Abstract
Locomotion, as a fundamental function in mammals directly associated with the use of ecological resources, is expected to have anatomical structures functionally committed that evolved under intense selective pressure, possibly carrying specializations for different locomotor habits. Among caviomorph rodents, the family Echimyidae stands out for having the greatest species richness, with relatively well-resolved phylogenetic relationships, wide variation in body mass, and remarkable diversity of locomotor habits, including arboreal, scansorial, semi-aquatic, semifossorial, and terrestrial forms. Thus, Echimyidae constitutes a promising model for understanding how phylogenetic, allometric, and ecological factors affect the evolution of postcranial structures directly linked to locomotor function. We investigated the influence of these three factors on scapular and humeral morphological variation in 38 echimyid species using two-dimensional geometric morphometry and phylogenetically informed comparative methods. Scapular and humeral shape variation had a low correlation with body mass and structure size, conveying a small or negligible allometric effect. Conversely, a significant moderate to strong phylogenetic signal was detected in both structures, suggesting that an important part of their morphometric variation results from shared evolutionary history. Notably, morphological variation of the scapula was extensively structured by phylogeny, without the marked influence of locomotor habits, suggesting that its shape may be a suitable taxonomic marker. Finally, locomotor habits were important in structuring the morphological variation of the humerus. Our results suggest that the morphologies of the scapula and humerus, despite being anatomically and functionally interconnected, were differentially shaped by ecological factors associated with locomotor habits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Locomotion is a fundamental ability of animals, necessary for an extensive variety of actions, such as foraging, searching for partners or shelter, and escaping from predators. Locomotion can take several forms, depending on the environment, including swimming, crawling, walking, as well as some more idiosyncratic mechanisms, such as jumping, brachiation, and digging (Ijspeert 2002). Since the variation in the performance of these actions directly affects the fitness of individuals, natural selection is expected to act on any phenotypic traits related to locomotion (Irschick and Garland 2001; Orr 2009). Because of this, the study of locomotion has always played a significant role in adaptation discussions (Bennett and Huey 1990; Garland and Losos 1994; Dickinson et al. 2000; Irschick and Garland 2001; Pough et al. 2008; Shaw 2020). On a microevolutionary scale, these discussions helped to understand the causes and consequences of individual variation on animal movement (Scales and Butler 2016; Shaw 2020), while on a macroevolutionary scale, these studies helped to elucidate the relationships between the shape of structures and their functions (Irschick and Garland 2001; Scales and Butler 2016), shedding light on the origin and diversification of specialized locomotor habits.
The emergence of distinct locomotor habits from a generalist ancestor often occurs via natural selection, resulting in improved use of available environmental resources and niche partitioning. It is estimated that terrestrial generalist locomotion was present in the ancestor of rodents (Lovegrove and Mowoe 2014; Hedrick et al. 2020), the largest group of living mammals, and has persisted in most of its descendents (Galewski et al. 2005; Hedrick et al. 2020). Consequently, and also due to their small body mass, most rodents, especially muroids, have only subtle morphological specializations for different locomotion strategies (Elissamburu and Vizcaíno 2004; Weisbecker and Schmid 2007; Coutinho et al. 2013; Coutinho and Oliveira 2017; Hedrick et al. 2020). On the other hand, some lineages have acquired specialized habits, with extreme examples that include gliding in flying squirrels (Pteromyini) and scaly-tailed squirrels (Anomaluridae), burrowing in mole-rats (Bathyergidae), swimming in beavers (Castoridae), and jumping in kangaroo rats (Dipodomyinae). In all these extreme cases, the acquisition of locomotor specializations was associated with the origin of marked behavioral, physiological, and/or morphological adaptations (Irschick and Garland 2001; Edut and Eilam 2003; Samuels and Van Valkenburgh 2008).
Natural selection also seems to have played a crucial role in the locomotor diversification of caviomorphs, the first rodents to colonize South America, in the middle Eocene, when this continent was isolated from the others (Antoine et al. 2012; Boivin et al. 2018; Arnal et al. 2020). In these animals, different bone structures such as skull, mandibles, vertebrae, humeri, carpi, metacarpi, pelvis, femora, tarsi, and metatarsi exhibit signs of adaptation to locomotor niches (Morgan and Verzi 2011; Morgan and Álvarez 2013; Candela et al. 2017; Carvalhaes et al. 2019; Tavares and Pessôa 2020; Ávarez et al. 2021; Netto and Tavares 2021). It is notable that in caviomorphs, the morphological adaptations of the appendicular skeleton to different locomotion strategies have become expressively more pronounced than in muroids, providing valuable ecomorphological markers even for paleontological reconstructions of life habits of already extinct species (Weisbecker and Schmid 2007; Candela and Picasso 2008; Olivares et al. 2020; Tavares and Pessôa 2020).
In addition to reflecting functional specializations (Smith and Savage 1955; Hildebrand and Goslow 2006; Polly 2007; Tague 2020), part of the variation in the shape of the appendicular skeleton is expected to be explained by its phylogenetic history (Morgan 2009; Morgan and Álvarez 2013; Martín-Serra et al. 2014; Gaudioso et al. 2020). It is important to consider that the phylogenetic component of morphological variation does not necessarily carry adaptive traits and may be the result of neutral evolution (Duret 2008; Kern and Hahn 2018). In addition, body size and/or bone structure size may also explain some variation in shape, because of evolutionarily conserved allometric constraints or structural rearrangements required to support body weight (Milne et al. 2009; Campione and Evans 2012; Walmsley et al. 2012; de Oliveira and Santos 2018). Therefore, attempts to identify locomotor adaptations should not disregard the possible existence of phylogenetic structuring and allometric effects on morphological variation. Thus, it is useful to compare forms in unrelated organisms that share similar strategies of environment use due to evolutionary convergence. This approach, when considering the phylogenetic history of the lineages investigated, has bolstered ecomorphological studies as it elucidates the evolutionary associations between forms, their functional properties, and their interactions with the environment (Galewski et al. 2005; Fabre et al. 2013a, 2017; Álvarez et al. 2017; Courcelle et al. 2019).
Given their extensive taxonomic diversity and disparate morphological evolution, associated with disparate morphological evolution associated with different locomotor habits, and availability of a reasonable phylogenetic framework based on molecular data (Fabre et al. 2013a; 2017; Álvarez et al. 2017; Courcelle et al. 2019), the family Echimyidae Gray, 1825 (spiny rats, nutria, and hutias) is an excellent group of caviomorphs to investigate morphological specializations in the appendicular skeleton (Elissamburu and Vizcaíno 2004; Seckel and Janis 2008; Morgan 2009; Morgan and Álvarez 2013; Tavares et al. 2020). There are approximately 100 species and 32 genera of living echimyids (Lacher et al. 2016; Burgin et al. 2018; Emmons and Fabre 2018), currently divided into four subfamilies: Capromyinae Smith, 1842 (exclusively Caribbean), Carterodontinae Courcelle, Tilak, Leite, Douzery, and Fabre, 2019, Euryzygomatomyinae Emmons, 2005 and Echimyinae Gray, 1825, the latter comprising two tribes: Echimyini Gray, 1825 and Myocastorini Ameghino, 1902 (Courcelle et al. 2019). Continental echimyids (all lineages except Capromyinae) probably evolved to both arboreal and semi-terrestrial habitats twice each, as well as once to a semi-aquatic habitat. The presumed ancestral terrestrial habitat is retained as symplesiomorphic in two extant lineages (Galewski et al. 2005; Fabre et al. 2013a; Courcelle et al. 2019). In addition, a clade may explore arboreal and shrub substrates and climb rocks, as well as move frequently on the ground, which is often classified as scansorial (Neves 2003; Hildebrand and Goslow 2006; Weisbecker and Schmid 2007; Karantanis 2017). It has been pointed out that the evolution of these different modes of locomotion in the Miocene, especially the acquisition of the arboreal habit, was a key factor in the origin of the remarkable echimyid species richness because it allowed access to a wide range of ecological resources previously unused by other rodents in South America, except erethizontids (Fabre et al. 2013a).
Generally, among mammals, the scapular appendicular skeleton concentrates some of the osteological structures that most intensely reflect locomotor specializations (Szalay and Sargis 2001; Polly 2007; Steiner-Souza et al. 2010; Morgan and Álvarez 2013; Pérez et al. 2021). Previous studies on caviomorphs, including some genera of echimyids, have shown that the morphology of part of their forelimbs, specifically humeri, ulnae, carpals, and metacarpals (Candela and Picasso 2008; Morgan and Verzi 2011; Morgan and Álvarez et al. 2013; Tavares et al. 2020), carries strong specializations associated with different locomotor habits. Contrarily, the shape of the scapulae of caviomorphs mostly reflects the phylogenetic history of the species, with no evidence of marked functional specializations (Morgan 2009). Considering the functional interdependence between scapula and humerus, it is notable that the evolution of these structures responds so differently to the functional demands imposed by different locomotor habits.
In the present study, our main goal was to understand how historical, allometric, and functional factors associated with locomotion shaped the evolution of the scapula and humerus morphology throughout the extraordinary phylogenetic and ecological diversification of the Echimyidae. Indirectly, our investigation will be a resource for studies that require functional and phylogenetic markers in the morphology of the postcranial skeleton of echimyids, with potential utility for other caviomorphs and rodents.
Material and Methods
Specimens and Taxa Examined
For this study, we captured images of a total of 186 scapulae and 181 humeri of 236 echimyids, belonging to 38 species, distributed in 15 genera (Figure 1, Online Resource 1). All specimens analyzed were intact and without damage or abnormalities that could prevent the digitalization of anatomical landmarks. Since none of the structures used presents sexual dimorphism in rodents (Coutinho et al. 2013; Coutinho and Oliveira 2017), we grouped individuals of both sexes in all analyses. Only adult individuals were used, all identified by having the fourth premolar and the three molars erupted, thus avoiding the inclusion of ontogenetic variation (Pessôa and Reis 1991; Leite 2003; Tavares and Pessôa 2010; Tavares et al. 2016). The species examined were allocated into five locomotor habits (terrestrial, semi-fossorial, semi-aquatic, arboreal, and scansorial) as per the literature (Galewski et al. 2005; Fabre et al. 2013a; Emmons et al. 2015a; Hannibal et al. 2019). Although some authors classify Thrichomys Trouessart, 1880 species as terrestrial (Galewski et al. 2005; Neves and Pessôa, 2011; Olivares et al. 2012; Tavares et al. 2018; Carvalhaes et al. 2019; Courcelle et al. 2019), we considered them as scansorial, following other studies (Lacher and Alho 1989; Weisbecker and Schmid 2007; Patterson and Velasco 2008) and because Thrichomys has also been captured in trees (Hannibal et al. 2019). The specimens examined are deposited in the collections of the Laboratório de Biologia e Parasitologia de Mamíferos Reservatórios Silvestres (LABPMR), the Museu Nacional da Universidade Federal do Rio de Janeiro (MN/UFRJ), the Museu de Zoologia da Universidade de São Paulo (MZUSP), and the Universidade Federal da Paraíba (UFPB) (Appendix 1).

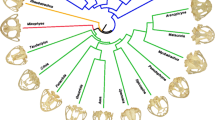
Adapted from Álvarez et al. (2017)
Phylogenetic tree and species list used as a framework for comparative analysis, highlighting locomotor habits and main clades. Green = arboreal, yellow = scansorial, blue = semiaquatic, brown = semi-fossorial, red = terrestrial.
Obtaining Morphometric Data
Morphological variation was assessed by two-dimensional morphometry based on digital photographs. The images of the structures were captured only by one author (JGC), with a SONY P520 digital camera with 16-megapixel resolution, positioned at a standard distance of 5 centimeters from the photographic plane. The structures were placed in a container containing dark sand to minimize shadows, and a grid-scale of 1x1 centimeter, subdivided into millimeters, was added.
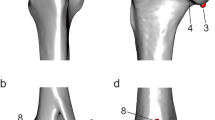
The two-dimensional morphological landmarks were mostly based on previous studies of the scapula (Morgan 2009) and the humerus of caviomorph rodents (Steiner-Souza et al. 2010; Morgan and Álvarez 2013), seeking to capture the maximum morphological variation observed during specimen handling and respecting the criteria of homology, consistency of relative position, repeatability, and coplanarity (Zelditch et al. 2004). We digitized 31 morphological characters for the scapula, including 14 landmarks and 17 semilandmarks; we digitized 23 morphological characters for the humerus, including 19 landmarks and 4 semilandmarks (Fig. 2; Table 1). All landmarks and semilandmarks were digitized by the same person, the author JGC (Online Resources 2 and 3). Specimens and their respective data used in analyses with the MorphoJ program can be found in Online Resource 4.
The digitized images were gathered and organized using the program tps-Util v. 1.78 (Rohlf 2019), and the anatomical landmarks were digitized with the program tps-Dig v. 2.31 (Rohlf 2017). The coordinates obtained from the anatomical landmarks were processed in the MorphoJ program (Klingenberg 2011), where General Procrustes Analysis Superimposition was performed to remove variation not corresponding to shape (size, position, and orientation; Debat et al. 2000).
Mean centroid size and Procrustes coordinates were calculated for each species examined and used in subsequent analyses. In addition, to each species, we assigned a mean body mass value obtained from the label of the examined specimens or the literature (Bonvicino et al. 2008).
Phylogenetically-informed Comparative Analyses
Despite the robust phylogeny proposed by Courcelle et al. (2019), in this study we used the phylogenetic framework of the calibrated phylogenetic tree obtained by Álvarez et al. (2017), through Bayesian Inference based on four mitochondrial and five nuclear genes, as it includes a more extensive taxonomic coverage. Using the program TreeGraph 2 (Stöver and Müller 2010), we made manual edits to the tree of Álvarez et al. (2017) to include four species not included in it (Fig. 1, Online Resource 5). While including species within the genus Proechimys Allen, 1899, we assumed that the currently recognized species groups are reasonable proxies to their phylogeny (Patton and Leite, 2015). Thus, P. cuvieri Petter, 1978 was included in a polytomic clade containing P. brevicauda (Günther, 1876) and P. longicaudatus (Rengger, 1830) and split at 5.39 Ma (Alvarez et al. 2017), as they are all allocated in the longicaudatus group. Proechimys goeldii Thomas, 1905 was inserted as sister to P. quadruplicatus Hershkovitz, 1948, as both belong to the goeldii group (Patton and Leite 2015). Given the absence of an estimated date for the divergence of P. goeldii, its branch was arbitrarily inserted halfway along the branch length separating its sister taxon, P. quadruplicatus, and its next most closely related taxon included in the backbone phylogeny, P. simonsi. We included Trinomys mirapitanga Lara, Patton, and Hingst, 2002 as sister to the clade formed by T. iheringi (Thomas, 1911) and T. dimidiatus (Günther, 1876) following Lara et al. (2002). Given the absence of an estimate date for the divergence of T. mirapitanga, its branch was arbitrarily inserted halfway along the branch length separating its sister taxa, T. iheringi and T. dimidiatus, and their next most closely related taxa included in the backbone phylogeny, T. gratiosus (Moojen 1948). We inserted T. moojeni (Pessôa, Oliveira, and Reis, 1992) as sister to T. gratiosus, diverging at 5.9 Ma (Tavares et al. 2015).
Aiming to understand the allometric effect on scapular and humeral shape in Echimyidae, phylogenetically informed regressions (phylogenetic generalized least squares – PGLS) was employed to test the correlation between body mass, centroid size, and Procrustes coordinates (Freckleton et al. 2002; Revell 2009, 2010). Residuals from regressions between centroid size and Procrustes variables were obtained and used in subsequent analyses to ensure the absence of allometric effect on analyses of shape variation (Revell 2009, 2010). Given the exceptionally large size of Myocastor coypus Molina, 1782 compared to other echimyids, the PGLS tests of correlation between body mass, centroid size and shape were run with two sets of data: including Myocastor and excluding this taxon.
Phylogenetically informed principal component analyses (pPCA) based on matrices of variance and covariance were implemented on the residuals of the Procrustes variables enabling the identification of the major sources of variation in the shape of the scapula and humerus of echimyids (Revel 2012). A broken-stick model was applied to determine the number of pPCs to be used in the subsequent analysis. The figure containing the pPC values resulting from the brocken stick model is available as Online Resource, (6). For each structure examined – i.e., scapula and humerus – the scores of each species along the informative pPCs according the broken-stick model were projected onto phylomorphospaces reconstructed by Maximum Likelihood.
Seeking to estimate the phylogenetic effect on the morphometric variation, the phylogenetic signal in shape variation was quantified and tested using Blomberg’s method (Blomberg et al. 2003; Adams 2014) with 10,000 permutations (Klingenberg and Gidaszewski 2010). Blomberg’s K-statistic quantifies the intensity of phylogenetic signals with values equal to or close to 1.0 when phenotypic variation is well explained by the Brownian motion model; with values greater than 1.0 when phenotypic attributes are phylogenetically more structured than expected by Brownian motion, and with values close to 0 in the absence of phylogenetic structure in phenotypic variation. K-statistics were estimated for the variation in the shape of each structure, as a whole using the multivariate approach of Adams (2014; Kmulti), and separately for each informative pPC using the conventional approach of Blomberg et al. (2003).
Morphometric differentiation among locomotor habits considering the phylogenetic history of taxa, reflecting possible morpho-functional specializations, was tested using phylogenetic ANOVA and MANOVA (phyANOVA and phyMANOVA) on species scores informative pPCs (Garland et al. 1993), run with 10,000 permutations. The phyANOVA, phyMANOVA, pPCA, PGLS, and phylomorphospace reconstruction were employed using the packages Geomorph 4.0 (Adams et al. 2021) and phytools (Revell 2012) in R computational environment R Core Team version 4.1.2 (R Core Team, 2021).
Results
Correlations Between Body Mass, Scapular and Humeral Size and Shape
Body mass was strongly correlated with humeral and scapular centroid sizes but not with the shapes of these structures (Table 2), regardless of whether Myocastor was included in the analysis. The centroid size of the scapular and humerus varied between Carterodon sulcidens Lund, 1838, Lonchothrix emiliae Thomas, 1920, and Mesomys hispidus Desmarest, 1817, with the smallest values, and Myocastor coypus with the largest value.
Variation in the Shape of the Scapula
The overall variation of scapular shape had low although significant phylogenetic signal (Kmulti-scapula = 0.488; p < 0.001), with no significant influence of locomotor habit (F < 4.6; p > 0.321; Table 3). The broken-stick model found that only the first four pPCs summarized more information than expected at random, accounting altogether for 79.4% of all scapular measured variation (Table 3). Strong and highly significant phylogenetic signal was found in species distribution along pPC1scapula and pPC2scapula, while weaker phylogenetic signal was found in pPC4scapula and non-significant phylogenetic signal was reported for pPC3scapula. Although along pPC1scapula and pPC4scapula species with different locomotor habits tended to occupy different regions of morphospace, phyANOVA did not find significant, phylogenetically independent differences between locomotor habits in any of the four major axes of variation. The variation summarized by the first two main components is described in detail below, as they are the only ones to present a strong and highly significant phylogenetic signal. The distribution of taxa along the morphospace formed by pPC3scapula and pPC4scapula can be found in Online Resource, (7).
The high pPC1scapula scores represented scapulae with narrow supraspinous and infraspinous fossae, long scapular spine, cranially projected coracoid process and infraglenoid tubercle, elongated coracoid process, and a narrow region between acromion and metacromion (Figure 3). Low pPC1scapula scores represented the opposite morphological conformation for these characters. The highest pPC1scapula value was found in Callistomys Emmons and Vucetich, 1998, while the lowest one was in Kannabateomys Jentink, 1891. It is noteworthy that most major clades were cohesively distributed along the pPC1scapula, reflecting strong phylogenetic signal (K = 0.769; p < 0.001). Echimyini had lower scores, while Euryzygomatomyinae had higher scores. Myocastorini exhibited a wide distribution, with Callistomys and Myocastor sharing the highest scores; most Proechimys and Thrichomys had intermediate scores, except for P. goeldii and T. apereoides Lund, 1839, with low scores. Carterodon Waterhouse, 1848 overlapped with Thrichomys and Echimyini, with intermediate scores.
Phylogenetic principal components analysis (pPCA) of the scapulae. Dark blue wireframes show changes in shape, while light blue wireframes show the average shape. Minimal convex polygons: Left, the distribution of each locomotor habit in morphospace: green = arboreal, yellow = scansorial, blue = semi-aquatic, gray = semi-fossorial, red = terrestrial. Right, the distribution of each taxon in morphospace: green = Echimyini, blue = Euryzygomatomyinae, red = Myocastorini, orange = Carterodontinae
The High pPC2scapula scores represented scapulae with a relatively expanded supraspinous fossa and reduced infraspinous fossa, supraspinous fossa more developed in its cranial region and retracted at the vertebral border, scapular spine short and caudally inclined, coracoid process and infraglenoid tubercle cranially projected, elongated coracoid process, and narrowing of the region between the acromion and metacromion (Figure 3). The low pPC2scapula scores represented the opposite morphological conformation. The highest pPC2scapula value was found in P. goeldii, while the lowest was in Dactylomys boliviensis Anthony, 1920. It is noteworthy that most major clades were cohesively distributed along pPC2scapula, reflecting the strong phylogenetic signal (KPC2scapula = 1.063; p < 0.001). Echimyini, especially the bamboo specialist clade, Carterodontinae, and Eurygygomatominae shared mostly low scores, while Myocastorini exhibited mostly high scores, with P. goeldii and P. brevicauda sharing the highest scores. However, most Thrichomys had intermediate scores, except for T. apereoides. (Fig. 3).
Variation in the Shape of the Humerus
The overall variation of humeral shape had strong and significant phylogenetic signal (Kmulti-humerus = 0.908; p < 0.001), and strong and significant influence of locomotor habits (F = 8.759; p = 0.014). The broken-stick model found that the first two pPCs summarized more information than expected at random, accounting altogether for 71.5% of all humeral measured variation (Table 3). Strong and highly significant phylogenetic signal was found in species distribution along both axes. Although along both pPC1humerus and pPC2humerus species with different locomotor habits tended to occupy different regions of morphospace, phyANOVA found significant, phylogenetically independent differences between locomotor habits only along pPC1humerus.
The high pPC1humerus scores represented humeri with an elongated diaphysis, reduced epiphyses, poorly developed and proximally positioned deltoid tuberosity (Fig. 4). The low pPC1humerus scores represented the opposite morphological conformation. The highest pPC1humerus value was found in Euryzygomatomys spinosus Fischer, 1814 while the lowest was in Proechimys cuvieri. Despite the overlap between tribes and subfamilies along pPC1humerus, the variation of scores along this axis denoted a strong signal (K = 1.673; p < 0.001). Notably, some taxa were strongly cohesive along with this axis: Proechimys, with low scores in most species; Echimyini, with high scores in most species; Thrichomys, with intermediate scores in most species; Trinomys Thomas, 1921¸ with intermediate to low scores; and the clade formed by Clyomys Thomas, 1916 and Euryzygomatomys Goeldi, 1901, with some of the highest scores. It is also noteworthy that some locomotor habits differed along pPC1humerus, showing that the distribution of species in the morphospace is strongly structured by locomotor habits, as found in the phyANOVA results (F = 15.845; p = 0.012). The semi-fossorial and most arboreal echimyids had high pPC1humerus values while terrestrial echimyids had low scores. Most scansorial scores were intermediate between arboreal and terrestrial along pPC1humerus. The high pPC2humerus scores represented humeri with a rounded and proximally projected head, deltoid tuberosity retracted and positioned in the medial region of the diaphysis, short distal epiphysis, a more elongated mid-laterally trochlea, more developed medial epicondyle, and less developed lateral epicondyle. The low pPC2humerus scores represented the opposite morphological conformation. The highest pPC2humerus value was found in Isothrix negrensis Thomas, 1920 while the lowest was in Euryzygomatomys. It is noteworthy that some of the major clades have differentiated along pPC2humerus, reflecting strong phylogenetic signal (K = 0.880; p < 0.001), with Echimyini showing high scores, Euryzygomatomyinae low scores, Carterodontinae intermediate score, and Myocastorini with a wide distribution along this axis. The distribution of scores along pPC2humerus was not significantly structured by locomotor habits according to phyANOVA (F = 8.014; p = 0.090); however, it was noticeable that some arboreal and scansorial species stood out by having relatively high scores, while the semi-fossorial genera Euryzygomatomys and Clyomys stood out with some of the lowest scores. Part of the terrestrial echimyids, mainly Proechimys, overlapped the arboreal ones with intermediate scores. Most species of the genus Trinomys had lower scores than the Proechimys species. The semi-aquatic Myocastor also had low pPC2humerus scores (Fig. 4).
Analysis of phylogenetic principal components (pPCA) of the humerus. Dark blue wireframes show changes in shape, while light blue wireframes show the average shape. Minimal convex polygons: Left, the distribution of each locomotor habit in morphospace: green = arboreal, yellow = scansorial, blue = semi-aquatic, gray = semi-fossorial, red = terrestrial. Right, the distribution of each taxon in morphospace: green = Echimyini, blue = Euryzygomatomyinae, red = Myocastorini, orange = Carterodontinae
Discussion
Allometric Effects on the Shape of the Scapula and Humerus
The evolution of body size and its allometric consequences on shape have played an important role in ecomorphological diversification in different mammalian taxa, such as artiodactyls, carnivores, primates, and rodents (Marroig and Cheverud 2005; Renaud et al. 2006; Meloro and Raia 2010; Raia et al. 2010; Meloro et al. 2015). Indeed, locomotor specialization through adaptive evolution may be achieved through an allometric change of shape and proportions (Schmidt-Kittler 2002, 2006; Meloro et al. 2015; Sansalone et al. 2018). Often body size and mass are correlated with several morphological, physiological, and life history characteristics in mammals (Biknevicius et al. 1993; Biknevicius 1999; Millien and Bovy 2010; Tavares and Pessôa 2020; Netto and Tavares 2021) and variation in body size is usually associated with biomechanical properties that influence the morphology and function of the appendicular skeleton (Biewener 2000; Morgan 2009). However, our results show a weak or negligible allometric effect on the shape of the scapula and humerus of echimyids (Table 3).
Our results are congruent with previous studies showing a low or negligible allometric effect on scapular shape variation of small eutherian mammals, such as bats (Gaudioso et al. 2020) and sciuromorph (Wölfer et al. 2019) and caviomorph rodents (Morgan 2009). Even in sciuromorph rodents, where this effect is shown to be somewhat more pronounced than in bats and caviomorphs, only 8.4% of the total variation in scapular shape is explained by variation in body mass (see Table S3 in Wölfer et al. 2019). The allometric effect on the scapula of didelphid marsupials is greater than that found in the eutherian small mammals, being more pronounced in larger species (Astúa 2009). The infraspinous fossa tends to be disproportionately more expanded in didelphids of greater body mass (Astúa 2009). Unlike didelphids, echimyids, as well as other caviomorph and sciuromorph rodents (Morgan 2009; Wölfer et al. 2019), tend to have a relationship between body size and infraspinous and supraspinous fossae sizes close to isometric. All these observations suggest absence of a generalized and conserved allometric pattern in the scapula of small therian mammals, although further studies are needed to verify this hypothesis.
Regarding the humerus, the relationship between body mass and diaphysis circumference remains highly conserved among the major clades of Mammalia, suggesting that the shape of the diaphysis of this weight-bearing bone is only weakly influenced by compressive forces on the limbs (Campione and Evans 2012). Concurrently, Christiansen (1999) found that mammals, on average, do not have limb bones that are disproportionately thicker or shorter than predicted for their body mass. On the other hand, studies restricted to medium and large sized mammals, such as felids and xenarthrans, show that the effect of body size and mass on the architecture of the humerus, especially its epiphyses, can be more evident (Milne et al. 2009; Walmsley et al. 2012; de Oliveira and Santos 2018). In contrast to these findings, among small mammals, the allometric effect is less common among small mammals. Although this effect has already been reported for moles (Sansalone et al. 2018), it should be considered that this the latter is a group with a highly specialized burrowing habit, and thus, the allometric effect may be enhanced by interaction with biomechanical demands of digging (Sansalone et al. 2018). Among muroid and geomyoid rodents, which are far more generalist than moles, this effect was shown to be small, although some sampling bias may have existed (Hedrick 2020). Similarly, in caviomorph rodents, the allometric effect on humerus shape has repeatedly been shown to be very low or negligible (Casinos et al. 1993; Fernández et al. 2000; Steiner-Souza et al. 2010; Morgan and Álvarez 2013). An exception is reported for octodontids, in which the diaphysis length and thickness scale isometrically, but the position and size of the deltoid tuberosity scale with positive allometry, although the correlation between deltoid tuberosity and forelimb size is low (Pérez et al. 2021).
The allometric effect concentrated in the humeral epiphyses of large mammals may be the result of specific functional demands for support and propulsion of their large body mass since these regions are among the main areas of insertion and muscle origin of the forelimb (Christiansen 1999; Biewener 2000; Campione and Evans 2012; Walmsley et al. 2012). On the other hand, the lack of allometric effect on humeral shape reported here for Echimyidae is congruent with those that have been observed for most other small mammals, as discussed above. Corroborating the assumption that low body mass imposes few structural constraints on the shape of supporting structures, it is noticeable that, similarly to what we observed for the humerus in echimyids and other small mammals, the effect of body size on the shape of lumbar vertebrae is negligible (Álvarez et al. 2013; Netto and Tavares 2021), contrasting with the strong effect on the same structures in large-sized mammalian taxa (Chen et al. 2005; Jones 2015; Randau et al. 2016).
Phylogenetic Effects on Scapular and Humeral Morphology
Our results demonstrated that the morphological variation in scapula and humerus of echimyids reflects the phylogenetic structure of the family. Moreover, the morphology of the humerus also reflects the functional demands associated with different locomotor habits, which was not evident in the scapula.
The significant value of K, notably high in the two major variation axes, and the absence of significant differences in the shape of the scapula associated with locomotor habits suggest that, in the spiny rats, this structure evolved approximately according to Brownian motion, without strong selective pressures associated with locomotion being necessary to explain its current variation. This evolutionary scenario is reflected in the unique morphology of some echimyid clades, independently of the locomotor habits of the species that constitute them.
As an example, the myocastorines Callistomys pictus Pictet, 1843 and Myocastor coypus, recovered as sister taxa by some studies (Fabre et al. 2017; Courcelle et al. 2019), share similar scapula shapes, despite having very distinct body sizes, external morphology, and ecology. The scapular shape of Callistomys (Fig. 5o), an arboreal taxon, differs greatly from the scapula of the other echimyids with similar locomotor habits. The other arboreal echimyids form the tribe Echimyini and share a cohesive scapular morphology (Fig. 5e-k). In addition, the terrestrial myocastorines (Proechimys spp.; Fig. 5n) have scapulae more like those of the scansorial myocastorines (Thrichomys spp.; Fig. 5l) than the other terrestrial echimyids (Trinomys spp.; Fig. 5a). Consistently, the scapulae of the semi-fossorial euryzygomatomyines (Euryzygomatomys and Clyomys; Fig. 5b-c) are more like those of the terrestrial euryzygomatomyines (Trinomys; Fig. 5a) than to that of the semi-fossorial carterodontine (Carterodon; Fig. 5d).
Echimyidae scapula specimens organized according to the taxa examined. Subfamily Euryzygomatomyinae: a, Trinomys iheringi LBCE 16,101; b, Euryzygomatomys spinosus MN 75,752; c, Clyomys laticeps LBCE 4877. Subfamily Carterodontinae: d, Carterodon sulcidens MN 54,368. Tribe Echimyini: e, Mesomys hispidus LBCE 18,058; f, Lonchothrix emiliae LBCE s/n (no identification); g, Isothrix negrensis MN 69,434; h, Makalata armata MN 69,325; i, Phyllomys pattoni MN 70,175; j, Kannabateomys amblyonyx MN 81,356; k, Dactylomys boliviensis LBCE 19,886. Tribe Myocastorini: l, Thrichomys fosteri LBCE 5372; m, Myocastor coypus MZUSP 32,353; n, Proechimys quadruplicatus MN 69,197; o, Callistomys pictus MZUSP 31,404. Scale bars = 1 cm
Within Echimyini, the small clade of the bamboo rats (Dactylomys I. Geoffroy St.-Hilaire, 1838 and Kannabateomys; Fig. 5 j-k), previously recognized as the subfamily Dactylomyinae (Emmons et al. 2015b), can also be recognized for exhibiting unique and cohesive morphologies, with an exceptionally well-developed infraspinous fossa. This general pattern suggests strong phylogenetic conservatism in the shape of the scapulae of spiny rats. It is necessary to recognize, however, that sampling of Callistomys, Carterodon and Myocastor, taxa with postcranial skeleton scarcely represented in scientific collections, was limited to few specimens. A larger sample would be needed to verify the robustness of our interpretations regarding phylogenetic or ecological effects on their morphology.
Despite the striking evidence of a strong phylogenetic structure of the scapular shape, it must be pointed out that in many cases (as in Fig. 1) locomotor habits accompany the phylogeny and, therefore, it is not easy to distinguish the influence of these factors to explain the scapular shape. That is, the acquisition of a certain habit early in a clade's history may have constrained the evolution of the scapular form, and later a particular scapular shape (clearly conservative in many mammals) may constrain ecological diversification.
Phylogenetic conservatism in the shape of the scapula, as well as in the muscles attached to it (Arnold et al. 2017), is remarkable in several mammalian groups, including marsupials, primates, rodents, carnivores, and bats (Young 2008; Morgan 2009; Martín-Serra et al. 2014; Gaudioso et al. 2020). Strong phylogenetic signal was also recognized for scapular morphology in other Neotropical small mammals, as didelphid marsupials and sigmodontine rodents (Astúa 2009; Coutinho et al. 2013; Coutinho and Oliveira 2017), being stronger in the former group (Bubadué et al. 2019). Despite conservatism of the scapula, Monteiro and Abe (1999) found a mixed contribution between function and phylogeny in xenarthrans, depending on the observed level. Young (2008) demonstrates that functional demands have a greater impact than the phylogenetic signal in primates.
Unusually, in some very specialized groups of burrowing species, such as Myrmecophagidae, Talpidae, Dasypodidae, Chlamyphoridae, and Chrysochloridae, scapular morphology seems to have been shaped by strong natural selection, showing evolutionary convergence in the elongation of the acromion, the expansion of the caudal angle and other conspicuous modifications (Smith and Savage 1955; Hildebrand and Goslow 2006). However, we did not observe shape changes in the scapulae of the semi-fossorial echimyids as significant as those found in the aforementioned families.
Similar to the scapula, shape variation in the humerus of echimyids exhibited strong phylogenetic signal, in congruence with previous studies with several mammalian groups, including marsupials, primates, rodents, carnivores and bats (O’Neill and Dobson 2008; Walmsley et al. 2012; Fabre et al. 2013b, 2019; Holliday and Friedl 2013; Morgan and Álvarez 2013; Martín-Serra et al. 2014; Janis et al. 2020; López-Aguirre et al. 2021). Even within less inclusive subclades, the phylogenetic structure could be recovered. As an example, within Echimyini, an exclusively arboreal taxon, the subclade formed by Isothrix Wagner, 1845 (Fig. 6g) and Lonchothrix Thomas, 1920 (Fig. 6f) shared humeri with especially short diaphysis and well-developed distal epiphysis, whereas the clade formed by Kannabateomys (Fig. 6j), Dactylomys (Fig. 6k), Phyllomys Lund, 1839 (Fig. 6l), and Makalata Husson, 1978 (Fig. 6h) had longer diaphysis and relatively smaller distal epiphysis. If this morphological difference is also reflected in different biomechanical abilities, it is possible to conjecture that the subclades of Echimyini have distinct locomotor behaviors, which can be further investigated with field and laboratory studies. However, certain biomechanical capacities associated to scapular and humeral shape and thus to particular clades, cannot be ruled out, and may be important to understand the ecomorphological diversification within each clade. Phylogenetic history and adaptive events occurred during that history are not mutually exclusive.
Echimyidae humerus specimens organized according to the taxa examined. Subfamily Euryzygomatomyinae: a, Trinomys iheringi LBCE 16,101; b, Euryzygomatomys spinosus MN 75,752; c, Clyomys laticeps LBCE 5326. Subfamily Carterodontinae: d, Carterodon sulcidens MN 54,368. Tribe Echimyini: e, Mesomys hispidus LBCE 19,843; f, Lonchothrix emiliae LBCE s/n (no identification); g, Isothrix negrensis MN 56,811; h, Makalata armata MN 70,179; i, Phyllomys pattoni MN 70,175; j, Kannabateomys amblyonyx MN 61,811; k, Dactylomys boliviensis LBCE 19,878. Tribe Myocastorini: l, Thrichomys apereoides LBCE 11,516; m, Myocastor coypus MZUSP 32,353; n, Proechimys quadruplicatus LBCE 14,994; o, Callistomys pictus MZUSP 31,404. Scale bars = 1 cm
In other mammalian groups, the phylogenetic structure is important to explain even variation in internal humeral architecture, including blood vessel distribution and bone density (Houssaye and Prévoteau 2020; Amson and Bibi 2021). These results warn us that analyses seeking to understand the morpho-functional factors guiding forelimb evolution must consider that phylogenetic history alone can explain a large amount of interspecific morphometric variation. Even forelimb structures strongly associated with functional specializations, such as the humerus, may have much of their interspecific variation explained by shared evolutionary history (Pérez et al. 2021). This consideration becomes especially important when we analyzed the placement of scansorial echimyids in the recovered morphospaces (Fig. 4). For both the humerus and scapula, scansorial echimyids occupied a region intermediate between most terrestrial and arboreal species, which could suggest that their shapes correspond to locomotor specializations, achieved and maintained via natural selection, that allow them to run and to climb. However, even though there are scansorial representatives in the subfamily Capromyinae (not analyzed), it should be noted that the scansorial echimyids in our sample belong to only one genus, Thrichomys. Meaning that, in our analysis, the scansorial habit conceivably resulted from a single evolutionary event, making it elusive to identify evolutionary convergences implying that natural selection shaped the form of Thrichomys. In addition, it should also be noted that in both recovered morphospaces, Thrichomys species were adjacent to Proechimys. Considering this, even if it is not possible to rule out an adaptive hypothesis for the shape of the humerus and scapula of Thrichomys, it could be argued that its shape is explained by phylogenetic history. Similar caution should be applied to explain the shape of the semi-aquatic habit in Echimyidae, limited to Myocastor.
Ecological Effects on Humerus Morphology
Unlike scansorial and semi-aquatic echimyids, there is consistent evidence that the humeral forms of terrestrial, arboreal, and semi-fossorial echimyids carry morpho-functional specializations partly shaped by natural selection. In these cases, similar locomotor habits have arisen more than once independently within Echimyidae and have been associated with evolutionary convergences or conspicuous changes in morphology, in line with previous studies that point to the humerus as possibly the postcranial structure containing the greatest amount of functional adaptive modifications (Morgan and Verzi 2006; Steiner-Souza et al. 2010; Tavares et al. 2020).
The terrestrial echimyids (Trinomys and Proechimys; Fig. 6a and n, respectively) have been characterized by elongation and tapering of the diaphysis, in addition to having small epiphyses, and a reduced deltoid tuberosity close to the proximal epiphysis. These features are the same as reported in other terrestrial or cursorial mammals, such as extinct kangaroos (Janis et al. 2020), carnivores (Taylor 1974; Heinrich and Rose 1997; Martín-Serra et al. 2014), felines (Walmsley et al 2012), marsupials (Argot 2001; Szalay and Sargis 2001), procyonids (Tarquini et al. 2017, 2019), rodents (Candela and Picasso 2008; Steiner-Souza et al. 2010; Morgan and Álvarez 2013; Coutinho and Oliveira 2017), tenrecoids (Salton and Sargis 2008), tupaids (Sargis 2002), and various small therian mammals (Janis and Martín-Serra 2020). The elongation of the humeral diaphysis results in a long stride, enabling greater travel speed, which is selectively favored in terrestrial mammals, for example, during an escape from predators (Wilson et al. 2015). In addition, the favoring of speed over strength is associated with the relatively underdeveloped epiphyses in terrestrial echimyids. These regions are the main insertion areas for muscles responsible for stabilizing joints under high stress and for flexing the digits (Woods 1972). These functions require relatively little strength and consequently small muscle insertion area in terrestrial species (Steiner-Souza et al. 2010; Morgan and Álvarez 2013). At the distal epiphysis, terrestrial echimyids tended to have the articular surface mediolaterally compressed, with the capitulum poorly developed, relatively to arboreal echimyids. This feature has also been reported as a locomotor specialization of terrestrial marsupials (Szalay and Sargis 2001), giving them more stability and amplitude of the parasagittal movements of the limbs (Candela and Picasso 2008).
Although less evident than in fossorial species, most species of Proechimys and Trinomys also shared reduced, flatter humeral heads compared to arboreal species, as indicated by their low scores along pPC2humerus. This recurrent feature in the humerus of terrestrial mammals restricts and stabilizes humeral movements along a parasagittal plane (Morgan and Álvarez 2013; Janis and Martín-Serra 2020).
The remarkable overlap of Proechimys and Trinomys in the humeral morphospace highlights that these two phylogenetically distant lineages share similar morpho-functional characters, in agreement with what has been previously reported in external, cranial, dental, femoral, pelvic, and lumbar morphology (Moojen 1948; Carvalho and Salles 2004; Perez et al. 2009; Tavares and Pessôa 2020; Netto and Tavares 2021), probably being thus reached and maintained by occupying similar ecological niches.
The humerus of arboreal echimyids, including Callistomys (Fig. 6o) and the Echimyini (Fig. 6e-k), was characterized by a wide diaphysis, intermediate in length between fossorial and terrestrial, well-developed epiphyses, with a larger and more rounded head than in other species, a well-developed medial epicondyle, and a medially wide distal articular surface, with an expanded capitulum approaching the apex of the lateral epicondyle, and the deltoid tuberosity poorly projected in most taxa. In general, these features are convergent with several other groups of arboreal mammals, including caviomorphs (Candela and Picasso 2008; Morgan and Álvarez 2013), sigmodontines (Coutinho and Oliveira 2017), tenrecoids (Salton and Sargis 2008), primates (Rose 1989; Szalay and Dagosto 1980), marsupials (Argot 2001; Szalay and Sargis 2001), and other arboreal therian mammals (Janis and Martín-Serra 2020). The rounded and well-developed humeral head in these animals gives them greater amplitude and stability of movement at the glenohumeral joint, necessary for climbing (Candela and Picasso 2008; Szalay and Sargis 2001; Rose and Chinnery 2004). The highly developed distal epiphysis and the expressive protrusion of the medial epicondyle allow for the insertion of robust forearm pronator and hand flexor muscles, necessary for grasping and climbing movements (Argot 2001; Szalay and Sargis, 2001; Candela and Picasso 2008; Janis and Martín-Serra 2020). The well-developed capitulum likely enhances rotational movements of the radial head during forearm flexion (Candela and Picasso 2008; Szalay and Dagosto 1980; Sargis 2002).
Remarkably, Callistomys pictus and Isothrix negrensis occupy extreme positions in the humeral morphospace (high pPC2humerus scores; Fig. 4), indicating that morphological characters typical of arboreality are well developed in these species. This result is congruent with previous findings, showing that the femur and lumbar vertebrae of Callistomys are among the most specialized within arboreal echimyids (Tavares and Pessôa 2020; Netto and Tavares 2021). At the other extreme among arboreal species are Phyllomys and the bamboo rats Dactylomys and Kannabateomys, whose humeral morphology was more like that of terrestrial species, especially by having a relatively elongated diaphysis and reduced epiphyses. Congruently, the femurs of bamboo rats and Phyllomys lamarum Thomas, 1916 also stood out for having elongated diaphysis and proximal epiphysis more like those of terrestrial species (Tavares and Pessôa 2020). Dactylomys and Kannabateomys display highly specialized cranial, dental, and external morphology for life into bamboo groves (Perez et al. 2009; Tavares et al. 2016; Candela et al. 2017) and their locomotion differs from other arboreal echimyids, by using the second and third digits to hold firmly onto thin bamboo branches, along which they move smoothly and cautiously (Emmons 1981; Candela et al. 2017). Although it cannot be ruled out that elongated humeri and femora, and scapulae with a wide infraspinous fossa, are phylogenetic traits in Dactylomys and Kannabateomys, it is reasonable to assume that such features are adaptations for their specialized locomotion, a proposition that should be investigated in the future.
Despite having an intermediate morphology between terrestrial and arboreal echimyids, the humerus of the scansorial Thrichomys had more general morphological similarities with that of terrestrial echimyids, overlapping with the latter along the axis of greatest variation (pPC1humerus). This greater similarity was concentrated in the elongated diaphysis. The similarity of Thrichomys with arboreal echimyids was concentrated in the broad, rounded head. These features suggest that Thrichomys species are capable of long and fast steps, favored by terrestrial locomotion, as well as wide movements of the glenohumeral joint, useful for climbing branches and rocks, a habit reported for this genus (Mares and Ojeda 1982; Hannibal et al. 2019).
The humerus of semi-fossorial echimyids had a well-developed deltoid tuberosity, besides having a short, although not markedly thick diaphysis, large and robust epiphyses, especially the distal epiphysis, with both epicondyles well projected. These features are congruent with those found by other authors in burrowing rodents (Candela and Picasso 2008; Steiner-Souza et al. 2010; Morgan and Álvarez 2013; Coutinho and Oliveira 2017; Tavares et al. 2020). A larger deltoid tuberosity is linked to the locomotor habit providing greater attachment of important muscles involved in arm protraction, retraction, and extension (acromiodeltoid, clavodeltoid, and spinodeltoid) and humeral flexion (pectoralis major and pectoralis minor) (Rabey et al. 2015). The shortening of the diaphysis favors strength over velocity and is congruent with the condition in several other fossorial rodents, which increases their resistance to bending and shear stresses during digging, increasing robustness and reducing humerus length (Samuels and Van Valkenburgh 2008; Hopkins and Davis 2009; Coutinho et al. 2013; Morgan and Álvarez 2013). To remove soil while tunneling with their claws, semi-fossorial rodents rely on well-developed muscles of the shoulder and arm retractors, elbow extensors, arm pronators, and carpal and digital flexors (Hildebrand and Goslow 2006; Lagaria and Youlatos 2006; Tavares et al. 2020). Their wider epicondyles suggest a larger area available for the origins of the flexor, pronator, and supinator muscles of the forearm and fingers (Woods 1972; McEvoy 1982; Coutinho et al. 2013; Tavares et al. 2020).
Strikingly, all the aforesaid characteristic traits of semi-fossorial echimyids were more prominent in Clyomys and Euryzygomatomys than in Carterodon, whose humeral shape somewhat resembles that found in arboreal echimyids. This result taken alone may suggest that Carterodon is less specialized for digging than semi-fossorial euryzygomatomyines. On the other hand, previous studies have shown that other morphological specializations associated with subterranean life, such as elongation of the olecranon process and enlargement of the auditory bulla, may be more prominent in Carterodon than in Euryzygomatomys (see Fig. 11 in Verzi et al. 2016; Fig. 4 in Tavares et al. 2020). In addition, the pelvis and penultimate lumbar vertebra of Carterodon present morpho-functional characters associated with fossoriality in a more pronounced way than in Euryzygomatomys (see Fig. 4 in Tavares and Pessôa 2020; Fig. 5 in Netto and Tavares 2021). This suggests that the different lineages of fossorial rats, Carterodontinae and the tribe Euryzygomatomyini Emmons, 2005 (i.e., Euryzygomatomys and Clyomys), evolved fossoriality through distinct morphological specializations, despite important evolutionary convergences. It should also be considered that Carterodon is poorly sampled in collections and all analyses carried out so far with its postcranial skeleton are based only on a single individual. Therefore, a more detailed understanding of the evolution of fossoriality in Echimyidae will require a larger sample to understand the limits of variation in this monospecific genus.
Conclusion
Our analyses showed that allometry is not an important active factor in the morphological evolution of the scapula and humerus of echimyids, similar to what has already been reported for other caviomorphs and other small mammals. Furthermore, the present study evidenced that phylogeny and locomotor habits are reflected in different ways on the scapular and humeral morphology. While phylogeny proved important in structuring the morphometric variation of both structures, locomotor habits seem to have been a relevant factor only in the differentiation of the humerus. The strong phylogenetic effect and the absence of the influence of locomotor habits on scapular morphology could make this structure a relevant taxonomic and phylogenetic marker in future studies. On the other hand, the presence of morpho-functional markers on the humerus shape makes it remarkably useful for studies that seek to reconstruct life habits from postcranial morphological data, especially paleontological investigations aiming at a better perspective on the evolution of ecomorphological disparity in caviomorphs. It is also worth emphasizing the importance of preserving postcranial structures to obtain larger sample sizes, thus increasing the robustness of these results with additional comparative analyses.
Data Availability
The datasets generated and analyzed during the current study are not publicly available as they are being continuously analyzed for further studies and are available from the corresponding author upon reasonable request.
References
Adams DC (2014) A generalized K statistic for estimating phylogenetic signal from shape and other high-dimensional multivariate data. Syst Biol 63:685–697. https://doi.org/10.1093/sysbio/syu030
Adams D, Collyer M, Kaliontzopoulou A, Baken E (2021) “Geomorph: Software for geometric morphometric analyses. R package version 4.0. https://cran.r-project.org/package=geomorph
Álvarez A, Arévalo RLM, Verzi DH. (2017) Diversification patterns and size evolution in caviomorph rodents. Biol J Linn Soc 121:907–922. https://doi.org/10.1093/biolinnean/blx026
Álvarez A, Ercoli MD, Olivares AI, De Santi NA, Verzi DH (2021) Evolutionary patterns of mandible shape diversification of caviomorph rodents. J Mammal Evol 28:47–58. https://doi.org/10.1007/s10914-020-09511-y
Álvarez A, Ercoli MD, Prevosti FJ (2013) Locomotion in some small to medium-sized mammals: a geometric morphometric analysis of the penultimate lumbar vertebra, pelvis and hindlimbs. Zoology 116:356–371. https://doi.org/10.1016/j.zool.2013.08.007
Amson E, Bibi F (2021) Differing effects of size and lifestyle on bone structure in mammals. BMC Biol 19:87. https://doi.org/10.1186/s12915-021-01016-1
Antoine PO, Marivaux L, Croft DA, Billet G, Ganerød M, Jaramillo C, Martin T, Orliac MJ, Tejada J, Altamirano AJ, Duranthon F, Fanjat G, Rousse S, Gismondi RS (2012) Middle Eocene rodents from Peruvian Amazonia reveal the pattern and timing of caviomorph origins and biogeography. Proc Roy Soc B 279:1319–1326. https://doi.org/10.1098/rspb.2011.1732
Argot C (2001) Functional-adaptive anatomy of the forelimb in the Didelphidae, and the paleobiology of the Paleocene marsupials Mayulestes ferox and Pucadelphys andinus. J Morphol 247:51–79. https://doi.org/10.1002/1097-4687(200101)247:1<51::AID-JMOR1003>3.0.CO;2-%23
Arnal M, Kramarz AG, Vucetich MG, Frailey CD, Campbell KEJr (2020) New Palaeogene caviomorphs (Rodentia, Hystricognathi) from Santa Rosa, Peru: systematics, biochronology, biogeography and early evolutionary trends. Pap Palaeontol 6(2):193-216. https://doi.org/10.1002/spp2.1264
Arnold P, Esteve-Altava B, Fischer MS (2017) Musculoskeletal networks reveal topological disparity in mammalian neck evolution. BMC Evol Biol 17, 251. https://doi.org/10.1186/s12862-017-1101-1
Astúa D (2009) Evolution of scapula size and shape in didelphid marsupials (Didelphimorphia: Didelphidae). Evolution 63(9):2438 56. https://doi.org/10.1111/j.1558-5646.2009.00720.x
Bennett AF, Huey RB (1990) Studying the evolution of physiological performance. Oxford Surv Evol Biol 7:251–84
Biewener AA (2000) Scaling of terrestrial support: differing solutions to mechanical constraints of size. In: Brown JH, West GB (eds) Scaling in Biology. Oxford University Press, Oxford, pp 51–66
Biknevicius AR (1999) Body mass estimation in armoured mammals: cautions and encouragements for the use of parameters from the appendicular skeleton. J Zool 248:179–187. https://doi.org/10.1111/j.1469-7998.1999.tb01194.x
Biknevicius AR, McFarlane DA, Macphee RDE (1993) Body size in Amblyrhiza inundata (Rodentia: Caviomorpha), an extinct megafaunal rodent from the Anguilla Bank, West Indies: estimates and implications. Am Mus Novit 3079:1–25
Blomberg SP, Garland T, Ives AR (2003) Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57:717–745. https://doi.org/10.1111/j.0014-3820.2003.tb00285.x
Boivin M, Ginot S, Marivaux L, Altamirano-Sierra AJ, Pujos F, Salas-Gismondi R, Tejada-Lara JV, Antoine PO (2018) Tarsal morphology and locomotor adaptation of some late middle Eocene caviomorph rodents from Peruvian Amazonia reveal early ecological diversity. J Vertebr Paleontol 38:e1555164. https://doi.org/10.1080/02724634.2018.1555164
Bonvicino CR, de Oliveira JA, D’andrea PS (2008) Guia dos Roedores do Brasil, com Chaves para Gêneros Baseadas em Caracteres Externos. Centro Pan-Americano de Febre Aftosa, Rio de Janeiro
Bubadué JM, Hendges CD, Cherem JJ, Cerezer FO, Falconí TP, Graipel ME, Cáceres NC (2019) Marsupial versus placental: assessing the evolutionary changes in the scapula of didelphids and sigmodontines, Biol J Soc 128(4):994-1007. https://doi.org/10.1093/biolinnean/blz134
Burgin CJ, Colella, JP, Kahn PL, Upham NS (2018) How many species of mammals are there? J Mammal 99(1):1–14. https://doi.org/10.1093/jmammal/gyx147
Campione NE, Evans DC (2012) A universal scaling relationship between body mass and proximal limb bone dimensions in quadrupedal terrestrial tetrapods. BMC Biol 10:60. https://doi.org/10.1186/1741-7007-10-60
Candela AM, Muñoz NA, García-Esponda CM (2017) The tarsal-metatarsal complex of caviomorph rodents: Anatomy and functional-adaptive analysis. J Morphol 278(6):828-847. https://doi.org/10.1002/jmor.20678
Candela AM, Picasso MBJ (2008) Functional anatomy of the limbs of Erethizontidae (Rodentia, Caviomorpha): indicators of locomotor behavior in Miocene porcupines. J Morphol 269:552–593. https://doi.org/10.1002/jmor.10606
Carvalhaes JG, Cordeiro-Estrela P, Hohl LSL, Vilela RV, D'Andrea PS, Rocha-Barbosa O (2019) Variation in the skull morphometry of four taxonomic units of Thrichomys (Rodentia: Echimyidae), from different Neotropical biomes. J Morphol 280:436–445. https://doi.org/10.1002/jmor.20955
Carvalho GAS, Salles LO (2004) Relationships among extant and fossil echimyids (Rodentia: Hystricognathi). Zool J Linn Soc 142:445–477. https://doi.org/10.1111/j.1096-3642.2004.00150.x
Casinos A, Quintana C, Viladiu C (1993) Allometry and adaptation in the long bones of a digging group of rodents (Ctenomyinae). Zool J Linn Soc 107:107–115. https://doi.org/10.1111/j.1096-3642.1993.tb00216.x
Chen X, Milne N, O’Higgins P (2005) Morphological variation of the thoracolumbar vertebrae in Macropodidae and its functional relevance. J Morphol 266:167–181. https://doi.org/10.1002/jmor.10370
Christiansen P (1999) Scaling of mammalian long bones: small and large mammals compared. J Zool Lond 247:333-348. https://doi.org/10.1111/j.1469-7998.1999.tb00996.x
Courcelle M, Tilak MK, Leite YLR, Douzery EJP, Fabre PH (2019) Digging for the spiny rat and hutia phylogeny using a gene capture approach, with the description of a new mammal subfamily. Mol Phylogenetics Evol 136:241–253. https://doi.org/10.1016/j.ympev.2019.03.007
Coutinho LC, de Oliveira JA, Pessôa LM (2013) Morphological variation in the appendicular skeleton of Atlantic Forest sigmodontine rodents. J Morphol 274: 779–792. https://doi.org/10.1002/jmor.20134
Coutinho LC, de Oliveira JA (2017) Relating appendicular skeletal variation of sigmodontine rodents to locomotion modes in a phylogenetic context. J Anat 231:543–567. https://doi.org/10.1111/joa.12665
Debat V, Alibert P, David P, Paradis E, Auffray JC (2000) Independence between developmental stability and canalization in the skull of the house mouse. Proc Roy Soc B 1442:423-30. https://doi.org/10.1098/rspb.2000.1017
de Oliveira AM, Santos CMD (2018) Functional morphology and paleoecology of Pilosa (Xenarthra, Mammalia) based on a two-dimensional geometric Morphometrics study of the Humerus. J Morphol 279(10):1455-1467. https://doi.org/10.1002/jmor.20882
Dickinson MH, Farley CT, Full RJ, Koehl MAR, Kram R, Lehman S (2000) How animals move: an integrative view. Science 288:100–6
Duret, L (2008) Neutral theory: the null hypothesis of molecular evolution. Nat Educ 1(1):218
Edut S, Eilam D (2003) Rodents in open space adjust their behavioral response to the different risk levels during barn-owl attack. BMC Ecol (3):10. https://doi.org/10.1186/1472-6785-3-10
Elissamburu A, Vizcaíno SF (2004) Limb proportions and adaptations in caviomorph rodents (Rodentia: Caviomorpha). J Zool 262:145–159. https://doi.org/10.1017/S0952836903004485
Emmons LH, Fabre PH (2018) A review of the Pattonomys/Toromys clade (Rodentia: Echimyidae), with descriptions of a new Toromys species and a new genus. Am Mus Novit 3894(3894):1–52. https://doi.org/10.1206/3894.1
Emmons LH, Leite YLR, Patton JL (2015a) Family Echimyidae Gray, 1825. In: Patton JL, Pardiñas UFJ, D’Elía G (eds) Mammals of South America, Volume 2 (rodents). The University of Chicago Press, Chicago pp 878–880
Emmons LH, Patton JL, Leite YLR (2015b) Subfamily Dactylomyinae Tate, 1935. In Patton JL, Pardiñas UFJ, D’Elía G (eds) Mammals of South America, Volume 2 (Rodents). The University of Chicago Press, Chicago pp 880–888
Emmons LH (1981) Morphological, ecological, and behavioral adaptations for arboreal browsing in Dactylomys dactylinus (Rodentia, Echimyidae). J Mammal 62(1):183–189. https://doi.org/10.2307/1380493
Fabre A-C, Cornette R, Peigné S, Goswami A (2013b) Influence of body mass on the shape of forelimb in musteloid carnivorans. Biol J Linn Soc 110(1):91–103.https://doi.org/10.1111/bij.12103
Fabre A-C, Peckre L, Pouydebat E, Wall CE (2019) Does the shape of forelimb long bones co-vary with grasping behaviour in strepsirrhine primates? Biol J Linn Soc 127(3):649–660. https://doi.org/10.1093/biolinnean/bly188
Fabre P-H, Galewski T, Tilak M-k, Douzery EJP (2013a) Diversification of South American spiny rats (Echimyidae): a multigene phylogenetic approach. Zool Scripta 42:117–134. https://doi.org/10.1111/j.1463-6409.2012.00572.x
Fabre P-H, Upham NS, Emmons LH, Justy F, Leite YLR, Loss AC, Orlando L, Tilak M-K, Patterson BD, Douzery EJP (2017) Mitogenomic phylogeny, diversification, and biogeography of South American spiny rats. Mol Biol Evol 34:613–633. https://doi.org/10.1093/molbev/msw261
Fernández ME, Vassallo AI, Zárate M (2000) Functional morphology and palaeobiology of the Pliocene rodent Actenomys (Caviomorpha: Octodontidae): the evolution to a subterranean mode of life. Bio J Linn Soc 71:71–90. https://doi.org/10.1111/j.1095-8312.2000.tb01243.x
Freckleton RP, Harvey PH, Pagel M (2002) Phylogenetic analysis and comparative data: A test and review of evidence. Am Nat 160:712-726. https://doi.org/10.1086/343873
Galewski T, Mauffrey JFF, Leite YLR, Patton JL, Douzery EJP (2005) Ecomorphological diversification among South American spiny rats (Rodentia; Echimyidae): A phylogenetic and chronological approach. Mol Phylogenetics Evol 34:601–615. https://doi.org/10.1016/j.ympev.2004.11.015
Garland TJr, Dickerman AW, Janis CM, Jones A (1993) Phylogenetic analysis of covariance by computer simulation. Syst Biol 42:265-292. https://doi.org/10.1093/sysbio/42.3.265
Garland T Jr, Losos JB (1994) Ecological morphology of locomotor performance in squamate reptiles. In: Wainwright PC, Reilly S (eds) Ecological Morphology: Integrative Organismal Biology, The University of Chicago Press, Chicago, pp 240–302
Gaudioso PJ, Martínez, JJ, Barquez, RM. Díaz MM (2020) Evolution of scapula shape in several families of bats (Chiroptera. Mammalia). J Zool Syst Evol Res 58:1374–1394. https://doi.org/10.1111/jzs.12383
Hannibal W, Arguelho WC, Moreira JC, Aoki C (2019) Use of understory for frugivory by Thrichomys fosteri (Rodentia, Echimyidae). Oecol Aust 23(4):1100-1103. https://doi.org/10.4257/oeco.2019.2304.30
Hedrick BP, Dickson BV, Dumont ER, Pierce SE (2020) The evolutionary diversity of locomotor innovation in rodents is not linked to proximal limb morphology. Sci Rep 10(1):717. https://doi.org/10.1038/s41598-019-57144-w
Heinrich RE, Rose KD (1997) Postcranial morphology and locomotor behaviour of two early Eocene miacoid carnivorans, Vulpavus and Didymictis. Palaeontology 40(2):279–305
Hildebrand M, Goslow G (2006) Análise da Estrutura dos Vertebrados. Atheneu Editora, São Paulo
Holliday TW, Friedl L (2013) Hominoid humeral morphology: 3D morphometric analysis. Am J Phys Anthropol 152(4):506-15. https://doi.org/10.1002/ajpa.22385
Hopkins SSB, Davis EB (2009) Quantitative morphological proxies for fossoriality in small mammals. J Mammal 90:1449–1460. https://doi.org/10.1644/08-MAMM-A-262R1.1
Houssaye A, Prévoteau J (2020) What about limb long bone nutrient canal(s)? A 3D investigation in mammals. J Anat 236(3):510-521. https://doi.org/10.1111/joa.13121
Ijspeert A (2002) Locomotion, Vertebrate. In The Handbook of Brain Theory and Neural Networks. 649–654
Irschick DJ, Garland TJr (2001) Integrating function and ecology in studies of adaptation: Investigations of locomotor capacity as a model system. Annu Rev Ecol Syst 32:367–96. https://doi.org/10.1146/annurev.ecolsys.32.081501.114048
Janis CM, Martín-Serra A (2020) Postcranial elements of small mammals as indicators of locomotion and habitat. PeerJ 2;8:e9634. https://doi.org/10.7717/peerj.9634
Janis CM, Napoli JG, Bilingham C, Martín-Serra A (2020) Proximal humerus morphology indicates divergent patterns of locomotion in extinct giant kangaroos. J Mammal Evol 27:627-647. https://doi.org/10.1007/s10914-019-09494-5
Jones KE (2015) Evolutionary allometry of lumbar shape in Felidae and Bovidae. Biol J Linn Soc 116:721–740. https://doi.org/10.1111/bij.12630
Karantanis N-E (2017) Adaptive Patterns and Processes in Mammalian Arboreality. Ph.D. Dissertation. Department of Zoology, School of Biology Aristotle University of Thessaloniki. 212
Kern AD, Hahn MW (2018) The neutral theory in light of natural selection. Mol Biol Evol 35(6):1366-1371. https://doi.org/10.1093/molbev/msy092
Klingenberg CP (2011) Morpho J: An integrated software package for geometric morphometrics. Mol Ecol Resour 11:353–357. https://doi.org/10.1111/j.1755-0998.2010.02924.x
Klingenberg CP, Gidaszewski NA (2010) Testing and quantifying phylogenetic signals and homoplasy in morphometric data. Syst Biol 59:245–261. https://doi.org/10.1093/sysbio/syp106
Lacher TE, Alho CJR (1989) Microhabitat use among small mammals in the Brazilian Pantanal. J Mammal 70(2):396–401
Lacher, TE, Murphy WJ, Rogan J, Smith AT, Upham NS (2016) Evolution, phylogeny, ecology, and conservation of the Clade Glires: lagomorpha and rodentia. In Wilson DE, Lacher JTE, Mittermeier RA (eds.), Handbook of Mammals of the World, Volume 6: Lagomorphs and Rodents. Barcelona: Lynx Edicions. pp. 15–26
Lagaria A, Youlatos D (2006) Anatomical correlates to scratch digging in the forelimb of European ground squirrels (Spermophilus citellus). J Mammal 87(3):563–570. https://doi.org/10.1644/05-MAMM-A-251R1.1
Lara M, Patton JL, Hingst-Zaher E (2002) Trinomys mirapitanga, a new species of spiny rat (Rodentia: Echimyidae) from the Brazilian Atlantic Forest. Mamm Biol 67:233–242. https://doi.org/10.1078/1616-5047-00034
Leite YLR (2003) Evolution and systematics of the Atlantic tree rats, genus Phyllomys (Rodentia, Echimyidae), with description of two new species. Univ Calif Publ 132:1–118. https://doi.org/10.1525/california/9780520098497.001.0001
López-Aguirre C, Hand SJ, Koyabu D, Vuong TT, Wilson LAB (2021) Phylogeny and foraging behaviour shape modular morphological variation in bat humeri. J Anat 238(6):1312-1329. https://doi.org/10.1111/joa.13380
Lovegrove BG, Mowoe MO (2014) The evolution of micro-cursoriality in mammals. J Exp Biol 217(15):1316-1325. https://doi.org/10.1242/jeb.095737
Mares MA, Ojeda A (1982) Patterns of diversity and adaptation in South American hystricognath rodents. In: Mares MA, Genoways HH (eds) Mammalian Biology in South America, Pymatuning Laboratory of Ecology, Pennsylvania, pp 185‑192
Marroig G, Cheverud JM (2005) Size as a line of least evolutionary resistance: diet and adaptive morphological radiation in New World monkeys. Evolution 59:1128-1142. https://doi.org/10.1111/j.0014-3820.2005.tb01049.x
Martín-Serra A, Figueirido B, Palmqvist P (2014) A three-dimensional analysis of morphological evolution and locomotor performance of the carnivoran forelimb. PLoS One 9(1):e85574. https://doi.org/10.1371/journal.pone.0085574
McEvoy JS (1982) Comparative myology of the pectoral and pelvic appendages of the North American porcupine (Erethizon dorsatum) and the prehensile-tailed porcupine (Coendou prehensilis). Bull Am Mus Nat Hist 173:337–421
Meloro C, Cáceres NC, Carotenuto F, Sponchiado J, Melo GL, Passaro F, Raia P (2015) Chewing on the trees: constraints and adaptation in the evolution of the primate mandible. Evolution 69:1690–1700. https://doi.org/10.1111/evo.12694
Meloro C, Raia P (2010) Cats and dogs down the tree: the tempo and mode of evolution in the lower carnassial of fossil and living Carnivora. Evol Biol 37:177–186. https://doi.org/10.1007/s11692-010-9094-3
Millien V, Bovy H (2010) When teeth and bones disagree: body mass estimation of a giant extinct rodent. J Mammal 91:11–18. https://doi.org/10.1644/08-MAMM-A-347R1.1
Milne N, Vizcaíno SF, Fernicola JC (2009) A 3D geometric morphometric analysis of digging ability in the extant and fossil cingulate humerus. J Zool 278(1):48–56. https://doi.org/10.1111/j.1469-7998.2008.00548.x
Monteiro LR, Abe AS (1999) Functional and historical determinants of shape in the scapula of xenarthran mammals: Evolution of a complex morphological structure. J Morphol 241(3):251–263. https://doi.org/10.1002/(SICI)1097-4687(199909)241:3<251::AID-JMOR7>3.0.CO;2-7
Moojen J (1948) Speciation in Brazilian spiny-rats (genus Proechimys, family Echimyidae). Univ Kansas Publ Mus Nat Hist 1:301–406
Morgan CC (2009) Geometric morphometrics of the scapula of South American caviomorph rodents (Rodentia: Hystricognathi): form, function and phylogeny. Mamm Biol 74:497–506. https://doi.org/10.1016/j.mambio.2008.09.006
Morgan CC, Álvarez A (2013) The humerus of South American caviomorph rodents: shape, function and size in a phylogenetic context. J Zool 290:107–116. https://doi.org/10.1111/jzo.12017
Morgan CC, Verzi DH (2011) Carpal-metacarpal specializations for burrowing in South American octodontoid rodents. J Anat 219(2):167-75. https://doi.org/10.1111/j.1469-7580.2011.01391.x
Morgan CC, Verzi DH (2006) Morphological diversity of the humerus of the South American subterranean rodent Ctenomys (Rodentia, Ctenomyidae). J Mammal 87:1252–1260. https://doi.org/10.1644/06-MAMM-A-033R1.1
Netto TFS, Tavares WC (2021) Historical, allometric and ecological effects on the shape of the lumbar vertebrae of spiny rats (Rodentia: Echimyidae). Biol J Linn Soc 132:789-810. https://doi.org/10.1093/biolinnean/blaa231
Neves AC, Pessôa LM (2011) Morphological distinction of species of Thrichomys (Rodentia: Echimyidae) through ontogeny of cranial and dental characters. Zootaxa, 24:15–24. https://doi.org/10.11646/zootaxa.2804.1.2
Neves RMB (2003) Heterogeneidade morfológica escapular e umeral em mamíferos terrestres (Rodentia: Sigmodontinae): relações com as estratégias de uso dos hábitats. Rio de Janeiro. Dissertação de Mestrado 167p
Olivares AI, Verzi DH, Vucetich MG, Montalvo CI (2012) Phylogenetic affinities of the Late Miocene echimyid †Pampamys and the age of Thrichomys (Rodentia, Hystricognathi). J Mammal 93(1):76–86. https://doi.org/10.1644/11-MAMM-A-176.1
Olivares AI, Álvarez A, Verzi DH, Perez SI, De Santi NA (2020) Unravelling the distinctive craniomandibular morphology of the Plio-Pleistocene Eumysops in the evolutionary setting of South American octodontoid rodents (Hystricomorpha). Palaeontology 63:443-458. https://doi.org/10.5061/dryad.s9b6070
O’Neill MC, Dobson SD (2008) The degree and pattern of phylogenetic signal in primate long-bone structure. J Hum Evol 54(3):309-322. https://doi.org/10.1016/j.jhevol.2007.08.008
Orr HA (2009) Fitness and its role in evolutionary genetics. Nat Rev Genet 10(8):531-539. https://doi.org/10.1038/nrg2603
Patterson BD, Velazco PM (2008) Phylogeny of the rodent genus Isothrix (Hystricognathi, Echimyidae) and its diversification in Amazonia and the eastern Andes. J Mammal Evol 15(3):181–201. https://doi.org/10.1007/s10914-007-9070-6
Patton JL, Leite RN (2015) Genus Proechimys. In: Patton JL, Pardiñas UFJ, D’Elía G (eds) Mammals of South America, Volume 2: Rodents, University of Chicago Press, Chicago, pp 950-988
Pérez MJ, Cassini GH, Díaz MM (2021) The forelimbs of Octodontidae (Rodentia: Mammalia): substrate use, morphology, and phylogenetic signal. Zoology (Jena). 144:125879. https://doi.org/10.1016/j.zool.2020.125879
Perez SI, Diniz-Filho JAF, Rohlf JA, dos Reis SF (2009) Ecological and evolutionary factors in the morphological diversification of South American spiny rats. Biol J Linn Soc 98(3):646–660. https://doi.org/10.1111/j.1095-8312.2009.01307.x
Pessôa LM, dos Reis SF (1991) The contribution of cranial indeterminate growth to non-geographic variation in adult Proechimys albispinus (Is. Geoffroy) (Rodentia: Echimyidae). Z Saugetierkd 56:219–224
Pessôa LM, de Oliveira JA, dos Reis SF (1992) A new species of spiny rat genus Proechimys, subgenus Trinomys (Rodentia: Echimyidae). Zeitschrift für Säugetierkunde, Jena, 57:39-46
Polly PD (2007) Limbs in mammalian evolution. In: Hall BK (ed) Fins Into Limbs: Evolution, Development, and Transformation. University of Chicago Press, Chicago, pp 245–268
Pough FH, Janis CM, Heiser JB (2008) A Vida dos Vertebrados. Atheneu, São Paulo, 4ª Edição
Rabey KN, Green DJ, Taylor AB, Begun DR, Richmond BG, McFarlin SC (2015) Locomotor activity influences muscle architecture and bone growth but not muscle attachment site morphology. J Hum Evol 78:91–102. https://doi.org/10.1016/j.jhevol.2014.10.010
R Core Team (2021) R: A Language and Environmental for Statical Computing. Vienna, Austria, R Foundation for Statical Computing. Available in: https://www.r-project.org
Raia P, Carotenuto F, Meloro C, Piras P, Pushkina D (2010) The shape of contention: adaptation, history, and contingency in ungulate mandibles. Evolution 64:1489–1503. https://doi.org/10.1111/j.1558-5646.2009.00921.x
Randau M, Goswami A, Hutchinson JR, Cuff AR, Pierce SE (2016) Cryptic complexity in felid vertebral evolution: shape differentiation and allometry of the axial skeleton. Zool J Linnean Soc 178:183–202. https://doi.org/10.1111/zoj.12403
Renaud S, Auffray JC, Michaux J (2006) Conserved phenotypic variation patterns, evolution along lines of least resistance, and departure due to selection in fossil rodents. Evolution 60:1701–1717. https://hal.archives-ouvertes.fr/hal-00698160
Revell LJ. (2010) Phylogenetic signal and linear regression on species data. Methods Ecol Evol 1:319-329. https://doi.org/10.1111/j.2041-210X.2010.00044.x
Revell LJ (2012) Phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecol Evol 3:217–223. https://doi.org/10.1111/j.2041-210X.2011.00169.x
Revell LJ (2009) Size-correction and principal components for interspecific comparative studies. Evolution 63:3258-3268. https://doi.org/10.1111/j.1558-5646.2009.00804.x
Rohlf FJ (2017) TpsDig2, version 2.31. Department of Ecology and Evolution, State University of New York, Stony Brook
Rohlf FJ (2019) TpsUtil, version 1.78, Software. Department of Ecology and Evolution, State University of New York, Stony Brook
Rose KD, Chinnery BJ (2004) The postcranial skeleton of early Eocene rodents. Bull Carnegie Mus Nat Hist 36:211–244. http://dx.doi.org/https://doi.org/10.2992/0145-9058(2004)36[211:TPSOEE]2.0.CO;2
Rose MD (1989) New postcranial specimens of catarrhines from the Middle Miocene Chinji Formation, Pakistan: descriptions and a discussion of proximal humeral functional morphology in anthropoids. J Hum Evol 18:131-161
Salton JA, Sargis EJ (2008) Evolutionary Morphology of the Tenrecoidea (Mammalia) Forelimb Skeleton. In: Sargis EJ, Dagosto M (eds) Mammalian Evolutionary Morphology. Vertebrate Paleobiology and Paleoantropology Series. Springer, Dordrecht. Mammal Evol Morphol 51–71. https://doi.org/10.1111/j.1095-8312.2007.00908.x
Samuels JX, Van Valkenburgh B (2008) Skeletal indicators of locomotor adaptations in living and extinct rodents. J Morphol 269(11):1387-411. https://doi.org/10.1002/jmor.10662
Sansalone G, Colangelo P, Kotsakis T, Loy A, Castiglia R, Bannikova AA, Zemlemerova ED, Piras P (2018) Influence of Evolutionary Allometry on Rates of Morphological Evolution and Disparity in strictly Subterranean Moles (Talpinae, Talpidae, Lipotyphla, Mammalia). J Mammal Evol 25:1–14. https://doi.org/10.1007/s10914-016-9370-9
Sargis EJ (2002) Functional morphology of the hindlimb of tupaiids (Mammalia, Scandentia) and its phylogenetic implications. 254(2):149–185. https://doi.org/10.1002/jmor.10025
Scales JA, Butler MA (2016) Adaptive evolution in locomotor performance: How selective pressures and functional relationships produce diversity. Evolution 70(1):48-61. https://doi.org/10.1111/evo.12825
Schmidt-Kittler N (2002) Feeding specializations in rodents. Senckenberg Leth 82:141–152
Schmidt-Kittler N (2006) Microdonty and macrodonty in herbivorous mammals. Palaeontogr Abt A 278:163–179. https://doi.org/10.1127/pala/278/2006/163
Seckel L, Janis C (2008) Convergences in scapula morphology among small cursorial mammals: an osteological correlate for locomotory specialization. J Mamm Evol 15:261–279. https://doi.org/10.1007/s10914-008-9085-7
Shaw AK (2020) Causes and consequences of individual variation in animal movement. Mov Ecol 8:12. https://doi.org/10.1186/s40462-020-0197-x
Smith JM, Savage RJG (1955) Some locomotory adaptations in mammals. Zool J Linn Soc 42:603-622. https://doi.org/10.1111/j.1096-3642.1956.tb02220.x
Steiner-Souza F; de Freitas TRO, Cordeiro-Estrela P (2010) Inferring adaptation within shape diversity of the humerus of subterranean rodent Ctenomys. Biol J Linn Soc 100:353–367. https://doi.org/10.1111/j.1095-8312.2010.01400.x
Stöver BC, Müller KF (2010) TreeGraph 2: Combining and visualizing evidence from different phylogenetic analyses. BMC Bioinformatics 11(1):7. https://doi.org/10.1186/1471-2105-11-7
Szalay FS, Dagosto M (1980) Locomotor adaptations as reflected on the humerus of Paleogene primates. Folia Primatol 34:1-45. https://doi.org/10.1159/000155946
Szalay FS, Sargis E (2001) Model-based analysis of postcranial osteology of marsupials from the Palaeocene of Itaboraí (Brazil) and the phylogenetics and biogeography of Metatheria. Geodiversitas 23:139–302. https://sciencepress.mnhn.fr/en/periodiques/geodiversitas/23/2/analyse-modelisee-du-squelette-postcranien-des-marsupiaux-du-paleocene-d-itaborai-bresil-et-la-phylogenie-et-biogeographie-des-metatheria
Tague RG (2020) Commonality in pelvic anatomy among three fossorial, scratch-digging, mammalian species. J Mammal Evol 27:315–327. https://doi.org/10.1007/s10914-019-09463-y
Tarquini J, Toledo N, Morgan CC, Soibelzon, LH (2017) The forelimb of †Cyonasua sp. (Procyonidae, Carnivora): ecomorphological interpretation in the context of carnivorans. Earth Environ Sci Trans R Soc Edinb 106(04):325–335. https://doi.org/10.1017/S1755691016000207
Tarquini J, Morgan CC, Toledo N, Soibelzon LH. (2019) Comparative osteology and functional morphology of the forelimb of Cyonasua (Mammalia, Procyonidae), the first South American carnivoran. J Morphol 280(3):446–470. https://doi.org/10.1002/jmor.20956
Tavares WC, Abi-Rezik P, Seuánez HN (2018) Historical and ecological influence in the evolutionary diversification of external morphology of Neotropical spiny rats (Echimyidae, Rodentia). J Zool Syst Evol Res 56:453–465. https://doi.org/10.1111/jzs.12215
Tavares WC, Pessôa LM (2010) Variação Morfológica em Populações de Trinomys (Thomas, 1921) de Restingas e Matas de Baixada no Estado do Rio de Janeiro. In: Pessôa LM, Tavares WC, Siciliano S, (eds) Mamíferos de Restingas e Manguezais do Brasil, Sociedade Brasileira de Mastozoologia, Rio de Janeiro, pp 128–154
Tavares WC, Pessôa LM (2020) Effects of size, phylogeny and locomotor habits on the pelvic and femoral morphology of South American spiny rats (Rodentia: Echimyidae). Biol J Linn Soc 131:835–869. https://doi.org/10.1093/biolinnean/blaa150
Tavares WC, Pessôa LM, Seuánez HN (2016) Phylogenetic and size constraints on cranial ontogenetic allometry of spiny rats (Echimyidae, Rodentia). J Evol Biol 29:1752–1765. https://doi.org/10.1111/jeb.12905
Tavares WC, Pessôa LM, Seuánez HN (2015) Plio-Pleistocene history of the endangered spiny rat Trinomys eliasi (Echimyidae) from Rio de Janeiro, Brazil. J Mammal 96:94–106. https://doi.org/10.1093/jmammal/gyu010
Tavares WC, Vozniak JH, Pessôa LM (2020) Evolution of appendicular specializations for fossoriality in euryzygomatomyine spiny rats across different Brazilian biomes (Echimyidae, Hystricognathi, Rodentia). J Mammal Evol 27:299–314. https://doi.org/10.1007/s10914-019-09459-8
Taylor ME (1974) The functional anatomy of the forelimb of some African Viverridae (Carnivora). J Morphol 143(3):307-35. https://doi.org/10.1002/jmor.1051430305
Verzi DH, Olivares AI, Morgan CC, Álvarez A (2016) Contrasting phylogenetic and diversity patterns in octodontoid rodents and a new definition of the family Abrocomidae. J Mammal Evol 23:93–115. https://doi.org/10.1007/s10914-015-9301-1
Walmsley A, Elton S, Louys J, Bishop LC, Meloro C (2012) Humeral epiphyseal shape in the Felidae: The influence of phylogeny, allometry, and locomotion. J Morphol 273(12):1424–1438. https://doi.org/10.1002/jmor.20084
Weisbecker V, Schmid S (2007) Autopodial skeletal diversity in hystricognath rodents: Functional and phylogenetic aspects. Mamm Biol 72(1):27–44. https://doi.org/10.1016/j.mambio.2006.03.005
Wölfer J, Arnold P, Nyakatura JA (2019) Effects of scaling and locomotor ecology suggest a complex evolution of scapular morphology in sciuromorph rodents. Biol J Linn Soc 127(2):175–196. https://doi.org/10.1093/biolinnean/blz042
Woods CA (1972) Comparative myology of jaw, hyoid, and pectoral appendicular regions of New and Old World hystricomorph rodents. Bull Am Mus Nat Hist 147(3):117-198
Young NM (2008) A comparison of the ontogeny of shape variation in the anthropoid scapula: Functional and phylogenetic signal. Am J Phys Anthropol 136(3):247-264. https://doi.org/10.1002/ajpa.20799
Zelditch ML, Swiderski DL, Sheets HD, Fink WL (2004) Geometric Morphometrics for Biologists: a primer. Elsevier Academic Press: London
Acknowledgements
We would like to thank the curators and staff responsible for the collections accessed, Dr. Cibele Rodrigues Bonvicino and Dr. Daniela Dias (LABPMR/IOC/FIOCRUZ), Dr. João Alves de Oliveira (MN/UFRJ), Dr. Juliana Gualda de Barros (MZUSP), Dr. Pedro Cordeiro Estrela de Andrade Pinto (UFPB), for assistance in accessing the echimyids under their incumbency. We are also grateful to the Fundação Oswaldo Cruz (FIOCRUZ), through the Instituto Oswaldo Cruz (IOC).
Funding
JGC was financially supported by the Coordination for the Improvement of Higher Education Personnel (CAPES).
Author information
Authors and Affiliations
Contributions
This study is part of the Doctoral Thesis by JGC, who conceived the idea of the study, captured and classified the images, conducted the analyses, drafted the manuscript, and prepared the illustrations. WCT conducted tests and analyses and made substantial contributions to the manuscript. RVV helped conceive the study, assisted in collecting data and images, and made major contributions to the manuscript. PSD helped conceive the study and made substantial contributions to the manuscript. All authors read, contributed to, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest/Competing Interests
The authors declare no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carvalhaes, J.G., Tavares, W.C., do Val Vilela, R. et al. Phylogenetic, Allometric, and Ecological Factors Affecting Morphological Variation in the Scapula and Humerus of Spiny Rats (Rodentia: Echimyidae). J Mammal Evol 29, 997–1014 (2022). https://doi.org/10.1007/s10914-022-09617-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-022-09617-5