Abstract
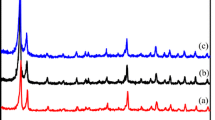
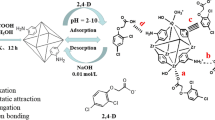
As a famous metal–organic framework (MOF) material, HKUST-1 has a high density of open metal sites and a moderate pore size. Moreover, it is usually used for liquid phase adsorption because of its good water resistance. However, the traditional hydrothermal method has the disadvantages of slow synthesis speed, harsh experimental processes and unsafe experimental conditions. Herein, HKUST-1 had been synthesized under room temperature through a quickly and convenient way. Synthesized HKUST-1 showed good adsorption capacity of europium(III) (135.32 mg/g) at pH = 6. The results indicated that the adsorption procedure obeys quasi-second-order kinetic model and Langmuir isothermal model.















Similar content being viewed by others
References
Hu BW, Hu QY, Li X, Pan H, Tang XP, Chen CG, Huang CC (2017) Rapid and highly efficient removal of Eu(III) from aqueous solutions using graphene oxide. J Mol Liq 229:6–14. https://doi.org/10.1016/j.molliq.2016.12.030
Feng XF, Long RX, Wang LL, Liu CC, Bai ZX, Liu XB (2022) A review on heavy metal ions adsorption from water by layered double hydroxide and its composites. Separ Purification Technol. https://doi.org/10.1016/j.seppur.2021.120099
Yang W, Pan Q, Song S, Zhang H (2019) Metal–organic framework-based materials for the recovery of uranium from aqueous solutions. Inor Chem Front 6:1924–1937. https://doi.org/10.1039/c9qi00386j
Zhao B, Yuan L, Wang Y, Duan T, Shi W (2021) Carboxylated UiO-66 tailored for U(VI) and Eu(III) trapping: from batch adsorption to dynamic column separation. ACS Appl Mater Int 13:16300–16308. https://doi.org/10.1021/acsami.1c00364
Yan J, Liu HJ, Xie L, Liu Z, Liu PF, Wen HX (2022) Europium(III) removal from aqueous solution using citric acid modified alkalized Mxene as an adsorbent. J Radioanalytical Nuclear Chem 331:1063–1073. https://doi.org/10.1007/s10967-021-08154-4
Tang T, Liu H, Liu J, Jiang W, Liu Z, Yan J, Xie L, Li L (2021) Facile synthesis of an environment-friendly cyclodextrin-based polycarboxylic acid polymer for efficient removal of U(VI) and Eu(III). J Radioanal Nucl Chem 329:1247–1260. https://doi.org/10.1007/s10967-021-07885-8
Sheng GD, Yang ST, Li YM, Gao X, Huang YY, Hu J, Wang XK (2014) Retention mechanisms and microstructure of Eu(III) on manganese dioxide studied by batch and high resolution EXAFS technique. Radiochim Acta 102:155–167. https://doi.org/10.1515/ract-2014-2088
Zhu YL, Zheng C, Wu SY, Song YZ, Hu BW (2018) Interaction of Eu(III) on magnetic biochar investigated by batch, spectroscopic and modeling techniques. J Radioanalytical Nuclear Chem 316:1337–1346. https://doi.org/10.1007/s10967-018-5839-8
Chen Y, Zhu B, Wu D, Wang Q, Yang Y, Ye W, Guo J (2012) Eu(III) adsorption using di(2-thylhexly) phosphoric acid-immobilized magnetic GMZ bentonite. Chem Eng J 181–182:387–396. https://doi.org/10.1016/j.cej.2011.11.100
Lu S, Xu J, Zhang C, Niu Z (2010) Adsorption and desorption of radionuclide europium(III) on multiwalled carbon nanotubes studied by batch techniques. J Radioanal Nucl Chem 287:893–898. https://doi.org/10.1007/s10967-010-0849-1
Hu J, Xie Z, He B, Sheng G, Chen C, Li J, Chen Y, Wang X (2010) Sorption of Eu(III) on GMZ bentonite in the absence/presence of humic acid studied by batch and XAFS techniques. Sci China Chem 53:1420–1428. https://doi.org/10.1007/s11426-010-3064-6
Carlos LD, Ferreira RA, Bermudez Vde Z, Ribeiro SJ (2009) Lanthanide-containing light-emitting organic-inorganic hybrids: a bet on the future. Adv Mater 21:509–534. https://doi.org/10.1002/adma.200801635
Cadogan EI, Lee C-H, Popuri SR, Lin H-Y (2014) Efficiencies of chitosan nanoparticles and crab shell particles in europium uptake from aqueous solutions through biosorption: synthesis and characterization. Int Biodeterior Biodegradation 95:232–240. https://doi.org/10.1016/j.ibiod.2014.06.003
Franville AC, Mahiou R, Zambon D, Cousseins JC (2001) Molecular design of luminescent organic-inorganic hybrid materials activated by europium (III) ions. Solid State Sci 3:211–222
Wang Z, Wang J, Zhang H (2004) Luminescent sol–gel thin films based on europium-substituted heteropolytungstates. Mater Chem Phys 87:44–48. https://doi.org/10.1016/j.matchemphys.2004.04.016
Dong L, Li S, Jin Y, Hu B, Sheng G (2021) Enhanced adsorption of Eu(III) from wastewater using Solidago canadensis-derived biochar functionalized by Ca/Al-LDH and hydroxyapatite. Appl Surf Sci 567:150794. https://doi.org/10.1016/j.apsusc.2021.150794
Burnett JL, Croudace IW, Warwick PE (2011) Pre-concentration of short-lived radionuclides using manganese dioxide precipitation from surface waters. J Radioanal Nucl Chem 292:25–28. https://doi.org/10.1007/s10967-011-1392-4
Prakash D, Gabani P, Chandel AK, Ronen Z, Singh OV (2013) Bioremediation: a genuine technology to remediate radionuclides from the environment. Microb Biotechnol 6:349–360. https://doi.org/10.1111/1751-7915.12059
Hassan KF, Spellerberg S, Scholten B, Saleh ZA, Qaim SM (2014) Development of an ion-exchange method for separation of radioiodine from tellurium and antimony and its application to the production of 124I via the 121Sb(α, n)-process. J Radioanal Nucl Chem 302:689–694. https://doi.org/10.1007/s10967-014-3270-3
Ma H, Wei X, Xiong X, Yuan F, Wei G, Wang Y (2016) Research progress of application of ion exchange technology on the removal of trace radionuclides from liquid radioactive waste innuclear power plant. Technol Water Treatment
Wang XX, Yu SJ, Wang XK (2019) Removal of radionuclides by metal-organic framework-based materials. J Inor Mater 34:17–26. https://doi.org/10.15541/jim20180211
Wang N, Pang H, Yu S, Gu P, Song S, Wang H, Wang X (2019) Investigation of adsorption mechanism of layered double hydroxides and their composites on radioactive uranium: a review. Acta Chim Sinica 77:143–152. https://doi.org/10.6023/a18090404
Sheng G, Dong H, Shen R, Li Y (2013) Microscopic insights into the temperature-dependent adsorption of Eu(III) onto titanate nanotubes studied by FTIR, XPS, XAFS and batch technique. Chem Eng J 217:486–494. https://doi.org/10.1016/j.cej.2012.10.076
Sheng GD, Yang ST, Zhao DL, Sheng J, Wang XK (2012) Adsorption of Eu(III) on titanate nanotubes studied by a combination of batch and EXAFS technique. Sci China-Chem 55:182–194. https://doi.org/10.1007/s11426-011-4370-3
Liu X, Wu J, Zhang SW, Ding CC, Sheng GD, Alsaedi A, Hayat T, Li JX, Song YT (2019) Amidoxime-functionalized hollow carbon spheres for efficient removal of uranium from wastewater. Acs Sustain Chem Eng 7:10800–10807. https://doi.org/10.1021/acssuschemeng.9b01616
Pei LA, Wang LM, Yu GQ (2011) Separation of Eu(III) with supported dispersion liquid membrane system containing D2EHPA as carrier and HNO3 solution as stripping solution. J Rare Earths 29:7–14. https://doi.org/10.1016/s1002-0721(10)60394-8
Kim KW, Baek YJ, Lee KY, Chung DY, Moon JK (2016) Treatment of radioactive waste seawater by coagulation-flocculation method using ferric hydroxide and poly acrylamide. J Nuclear Sci Technol 53:439–450. https://doi.org/10.1080/00223131.2015.1055313
Luo X, Zhang G, Wang X, Gu P (2013) Research on a pellet co-precipitation micro-filtration process for the treatment of liquid waste containing strontium. J Radioanal Nucl Chem 298:931–939. https://doi.org/10.1007/s10967-013-2495-x
Naveau A, Monteil-Rivera F, Dumonceau J, Boudesocque S (2005) Sorption of europium on a goethite surface: influence of background electrolyte. J Contam Hydrol 77:1–16. https://doi.org/10.1016/j.jconhyd.2004.10.002
Zhang P, Wang L, Du K, Wang S, Huang Z, Yuan L, Li Z, Wang H, Zheng L, Chai Z, Shi W (2020) Effective removal of U(VI) and Eu(III) by carboxyl functionalized MXene nanosheets. J Hazard Mater 396:122731. https://doi.org/10.1016/j.jhazmat.2020.122731
Xu Z, Niu Z, Tang Q, Wei X, Chen X, Pan D, Wu W (2021) Adsorption characteristics of Eu(III) on colloidal bentonite particles in aqueous solution: impact of colloid concentration, pH, foreign ions, and temperature. J Radioanal Nucl Chem 330:765–773. https://doi.org/10.1007/s10967-021-07976-6
Zhang S, Wang JQ, Zhang Y, Ma JZ, Huang LT, Yu SJ, Chen L, Song G, Qiu MQ, Wang XX (2021) Applications of water-stable metal-organic frameworks in the removal of water pollutants: a review. Environ Pollut. https://doi.org/10.1016/j.envpol.2021.118076
Feng YF, Jiang H, Li SN, Wang J, Jing XY, Wang YR, Chen M (2013) Metal-organic frameworks HKUST-1 for liquid-phase adsorption of uranium. Colloids Surfaces a-Physicochem Eng Aspects 431:87–92. https://doi.org/10.1016/j.colsurfa.2013.04.032
Zhao L, Azhar MR, Li X, Duan X, Sun H, Wang S, Fang X (2019) Adsorption of cerium (III) by HKUST-1 metal-organic framework from aqueous solution. J Colloid Interface Sci 542:421–428. https://doi.org/10.1016/j.jcis.2019.01.117
Yang AL, Wang ZJ, Zhu YK (2020) Facile preparation and adsorption performance of low-cost MOF@cotton fibre composite for uranium removal. Sci Rep. https://doi.org/10.1038/s41598-020-76173-4
Li Z-Q, Qiu L-G, Xu T, Wu Y, Wang W, Wu Z-Y, Jiang X (2009) Ultrasonic synthesis of the microporous metal-organic framework Cu-3(BTC)(2) at ambient temperature and pressure: An efficient and environmentally friendly method. Mater Lett 63:78–80. https://doi.org/10.1016/j.matlet.2008.09.010
Diring S, Furukawa S, Takashima Y, Tsuruoka T, Kitagawa S (2010) Controlled Multiscale Synthesis of Porous Coordination Polymer in Nano/Micro Regimes. Chem Mater 22:4531–4538. https://doi.org/10.1021/cm101778g
Hartmann M, Kunz S, Himsl D, Tangermann O, Ernst S, Wagener A (2008) Adsorptive separation of isobutene and isobutane on Cu-3(BTC)(2). Langmuir 24:8634–8642. https://doi.org/10.1021/la8008656
Chui SSY, Lo SMF, Charmant JP, Orpen AG, Williams ID (1999) A chemically functionalizable nanoporous material. Sci 283:1148–1150. https://doi.org/10.1126/science.283.5405.1148
Ameloot R, Gobechiya E, Uji-i H, Martens JA, Hofkens J, Alaerts L, Sels BF, De Vos DE (2010) Direct patterning of oriented metal-organic framework crystals via control over crystallization kinetics in clear precursor solutions. Adv Mater 22:2685. https://doi.org/10.1002/adma.200903867
Krawiec P, Kramer M, Sabo M, Kunschke R, Froede H, Kaskel S (2006) Improved hydrogen storage in the metal-organic framework Cu-3(BTC)(2). Adv Eng Mater 8:293–296. https://doi.org/10.1002/adem.200500223
Wee LH, Lohe MR, Janssens N, Kaskel S, Martens JA (2012) Fine tuning of the metal–organic framework Cu3(BTC)2 HKUST-1 crystal size in the 100 nm to 5 micron range. J Mater Chem 22:13742. https://doi.org/10.1039/c2jm31536j
Zhao J, Nunn WT, Lemaire PC, Lin Y, Dickey MD, Oldham CJ, Walls HJ, Peterson GW, Losego MD, Parsons GN (2015) Facile Conversion of hydroxy double salts to metal-organic frameworks using metal oxide particles and atomic layer deposition thin-film templates. J Am Chem Soc 137:13756–13759. https://doi.org/10.1021/jacs.5b08752
Kapnisti MG, Noli FG, Papastergiadis ES, Pavlidou EG (2018) Exploration of the parameters affecting the europium removal from aqueous solutions by novel synthesized titanium phosphates. J Environ Chem Eng 6:3408–3417. https://doi.org/10.1016/j.jece.2018.05.010
Chen L, Yu XJ, Zhao ZD (2007) Effect of humic acid, pH and ionic strength on the sorption of Eu(III) on red earth and its solid component. J Radioanalytical Nuclear Chem 274:187–193. https://doi.org/10.1007/s10967-006-6814-0
Zhu Y, Chen T, Liu H, Xu B, Xie J (2016) Kinetics and thermodynamics of Eu(III) and U(VI) adsorption onto palygorskite. J Mol Liq 219:272–278. https://doi.org/10.1016/j.molliq.2016.03.034
Xu B, Zhu Y, Liu H, Jin Z, Chen T (2016) The kinetic and thermodynamic adsorption of Eu(III) on synthetic maghemite. J Mol Liq 221:171–178. https://doi.org/10.1016/j.molliq.2016.05.055
Wu C, Cai Y, Xu L, Xie J, Liu Z, Yang S, Wang S (2018) Macroscopic and spectral exploration on the removal performance of pristine and phytic acid-decorated titanate nanotubes towards Eu(III). J Mol Liq 258:66–73. https://doi.org/10.1016/j.molliq.2018.02.110
Yu T, Yang J, Wang Z-Y (2019) Adsorption behaviour of Eu(III) on natural bamboo fibres: effects of pH, humic acid, contact time, and temperature. Nucl Sci Tech. https://doi.org/10.1007/s41365-019-0710-3
Hu SZ, Huang T, Zhang N, Lei YZ, Wang Y (2022) Chitosan-assisted MOFs dispersion via covalent bonding interaction toward highly efficient removal of heavy metal ions from wastewater. Carbohyd Polym. https://doi.org/10.1016/j.carbpol.2021.118809
Hu K, Liu ZR, Xiu TY, Zhou LM, Wang Y (2020) Removal of thorium from aqueous solution by adsorption with Cu-3(BTC)(2). J Radioanalytical Nuclear Chem 326:185–192. https://doi.org/10.1007/s10967-020-07310-6
Ma JP, Zhao QY, Zhou LJ, Wen T, Wang JJ (2019) Mutual effects of U(VI) and Eu(III) immobilization on interpenetrating 3-dimensional MnO2/graphene oxide composites. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2019.133696
Lin KYA, Hsieh YT (2015) Copper-based metal organic framework (MOF), HKUST-1, as an efficient adsorbent to remove p-nitrophenol from water. J Taiwan Inst Chem Eng 50:223–228. https://doi.org/10.1016/j.jtice.2014.12.008
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.11375084), and Natural Science Foundation of Hunan Province (No.2021JJ50092).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, P., Wen, H., Jiang, Z. et al. One-step rapid synthesis of HKUST-1 and the application for europium(III) adsorbing in solution. J Radioanal Nucl Chem 331, 4309–4321 (2022). https://doi.org/10.1007/s10967-022-08510-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08510-y