circ Res 94(10):1290–1300
Mudalige WA, Tao TC, Lehrer SS (2009) Ca2+-dependent photocrosslinking of tropomyosin residue 146 to residues 157–163 in the C-terminal domain of troponin I in reconstituted skeletal muscle thin filaments. J Mol Biol 389(3):575–583
Nagashima H, Asakura S (1982) Studies on cooperative properties of tropomyosin-actin and tropomyosin-troponin-actin complexes by the use of N-ethyl maleimide-treated and untreated species of myosin subfragment 1. J Mol Biol 155:409–428
Perry SV (1999) Troponin I: inhibitor or facilitator. Mol Cell Biochem 190(1–2):9–32
Potter JD, Gergely J (1974) Troponin, tropomyosin and actin interactions in the regulation of muscle contraction. Biochemistry 13:2697–2703
Potter JD, Sheng Z, Pan BS, Zhao J (1995) A direct regulatory role for troponin T and a dual role for troponin C in the Ca2+ regulation of muscle contraction. J Biol Chem 270(6):2557–2562
Prochniewicz E, Walseth TF, Thomas DD (2004) Structural dynamics of actin during active interaction with myosin: different effects of weakly and strongly bound myosin heads. Biochemistry 43(33):10642–10652
Robinson P, Griffiths PJ, Watkins H, Redwood CS (2007) Dilated and Hypertrophic Cardiomyopathy Mutations in Troponin and {alpha}-Tropomyosin Have Opposing Effects on the Calcium Affinity of Cardiac Thin Filaments. Circ Res 101(12):1266–1273
Singh A, Hitchcock-DeGregori SE (2006) Dual requirement for flexibility and specificity for binding of the coiled-coil tropomyosin to its target, actin. Structure 14(1):43–50
Solaro RJ, Moss RL (eds) (2002) Molecular control mechanisms in striated muscle contraction, vol 1. Advances in Muscle Research, Kluwer, Dordrecht
Stelzer JE, Larsson L, Fitzsimons DP, Moss RL (2006) Activation dependence of stretch activation in mouse skinned myocardium: implications for ventricular function. J Gen Physiol 127(2):95–107
Sun YB, Irving M (2009) The molecular basis of the steep force-calcium relation in heart muscle. J Mol Cell Cardiol 48(5):859–865
Tesi C, Piroddi N, Colomo F, Poggesi C (2002) Relaxation kinetics following sudden Ca(2+) reduction in single myofibrils from skeletal muscle. Biophys J 83(4):2142–2151
Tobacman LS, Butters CA (2000) A new model of cooperative myosin-thin filament binding. J Biol Chem 275(36):27587–27593
Van Eyk JE, Thomas LT, Tripet B, Wiesner RJ, Pearlstone J, Farah CS, Reinach FC, Hodges RS (1997) Distinct region of troponin I regulate Ca2+−dependent activation and Ca2+−sensitivity of the acto-S1-Tm ATPase activity of the thin filament. J Biol Chem 272:10529–10537
Vibert P, Craig R, Lehman W (1997) Steric-model for activation of muscle thin filaments. J Mol Biol 266(1):8–14
Wang CK, Cheung HC (1985) Energetics of the binding of calcium and troponin I to troponin C from rabbit skeletal muscle. Biophys J 48(5):727–739
Zhou X, Morris EP, Lehrer SS (2000) Binding of troponin I and the troponin I-troponin C complex to actin-tropomyosin. Dissociation by myosin subfragment 1. Biochemistry 39(5):1128–1132
Acknowledgments
I greatly appreciate input on drafts of this review and helpful critical discussions with my colleagues, Drs. Franklin Fuchs, Michael Geeves, and Zenon Grabarek. Support from NIH HL9116 is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lehrer, S.S. The 3-state model of muscle regulation revisited: is a fourth state involved?. J Muscle Res Cell Motil 32, 203–208 (2011). https://doi.org/10.1007/s10974-011-9263-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-011-9263-8