Abstract
Interest in polymer-based biomaterials such as chitosan and its modifications and also the methods of their application in various fields of science is uninterruptedly growing. Owing to unique physicochemical, biological, ecological, physiological properties, such as biocompatibility, biodegradability, stability in the natural environment, non-toxicity, high biological activity, economic affordability, chelating of metal ions, high sorption properties, chitosan is used in various biomedical and industrial processes. The reactivity of the amino and hydroxyl groups in the structure makes it more interesting for diverse applications in drug delivery, tissue engineering, wound healing, regenerative medicine, blood anticoagulation and bone, tendon or blood vessel engineering, dentistry, biotechnology, biosensing, cosmetics, water treatment, agriculture. Taking into account the current situation in the world with COVID-19 and other viruses, chitosan is also active in the form of a vaccine system, it can deliver antibodies to the nasal mucosa and load gene drugs that prevent or disrupt the replication of viral DNA/RNA, and deliver them to infected cells. The presented article is an overview of the nowaday state of the application of chitosan, based on literature of recent years, showing importance of fundamental and applied studies aimed to expand application of chitosan-based polymers in many fields of science.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biopolymers, having unique properties, ease of using and processing, variety in combination with economy and environmental friendliness, differ from other classes of materials. These are high molecular weight natural materials that constitute the structural basis of all living organisms and play a significant role in vital processes [1]. They can be obtained both from living organisms (plants, animals, bacteria, fungi) and by synthesis method. New developments in the production of biopolymers are aimed at using these biomaterials as medical materials, food additives, adsorbents, packaging, cosmetics, fabrics for clothes, chemicals for water purification, industrial plastics, biosensors, etc. [2].
Production of biodegradable carbohydrate biopolymers, which are both a structural material (cellulose, chitin), an energy reserve (starch, glycogen), and also perform numerous biological functions, shows a special interest [3, 4]. Thus, the role of biomaterials synthesized on the basis of carbohydrate biopolymers has been studied in intercellular interactions, cell differentiation, the formation of multicellular systems, the development of malignant neoplasms, etc. [5]. Cellulose-, chitin-, and chitosan-based materials in the form of fibers, membranes, hydrogels, sponges have been developed and implemented in such important areas as pharmaceuticals, biomedicine, the food industry, etc. [6,7,8,9,10,11,12]. The combination of properties such as solubility, viscosity, gelation, mechanical, surface and interfacial properties, composition, degree of polymerization, types of bonds and structure allows to create biomaterials that are promising compounds which meet the requirements of environmental friendliness and economic sustainability for a variety of applications [13].
The aim of some modern studies is to obtain highly effective drugs with sorption activity towards toxic metals, which are dangerous environmental pollutants that can be accumulated and negatively affect the vital functions of the human organism, leading to various pathologies. From the literature data it is known that enterosorbents (drugs of various structures that bind exo- and endogenous substances in the gastrointestinal tract by adsorption) based on polysaccharides and used for purification and binding of various toxins in the internal medium of the organism are very promising for these purposes [14]. In addition, these compounds possess a wide range of pharmacological properties. Removal of toxic metals from the organism is one of the important directions of modern science as well. In this regard, our research carried out on synthesis and application of enterosorbents obtained on the base of chitosan and poly-N-vinylpyrrolidone, with the aim of removal of toxic metals from the human organism is very topical and practical; we are going to present the obtained results in our further publications.
The aim of this review is to present the recent scientific advances in properties and applications of various chitosan-based polymers. Synthesis, study and practical application of chitosan-based polymers in many biomedical fields and as the important environmental treatment materials for removal of toxic metals from different media are one of the achievements of scientific progress in the search of new promising materials in recent years.
Production and structure of chitosan
Source and production of chitosan
Chitosan is produced from chitin, which is present in the bodies of crustacean, molluscs, insects, fungi, etc., by the chemical or enzymatic partial N-deacetylation process [15, 16]. Production process of chitosan (Fig. 1) consists of deproteinization (heat at 60–100 °C for 1–72 h in the presence of 0.125–2.5 M of NaOH, Na2CO3, KOH, K2CO3, Ca(OH)2, Na2SO3); demineralization (HCl, HNO3, H2SO4, CH3COOH and HCOOH at 100 °C for 1–48 h); decolouration (dissolve in organic solvents, bleach with KMnO4, heat at 20–60 °C for 0.25–12 h); and deacetylation (30–50% solution of NaOH) [17,18,19,20].
Scheme of chitosan production from chitin [21]
Structure of chitosan
Chitosan is a natural linear polysaccharide composed of randomly distributed β-(1–4)-linked D-glucosamine and N-acetyl-D-glucosamine (Fig. 2a), namely it is consisted of two monosaccharide units: 2-amino-2-deoxy-β-D-glucopyranose and 2-acetamido-2-deoxy-β-D-glucopyranose linked by β-(1–4) glycosidic bonds, in which about 50% of the acetyl groups will be removed from the chitin by a hydration process or enzyme hydrolysis [22, 23].When the deacetylation degree is higher than 50%, the polymer is called chitosan (in case of less than 50% it is called chitin) [24,25,26]. To avoid depolymerization and the formation of reactive particles under the influence of oxygen, sodium borohydride is added or the system is purged with nitrogen [27]. Jang et al. [28] found that chitosan has α, β and γ crystal structures (Fig. 2b).
α-Chitosan (main form) has a tight structure with strong intermolecular forces and is formed by two parallel and inversely arranged polysaccharide chains [29]. β-Chitosan is formed by two parallel and aligned polysaccharide chains with poor intermolecular hydrogen bonds [30]. γ-Chitosan is composed of three parallel polysaccharide chains, two of which were aligned in the same direction and the other was arranged in the opposite direction [31]. The sources of α-chitosan are crabs and shrimps, β-chitosan are squids, and γ-chitosan are loligos [32].
Properties and modifications of chitosan biopolymers
Properties of chitosan biopolymers
Chitosan is white odourless powder (or flakes) with different molecular weight (MW), degree of deacetylation (DD), insoluble in water and organic solvents, soluble in dilute hydrochloric, formic and acetic acids. Melting point is approximately 290 °C [33, 34]. Thus, the reason for the dissolution of chitosan in dilute hydrochloric acid is explained by the interaction of amino groups with hydrogen cations and converting it into a positively charged polyelectrolyte [35, 36].
Cations in the composition damage hydrogen bonds among the chitosan molecules, and it leads to dissolving them in water. The solubility of chitosan depends on MW and DD. The higher the DD of chitosan, the higher the degree of protonation of amino groups in the molecule, and the easier it dissolves. The larger MW of chitosan, the large number of hydrogen bonds formed in its polymer chain, and more difficult it dissolves [37, 38]. Solubility in water increases, biodegradability and biocompatibility enhance at partial removal of the acetyl groups [39]. DD and MW greatly determine many properties of chitosan, in particular, antimicrobial and anti-biofilm activities, DD determines chitosan solubility and viscosity [40]. Therefore, at the application of chitosan biopolymers in practice (for example, as biomaterials, biopesticides, in drug delivery, immunology, etc.), it is necessary to have information about the main characteristics and control some parameters, such as the content of heavy metals, radionuclides, residual protein content, the presence of endotoxins, allergens bacteria and yeast, and other impurities [41].
Unlike other representatives of polysaccharides (cellulose, pectin, agar, dextran, etc.) chitosan possesses many important properties (Fig. 3), including non-toxicity, chelating activities, biocompatibility, biodegradability, adsorption capacities, film-forming ability, bacteriostatic action [42].
Various physical and chemical properties of chitosan [47]
The antiviral, antibacterial activity of chitosan has been proven, the immunostimulating, adjuvant, adaptogenic, antihypoxic, cholestric, radioprotective, hemostatic effects of chitosan and its derivatives have been confirmed [43,44,45]. The antibacterial effect of chitosan is explained by the interaction of its positively charged amino groups with negatively charged phosphoryl groups of phospholipids of the bacterial cell wall, changes in metabolism, which leads to cell death [46].
It is known that chitosan is capable of interacting with nucleic acids, which, in turn, leads to the disturbance of synthesis of vital proteins and enzymes, and damaging the structure and function of the bacterial cell [48]. The fungicidal properties of chitosan are described by identical mechanisms [49]. The analgesic effect of chitosan has been established due to its ability to absorb bradykinin [50]. Chitosan sulfate, the structural analogue of chitosan, is similar in structure to the heparin—natural blood anticoagulant [51, 52]; the possibility of a synergistic effect of chitosan allows to create the drugs with anticoagulant and anti-sclerotic action [53]. Furthermore, sulfated chitosan is a natural antioxidant, which absorbs hydroxyl and superoxide anion radicals, and can be a substrate for creating drugs and biologically active additives as well [51, 54]. Chitosan can be used for treatment of diabetes because it increases insulin levels [55]. Possibility of using as a polymer matrix for the delivery and dosage release of drugs and anti-allergic properties of chitosan are proven [56]. Application of chitosan in immunotherapy is proposed as an antitumor agent that suppresses the growth of tumor cells, pathogens, stimulates humoral and cellular immunity, for gene therapy with the aim of targeted delivery of genetic material [45]. Chitosan has wound healing properties, stimulates the formation of granulation tissue and the activity of fibroblast proliferation [57, 58] and suppresses fibrosis [44]. Chitosan and its derivatives can be used to create biodegradable carriers of pharmaceuticals in the form of films, which provides the prolonging effect of their action [53, 59, 60].
Modifications of chitosan biopolymers
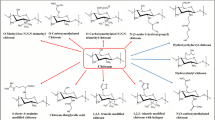
In order to improve the solubility, rheological properties, thermal stability, and oxidation resistance, chitosan is subjected to chemical modifications (Fig. 4) [61].
Different modifications of chitosan biopolymers [62]. Modifications can be used to attach different functional groups and to regulate hydrophobic, cationic and anionic properties of the obtained derivatives of chitosan demonstrating unlimited potential for application in various fields of science
Amino groups, hydroxyl groups at C3 and C6 positions are the active groups in the chemical structure of chitosan. As a rule, the NH2-amino group is more reactive than the C6-OH primary hydroxyl group (due to the free rotation), and the primary hydroxyl group is more reactive than the C3-OH secondary hydroxyl group. Chemical modification of chitosan could be carried out on amino, hydroxyl, or both amino and hydroxyl groups to form N-, O- or N,O-modified chitosan derivatives [63, 64]. Etherification, esterification, crosslinking, graft copolymerization and O-acetylation are reactions carried out on hydroxyl groups, while acetylation, quaternization, Schiff's base reaction and grafting are carried out on amino groups [65].
Schiff bases formation reactions
Modifications of chitosan biopolymers via reactions of Schiff bases formation are well-known. Chitosan reacts readily with most aliphatic and aromatic aldehydes to produce Schiff bases—imines. The Schiff base formed after the reaction of aldehyde and chitosan could be reduced by sodium borohydride to synthesize N-derivatives of chitosan [66, 67]. They could chelate transition metal ions in aqueous solution to form insoluble metal chelates, which could be separated. This reaction is very useful for the application of chitosan for removal of toxic metals.
Quaternization reactions
Modifications of chitosan biopolymers via quaternization reaction are carried out by means of a free amino group on the chitosan. It includes introduction of quaternary ammonium groups or small molecule quaternary ammonium salts on the amino group of chitosan. These groups have strong hydration ability and large steric hindrance. The quaternized chitosan has increased solubility in water and good antibacterial properties [68,69,70].
Alkylation and acylation reactions
Modifications of chitosan biopolymers via alkylation and acylation reactions are carried out with halogenated hydrocarbons, anhydrides, acid halides as acylating agents in a certain reaction medium. Synthesized compounds destroy the hydrogen bonds among chitosan molecules, change the original crystal structure, greatly improving the solubility and widening application range of chitosan [71,72,73,74].
Carboxylation and carboxymethylation reactions
Modifications of chitosan biopolymers via carboxylation and carboxymethylation reactions involve the introduction of acid groups into the main chain of chitosan in order to improve the solubility, moisturizing and film-forming properties of the compound [75, 76]. Carboxymethylation can occur both at the hydroxyl and amino groups of chitosan with the formation of O-carboxymethyl and N-carboxymethyl derivatives, respectively. Carboxymethyl chitosan, a water-soluble anionic polymer was selectively modified to prepare antitumour drug conjugates [77, 78], also was reported as a potential vehicle for targeted drug delivery to the liver due to its preferentially located and long retainment in the liver and spleen after intravenous injection [79]. The modification of chitosan with sugars on amino groups allows to introduce cell-specific sugars recognized by cells, viruses and bacteria into carriers of specific drugs, DNA and antibodies [80, 81].
Graft copolymerization reactions
Modifications of chitosan biopolymers via graft copolymerization reaction improve the solubility and biological activity of the polymer [82] and are used for medical and pharmaceutical applications as orthopedic/periodontal, wound-dressing materials, tissue engineering and controlled drug/gene delivery [83,84,85,86]. Based on this modification and a molecular imprinting technique, chitosan could be used for special absorption of template molecules mimicking natural recognition materials such as antibodies for diagnostics [87]. Recently composites of chitosan with various polymers (polyethylene glycol, polylactic acid, polypyrrole, collagen, starch) and with inorganic materials (bioactive glass, ceramics) have been intensively studied for drug delivery systems, tissue engineering, and other medical applications [88,89,90,91]. Hyaluronic acid, alginate, chondroitin sulfate, hydroxyapatite are used with chitosan for preparation of multilayer-structured biomaterials based on the layer-by-layer technique for applications in tissue engineering [92,93,94,95,96].
Cross-linking reactions
Modifications of chitosan biopolymers via cross-linking allow to obtain chitosan derivatives with stable chemical properties, insoluble in acids and bases and which are used as a carrier for the adsorption of drugs, immobilized enzymes, heavy metal adsorbents, etc. Researchers have compared the composition of metal complexes formed by the coordination of chitosan with some heavy metal ions before and after cross-linking. The ability of chitosan to adsorb metal ions was as follows: Hg > Cu > Pb > Zn > Cd > Mn [97].
Other chemical modifications of chitosan such as esterification, hydroxyalkylation, sulfonation, etc. are known and studied [98,99,100,101]. At present, chitosan is also physically modified through mechanical grinding, ionizing radiation and ultrasonic treatment to prepare biomaterials for the various applications [102].
Recent researches of chitosan biopolymers
The presence of amino and hydroxyl groups in chitosan opens the great opportunities for many industrial and biomedical applications. Use of chitosan biopolymers is uninterruptedly growing in such fields as medicine, pharmaceutical research, paper, textile, agriculture and food industries, cosmetology, tissue engineering, ecology, biotechnology, wastewater treatment (Fig. 5). Chitosan-based materials have also found application in veterinary medicine, medical nutrition, production of dietary supplements, biopesticides, biosensors, chromatographic materials [103,104,105,106,107,108,109,110,111,112]. The use of chitosan has been described in direct tablet compression, as tablet disintegrant, for the production of controlled release dosage form or for the improvement of drug dissolution [113].
Application of chitosan biopolymers in biomedical practice
Recent applications are in ophthalmic, nasal, sublingual, buccal, periodontal, gastrointestinal, colon-specific, vaginal, mucosal-vaccine and gene carrier fields. Chitosan, being an adsorbable and nontoxic polymer, is favored in drug delivery because of antiulcer and antacid properties, which help in preventing drug irritation [114, 115]. During the last years the use of chitosan composite-based scaffolds as a biomaterial has been reported for tissue engineering [116, 117] due to the cationic nature and ability to form interconnected porous structures. Chitosan with other biomaterials such as hydroxyapatite, bioactive glass ceramic are used for bone repair and reconstruction to form a carbonated apatite layer to enhance the mechanical properties [118,119,120,121,122]. Owing to unique properties (toughness, biocompatibility, oxygen permeability) chitosan-based biomaterials in the form of fibers, mats, sponges have been used for burn treatment and wound dressings [123]. Influence of chitosan biomaterials on the synthesis of collagen for wound healing was studied [124]. Chitosan has been modified by authors [125] for using as a dressing material for treatment of wounds and burns. It was found that dressing materials based on chitosan and its modified forms, having haemostatic and analgesic properties, and also possessing properties of high strength, non-toxicity, good water absorption capacity and biocompatibility, together with other polymers (both synthetic and natural) accelerate the process of wound contraction and healing [126].
Researches carried out in the field of infectious diseases show the effectiveness of the use of chitosan in this area. Systems developed on the base of chitosan with different properties have been proposed [127]. It has been shown that these systems reduce the side effects of drugs and increase the effectiveness of treatment. Taking account the current situation in the world with COVID-19 and other viruses, chitosan is also active in the form of a vaccine system, for example, it can deliver antibodies to the nasal mucosa and load gene drugs that prevent or disrupt the replication of viral DNA/RNA, and deliver them to infected cells. Further work on the development of systems is proposed that will be widely used in clinical practice, in particular, for the treatment of infectious diseases (Fig. 6).
Applications of chitosan-based biomaterials in infection diseases [127]. Chitosan biomaterials having good biocompatibility, bioactivity and biosafety, demonstrate great potential in the field of infection control
From year to year, the spread of dangerous pathogenic bacteria is very serious for all mankind and that requires the creation of new materials for the treatment of bacterial infections. Thus, antibacterial and antibiotic properties of the chitosan biomaterial with grafted ferulic acid (CFA) against Listeria monocytogenes (LM), Pseudomonas aeruginosa (PA), and Staphylococcus aureus (SA) were studied [128]. It was found that CFA exhibits bactericidal action against LM and SA and bacteriostatic action against PA within 24 h of incubation. In dependence on the concentration it suppresses the viability of pathogenic bacteria, which was associated with a change in membrane properties.
Silver nanoparticles functionalized with chitosan (CS-AgNP) using ethanolic buds extract of Sygyzium aromaticum have been studied by authors of the given research [129]. Decrease in the level of fibrinogen was observed, platelet aggregation was decreased at relatively high concentrations of CS-AgNP. It has been shown, that due to the stable nature, antibacterial, anticoagulant, antiplatelet and thrombolytic activity, CS-AgNP can be used as effective antibacterial agents and anticoagulants with low toxicity in the biomedical field.
The antibacterial efficacy of chitosan has been confirmed as a drug for pulpectomy of infectious teeth [130]. Chitosan can play an important role in preventive dentistry as an agent to prevent dental diseases (caries, periodontitis), an ingredient in dentifrices (toothpaste, chewing gum) having antibacterial effects, increasing salivary secretion, dental adhesives, etc. [131]. Blend hydrogels based on poly(vinyl alcohol) and carboxymethylated chitosan were prepared by electron beam irradiation at room temperature. The antibacterial activity of the hydrogels was studied by optical density method. It was found that the hydrogels exhibited satisfying antibacterial activity against E.coli. and can be widely used in the field of biomedicine and pharmacy [132].
A new antifungal denture base material was proposed by modifying polymethyl methacrylate (PMMA) with chitosan salt (chitosan hydrochloride (CS-HCl) or chitosan glutamate (CS-G)) [133]. When studying its properties in vitro, the analyses carried out showed that, despite the antifungal effect of CS salts in solution, modification of the PMMA polymer with these CS salts does not improve the antifungal, antibiofilm and antiadhesive properties of the base material of PMMA dentures.
Possible applications of biomaterials based on chitosan, antibiotics and antifungal drugs, considering the factors and mechanisms of the antimicrobial and antifungal action of chitosan, and also clarifying the question of the genetic response of microorganisms to chitosan are described [134, 135]. It was established that there are electrostatic interactions between positively charged chitosan and negatively charged cell surface of the microorganism (teichoic acid in gram-positive bacteria, lipopolysaccharide (LPS) in gram-negative bacteria and phosphorylated mannosyl in fungi). In addition, chitosan chelates environmental ions and nutrients which are necessary for the survival of bacteria. It was found that low-molecular-weight chitosan and oligo-chitosan exhibit an extracellular antifungal action, inhibit mitochondrial activity and ATP production, and are also able to penetrate the cell wall, inhibiting DNA/RNA and protein synthesis. The research indicates that despite the fact that chitosan exhibits a high antimicrobial effect, its use on a large scale is limited by some of its properties, such as low solubility in water, lack of a certain molecular weight and purity.
Nanoparticles based on chitosan and its modified forms are widely tested as drug carriers in ophthalmology for the treatment of bacterial and viral infections, glaucoma, age-related macular degeneration and diabetic retinopathy. Authors summarize recent advances in chitosan-based nanotherapy for drug delivery to the eye and the problems that arise during this process [136]. It has been shown that a high degree of cross-linking in chitosan nanoparticles allows to increase drug retention and facilitates penetration into the eyes.
The following research describes in detail the recent developments of chitosan blends with an emphasis on electrospun nanofibers, which represent a new class of biomaterials, in the field of biomedical applications (drug delivery, wound healing, tissue engineering, biosensing, regenerative medicine) (Fig. 7) [137].
Electrospun nanofibers [137] as a novel class of materials that can be used in various biomedical applications
A new method (electrospinning) for the production of chitosan nanofibers with a large surface area and porosity was considered [138]. Specialists working with this material can optimize the properties of these fibers and expand their range of applications. Thus, it is indicated that the development of complex organ structures will be achieved by the method of electrospinning in combination with 3D printing technology, three-dimensional scaffolds will be designed, integrated with growth factors and cells with high viability. It is noted that despite the fact that specialists were able to simulate the structure and morphology of natural tissue, these studies need further clinical trials until they can be reliably applied in medical practice.
Chitosan-g-poly(acrylic acid)/attapulgite/sodium alginate composites were synthesized as drug delivery matrices [139]. It was found that the composite hydrogels displayed high pH-sensitivity. The cumulative release ratios of diclofenac sodium from the hydrogel were 3.76% at pH = 2.1 and 100% at pH = 6.8 within 24 h, respectively. It has been noted that such pH-sensitive polymeric materials can be offered for the development of new controlled drug delivery systems.
Hydrogels based on different ratios of chitosan and sodium alginate were synthesized by gamma irradiation in the presence of glutaraldehyde, as a cross-linking agent. It was found that these blend hydrogels exhibited high water swelling and showed high thermal stability. Also, pH responsive release character of ketoprofen drug was studied in this research [140].
The recent developments in chitosan delivery systems for the treatment of brain tumors and neurodegenerative diseases are presented [141]. It has been found that chitosan nanoparticles improve therapeutic efficacy in various brain diseases due to their biocompatibility, biodegradability, low toxicity, controlled release, mucoadhesiveness and effective absorption by nasal mucosa and tumor cells. Chitosan nanoparticles are also often used as carriers for the delivery of therapeutic agents, successfully increasing their concentration in the brain, and when administered intranasally chitosan nanoparticles are commonly used to deliver drugs to the brain and can increase nasal residence time and absorption by the nasal mucosa.
It is known that chitosan composites are widely used in medical practice (treatment of burns, artificial kidneys, blood anticoagulation and bone, tendon or blood vessel engineering), and also developed for use in biosensors, packaging, separation processes, food or agricultural industries, and catalytic processes. It is planned to create modulated three-dimensional structures of chitosan using cross-linking processes that improve its use in various fields of medicine, as well as the development of porous catalysts based on chitosan in order to increase the efficiency of catalytic processes by increasing the number of available active sites [142].
The presence of electron-donating amino and hydroxyl groups allows to use chitosan biopolymers in the separation and purification of biologically active compounds (nucleic acids and products of their hydrolysis, steroids, amino acids). Recent studies have indicated usage of chitosan-based compounds as effective materials to inhibit biofilm formation and attenuate of virulence properties by various pathogenic bacteria [143].
Application of chitosan biopolymers in environmental protection
Environmental pollution with heavy toxic metals is dangerous for all living organisms. Currently, methods (such as bioadsorption, solvent extraction, remediation by plants and microbial communities, green separation by hydrogel polymers, immobilization, and others) are being developed for the extraction of heavy metals from soil and wastewater. Taking into account the ingestion of heavy metals by humans with food and to prevent serious risks to human health, development of effective methods for removal of heavy toxic metals and to eliminate the toxicity of these metals in air, soil, and water is of great importance. The food chain of the adsorption process of heavy toxic metals by humans is shown in Fig. 8 [144, 145].
Adsorption process of heavy metals from water, soil, air to food chain and finally to human [146]
In work [147] it was shown that chitosan hydrolysates obtained by hydrolysis of high-molecular-weight chitosan by the fenton reaction can be used as potent agents that block or form tight complexes with fine dust in the air, containing some solid particles and unknown species of microorganisms. This data can be used in the future for the production of various dust-proof masks and filters for the purpose of human healthcare.
Nanomaterials prepared on the basis of chitosan and its modified forms together with carbon nanotubes have been used as bacterial disinfectors of various pollutants in the field of water purification [148]. The use of these materials compared to ozonation, chlorination and other disinfection methods has demonstrated the absence of treatment by-products. In the future, the authors plan to develop materials with increased stability and low toxicity, and pay special attention to the design of nanomaterials, which affects the properties and efficiency of the material, in order to eliminate undesired adsorption of biomolecules and increase antibacterial activity.
A promising direction for application of chitosan biopolymers is the sphere of environmental protection, for development of drugs with radioprotective properties, sorbents for the isolation of radionuclides [149]. Chitosan can also be used as a flocculant for water treatment, surfactants and membranes in ultrafiltration, reverse osmosis and evaporation, purification of industrial effluents containing heavy metal ions [150,151,152,153,154]. Chitosan is capable of forming complexes with transition metals [155, 156]. The heavy metal complexes are formed as a result of donation of a nonbonding pair of nitrogen or oxygen electrons on the -NH2 and/or -OH groups, respectively, to a heavy metal ion. Chitosan granules obtained by cross-linking chitosan with tripolyphosphate have significant adsorption properties towards the metal ions and could be effectively used in wastewater treatment [157,158,159]. The nature of the cation is very important in the mechanism of interaction; the affinity of chitosan for cations absorbed on film shows selectivity in the following order [160]:
One of the important applications of chitosan biopolymers is connected with their ability to bind heavy and toxic metal ions. The adsorption capacity values of modified chitosans (MChs) for metal ions removal were reported by Zhang et al. [161]. It has been noted that adsorption process depends not only on adsorbent structure (modifications of chitosan) but also on conditions of the process (pH, temperature, adsorbent dosage, contact time, co-existing ions). The following results for Cu(II) ions adsorption were observed on various MChs (Table 1).
Authors of research [162] developed monodisperse microspheres of chitosan by the microfluidic method and carried out experiments to study the adsorption characteristics to remove copper ions from waste water. The adsorption mechanism was developed based on various adsorption kinetics and isotherms models. The research results showed a high adsorption capacity (75.52 mg/g) and a readsorption efficiency of 74% after 5 cycles. The adsorption capacity in the presence of other competing ions was also studied by the density functional theory (DFT) analysis. It was shown that the most energetically favorable structure of the studied metal complexes is the central model, where metal ions are coordinatedly bound to several amino groups (Fig. 9).
Structures of investigated divalent metal-CS complexes [162]
Pb(II) imprinted magnetic biosorbent was prepared by means of lead ion imprinting technology and cross-linking reactions between chitosan, Fe3O4 and Serratia marcescens in order to remove of Pb2+ ions. The influence of solution pH, adsorbent dosage, selectivity of sorption and desorption processes were studied on the adsorption of lead ion. Kinetics and thermodynamics of adsorption process were investigated and adsorbent was studied by XRD, VSM, SEM, EDS, FTIR, XPS and BET analyses. It has been established that nitrogen of amino group and oxygen of hydroxyl group in Pb(II) imprinted magnetic biosorbent were coordination atoms [163].
A method of heavy metal ions removal by bioadsorption with hybrid 3D printing technology was proposed [164]. For this purpose, 3D chitosan composite of a monolithic structure of reusable application was prepared, which showed high efficiency in contrast to conventional biosorbents. The adsorption capacity of this material was about 13.7 mg/g at T = 25 °C and pH = 5.5. The analyses performed showed that the –NH2 and –OH functional groups of chitosan are actively involved in the adsorption process, which indicates the possibility of this sorbent using to remove numerous metal ions from different solutions.
In work [165, 166] recent data on removal of lead (Pb), cadmium (Cd), mercury (Hg) and arsenic (As) by chitosan-based magnetic adsorbents from various aqueous solutions are presented. It has been shown that these adsorbents have a high adsorptive capacity towards toxic metals and can be reused in consecutive adsorption–desorption cycles. Langmuir isotherm model confirms good monolayer capacity values of 341.7 mg/g for lead, 152 mg/g for mercury, 321.9 mg/g for cadmium and 65.5 mg/g for arsenic (Fig. 10).
Mechanism of monolayer chemical adsorption of toxic metal ions on the surface of chitosan-based magnetic adsorbent [165]. Metal ions, marked by red circles, are gradually adsorbed on the surface of the magnetic adsorbent
Removal of cadmium ions from waste water was studied using polypropylene/sisal fiber/banana fiber (PP/SF/BF) and chitosan/sisal fiber/banana fiber (CS/SF/BF) composite materials as adsorbents. It has been established that sorption capacity of CS/SF/BF composite (419 mg/g) is higher than PP/SF/BF composite (304 mg/g), and permits multilayer adsorption. The carried out tests have shown that adsorption process was best satisfied with the Freundlich isotherm [167].
Modified chitosan-based nanocomposites (MCS/GO-PEI) were prepared for removal toxic heavy metals and organic compounds from environmental water. The results of research showed that sorption process was characterized by pseudo-second-order kinetic and Langmuir isotherm model. High adsorptive capacities of these samples for arsenic, mercury ions, congo red, amaranth (220.26, 124.84, 162.07, 93.81 mg/g, respectively) were presented and the possibility of re-using these nanocomposites as promising adsorbents was shown [168]. Preparation of graphene oxide/chitosan (GO/CS) composites as new promising sorbent materials for removal of heavy metal ions, dyes and other organic molecules from aquatic environment is presented in paper [169, 170]. Sorption of copper (II), cobalt (II) and iron (III) ions, using chitosan composite sponges prepared by ice-segregation procedure, was studied for purification of waste water [171]. It has been determined that iron (III) ions were mainly adsorbed from two-component mixtures with cobalt (II) ions at pH = 4, whereas copper (II) ions were removed from two-component mixtures with cobalt (II) ions at pH = 6. Carried out experiments showed high chemical stability and reusability of these sponges in sorption–desorption processes.
Nitrogen-enriched chitosan-based activated carbon biosorbent was prepared for separation of Cr(VI) and Pb(II) ions from contaminated water. Thermodynamic parameters have been studied, and kinetics of adsorption of these metal ions is well-fitted by a pseudo-second-order model. High efficiency, availability, recyclability, and cost effectiveness make it possible to use this biosorbent for wastewater treatment [172, 173].
Magnetic phosphorylated chitosan composite (P-MCS) as an adsorbent for Co(II) ions was prepared by authors [174]. Adsorption capacity for Co(II) was equal to 46.1 mg/g. Adsorption isotherms and kinetic models of these ions well fitted the Langmuir model and the pseudo-second-order model, respectively. The carried out experiments have shown dependence of Co(II) adsorption process on surface chelation between functional groups and metal ions, and possibility of use P-MCS for treatment of wastewater.
In order to eliminate the limitations in the use of chitosan as an adsorbent for the removal of heavy metals, such modifications as cross-linking, grafting, and the use of magnetic chitosan (modified with Fe3O4) were carried out [175]. It was suggested in further studies to focus attention on: issues of regeneration and desorption; replacing glutaraldehyde and epichlorohydrin as crosslinking agents with less toxic ones; the use of an adsorbent that does not depend on pH; the use of various optimization tools (for example, the response surface methodology) and other issues in order to use chitosan on an industrial scale.
New class of crystalline porous composite consisting of metal ions and multidentate organic ligands is metal organic framework (MOF), which showed an appreciable capability in wastewater treatment for the removal of heavy metal ions. Functionalization of chitosan with ionic liquids (new class of salts with combination of organic and inorganic ions and with very unique and novel properties) was found to have increased adsorption capacity. They are immobilized on a solid support or they chemically react due to their high reactivity in adsorption process. Analyses carried out in work [176] showed that introduction of ionic liquids in chitosan improves thermal stability and heavy metal uptake properties.
Chitosan conjugated magnetite nanoparticle (CH-MNP) as an effective adsorbent was synthesized for the removal of Pb(II) ions by means of controlled co-precipitation technique and studied by response surface methodology (RSM) for optimization of process parameters [177]. Optimum value of pH, adsorbent concentration and contact time were obtained as 5.1, 1.04 g/L, and 59.9 min, respectively. Adsorption isotherm data were correlated well with the Langmuir adsorption isotherm model, and the equilibrium data followed the pseudo-second-order kinetics and intraparticle diffusion kinetic model.
New EDTA modified γ-MnO2/chitosan/Fe3O4 nanocomposite was produced for the removal of heavy ions from aqueous solutions. Experiments data have been shown high adsorption capacities for Pb(II) and Zn(II) (310.4 and 136 mg/g, respectively). Results of thermodynamic tests (ΔG° < 0, ΔH° > 0, and ΔS° > 0) showed that the nature of adsorption by this nanocomposite for Pb(II) and Zn(II) ions is spontaneous and endothermic, and is favored at higher temperatures [178].
Adsorption and removal of chromium (VI) ions from aqueous solutions, using chitosan hydrogel cross-linked with polyacrylic acid and N, N'-methylenebisacrylamide, has been studied in paper [173]. Evaluation of adsorption mechanism was carried out using Langmuir, Freundlich, Redlich-Peterson, and Sips nonlinear isotherms. The removal of chromium (VI) at pH 4.5 and an initial metal concentration of 100 mg/L was 94.72%. It was proposed to use chitosan hydrogel as an economical and environmentally friendly adsorbent of heavy metal ions for water and wastewater treatment.
A new efficient method of adsorption and removal of heavy metal ions with electric field-driven from wastewater has been proposed [179]. A composite adsorbent based on chitosan (CS) and sodium phytate (SP) deposited on a polyethylene glycol terephthalate (PET) material was used and placed near the cathode in a pair of titanium plate electrodes. Experiments have shown that the rate of copper ions removal adsorbed on the CS-SP/PET adsorbent increased from 56 to 88% for 10 mg Cu (II) solution per liter when the applied voltage was from 0 to 1.2 V (energy consumption was economical). The adsorption mechanism was correlated to the Langmuir isotherm model and the kinetic equation of the pseudo-second order.
Chitosan and silica gel-based composite was prepared with the purpose to study the adsorption of heavy metal ions in various solutions [180]. This composite was studied by FTIR and SEM–EDS methods in order to obtain information about the presence of active sites and surface morphology. The study of the adsorption process by this material showed the maximum percentage of removal of Cu (89.78%), Pb (96.9%) and Ni (69.33%) at pH = 5, Hg (92.78%) at pH = 6 with adsorbent mass of 1.5 g, temperature 30 °C and 120 min contact time. Adsorption of Pb is best satisfied to pseudo-first order, whereas pseudo-second order is best fitted to the adsorption of Cu, Ni and Hg. Obtained values of change in enthalpy testify to the effect that both physical and chemical adsorption occur in this process.
A highly adsorptive cross-linked carboxymethyl chitosan (CMC)/2,3-dimethoxybenzaldehyde Schiff base complex was synthesized for removal of heavy metals such as lead (II) and cadmium (II) ions from aqueous solutions and characterized using FTIR, XRD and SEM analysis [181]. It was confirmed that adsorption follows the Freundlich model and the pseudo-second order kinetic model. The cross-linked Schiff base has been found to be an effective, environmentally friendly and inexpensive adsorbent.
Development of a new economical and environmentally friendly chitosan nanoadsorbent has been proposed for water purification [182]. Use of inorganic nanomaterials, agricultural waste, adsorbents based on polymer nanocomposites for removing of heavy metal ions such as Hg (II), Cu (II), Cr (VI), Zn (II), Co (II), Cd (II), Pb (II) from wastewater has been studied. Experiments have shown that polymer-based materials have a strong chelating ability towards heavy metal ions, fast adsorption kinetics, and are well regenerated due to the synergistic effect of polymers and various nanofillers present in nanocomposites.
Hydrogels based on different ratios of carboxymethyl cellulose (CMC) and carboxymethyl chitosan (CMCh) and prepared by γ-irradiation showed high adsorption capacities for Pb and Au ions. It has been established that the effective sorption of these metal ions occurred with amino groups of the hydrogel with (CMC/CMCh) composition of 75/25 or 50/50. Properties of the obtained hydrogels (gel fraction, swelling ratio, gel strength) were also studied [183].
Carboxymethylated chitosan hydrogels were obtained by γ-ray irradiation crosslinking method. Kinetic studies of sorption process were carried out with a purpose to determine favourable conditions for the adsorption of Fe(III) ions on these hydrogels and showed that maximum uptake of Fe(III) ions was equal to 18.5 mg/g at pH = 4.7 [184]. Favorable adsorption behavior was explained due to the coordination of Fe(III) ions with amino, hydroxyl and carboxyl groups in the structures of the proposed hydrogels.
Application of chitosan biopolymers in other spheres
Chitosan is widely used in cosmetology as a moisturizer, emulsifier, antistatic, emollient for hair and skin care. Chitosan biopolymers are polycation hydrocolloids that become viscous at interaction with acid and can act as abrasive film formers interacting with integuments and hair. Its use as an antioxidant agent and gelling agent in the food industry has also been proven [185, 186]. This biopolymer is used as a food wrap owing to its ability to form semipermeable tough, long-lasting, flexible films, thus extending the shelf life of food [187, 188], inhibiting microbial growth [189, 190]. Chitosan has been used in agriculture as antifungal agents and also to accelerate the growth of plant and decelerate root knot worm infestations [191].
In the paper and textile industry, chitosan is applied to cellulose fiber during the formation of paper, while the strength of the paper sheet is significantly increased, the resistance to bursting, tearing, and image stability are improved. Chitosan is used to improve the dyeing quality of fabrics made from various fibers. There are known data on the use of this biopolymer for the preparation of antistatic, stain-resistant, printing and finishing materials, for the removal of dyes and the manufacture of textile seams, threads and fibers as well [192].
Commercial chitosan products
Chitosan can be produced from different sources and the most traditional source of chitosan is from waste crustaceans’ shells from the seafood processing industry, such as crab or shrimp shells. While research has indicated the availability of other sources, these are currently the most sources actively explored on a commercial scale. Chitosan market volume is expected to reach 2.55 × 109 US dollar by 2022. Although many articles have been published during the last twenty years, chitosan applications in the biomedical field are still limited, mainly due to the difficulty of obtaining of the biopolymer with high purity and reliability at its source. Furthermore, production of new chitosan-based materials is quite limited, mainly due to their cost, which remains higher than that of petroleum-based polymers with similar properties [131]. It is required to develop more economical and environmentally friendly methods in order to obtain chitosan and convert it into useful products. On the other hand, the production cost of crustaceans based chitosan is cheap compared with fungal based chitosan. Crustaceans raw materials are readily available and cheap whereas the cost of raw materials is the main bottleneck for fungal chitosan production. Crustaceans chitosan can be found from 10 US dollar per kg to 1000 US dollar per kg. It also depends on product quality and application [193].
It should be noted that some commercial products of chitosan are known in the world market. Different forms of chitosan-based materials are used as wound dressing (HemCon® Bandage, ChitoGauze® PRO, ChitoFlex® PRO, ChitoSam™, Syvek-Patch®, Chitopack C® and Chitopack S®, Chitodine®, ChitosanSkin®, TraumaStat®, TraumaDEX®, Celox™), as hemostatic sealants (ChitoSeat™) in biomedical practice. Reaxon® (Medovent, Germany) is a chitosan-based nerve conduit which is resistant to destruction, prevents irritation, inflammation and infection, inhibits scar tissue and neuroma formation. Chitosan-based nutritional supplements (Epakitin™, Nutri + Gen®) are commercially available for use in chronic kidney disease in pets. Various chitosan-based products (ChitoClear®, Chitoseen™-F, MicroChitosan NutriCology®, etc.) are for sale as safe weight loss supplement, cholesterol-reducing agents, and also as antioxidant agents.
Many chitosan-containing products (Curasan™, Hydamer™, Zenvivo™, Ritachitosan®, Chitosan MM222, Chitoseen™-K, ChitoCure®, ChitoClear®, etc.) are also commercially available for cosmetic and hygienic usage. [131].
Conclusion
At present, chitosan due to the availability, renewability of raw material and the unique properties is a subject of researches and is widely used in various fields of biotechnology, medicine, pharmacy and industry.
In the coming years, demand for polymer-based biomaterials with better performance will be unquestionably the highest. Distribution of chitosan-based biomaterials at the larger scale can contribute as a sustainable and renewable material for the scientific developments in future. Furthermore, in the past decade in various fields of researches significant advancement has submitted but is still incomplete and applications of chitosan in the biomedical area are still limited. There are still many unresolved issues and challenges. Bioactivity of chitosan-based polymers has been studied for many years, however, the structure activity relationship and the mechanism of activity needs further investigation. This might be connected with poor bioavailability, and lacked of human clinical trials, and all these factors required further analysis.
At present time, there is not enough literature information on the application of polymer-based enterosorbents in medical practice, which is considered as one of the promising directions in the treatment and prevention of diseases of various etiologies [194, 195]. Preparation and application of enterosorbents reduces the intensity of antibiotic and hormone therapy. The development of this direction depends on both technological possibilities and the state of the environment.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
References
Pattanashetti NA, Heggannavar GB, Kariduraganava MY (2017) Smart biopolymers and their biomedical applications. Procedia Manuf 12:263–279. https://doi.org/10.1016/J.PROMFG.2017.08.030
Augustine R, Rajakumari R, Cvelbar U, Mozetic M, George A (2013) In: Thomas S (ed) Biopolymers for health, food, and cosmetic applications. Handbook of biopolymer-based materials. 1st edn, Wiley-VCH Verlag GmbH & Co. KGaA 801–851
Soni S, Gupta H, Kumar N, Nishad D, Mittal G, Bhatnagar A (2010) Biodegradable biomaterials. Recent Patents Biomed Eng 3:30–40
Tunakova YA, Mukhametshina ES, Shmakova YA (2011) Assessment of the sorption capacity of biopolymer sorbents based on chitosan in relation to metals. Bullet Kazan Technol Univ 10:96–100
Varlamov VP, Ilyina AV, Shagdarova BT, Lunkov AP, Mysyakina IS (2020) Chitin/chitosan and its derivatives: fundamental problems and practical approaches. Biochemistry 60:317–368. https://doi.org/10.1134/S0006297920140084
Hu Y, Abidi N (2016) Distinct chiral nematic self-assembling behavior caused by different size-unified cellulose nanocrystals via a multistage separation. Langmuir 32(38):9863–9872. https://doi.org/10.1021/acs.langmuir.6b02861
HuY, Catchmark JM (2011) In vitro biodegradability and mechanical properties of bioabsorbable bacterial cellulose incorporating cellulases. Acta Biomaterialia 7(7):2835–2845. https://doi.org/10.1016/j.actbio.2011.03.028
Hu Y, Catchmark JM, Vogler EA (2013) Factors impacting the formation of sphere-like bacterial cellulose particles and their biocompatibility for human osteoblast growth. Biomacromolecules 14(10):3444–3452. https://doi.org/10.1021/bm400744a
Hu Y et al (2014) Engineering of porous bacterial cellulose toward human fibroblasts in growth for tissue engineering. J Mater Res 29(22):2682–2693. https://doi.org/10.1557/jmr.2014.315
Hu Y et al (2016) Preparation, characterization, and cationic functionalization of cellulose-based aerogels for wastewater clarification. J Mater. Article ID:3186589. https://doi.org/10.1155/2016/3186589
Hu Y et al (2016) Bioabsorbable cellulose composites prepared by an improved mineral-binding process for bone defect repair. J Mater Chem B 4(7):1235–1246. https://doi.org/10.1039/C5TB02091C
Wang S, Lu A, Zhang L (2016) Recent advances in regenerated cellulose materials. Prog Polym Sci (53):169–206. https://doi.org/10.1016/j.progpolymsci.2015.07.003
Dassanayake RS, Acharya S, Abidi N (2019) Biopolymer-based materials from polysaccharides: properties, processing, characterization and sorption applications. Sorption 1–24. Intechopen. https://doi.org/10.5772/intechopen.80898
Fatullayeva S, Tagiyev D, Zeynalov N (2021) A review on enterosorbents and their application in clinical practice: Removal of toxic metals. Colloid Interf Sci Commun 45:100545. https://doi.org/10.1016/j.colcom.2021.100545
Elchinger PH, Delattre C, Faure S, Roy O, Badel S, Bernardi T, Taillefumier C, Michaud P (2015) Immobilization of proteases on chitosan for the development of films with anti-biofilm properties, Int J Biol Macromol 72:1063–1068. https://doi.org/10.1016/j.ijbiomac.2014.09.061
Jung WJ, Park RD (2014) Bioproduction of chitooligosaccharides: present and perspectives. Mar Drugs 12:5328–5356. https://doi.org/10.3390/md12115328
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31:603–632. https://doi.org/10.1016/j.progpolymsci.2006.06.001
Knidri H, Belaabed R, Addaou A, Laajeb A, Lahsini A (2018) Extraction, chemical modification and characterization of chitin and chitosan. Int J Biol Macromol 120:1181–1189. https://doi.org/10.1016/j.ijbiomac.2018.08.139
Kumari S, Rath P, Kumar A, Tiwari T (2015) Extraction and characterization of chitin and chitosan from fishery waste by chemical method. Environ Technol Innov 3:77–85. https://doi.org/10.1016/j.eti.2015.01.002
Cai J, Yang JH, Du YM, Fan LH, Qiu YL, Li J, Kennedy JF (2006) Enzymatic preparation of chitosan from the waste Aspergillusniger mycelium of citric acid production plant. Carbohydr Polym 64(2):151–157. https://doi.org/10.1016/j.carbpol.2005.11.004
Razaa ZA, Khalila S, Ayuba A, Banat IM (2020) Recent developments in chitosan encapsulation of various active ingredients for multifunctional applications. Carbohydr Res 492:108004. https://doi.org/10.1016/j.carres.2020.108004
Zhao Y, Ma L, Zeng R, Tu M, Zhao J (2016) Preparation, characterization and protein sorption of photo-crosslinked cell membrane-mimicking chitosan-based hydrogels. Carbohydr Polym 151:237–244. https://doi.org/10.1016/j.carbpol.2016.05.067
Pereira L, Alves M (2012) Dyes-environmental impact and remediation Environmental Protection Strategies for Sustainable Development. 111–162. https://doi.org/10.1007/978-94-007-1591-2-4
Bajaj M, Winter J, Gallert C(2011) Effect of deproteination and deacetylation conditions on viscosity of chitin and chitosan extracted from Crangoncrangon shrimp waste. Biochem Eng J 56:51–62. https://doi.org/10.1016/j.bej.2011.05.006
Mochalova AE (2017) Target functional modification of chitosan. Dissertation for the degree of Doctor of Chemical Sciences. Nizhny Novgorod 257
Yong SK, Shrivastava M, Srivastava P, Kunhikrishnan A, Bolan N (2015) Environmental applications of chitosan and its derivatives. Rev Environ Contamination Toxicol 233:1–43. https://doi.org/10.1007/978-3-319-10479-9_1
Jolles P, Muzzarelli RAA (1999) Chitin and Chitinases. Springer Basel AG
Jang MK, Kong BG, Jeong YI, Lee CH, Nah JW (2004) Physicochemical characterization of α-chitin, β-chitin, and γ-chitin separated from natural resources. J Polym Sci A Polym Chem 42(14):3423–3432. https://doi.org/10.1002/pola.20176
Faria RR, Guerra RF, Neto LR, Motta LF, Franca EF (2016) Computational study of polymorphic structures of α-and β-chitin and chitosan in aqueous solution. J Mol Graph Model 63:78–84. https://doi.org/10.1016/j.jmgm.2015.11.001
Subhapradha N, Shanmugam A (2017) Fabrication of β-chitosan nanoparticles and its anticancer potential against human hepatoma cells. Int J Biol Macromol 94:194–201. https://doi.org/10.1016/j.ijbiomac.2016.10.016
Kaya M, Mujtaba M, Ehrlich H, Salaberria AM, Baran T, Amemiya CT, Galli R, Akyuz L, Sargin I, Labidi J (2017) On chemistry of γ-chitin. Carbohydr Polym 176:177–186. https://doi.org/10.1016/j.carbpol.2017.08.076
Anitha A, Sowmyaa S, Kumar PT, Deepthi S, Chennazhi KP, Ehrlich H, Tsurkan M, Jayakumar R (2014) Chitin and chitosan in selected biomedical applications. Prog Polym Sci 39:1644–1667. https://doi.org/10.1016/j.progpolymsci.2014.02.008
Sivaramakrishna D, Bhuvanachandra B, Mallakuntla MK, Das SN, Ramakrishna B, Podile AR (2020) Pretreatment with KOH and KOH-urea enhanced hydrolysis of α-chitin by an endo-chitinase from Enterobacter cloacae subsp. cloacae. Carbohydr Polym 235:115952. https://doi.org/10.1016/j.carbpol.2020.115952
Goy RC, Britto DD, Assis OB (2009) A review of the antimicrobial activity of chitosan. Polimeros 19:241–247. https://doi.org/10.1590/S0104-14282009000300013
Islam S, Bhuiyan MR, Islam MN (2017) Chitin and chitosan: structure, properties and applications in biomedical engineering. J Polym Environ 25(3):854–866. https://doi.org/10.1007/s10924-016-0865-5
Younes I, Rinaudo M (2015) Chitin and chitosan preparation frommarine sources. Structure, properties and applications, Marine Drugs 13 (3):1133–1174. https://doi.org/10.3390/md13031133
Zargar V, Asghari M, DashtiA (2015) A review on chitin and chitosan polymers: structure, chemistry, solubility, derivatives, and applications. ChemBioEng Rev 2(3):204–226. https://doi.org/10.1002/cben.201400025
Mourya VK, Inamdar N (2012) Details on chitosan, its chemistry, physiochemical properties and chitosan derivatives with their applications. Chapter 1: Chitosan and Chemically Engineered Chitosan, in: Comprehensive Chemistry and Biomedical Applications of Chitosan and Chemically Engineered Chitosan. Career Publishers, India, 1st ed. 1–24
Wang W, Xue C, Mao X (2020) Chitosan: structural modification, biological activity and application. Int J Biol Macromol 164:4532–4546. https://doi.org/10.1016/j.ijbiomac.2020.09.042
Perinelli DR, Fagioli L, Campana R, Lam JKW, Baffone W, Palmieri GF, Casettari L, Bonacucina G (2018) Chitosan-based nanosystems and their exploited antimicrobial activity. Eur J Pharm Sci 117:8–20. https://doi.org/10.1016/j.ejps.2018.01.046
Kurek D (2013) Sovremennoye sostoyaniye fiziko-khimicheskix metodov opredeleniya osnovnix kharakteristik khitozana i ego proizvodnix. Khitozan/ pod red. K.Q. Skryabina. M.: Sentr «Bioingeneriya» RAN (in Russian) 61‒70
Richter T, Gulich M, Richter K (2012) Quality control and good manufacturing practice (GMP) for chitosan-based biopharmaceutical products, Chitosan-based biopharmaceutical delivery, targeting and polymer therapeutics / Eds. B. Sarmento, J. Neves, John Wiley & Sons 503‒542
Rechkina OY, Koryagin AS, Mochalova AY, Salomatina YV, Smirnova LA (2012) Adaptogenniye effekti nanopreparata “khitozan-zoloto” v usloviyax hipoksii. Perspektivniye Materiali (in Russian) 5:53–57
Slivkin AI, Lapenko VL, Arzamastsev AP, Bolgov AA (2005) Aminoglukani v kachestve biologicheski aktivnix komponentov lekarstvennix sredstv. Vestnik VGU (in Russian) 2:73–87
Li X, Min M, Du N, Gu Y, Hode T, Naylor M, Chen D, Nordquist RE, Chen WR (2013) Chitin, chitosan, and glycated chitosan regulate immune responses: the novel adjuvants for cancer vaccine. Clin Dev Immunol 2013:1–6. https://doi.org/10.1155/2013/387023
Ivanushko LA, Solovyeva TF, Zaporojets TS, Somova LM, Gorbach VI (2009) Antibakterialniye i antitoksicheskiye svoystva khitozana i ego proizvodnix. Tikhookeanskiy Medisinskiy Jurnal (in Russian) 3:82–85
Ahmed S, Ikram S (2015) Chitosan & Its Derivatives: A review in recent innovations. Int J Pharm Sci Res 6(22):14–30. https://doi.org/10.13040/IJPSR.0975-8232.6 (1):14–30
Chung YC, Chen CY (2008) Antibacterial characteristics and activity of acid-soluble chitosan. Bioresour Technol 99(8):2806–2814. https://doi.org/10.1016/j.biortech.2007.06.044
Leuba JL, Stossel P (1986) Chitosan and other polyamines: antifungal activity and interaction with biological membranes. Chitin in Nature and Technology. Plenum Press, New York, pp 215–221
Okamoto Y, Kawakami K, Miyatake K, Morimoto M, Shigemasa Y, Minami S (2002) Analgesic effects of chitin and chitosan. Carbohydr Polym 49(3):249–252. https://doi.org/10.1016/S0144-8617(01)00316-2
Subhapradha N, Suman S, Ramasamy P, Saravanan R, Shanmugam V, Srinivasan A, Shanmugam A (2013) Anticoagulant and antioxidant activity of sulfated chitosan from the shell of donacid clam Donaxscortum (Linnaeus, 1758). Int J Nutr Pharm Neurol Dis 3(1):39–45. https://doi.org/10.4103/2231-0738.106990
Budovskaya KE (2000) Izucheniye vzaimosvyazi stroyeniya i svoystv proizvodnix khitozana s ionogennimi gruppami razlichnix tipov. Dissertation (PhD). Moscow. MGTU (in Russian)
Galbraykh LC (2001) Khitin i khitozan: stroyeniye, svoystva, primeneniye. Sorosovskiy obrazovatelniy jurnal (in Russian) 7(1):51–56
Charernsriwilaiwat N, Opanasopit P, Rojanarata T, Ngawhirunpat T (2012) In vitro antioxidant activity of chitosan aqueous solution: effect of salt form. Trop J Pharm Res 11(2):235–242. https://doi.org/10.4314/tjpr.v11i2.9
Prabu K, Natarajan E (2013) Antihyperglycemic effect of chitosan of podophthalmus vigil in streptozotocin induced diabetic rats. Int J Pharm Sci Res 4(1):352–359. https://doi.org/10.13040/IJPSR.0975-8232.4(1).352-59
Kulikov SN, Tyurin YA, Fassakhov RS, Varlamov VP (2009) Perspektivi primeneniya khitina i khitozana v lechenii razlichnix form allergicheskix zabolevaniy. Prakticheskaya medisina(in Russian) 3:92–97
Alemdaroglu C, Degim Z, Çelebi N, Zor F, Öztürk S, Erdoğan D (2006) An investigation on burn wound healing in rats with chitosan gel formulation containing epidermal growth factor. Burns 32(3):319–327. https://doi.org/10.1016/j.burns.2005.10.015
Ueno H, Mori T, Fujinaga T (2001) Topical formulations and wound healing applications of chitosan. Adv Drug Deliv Rev 52(2):105–115. https://doi.org/10.1016/s0169-409x(01)00189-2
Lozbina NV, Bolshakov IN, Lazarenko VI (2015) Properties of chitosan and its using in ophthalmology. Siberian Med Rev 5:5–13
Shahidi F, Abuzaytoun R (2005) Chitin, chitosan, and co-products: chemistry, production, applications, and health effects. Adv Food Nutr Res 49:93–135. https://doi.org/10.1016/S1043-4526(05)49003-8
Azmy EA, Hashem HE, Mohamed EA, Negm NA (2019) Synthesis, characterization, swelling and antimicrobial efficacies of chemically modified chitosan biopolymer. J Mol Liq 284:748–754. https://doi.org/10.1016/j.molliq.2019.04.054
Morin-Crini N, Lichtfouse E, Torri G, Crini G (2019) Fundamentals and Applications of Chitosan, in: Sustainable Agriculture Reviews. Springer Int Publ AG 338:49–123. https://doi.org/10.1007/978-3-030-16538-3_2
Dimassi S, Tabary N, Chai F, Blanchemain N, Martel B (2018) Sulfonated and sulfated chitosan derivatives for biomedical applications: a review. Carbohydr Polym 202:382–396. https://doi.org/10.1016/j.carbpol.2018.09.011
Sahariah P, Masson M (2017) Antimicrobial chitosan and chitosan derivatives: a review of the structure–activity relationship. Biomacromolecules 18 (11):3846–3868. https://doi.org/10.1021/acs.biomac.7b01058
Wang K, Liu Q (2014) Chemical structure analyses of phosphorylated chitosan. Carbohydr Res 386:48–56. https://doi.org/10.1016/j.carres.2013.12.021
Baran T (2017) A new chitosan Schiff base supported Pd (II) complex for microwave-assisted synthesis of biaryls compounds. J Mol Struct 1141:535–541. https://doi.org/10.1016/j.molstruc.2017.03.122
Tapdigov SZ, Safaraliyeva SF, Theato P, Zeynalov NA, Tagiyev DB, Raucci MG, Hasanova MX (2018) Synthesis of N, N-diethyl N-methyl chitosan chloride with certain quaternization degree and molecular spectroscopic and thermo-morphological study of the alkylation. J Biomim Biomater Biomed Eng 39:77–88. https://doi.org/10.4028/www.scientific.net/JBBBE.39.77
Liu B, Shen S, Luo J, Wang XY, Sun R (2013) One-pot green synthesis and antimicrobial activity of exfoliated Ag NP-loaded quaternized chitosan/clay nanocomposites. RSC Adv 3(25):9714–9722. https://doi.org/10.1039/C3RA41270A
Ling Y, Li X, Zhou S, Wang XY, Sun R (2015) Multifunctional cellulosic paper based on quaternized chitosan and gold nanoparticle–reduced graphene oxide via electrostatic self-assembly. J Mater Chem A 3(14):7422–7428. https://doi.org/10.1039/C4TA07160C
Rúnarsson ÖV, Holappa J, Nevalainen T, Hjálmarsdóttir M, Järvinen T, Loftsson T, Einarsson JM, Jónsdóttir S, Valdimarsdóttir M, Másson M (2007) Antibacterial activity of methylated chitosan and chitooligomer derivatives: synthesis and structure activity relationships. Eur Polym J 43(6):2660–2671. https://doi.org/10.1016/j.eurpolymj.2007.03.046
Prakash G, Viswanathamurthi P (2014) New ruthenium (II) carbonyl complexes bearing disulfide Schiff base ligands and their applications as catalyst for some organic transformations. Spectrochim ActaAMol Biomol Spectrosc 129:352–358. https://doi.org/10.1016/j.saa.2014.03.086
Ma GP, Yang DZ, Kennedy JF, Nie J (2009) Synthesize and characterization of organic-solubleacylated chitosan. Carbohydr Polym 75(3):390–394. https://doi.org/10.1016/j.carbpol.2008.07.035
Wang JT, Wang HD (2011) Preparation of soluble p-aminobenzoyl chitosan ester by Schiff’s base and antibacterial activity of the derivatives. Int J Biol Macromol 48(3):523–529. https://doi.org/10.1016/j.ijbiomac.2011.01.016
Kurita K, Ikeda H, Yoshida Y, Shimojoh M, Harata M (2002) Chemoselective protection of the amino groups of chitosan by controlled phthaloylation: facile preparation of a precursor useful for chemical modifications. Biomacromolecules 3(1):1–4. https://doi.org/10.1021/bm0101163
Jayakumar R, Prabaharan M, Nair SV, Tokura S, Tamura H, Selvamurugan N (2010) Novel carboxymethyl derivatives of chitin and chitosan materials and their biomedical applications. Prog Mater Sci 55(7):675–709. https://doi.org/10.1016/j.pmatsci.2010.03.001
Fonseca-Santos B, Chorilli M (2017) An overview of carboxymethyl derivatives of chitosan: their use as biomaterials and drug delivery systems. Mater Sci Eng C 77:1349–1362. https://doi.org/10.1016/j.msec.2017.03.198
Alves NM, Mano JF (2008) Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int J Biol Macromol 43(5):401–414. https://doi.org/10.1016/j.ijbiomac.2008.09.007
Rinaudo M (2008) Main properties and current applications of some polysaccharides as biomaterials. Polym Int 57(3):397–430. https://doi.org/10.1002/pi.2378
Hata H, Onishi H, Machida Y (2000) Preparation of CM-chitin microspheres by complexation with iron(III) in w/o emulsion and their biodisposition characteristics in mice. Biomaterials 21(17):1779–1788. https://doi.org/10.1016/s0142-9612(00)00069-7
Khan F, Ahmad SR (2013) Polysaccharides and their derivatives for versatile tissue engineering application. Macromol Biosci 13(4):395–421. https://doi.org/10.1002/mabi.201200409
Liun J, Willfor S, Xu C (2015) A review of bioactive plant polysaccharides: Biological activities, functionalization and biomedical applications. Bioactive Carbohydr Dietary Fibre 5:31–61. https://doi.org/10.1016/j.bcdf.2014.12.001
Liu J, Pu HM, Liu S, Kan J, Jin CH (2017) Synthesis, characterization, bioactivity and potential application of phenolic acid grafted chitosan: a review. Carbohydr Polym 174:999–1017. https://doi.org/10.1016/j.carbpol.2017.07.014
Ito T, Yoshida C, Murakami Y (2013) Design of novel sheetshaped chitosan hydrogel for wound healing: A hybrid biomaterial consisting of both PEG-grafted chitosan and crosslinkable polymeric micelles acting as drug containers. Mater Sci Eng, C Mater Biol Appl 33(7):3697–3703. https://doi.org/10.1016/j.msec.2013.04.056
Sun T, Xu PX, Liu Q, Xue J, Xie WM (2003) Graft copolymerization of methacrylic acid onto carboxymethyl chitosan. Eur Polym J 39(1):189–192. https://doi.org/10.1016/S0014-3057(02)00174-X
Lu B, Xu XD, Zhang XZ, Cheng SX, Zhuo RX (2008) Low molecular weight polyethylenimine grafted N-maleated chitosan for gene delivery: Properties and in vitro transfection studies. Biomacromolecules 9(10):2594–2600. https://doi.org/10.1021/bm8004676
Kumar P, Choonara YE, Toit LC, Modi G, Naidoo D, Pillay V (2012) Novel high-viscosity polyacrylamidated chitosan for neural tissue engineering: Fabrication of anisotropic neurodurable scaffold via molecular disposition of persulfate-mediated polymer slicing and complexation. Int J Mol Sci 13(11):13966–13984. https://doi.org/10.3390/ijms131113966
Kyzas GZ, Lazaridis NK, Bikiaris DN (2013) Optimization of chitosan and beta-cyclodextrin molecularly imprinted polymer synthesis for dye adsorption. Carbohyd Polym 91(1):198–208. https://doi.org/10.1016/j.carbpol.2012.08.016
Dan R, Wang Y, Du L, Du S, Huang M, Yang S, Zhang M (2013) The synthesis of molecular imprinted chitosan-gels copolymerized with multiform functional monomers at three different temperatures and the recognition for the template ovalbumin. Analyst 138(12):3433–3443. https://doi.org/10.1039/C3AN36930G
Jayakumar R, Chennazhi KP, Srinivasan S, Nair SV, Furuike T, Tamura H (2011) Chitin scaffolds in tissue engineering. Int J Mol Sci 12(3):1876–1887. https://doi.org/10.3390/ijms12031876
Kumar MR, Muzzarelli RA, Muzzarelli CR, Sashiva H, Domb AJ (2004) Chitosan chemistry and pharmaceutical perspectives. Chem Rev 104:6017‒6084. https://doi.org/10.1021/cr030441b
Nakamatsu J, Torres FG, Troncoso OP, Min-Lin Y, Boccaccini AR (2006) Processing and characterization of porous structures from chitosan and starch for tissue engineering scaffolds. Biomacromol 7(12):3345–3355. https://doi.org/10.1021/bm0605311
Miranda ES, Silva TH, Reis RL, Mano JF (2011) Nanostructured natural-based polyelectrolyte multilayers to agglomerate chitosan particles into scaffolds for tissue engineering. Tissue Eng Part A 17(21–22):2663–2674. https://doi.org/10.1089/ten.tea.2010.0635
Santo VE, Gomes ME, Mano JF, Reis RL (2012) Chitosan-chondroitin sulphate nanoparticles for controlled delivery of platelet lysates in bone regenerative medicine. J Tissue Eng Regen Med 6(3):47–59. https://doi.org/10.1002/term.1519
Schneider A, Richert L, Francius G, Voegel JC, Picart C (2007) Elasticity, biodegradability and cell adhesive properties of chitosan/hyaluronan multilayer films. Biomed Mater 2(1):45–51. https://doi.org/10.1088/1748-6041/2/1/S07
Silva JM, Duarte AR, Custodio CA, Sher P, Neto AI, Pinho AC, Fonseca J, Reis RL, Mano JF (2013) Nanostructured hollow tubes based on chitosan and alginate multilayers. Adv Healthcare Mater 3(3):433–440. https://doi.org/10.1002/adhm.201300265
Silva JM, Georgi N, Costa R, Sher P, Reis RL, Van Blitterswijk CA, Karperien M, Mano JF (2013) Nanostructured 3D constructs based on chitosan and chondroitin sulphate multilayers for cartilage tissue engineering. PLoS ONE 8(2):e55451. https://doi.org/10.1371/journal.pone.0055451
Trimukhe KD, Varma AJ (2008) Amorphological study of heavy metal complexes of chitosan and crosslinkedchitosans by SEM and WAXRD. Carbohydr Polym 71(4):698–702. https://doi.org/10.1016/j.carbpol.2007.07.010
Ngah WW, Fatinathan S (2010) Adsorption characterization of Pb (II) and Cu (II) ions onto chitosan-tripolyphosphate beads: kinetic, equilibrium and thermodynamic studies. J Environ Manag 91(4):958–969. https://doi.org/10.1016/j.jenvman.2009.12.003
Han GJ, Liu SY, Pan ZC, Lin YC, Ding S, Li LH, Luo BH, Jiao YP, Zhou CG (2020) Sulfonated chitosan and phosphorylated chitosan coated polylactide membrane by polydopamine-assisting for the growth and osteogenic differentiation of MC3T3-E1s. Carbohydr Polym 229:115517. https://doi.org/10.1016/j.carbpol.2019.115517
Hu WQ, Liu M, Yang XS, Zhang C, Zhou HY, Xie WG, Fan LH, Nie M (2019) Modification of chitosan grafted with collagen peptide by enzyme crosslinking. Carbohydr Polym 206:468–475. https://doi.org/10.1016/j.carbpol.2018.09.023
Mahmood A, Lanthaler M, Laffleur F, Huck CW, Bernkop-Schnürch A (2017) Thiolated chitosan micelles: highly mucoadhesive drug carriers. Carbohydr Polym 167:250–258. https://doi.org/10.1016/j.carbpol.2017.03.019
Mutreja R, Thakur A, Goyal A (2020) Chitin and chitosan: current status and future opportunities.in: Handbook of Chitin and Chitosan. Chapter 13:401–417. https://doi.org/10.1016/B978-0-12-817970-3.00013-4
Skryabin KG, Mikhaylov SN, Varlamov VP (2013) Khitozan. M: Izd.Sentr "Bioinjeneria" (in Russian) 593
Doshi B, Repo E, Heiskanen JP, Sirvio JA, Sillanpaa M (2018) Sodium salt of oleoylcarboxymethyl chitosan: a sustainable adsorbent in the oil spill treatment. J Clean Prod 170:339–350. https://doi.org/10.1016/J.JCLEPRO.2017.09.163
Rao MS, Kanatt SR, Chawla SP, Sharma A (2010) Chitosan and guar gum composite films: preparation, physical, mechanical and antimicrobial properties. Carbohydr Polym 82:1243–1247. https://doi.org/10.1016/j.carbpol.2010.06.058
Reddy KM, Babu VR, Sairam M, Subha MC, Mallikarjuna NN, Kulkarni PV et al (2006) Development of chitosan-guar gum semi-interpenetrating polymer network microspheres for controlled release of cefadroxil. Des Monomers Polym 9:491–501. https://doi.org/10.36468/pharmaceutical-sciences.796
Dai T, Tanaka M, Huang YY, Hamblin MR (2011) Chitosan preparations for wounds and burns: antimicrobial and wound-healing effects. Expert Rev Anti Infect Ther 9:857–879. https://doi.org/10.1586/eri.11.59
Morris GA, Kok SM, Harding SE, Adams GG (2010) Polysaccharide drug delivery systems based on pectin and chitosan. Biotechnol Genet Eng Rev 27:257–284. https://doi.org/10.1080/02648725.2010.10648153
Sivashankari PR, Prabaharan M (2016) Prospects of chitosan-based scaffolds for growth factor release in tissue engineering. Int J Biol Macromol 93:1382–1389. https://doi.org/10.1016/j.ijbiomac.2016.02.043
Christy EJS, Rajeswari A, Gopi S, Pius A (2020) Chitin and chitosan-based aerogels. Chapter 10 In: Handbook of Chitin and Chitosan 285–334. https://doi.org/10.1016/B978-0-12-817970-3.00016-X
Muzzarelli RAA, Tanfani F, Emanuelli M, Bolognini L (1985) Aspartate glucan, glycine glucan, and serine glucan for the removal of cobalt and copper from solutions and brines. Biotechnol Bioeng 27(8):1115–1121
Mathur NK, Narang CK (1990) Chitin and chitosan, versatile polysaccharides from marine animals. J Chem Educ 67:938
Ahmed S, Ikram S (2015) Chitosan and its derivatives: a review in recent innovations. Int J Pharm Sci Res 6(1):14–30
Miyazaki S, Ishii K, Nadai T (1981) The use of chitin and chitosan as drug carriers. Chem Pharm Bull 29:3067–3069. https://doi.org/10.1248/cpb.29.3067
Humbatova SF, Zeynalov NA, Taghiyev DB, Tapdiqov SZ, Mammedova SM (2016) Chitosan polymer composite material containing of silver nanoparticle. Dig J Nanomater Biostruct 11(1):39–44
Kim I-Y, Seo S-J, Moon H-S, Yoo M-K, Park I-Y, Kim A-C et al (2008) Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv 26:1–21. https://doi.org/10.1016/j.biotechadv.2007.07.009
Chow KS, Khor E (2000) Novel fabrication of open-pore chitin matrixes. Biomacromolecules 1:61–67. https://doi.org/10.1021/bm005503b
Kokubo T (1991) Bioactive glass ceramics: properties and applications. Biomaterials 12:155–163. https://doi.org/10.1016/0142-9612(91)90194-F
Huang Y, Onyeri S, Siewe M, Moshfeghian A, Madihally SV (2005) In vitro characterization of chitosangelatin scaffolds for tissue engineering. Biomaterials 26:7616–7627. https://doi.org/10.1016/j.biomaterials.2005.05.036
Sarasam A, Madihally SV (2005) Characterization of chitosan_polycaprolactone blends for tissue engineering applications. Biomaterials 26:5500–5508. https://doi.org/10.1016/j.biomaterials.2005.01.071
Hsieh C-Y, Tsai S-P, Wang D-M, Chang Y-N, Hsieh H-J (2005) Preparation of γ-PGA/chitosan composite tissue engineering matrices. Biomaterials 26:5617–5623. https://doi.org/10.1016/j.biomaterials.2005.02.012
Chung TW, Yang J, Akaike T, Cho KY, Nah JW, Kim SI et al (2002) Preparation of alginate/galactosylated chitosan scaffold for hepatocyte attachment. Biomaterials 23:2827–2834. https://doi.org/10.1016/s0142-9612(01)00399-4
Azuma K, Izumi R, Osaki T, Ifuku S, Morimoto M, Saimoto H et al (2015) Chitin, chitosan, and its derivatives for wound healing: old and new materials. J Funct Biomater 6:104–142. https://doi.org/10.3390/jfb6010104
Kojima K, Okamoto Y, Kojima K, Miyatake K, Fujise H, Shigemasa Y et al (2004) Effects of chitin and chitosan on collagen synthesis in wound healing. J Vet Med Sci 66:1595–1598. https://doi.org/10.1292/jvms.66.1595
Bano I, Arshad M, Yasin T, Ghauri MA, Younus M (2017) Chitosan: A potential biopolymer for wound management. Int J Biol Macromol 102:380–383. https://doi.org/10.1016/j.ijbiomac.2017.04.047
Nowroozi N, Faraji S, Nouralishahi A, Shahrousvand M (2021) Biological and structural properties of graphene oxide/curcumin nanocomposite incorporated chitosan as a scaffold for wound healing application. Life Sci 264:118640. https://doi.org/10.1016/j.lfs.2020.118640
Meng Q, Sun Y, Cong H, Hu H, Xu F-J (2021) An overview of chitosan and its application in infectious diseases. Drug Deliv Transl Res 11:1340–1351. https://doi.org/10.1007/s13346-021-00913-w
Dasagrandhi CH, Park S, Jung W, KimY (2018) Antibacterial and biofilm modulating potential of ferulic acid-grafted chitosan against human pathogenic bacteria. Int J Mol Sci 19:2157. https://doi.org/10.3390/ijms19082157
Asghar MA, Yousuf RI, Shoaib MH, Asghar MA (2020) Antibacterial, anticoagulant and cytotoxic evaluation of biocompatible nanocomposite of chitosan loaded green synthesized bioinspired silver nanoparticles. Int J Biol Macromol 160:934–943. https://doi.org/10.1016/j.ijbiomac.2020.05.197
Imani Zh, Imani Z, Basir L, Shayeste M, Montazeri EA, Rakhshan V (2018) Antibacterial effects of chitosan, formocresol and CMCP as pulpectomy medicament on Enterococcus faecalis, Staphylococcus aureus and Streptococcus mutans. Iranian Endodontic J 13(3):342–350. https://doi.org/10.22037/iej.v13i3.20791
Morin-Crini N, Lichtfouse E, Torri G, Crini G (2019) Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ Chem Lett 17:1667–1692. https://doi.org/10.1007/s10311-019-00904-x
Zhao L, Mitomo H, Zhai M, Yoshiic F, Nagasawa N, Kume T (2003) Synthesis of antibacterial PVA/CM-chitosan blend hydrogels with electron beam irradiation. Carbohyd Polym 53:439–446. https://doi.org/10.1016/S0144-8617(03)00103-6
Walczak K, Schierz G, Basche S, Petto C, Boening K, Wieckiewicz M (2020) Antifungal and surface properties of chitosan-salts modified PMMA denture base material. Molecules 25:5899. https://doi.org/10.3390/molecules25245899
Ke C, Deng F, Chuang C, Lin C (2021) Antimicrobial actions and applications of chitosan. Polymers 13:904. https://doi.org/10.3390/polym13060904
Rajoka MS, Mehwish H, Wa Y, Zhao L, Arfat Y, Majeed K, Anwaar S (2020) Chitin/chitosan derivatives and their interactions with microorganisms: a comprehensive review and future perspectives. Crit Rev Biotechnol 40(3):365–379. https://doi.org/10.1080/07388551.2020.1713719
Vichare R, Garner I, Paulson RJ, Tzekov R, Sahiner N, Panguluri SK, Mohapatra S, Mohapatra SS, Ayyala R, Sneed KB, Biswal MR (2020) Biofabrication of chitosan-based nanomedicines and its potential use for translational ophthalmic applications. Appl Sci 10:4189. https://doi.org/10.3390/app10124189
Kalantari K, Afifi AM, Jahangirian H, Webster TJ (2019) Biomedical applications of chitosan electrospun nanofibers as a green polymer – Review. Carbohyd Polym 207:588–600. https://doi.org/10.1016/j.carbpol.2018.12.011
Ghosh T, Das T, Purwar R (2021) Review of electrospun hydrogel nanofiber system: synthesis, properties and applications. Polym Eng Sci 61:1887–1911. https://doi.org/10.1002/pen.25709
Wang Q, Zhang J, Wang A (2009) Preparation and characterization of a novel pH-sensitive chitosan-g-poly(acrylic acid)/attapulgite/sodium alginate composite hydrogel bead for controlled release of diclofenac sodium. Carbohyd Polym 78:731–737. https://doi.org/10.1016/j.carbpol.2009.06.010
El-Din HM, El-Naggar AW (2011) Characterization of Gamma Irradiated Concentrated Aqueous Solutions of Chitosan/Sodium Alginate Blends and Their Drug Uptake-Release Characters. J Appl Polym Sci 122:2383–2390. https://doi.org/10.1002/app.34320
Yu Sh, Xu X, Feng J, Liu M, Hu K (2019) Chitosan and chitosan coating nanoparticles for the treatment of brain disease. Int J Pharm 560:282–293. https://doi.org/10.1016/j.ijpharm.2019.02.012
Jiménez-Gómez CP, Cecilia JA (2020) Chitosan: a natural biopolymer with a wide and varied range of applications. Molecules 25:3981. https://doi.org/10.3390/molecules25173981
Khan F, Pham DT, Oloketuyi SF, Manivasagan P, Oh J, Kim Y-M (2019) Chitosan and their derivatives: antibiofilm drugs against pathogenic bacteria. Colloids Surf B Biointerf 5(185):110627. https://doi.org/10.1016/j.colsurfb.2019.110627
Ramezani M, Enayati M, Ramezani M, Ghorbani A (2021) A study of different strategical views into heavy metal(oid) removal in the environment. Arab J Geosci 14:2225. https://doi.org/10.1007/s12517-021-08572-4
Escudero LB, Quintas PY, Wuilloud RG, Dotto GL (2019) Recent advances on elemental biosorption. Environ Chem Lett 17:409–427. https://doi.org/10.1007/s10311-018-0816-6
Mahl CRA, Taketa TB, Rocha-Neto JBM, Almeida WP, Beppu MM (2020) Copper ion uptake by chitosan in the presence of amyloid-β and histidine. Appl Biochem Biotechnol 190(3):949–965. https://doi.org/10.1007/s12010-019-03120-z
Lee YH, Park SY, Park JE, Jung BO, Park JE, Park JK, Hwang YJ (2019) Anti-oxidant activity and dust-proof effect of chitosan with different molecular weights. Int J Mol Sci 20:3085. https://doi.org/10.3390/ijms20123085
Kassem A, Ayoub GM, Malaeb L (2019) Antibacterial activity of chitosan nano-composites and carbon nanotubes: a review. Sci Total Environ 668:566–576. https://doi.org/10.1016/j.scitotenv.2019.02.446
Veleshko IY (2010) Perspektivi primeneiya khitozana lkya videleniya i konsentrirovaniya radionuklidov v rastvorax, Ribprom: tekhnologii i oborudovaniye dlya pererabotki vodnix resursov (in Russian) 2:53–57
Pokhrel S, Yadav PN, Adhikari R (2015) Applications of chitin and chitosan in industry and medical science: a review. Nepal J Sci Technol 16:99–104. https://doi.org/10.3126/njst.v16i1.14363
Sridhari TR, Dutta PK (2000) Synthesis and characterization of maleilated chitosan for dye house effluent. Indian J Chem Technol 7:198–201. http://hdl.handle.net/123456789/30422
Crini G (2005) Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog Polym Sci 30:38–70. https://doi.org/10.1016/j.progpolymsci.2004.11.002
Bratskaya SY, Chervonetskiy DV, Perfilyev AV, Yudakov AV, Avramenko VA (2010) Primeneniye khitozana i ego proizvodnix v pityevom vodosnabjenii, Ribprom: texnologii i oborudovaniye dlya pererabotki vodnix bioresursov (in Russian) 2:58–63
Shagdarova BT, Levov AN, Varlamov VP (2013) Obtaining low molecular weight chitosan and its derivatives. Izvestia Samara Sci Center Russian Acad Sci 15(5):1694–1696
Muzzarelli RAA (1973) Natural Chelating Polymers: Alginic acid. Chitin and Chitosan, Pergamon Press, Oxford, UK 55:254p
Varma AJ, Deshpande SV, Kennedy JF (2004) Metal complexation by chitosan and its derivatives: a review. Carbohydr Polym 55(1):77–93. https://doi.org/10.1016/j.carbpol.2003.08.005
Zahid AA, Ahmed R, Rehman SR, Augustine R, Tariq M, Hasan A (2019) Nitric oxide releasing chitosan-poly (vinyl alcohol) hydrogel promotes angiogenesis in chick embryo model. Int J Biol Macromol 136:901–910. https://doi.org/10.1016/j.ijbiomac.2019.06.136
Bikova VM (2004) Nekotoriye aspekti ispolzovaniya khitina i khitozana v kachestve flokulyantov. Agrarnaya Rossiya (in Russian) 5:30–31
Zubair M,Arshad M, Ullah A (2020) Chitosan-based materials for water and wastewater treatment, in: Handbook of Chitin and Chitosan. 1st edition. 25:773–809
Rhazi M, Desbrie`res J, Tolaimate A, Rinaudo M, Vottero P, Alagui A et al (2002) Influence of the nature of the metal ions on the complexation with chitosan. Eur Polym J 38:1523–1530. https://doi.org/10.1016/S0014-3057(02)00026-5
Zhang L, Zeng Y, Cheng Z (2016) Removal of heavy metal ions using chitosan and modified chitosan: A review. J Mol Liq 214:175–191. https://doi.org/10.1016/j.molliq.2015.12.013
Wang B, Bai Z, Jiang H, Prinsen P, Luque R, Zhao S, Xuan J (2019) Selective heavy metal removal and water purification by microfluidically-generated chitosan microspheres: Characteristics, modeling and application. J Hazard Mater 364:192–205. https://doi.org/10.1016/j.jhazmat.2018.10.024
He Y, Wu P, Xiao W, Li G, Yi J, He Y et al (2019) Efficient removal of Pb(II) from aqueous solution by a novel ion imprinted magnetic biosorbent: Adsorption kinetics and mechanisms. PLoS ONE 14(3):e0213377. https://doi.org/10.1371/journal.pone.0213377
Zhang D, Xiao J, Guo Q, Yang J (2019) 3D-printed highly porous and reusable chitosan monoliths for Cu (II) removal. J Mater Sci 54:6728–6741. https://doi.org/10.1007/s10853-019-03332-y
Briao G, Andrade J, Silva M, Vieira M (2020) Removal of toxic metals from water using chitosan-based magnetic adsorbents: A review. Environ Chem Lett 18:1145–1168. https://doi.org/10.1007/s10311-020-01003-y
Ganesan J, Ponnusamy S, Carolin C, Balji G (2019) Insights of CMNPs in water pollution control. IET Nanobiotechnol 13(6):553–559. https://doi.org/10.1049/iet-nbt.2019.0030
Alaswad SO, Lakshmi KB, Sudha PN, Gomathi T, Arunachalam P (2020) Toxic heavy metal cadmium removal using chitosan and polypropylene based fiber composite. Int J Biol Macromol 164:1809–1824. https://doi.org/10.1016/j.ijbiomac.2020.07.252
Li Y, Dong X, Zhao L (2021) Application of magnetic chitosan nanocomposites modified by graphene oxide and polyethyleneimine for removal of toxic heavy metals and dyes from water. Int J Biol Macromol 192:118–125. https://doi.org/10.1016/j.ijbiomac.2021.09.202
Silva Alves DC, Healy B, Yu T, Breslin CB (2021) Graphene-based materials immobilized within chitosan: applications as adsorbents for the removal of aquatic pollutants. Materials 14:3655. https://doi.org/10.3390/ma14133655
Croitoru A, Ficai A, Ficai D, Trusca R, Dolete G, Andronescu E, Turculet SC (2020) Chitosan/graphene oxide nanocomposite membranes as adsorbents with applications in water purification. Materials 13:1687. https://doi.org/10.3390/ma13071687
Dinu MV, Humelnicu D, Lazar MM (2021) Analysis of copper (II), cobalt (II) and iron (III) sorption in binary and ternary systems by chitosan-based composite sponges obtained by ice-segregation approach. Gels 7(3):103. https://doi.org/10.3390/gels7030103
Emamy FH, Bumajdad A, Lukaszewicz JP (2021) Adsorption of hexavalent chromium and divalent lead ions on the nitrogen-enriched chitosan-based activated carbon. Nanomaterials 11(8):1907. https://doi.org/10.3390/nano11081907
Vilela PB, Dalalibera A, Duminelli E, Becegato VA, Paulino A (2019) Adsorption and removal of chromium (VI) contained in aqueous solutions using a chitosan-based hydrogel. Environ Sci Pollut Res 26:28481–28489. https://doi.org/10.1007/s11356-018-3208-3
Yuan D, Zhang W, Cui J, He L, Wang J, Yan C, Kou Y, Li J (2020) Facile fabrication of magnetic phosphorylated chitosan for the removal of Co(II) in water treatment: separation properties and adsorption mechanisms. Environ Sci Pollut Res 27:2588–2598. https://doi.org/10.1007/s11356-019-07026-5
Sheth Y, Dharaskar S, Khalid M, Sonawane S (2021) An environment friendly approach for heavy metal removal from industrial wastewater using chitosan based biosorbent: A review. Sustainable Energy Technol Assess 43:100951. https://doi.org/10.1016/j.seta.2020.100951
Cunha BS, Bataglioli RA, Taketa TB, Lopes LM, Beppu MM (2019) Ionic liquid functionalization of chitosan beads for improving thermal stability and copper ions uptake from aqueous solution. J Environ Chem Eng 7(3):103181. https://doi.org/10.1016/j.jece:2019.103181
Cheraghipour E, Pakshir M (2020) Process optimization and modeling of Pb(II) ions adsorption on chitosan-conjugated magnetite nano-biocomposite using response surface methodology. Chemosphere 260:127560. https://doi.org/10.1016/j.chemosphere.2020.127560
Panahandeh A, Parvareh A, Moraveji MK (2021) Synthesis and characterization of γ-MnO2/chitosan/Fe3O4 cross-linked with EDTA and the study of its efficiency for the elimination of zinc(II) and lead(II) from wastewater. Environ Sci Pollut Res 28:9235–9254. https://doi.org/10.1007/s11356-020-11359-x
Song Y, Kong A, Ji Y, He B, Wang H, Li J (2019) Adsorption for copper (II) ion with chitosan-SP / PET composite adsorbent enhanced by electric field. Adsorption Sci Technol 37(3–4):274–287. https://doi.org/10.1177/0263617419825505
Joshi S, Srivastava RK (2019) Adsorptive removal of lead (Pb), copper (Cu), nickel (Ni) and mercury (Hg) ions from water using chitosan silica gel composite. Environ Monit Assess 191(10):615. https://doi.org/10.1007/s10661-019-7777-5
Moganavally P, Deepa M, Sudha PN, Suresh R (2016) Adsorptive Removal of Lead and Cadmium Ions using Cross-Linked CMC Schiff Base: Isotherm, Kinetics and Catalytic Activity. Orient J Chem 32(1):441–453. https://doi.org/10.13005/ojc/320150
Bhandari H, Garg S, Gaba R (2021) Advanced nanocomposites for removal of heavy metals from wastewater. Macromol Symp 397:2000337. https://doi.org/10.1002/masy.202000337
Hiroki A, Tran HT, Nagasawa N, Yagi T, Tamada M (2009) Metal adsorption of carboxymethyl cellulose/carboxymethyl chitosan blend hydrogels prepared by Gamma irradiation. Radiat Phys Chem 78:1076–1080. https://doi.org/10.1016/j.radphyschem.2009.05.003
Wang M, Xu L, Zhai M, Peng J, Li J, Wei G (2008) γ-ray radiation-induced synthesis and Fe(III) ion adsorption of carboxymethylated chitosan hydrogels. Carbohyd Polym 74:498–503. https://doi.org/10.1016/j.carbpol.2008.04.008
Aranaz I, Acosta N, Civera C, Elorza B, Mingo J, Castro C et al (2018) Cosmetics and cosmeceutical applications of chitin, chitosan and their derivatives. Polymers 10(2):213. https://doi.org/10.3390/polym10020213
Li K, Hwang Y, Tsai T, Chi S (1996) Chelation of iron ion and antioxidative effect on cooked salted ground pork by N-carboxymethylchitosan (NCMC). Food Sci Taiwan 23:608–616
Muzzarelli RAA, Aiba S, Fujiwara Y, Hideshima T, Hwang C, Kakizaki M et al (1986) Filmogenic properties of chitin/chitosan, in: Chitin in Nature and Technology, Springer 389–402
Shin MS, Kim SI, Kim IY, Kim NG, Song CG, Kim SJ (2002) Characterization of hydrogels based on chitosan and copolymer of poly (dimethylsiloxane) and poly(vinyl alcohol). J Appl Polym Sci 84(14):2591–2596. https://doi.org/10.1002/app.10365
Bai R-K, Huang M-Y, Jiang Y-Y (1988) Selective permeabilities of chitosan-acetic acid complex membrane and chitosan-polymer complex membranes for oxygen and carbon dioxide. Polym Bull 20:83–88. https://doi.org/10.1007/BF00262253
Dumitriu S, Chornet E (1997) Immobilization of xylanase in chitosan—xanthan hydrogels. Biotechnol Prog 13(5):539–545. https://doi.org/10.1021/bp970059i
Sharp RG (2013) A review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agronomy 3(4):757–793. https://doi.org/10.3390/agronomy3040757
Hamed I, Zogul FO, Regenstein JM (2016) Industrial applications of crustacean byproducts (chitin, chitosan, and chitooligosaccharides): a review. Trends Food Sci & Technol 48:40–50. https://doi.org/10.1016/j.tifs.2015.11.007
Huq T, Khan A, Brown D, Dhayagude N, He Z, Ni Y (2022) Sources, production and commercial applications of fungal chitosan: A review. J Bioresour Bioprod 7:85–98. https://doi.org/10.1016/j.jobab.2022.01.002
Fillipova VA, Lysenko AV, Ignatenko VA, Dovnar AK (2016) Comparative characteristics of the adsorption properties of enterosorbents. Health and Environm.Issues (in Russian) 1(47):41–46. https://elib.gsmu.by/handle/GomSMU/949
Budnyak TM, Vlasova NN, Golovkova LP, Slabonb A, Tertykh VA (2019) Bile acids adsorption by chitosan-fumed silica enterosorbent. Colloid Interf Sci Commun 32:100194. https://doi.org/10.1016/j.colcom.2019.100194
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and analysis were performed by [Sevda Fatullayeva], data collection by [Samira Mammadova] and [Elmira Aliyeva], review and editing by [Nizami Zeynalov] and [Dilgam Tagiyev]. The first draft of the manuscript was written by [Sevda Fatullayeva] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fatullayeva, S., Tagiyev, D., Zeynalov, N. et al. Recent advances of chitosan-based polymers in biomedical applications and environmental protection. J Polym Res 29, 259 (2022). https://doi.org/10.1007/s10965-022-03121-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-022-03121-3