Abstract
Possessing toxins can contribute to an efficient defence against various threats in nature. However, we generally know little about the energy- and time-demands of developing toxicity in animals, which determines the efficiency of chemical defence and its trade-off with other risk-induced phenotypic responses. In this study we examined how immersion into norepinephrine solution inducing the release of stored toxins, administration of mild stress mimicking predator attack or simple handling during experimental procedure affected the quantity and number of toxin compounds present in common toad (Bufo bufo) tadpoles as compared to undisturbed control individuals, and investigated how fast toxin reserves were restored. We found that total bufadienolide quantity (TBQ) significantly decreased only in the norepinephrine treatment group immediately after treatment compared to the control, but this difference disappeared after 12 h; there were no consistent differences in TBQ between treatments at later samplings. Interestingly, in the norepinephrine treatment approximately half of the compounds characterized by >700 m/z values showed the same changes in time as TBQ, but several bufadienolides characterized by <600 m/z values showed the opposite pattern: they were present in higher quantities immediately after treatment. The number of bufadienolide compounds was not affected by any treatments, but was positively related to TBQ. Our study represents the first experimental evidence that toxin quantities returned to the original level following induced toxin release within a very short period of time in common toad tadpoles and provide additional insights into the physiological background of chemical defence in this model vertebrate species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many organisms are known to use toxins to defend themselves against naturally occurring threats (Chen 2008; Kempken and Rohlfs 2010; Kicklighter 2012). If adaptive plasticity evolves in such chemical defences, the presence of pathogens/parasites, predators or competitors can induce a physiological response that results in enhanced toxin production (manifested either in the increased number of toxins or in higher amounts of effective compounds), leading to better chances of survival in more risky environments (Harvell 1990; West-Eberhard 1989). Plasticity in chemical defence is well known in plants (Ahuja et al. 2012; Mithöfer and Boland 2012; War et al. 2012), and previous studies have also shown that in several animal species individuals can adjust their defensive toxin production in response to the risk of predation, competition or infection (reviewed in Hettyey et al. 2014). However, unlike in many plants (Ali and Agrawal 2012; Gonzales-Vigil et al. 2011) and (cyano)bacteria (Dittmann et al. 2013; Wang et al. 2016), the biosynthetic pathways and the enzymes involved in the production of toxins are often unknown in animals (Bane et al. 2014; Mebs 2001, but see McGugan et al. 2016). Yet investigations on the proximate mechanisms of toxin production are important because physiological constraints and limits of toxicity may fundamentally influence the effectiveness of toxins and affect its trade-offs with other forms of inducible defence, such as plastic responses in morphology, development or behaviour.
In species where de novo biosynthesis takes place through a complex metabolic pathway and specific enzymes are involved in toxin production (i.e. toxic compounds are not produced by symbiotic bacteria or uptaken from the diet), physiological constraints are simply definable. Such constraints may be the time, energy and precursor availability that are required for toxin synthesis and, thus, for the build-up of baseline toxicity, for the replenishment of depleted toxin reserves and for the production of an increased quantity of toxins if induced by environmental cues. For instance, Richelle-Maurer et al. (2003) found that in the sponge Agelas conifera individuals exhibited a three- to four-fold rise in levels of endogenous bromopyrrole alkaloids in response to simulated predator attacks compared to control conditions. More interestingly, one of the two predominant compounds’ concentration increased 12 h after the inflicted damage, whereas the increase in the other compound’s concentration was delayed by six days. This finding implies that different compounds within a toxic blend may have different production costs or time requirements, which may also substantially affect their function or deterrence efficiency. However, in most species physiological constraints related to toxin production were rarely studied, although these are especially relevant in species where toxins are excreted during antagonistic encounters and, thus, their reserves need to be restored.
Amphibians are popular model organisms for the study of various aspects of inducible responses, including chemical defence (Mangoni et al. 2001; Toledo and Jared 1995). Most bufonid species produce bufadienolides (Hayes et al. 2009; Mebs et al. 2007; Sciani et al. 2013), cardiotoxic steroids that inhibit Na+/K+-ATPases (Steyn and van Heerden 1998) and make these animals more or less unpalatable to most vertebrate predators (Gunzburger and Travis 2005). In toads, more than 100 different bufadienolide compounds have been identified so far, some of which may be the result of in situ bacterial biotransformation (Hayes et al. 2009). The biosynthesis of bufadienolides starts with cholesterol, but the intermediate compounds and associated enzymes along the biosynthetic pathway are not yet known, although a novel “acidic” bile acid pathway has been proposed to be involved in the synthesis of marinobufagenin, an endogenous Na+/K+-ATPase inhibitor also present in mammals (Fedorova et al. 2015). Bufadienolide compounds are usually classified as either free type bufogenins or conjugated type bufotoxins (although a “bufolipin” sub-class has also been identified in cane toad eggs and ovaries; Crossland et al. 2012), according to the esterification of the C-3 hydroxyl group of the steroid nucleus (Rodríguez et al. 2017). While bufogenins possess a free hydroxyl group at C-3, bufotoxins are typified by the conjugation to this ligand to form various esters (Wang et al. 2011), which generally results in a detectable increase in their mass-to-charge ratio (m/z value); however, sulphate conjugates can have m/z values similar to that of the bufogenins (Meng et al. 2016). Previous studies, which investigated the structure-activity relationship in bufadienolide compounds, found that bufogenins are generally more potent than bufotoxins (Kamano et al. 1998; Lee et al. 1994; Meng et al. 2016; Shimada et al. 1987a), although some bufotoxins containing a suberoyl-arginine group in their side chain are more toxic than their respective bufogenin analogues (Shimada et al. 1985, 1986, 1987b). It has been proposed that an increasing structural diversity of bufadienolides could be advantageous in terms of survival if it enhances the probability of interfering with a wider subset of Na+/K+-ATPase isoforms (Hayes et al. 2009), however we still do not know how these compounds are related to each other in the bufadienolide biosynthetic pathway, and whether or not there are any functional differences between various compounds or variation in physiological limits related to their production.
In this study, we used common toad (Bufo bufo) tadpoles to examine how fast toxin quantities can be restored after experimentally induced toxin release. During this experiment we manipulated tadpoles’ bufadienolide reserves by either immersing them into a norepinephrine hormonal solution or by applying mild, but abrupt mechanical disturbance. Norepinephrine has been successfully applied in previous studies to induce toxin release from skin glands in tadpoles of several species (e.g. Calhoun et al. 2016), including the common toad (Kurali et al. 2016). We predicted that immersion into norepinephrine solution would lead to a decrease in the amount of bufadienolides in tadpoles’ toxin reserves due to enforced excretion, but expected bufadienolide reserves to become similar to that of untreated individuals within five days. Furthermore, if a mild, non-invasive stressor can also trigger active toxin release, the amount of detectable bufadienolides in tadpoles would also decrease, but to a lower extent, so that the induced difference between treated and untreated individuals would diminish more rapidly. Tadpoles kept in control conditions were expected to maintain a relatively constant level of toxicity throughout the experiment. The number of bufadienolide compounds was predicted to remain unaffected by the applied treatments as these do not hinder the production pathway of bufadienolides in any way according to our present knowledge.
Methods and Materials
Study Species
The common toad (Bufo bufo Linnaeus, 1758) is an anuran amphibian that is widespread across Europe (Gasc et al. 1997) and uses various types of waterbodies for breeding. Due to the high environmental variability of these aquatic habitats, offspring may be exposed to widely varying abundances of predators, competitors and pathogens during larval ontogeny (Bókony et al. 2016; Ujszegi et al. 2017). Common toad tadpoles have previously been found to exhibit plasticity in behaviour (Marquis et al. 2004; Nunes et al. 2013, but see Richter-Boix et al. 2007), life-history (e.g., Lardner 2000; Laurila et al. 1998; Nunes et al. 2014), and morphology (Nunes et al. 2014; Van Buskirk 2009), although the extent/intensity of these plastic responses is relatively weak compared to those of ranid tadpoles (e.g. Lardner 2000; Laurila et al. 1998). As with most bufonid species, common toads produce cardiotoxic steroids, called bufadienolides, de novo, and we have recently shown that tadpoles are capable of synthesizing their own bufadienolides (Üveges et al. 2017). Histological and ultrastructural studies on this species have also demonstrated that underlying secretory cells are already present in larvae and that they are not distributed evenly in the skin especially at earlier developmental stages (Delfino et al. 1995; Kulzer 1954). Secretions from these cells can also reach the exterior of tadpoles’ skin in bufonids, including this species (Kulzer 1954; Le Quang Trong 1973; Meyer 1962). Recent studies investigating various aspects of B. bufo tadpoles’ bufadienolide production have also revealed that individuals can respond plastically in the amount of produced bufadienolides to the perceived intensity of competition (Bókony et al. 2018) and to the presence of a glyphosate-based herbicide (Bókony et al. 2017). Moreover, experimentally induced toxin release was found not to be associated with high costs measured in several fitness-related traits (Kurali et al. 2016).
Animal Collection and Husbandry
We collected segments of ten different common toad egg strings from Lake Garancsi (47°37′25”N, 18°48′27″E) located in the Pilis Hills, Hungary, in early March 2016, and transported them to a laboratory of the Plant Protection Institute (PPI), Centre for Agricultural Research (CAR), Hungarian Academy of Sciences (HAS). Each egg string was kept separately until the embryos hatched. Two days after hatchlings reached the free-swimming state, we placed tadpoles individually into 2-L rearing containers filled with 0.7 L reconstituted soft water (RSW; APHA 1985). Ambient temperature was set to 21 °C during daylight hours, which was allowed to decrease to 18 °C at night. Lighting was set to a 11:13 dark:light cycle. Tadpoles were fed ad libitum twice a week with a 1:100 mixture of finely ground Spirulina (NaturPiac, Budapest, Hungary) and slightly boiled spinach. We changed water in the rearing containers every three days taking care to minimize disturbance to the animals: we carefully drained 90% of the water from the containers with a plastic pipe and refilled them with fresh RSW. All procedures involving animals in this study were approved by the national authority of the Middle-Danube-Valley Inspectorate for Environmental Protection, Nature Conservation and Water Management, Hungary (KTF: 3596–7/2016) and the Ethical Commission of the PPI CAR HAS. All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Experimental Design and Data Collection
During the setup of the experiment, we applied a randomized full-factorial block design with two factors: treatment (4 levels) and sampling (time since the application of treatments; 7 levels). We haphazardly allocated one tadpole from each of the 10 families to each treatment combination (280 tadpoles). We raised an additional tadpole from each family (10 tadpoles) and preserved it immediately before the treatments were applied to be able to determine initial bufadienolide quantities. We arranged all specimens of each family into a spatial block each, resulting in ten horizontal blocks in the vertical space of the laboratory shelves.
On the 14th day of the experiment, when tadpoles reached Gosner stage 35 (Gosner 1960), individuals were exposed to one of four different treatments: administration of norepinephrine, stressing, only handling and no disturbance. For the determination of initial bufadienolide amounts in tadpoles in baseline conditions, one tadpole from each family was fixed in 70% methanol prior to the application of treatments. To induce the depletion of bufadienolide stores in the tadpoles’ skin, we applied in vivo stimulation via hormonal treatment (administration of norepinephrine). Tadpoles in the norepinephrine treatment group were placed into a 3-ml, 100-μM norepinephrine-bitartrate (CAS 3414-63-9, Sigma-Aldrich, USA) solution for 15 min (Kurali et al. 2016; Maag et al. 2012). To facilitate the washing of norepinephrine and any toxins that had been released from the skin, we subsequently transferred individuals into a box filled with 700 ml RSW for one minute and finally placed them back into their rearing containers. For stressing tadpoles, we abruptly prodded them with a blunt glass rod while taking care not to damage the skin of treated animals; this was done only once and without removing animals from the rearing container. In the treatment group receiving only handling, we placed tadpoles into 3 ml of RSW and otherwise handled them the same way as those in the norepinephrine treatment group. Animals in the no disturbance group were left undisturbed throughout the experiment. Zero, 12, 24, 48, 72, 96, and 120 h after applying treatments, we haphazardly selected one tadpole from each family in each treatment (40 tadpoles/sampling) and fixed it in 70% methanol. The resulting sample size was similar to those of previous studies on bufadienolide production in common toad tadpoles (e.g. Bókony et al. 2017; Üveges et al. 2017).
Chemical Analysis
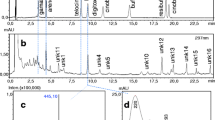
The collected samples were processed in the Department of Pathophysiology at the PPI, CAR, HAS. We homogenized tadpoles using a VWR VDI 12 homogenizer with an IKA S12 N-7S dispersing tool. After homogenization, samples were dried under vacuum at 45 °C using a rotary evaporator (Büchi Rotavapor R-134, Flawil, Switzerland), and dry mass measured to the nearest 0.0001 g using an Ohaus Pioneer PA-114 analytical balance (Ohaus Corp., Parsippany, NJ, USA). We re-dissolved the samples in 1 ml HPLC grade methanol, which was aided by brief use of ultrasound in a bath sonicator (Tesla UC005AJ1). Finally, we filtered samples using FilterBio nylon syringe filters (pore size = 0.22 μm). We identified toxin compounds as bufadienolides by inspecting the UV spectrum of peaks and by using commercially acquired bufalin, bufotalin, (resi)bufogenin, gamabufotalin, areno- and telocinobufagin (Biopurify Phytochemicals, Chengdu, China), cinobufagin (Chembest, Shanghai, China), cinobufotalin (Quality Phytochemicals, New Jersey, USA), digitoxigenin (Santa Cruz Biotechnology, Dallas, TX, USA) and marinobufotoxin (a courtesy of Prof. Rob J. Capon, Institute for Molecular Bioscience, University of Queensland, Australia) as standards. To identify and quantify the bufadienolide compounds in tadpoles, we used a high-performance liquid chromatography – mass spectrometry (HPLC-MS) system (Shimadzu LC-MS-2020, Shimadzu Corp., Kyoto, Japan) equipped with a binary gradient solvent pump, a vacuum degasser, a thermostated autosampler, a column oven, a diode array detector and a single-quadrupole mass analyser with electrospray ionization (ESI-MS). Chromatographic separations were carried out at 35 °C on a C18 2.6 μm column (Kinetex, 100 mm × 3 mm i.d.) in series with a C18 guard column (4 mm × 3 mm i.d.) using 10 μl injections. The mobile phase consisted of 5% aqueouos acetonitrile containing 0.05% formic acid (solvent A) and acetonitrile containing 0.05% formic acid (solvent B). The flow rate was 0.8 mL/min and the gradient was as follows: 0–2 min, 10.5–21.1% B; 2–15 min, 21.1–26.3% B; 15–24 min, 26.3–47.4% B; 24–25 min, 47.4–100% B; 25–30 min 100% B; 30–31 min 100–10.5% B; 31–35 min 10.5% B. ESI worked under the following conditions: desolvation line (DL) temperature: 250 °C; heat block temperature: 400 °C; drying N2 gas flow:15 l min−1; nebulizer N2 gas flow: 1.5 l min−1; positive ionization mode. We acquired and processed the data using the programme LabSolutions 5.42v (Shimadzu Corp.). The above optimization settings have been successfully used to identify and quantify bufadienolide compounds in previous studies (e.g. Bókony et al. 2016; Üveges et al. 2017).
Statistical Analyses
For calculating the number of bufadienolide compounds (NBC) present in each tadpole, we scored a compound to be present if it was detectable (the limit of detection was between 0.02 and 0.05 ng for the standards) in the chromatogram. The quantity of each compound was estimated from the area under the chromatographic peaks using the calibration curve of the bufotalin standard; these bufotalin-equivalent quantities were subsequently used in the statistical analysis. Both outcomes were measured by an investigator who was blinded to the group allocation during the experiment. We also calculated total bufadienolide quantity (TBQ) for each animal by summing the estimated quantities of each compound (for a similar approach see e.g., Benard and Fordyce 2003; Bókony et al. 2018; Hagman et al. 2009).
We fitted quasi-Poisson generalized linear mixed-effect model (GLMM) using penalized quasi-likelihood estimation to analyse the effects of treatment and sampling on NBC using the ‘MASS’ R package 2002). We used linear mixed-effect models (LMM) to analyse the effects of treatment and different sampling occasions on toxin quantities using the ‘nlme’ R package (Pinheiro et al. 2017). Into these latter models, we included TBQ or the quantity of a single compound as the dependent variable. We applied square-root transformation in the case of TBQ and seven compounds, and log transformation in the case of one compound to improve residuals’ fit to a normal distribution. In the case of another four components residual diagnostics of the initial models indicated insufficient fit due to their highly skewed distributions; here we entered rank-transformed quantities as the dependent variable (Table S4). Three compounds were absent in more than 50% of the tested individuals; so we refrained from analysing these components separately, but took them into account in the calculation of TBQ. We entered treatment and sampling occasion as fixed factors and their interaction into all fitted models, and also added dry weight of each tadpole (mean ± SD: 20.27 ± 6.6 mg) as a potential confounding variable. Deviation from the median NBC was also included as a covariate into the model fitted on TBQ to control for consistent differences between individuals in the number of compounds. Family was incorporated into all models as a random factor, and we allowed heterogeneous variance between treatments in the LMMs by adding the ‘weights’ parameter with ‘varIdent’ to the models. We used a backward elimination procedure for model selection, starting with fitting the full model first, then dropping the predictor with the highest P-value in each step and retaining only statistically significant effects (P ≤ 0.05) in the final models (Engqvist 2005; Grafen and Hails 2002). Test statistics (type III Wald χ2) and associated P-values were computed using the ‘Anova’ function in the ‘car’ R package (Fox and Weisberg 2011). The ‘weights’ variable was excluded from the model if it had negligible effect on model fit based on a likelihood ratio test; fulfilment of the requirements of the fitted LMMs was checked by plot diagnosis. Post hoc tests with Tukey adjustment were used for pairwise comparisons between treatments within each sampling occasion using the ‘lsmeans’ R package (Lenth 2016). We run all analyses in R 3.4.1 (R Core Team 2017). All tests were two-tailed with α set to 0.05. Data used in the statistical analyses are available from Figshare (https://figshare.com/s/0f4c347e1e4c3f412f92).
Results
In total, we found 21 putative bufadienolide compounds in the toad tadpoles, of which three could be unambiguously identified as bufotalin, arenobufagin and marinobufotoxin, respectively (Table S1).
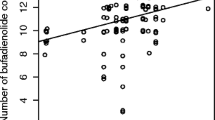
The number of bufadienolide compounds (overall median: 18 compounds/tadpole, range: 13–21) was significantly affected by sampling occasions (χ26 = 35.98, P < 0.001, Table 1), but not by the applied treatment (either by itself or in interaction with sampling; both P ≥ 0.502, Table 1). Immediately after treatments (0 h), the number of bufadienolide compounds was significantly lower compared to the third (24 h) sampling, and was also lower than on other sampling occasions except for the second (12 h) and fourth (48 h) samplings; for further details see Table S2. The number of bufadienolide compounds also increased slightly, but significantly with tadpoles’ dry mass (β ± SE = 0.002 ± 0.0007, Table 1).
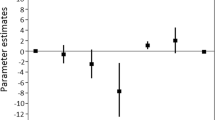
Total bufadienolide quantity (overall mean ± SD: 12040.54 ± 4697.37 bufotalin-equivalent ng/tadpole) was significantly affected by the interaction of treatment and sampling (χ218 = 33.18, P = 0.016; Table 1, Fig. 1): immediately after the application of treatments, TBQ was lower in tadpoles that were immersed into norepinephrine solution compared to individuals in other treatments, but this difference disappeared by the second sampling occasion (i.e. after 12 h; Table 2). Total bufadienolide quaility was also lower in the control than in the handling treatment group at the third (24 h) and the sixth (96 h) samplings. All other pairwise comparisons were non-significant (all P ≥ 0.400, Table 2). Deviation from the median number of compounds was positively related to TBQ (β ± SE = 2.27 ± 0.73, Table 1), suggesting that individuals which produced a greater diversity of compounds had overall greater quantities of bufadienolides, whereas dry mass had no significant effect on TBQ (Table 1).
Mean of the total bufadienolide quantity (TBQ) at various sampling occasions. The error bar indicates SE calculated from biological replicates (n = 10 tadpoles in each treatment-sampling combination). Tukey post hoc test performed within each sampling occasion showed that immediately after the application of treatments (0 h) TBQ was significantly lower than in any of the other treatments (all P ≤ 0.003; see also in Table 2)
Out of the 18 individually analysed bufadienolides, the quantity of 11 compounds was significantly affected by the interaction between treatment and the timing of sampling (Table S3). Six of these compounds were characterized by >700 m/z value and their amounts significantly decreased during the hormonal treatment, but were restored after 12 h similarly to TBQ (Table 3). The change in quantities of the other five compounds (all <600 m/z value) showed an opposite pattern, being significantly higher immediately after the norepinephrine treatment than in the control group (the quantity of four compounds was also higher than in the ‘handling’ treatment group, and for three compounds also higher than in the ‘stress’ treatment group), while this difference also disappeared after 12 h (Table 3). The rest of the compounds (seven bufadienolides) varied significantly among sampling occasions, but were not affected by treatment (either by itself or in interaction with sampling; all P ≥ 0.066, Table S3). Also, dry mass was positively related to the quantity of bufadienolides in eight out of the 18 compounds (Table S3).
Discussion
Our results provide experimental evidence for fast changes in the quantities of bufadienolides in common toad tadpoles. Total bufadienolide quantity was significantly lower in treated compared to untreated tadpoles immediately after stimulating bufadienolide release induced by immersion of treated individuals into norepinephrine solution, but it become similar in treated and untreated tadpoles within only 12 h. We observed this change only in hormone-treated individuals, but not in tadpoles from other treatment groups, which suggests that animals did not secrete bufadienolides onto their skin surface in response to stress stimuli. Interestingly, some compounds showed opposite changes in their quantity following induced bufadienolide release, while the amount of six out of ten bufadienolides characterized by >700 m/z values (most probably conjugate-type bufotoxins) decreased significantly, five out of 11 bufadienolides characterized by <600 m/z values (most likely free-type bufogenins) had higher quantities immediately after the applied hormonal treatment. The rest of the compounds were either not affected by the norepinephrine treatment (these could be present only in other tissues of the tadpoles; Matsukawa et al. 1996) or were not individually analysed due to their absence in more than half of the tadpoles. Nevertheless, after 12 h all examined bufadienolides were present in similar amounts as in the control. The number of bufadienolide compounds was, in accordance with our initial prediction, not affected by the applied treatments. In the following sections we provide possible explanations for this pattern and discuss the ecological relevance of our findings in the context of chemical defence.
We found that none of the 700 m/z bufadienolides showed an immediate increase in their quantity and none of the <600 m/z compounds had an immediate decrease in their amount in response to the norepinephrine stimulus, which implies a structure-related difference in these components’ role in the bufadienolide expression pathway. If free-type bufogenins are indeed more potent agents of toxicity in common toads (Meng et al. 2016; but see Shimada et al. 1985, 1986, 1987b), animals may store bufadienolides in the form of conjugated bufotoxins to facilitate their in situ transport and avoid self-poisoning during storage. Upon secretion, these compounds may be transformed into active bufogenins through enzymatic catalysis (either by their own specific enzymes or through bacterial biotransformation; Hayes et al. 2009; Kamalakkannan et al. 2017), to elicit a strong deterrent/toxic effect once shed onto the surface of the skin. As potent toxins are usually more lipophilic (enabling them to be transported by lipoproteins with blood and to be absorbed swiftly through lipid membranes; Dekant 2009), these compounds can provide protection to the producing individuals until the secreted toxic blend eventually washes off. This idea is supported by the fact that in baseline conditions <600 m/z compounds were present in much lower quantities in the toxin blend than >700 m/z bufadienolides in the tadpoles (mean ± SD: 562.2 ± 207.7 vs. 11,032.6 ± 4604.3 bufotalin-equivalent ng/tadpole; Wilcoxon signed rank test: V = 55, n = 10, P = 0.002). Alternatively, bufadienolides characterized by lower m/z values are, in fact, precursors of the stored, conjugated bufotoxins, and their quantity increased in our experiment because of rapid de novo synthesis during the applied hormonal treatment. In this case, both the biosynthesis of bufogenins and dissolution of bufotoxins into the water from the tadpoles’ skin surface took place within a mere 15 min during the experiment. Previous studies of experimentally induced toxin release in toads usually applied more invasive methods, were conducted on juveniles or adults in a much coarser time scale, and toxin replenishment inside the parotoid glands’ alveoli was only inferred, not explicitly quantified (e.g. Jared et al. 2009, 2014; Toledo et al. 1992). Thus, as long as we do not have more detailed information about the bufadienolide expression pathway in bufonids, further investigations on potential differences in toxicity and water solubility among these toxin compounds and on differences in their proportion in the secreted and stored toxin blend may indirectly support one of these alternative explanations.
We found that bufadienolide toxin levels became similar surprisingly fast, within 12 h, in the hormone-treated tadpoles compared to their control counterparts. Amphibian toxin glands are typically specialized for passive defence (Jared et al. 2009; Mailho-Fontana et al. 2014), and toxin release is induced by a sufficiently high external pressure (e.g. during predator attack), which may be also true for the much more simply structured unicellular skin glands in tadpoles (as also suggested in Delfino et al. 1995, p. 111). This may also explain why the total bufadienolide quantity changed only in the hormone-stimulated tadpoles in our experiment and not in response to physical disturbance caused by the handling procedure or the applied stress stimulus. The rapid offset of toxin reserves in treated and untreated tadpoles may indicate relatively relaxed resource requirements for bufadienolide production, which is consistent with our previous work, where we found that toxin release induced multiple times during larval development had low fitness costs in common toad tadpoles (Kurali et al. 2016). These findings indicate that common toad tadpoles are chemically well-defended at almost all times against recurring attacks of predators that are susceptible to bufadienolide toxins, and individuals can maintain their toxicity without considerable trade-offs with other predator-induced traits. However, this may only be true if such attacks do not frequently result in injuries, which can substantially alter the fitness costs associated with passive chemical defence, i.e. if toxins are secreted and become effective only once prey are attacked and grabbed by the predator. In a recent meta-analysis, Zvereva and Kozlov (2016) also found no detectable physiological costs of the production of chemical defence against predators in herbivorous insects, and concluded that ecological costs in those species may be more important in trade-offs associated with chemical defence than the costs of acquiring the necessary resources.
While we still have limited information about many aspects of the bufadienolide-based chemical defence in bufonids, other defensive toxins have been investigated in more detail both in amphibians and in other organisms. For example, many marine and terrestrial animals, like echinoderms, arthropods, gastropods, fish and amphibians are known to contain tetrodotoxin (TTX), a highly potent non-proteinaceous neurotoxin, as a chemical defence against predators (reviewed in Magarlamov et al. 2017). Although in many TTX-bearing organisms this compound is acquired either through dietary uptake or via symbiosis with TTX producing bacteria, some terrestrial newts like the rough-skin newt, Taricha granulosa, may be able to produce this toxin de novo (Gall et al. 2014). Previous findings in this species revealed that individual TTX concentrations show considerable fluctuation in newt populations in the wild (Bucciarelli et al. 2016), and population variation in TTX concentrations may, at least partly, be the result of a coevolutionary arms race between newts and a predatory snake (Brodie et al. 2002). In laboratory studies, adults were also found to be able to regenerate their toxin following experimentally induced secretion (Cardall et al. 2004) and to rapidly invest into toxin production as a result of simulated failed predator attack similarly to their larvae (Bucciarelli et al. 2017).
In conclusion, we showed that the significant decrease in total bufadienolide quantity induced by an experimentally stimulated toxin release vanished within 12 h in common toad tadpoles. We also uncovered a remarkable difference in the temporal dynamics of some bufadienolides within the toxin blend, emphasizing a different role of toxin compounds in tadpoles’ chemical defence depending on their structural complexity. Further studies are needed to clarify the functional differences between various bufadienolide compounds and the exact proximate mechanism of toxin expression in bufonids, one of the most important vertebrate model organisms of inducible chemical defence.
References
Ahuja I, Kissen R, Bones AM (2012) Phytoalexins in defense against pathogens. Trends Plant Sci 17:73–90
Ali JG, Agrawal AA (2012) Specialist versus generalist insect herbivores and plant defense. Trends Plant Sci 17:293–302
APHA (1985) Standard methods for the examination of wastewater, 16th edn. American Public Health Association, Washington DC
Bane V, Lehane M, Dikshit M, O’Riordan A, Furey A (2014) Tetrodotoxin: chemistry, toxicity, source, distribution and detection. Toxins 6:693–755
Benard MF, Fordyce JA (2003) Are induced defenses costly? Consequences of predator-induced defenses in western toads, Bufo boreas. Ecology 84:68–78
Bókony V, Móricz ÁM, Tóth Z, Gál Z, Kurali A, Mikó Z, Pásztor K, Szederkényi M, Tóth Z, Ujszegi J, Üveges B, Krüzselyi D, Capon RJ, Hoi H, Hettyey A (2016) Variation in chemical defense among natural populations of common toad (Bufo bufo) tadpoles: the role of environmental factors. J Chem Ecol 42:329–338
Bókony V, Mikó Z, Móricz ÁM, Krüzselyi D, Hettyey A (2017) Chronic exposure to a glyphosate-based herbicide makes toad larvae more toxic. Proc R Soc B Biol Sci 284:20170493
Bókony V, Üveges B, Móricz ÁM, Hettyey A (2018) Competition induces increased toxin production in toad larvae without allelopathic effects on heterospecific tadpoles. Funct Ecol 32:667–675
Brodie ED, Ridenhour BJ, Brodie ED (2002) The evolutionary response of predators to dangerous prey: hotspots and coldspots in the geographic mosaic of coevolution between garter snakes and newts. Evolution 56(10):2067-2082
Bucciarelli GM, Green DB, Shaffer HB, Kats LB (2016) Individual fluctuations in toxin levels affect breeding site fidelity in a chemically defended amphibian. Proc R Soc B 283(1831):20160468
Bucciarelli GM, Shaffer HB, Green DB, Kats LB (2017) An amphibian chemical defense phenotype is inducible across life history stages. Sci Rep 7:8185
Calhoun DM, Woodhams D, Howard C, LaFonte BE, Gregory JR, Johnson PT (2016) Role of antimicrobial peptides in amphibian defense against trematode infection. EcoHealth 13:383–391
Cardall BL, Brodie ED Jr, Brodie ED III, Hanifin CT (2004) Secretion and regeneration of tetrodotoxin in the rough-skin newt (Taricha granulosa). Toxicon 44:933–938
Chen MS (2008) Inducible direct plant defense against insect herbivores: a review. Insect Sci 15:101–114
Crossland MR, Haramura T, Salim AA, Capon RJ, Shine R (2012) Exploiting intraspecific competitive mechanisms to control invasive cane toads (Rhinella marina). Proc R Soc B Biol Sci 279(1742):3436–3442
Dekant W (2009) The role of biotransformation and bioactivation in toxicity. In: Luch A. (eds) Molecular, Clinical and Environmental Toxicology. Experientia Supplementum, vol 99. Birkhäuser, Basel. pp. 57–86
Delfino G, Brizzi R, Feri L (1995) Chemical skin defence in Bufo bufo: an ultrastructural study during ontogenesis. Zool Anz 234:101–111
Dittmann E, Fewer DP, Neilan BA (2013) Cyanobacterial toxins: biosynthetic routes and evolutionary roots. FEMS Microbiol Rev 37:23–43
Engqvist L (2005) The mistreatment of covariate interaction terms in linear model analyses of behavioural and evolutionary ecology studies. Anim Behav 70:967–971
Fedorova OV, Zernetkina VI, Shilova VY, Grigorova YN, Juhasz O, Wei W, Marshall CA, Lakatta EG, Bagrov AZ (2015) Synthesis of an endogenous steroidal Na pump inhibitor marinobufagenin, implicated in human cardiovascular diseases, is initiated by CYP27A1 via bile acid pathway. Circ Cardiovasc Genet 8:736–745
Fox J, Weisberg S (2011) An {R} companion to applied regression, Second edn. Sage, Thousand Oaks
Gall BG, Stokes AN, Pett JJ, Spivey KL, French SS, Brodie ED III, Brodie ED Jr (2014) Tetrodotoxin concentrations within a clutch and across embryonic development in eggs of the rough-skinned newts (Taricha granulosa). Toxicon 90:249–254
Gasc JP, Cabela A, Crnobrnja-Isailovic J, Dolmen D, Grossenbacher K, Haffner P, Lescure J, Martens H, Martínez Rica JP, Maurin H, Oliveira ME, Sofianidou TS, Veith M & Zuiderwijk A (eds) (1997) Atlas of amphibians and reptiles in Europe. Collection Patrimoines Naturels, 29, Societas Europaea Herpetologica, Muséum National d'Histoire Naturelle & Service du Patrimoine Naturel, Paris, 496 pp.
Gonzales-Vigil E, Bianchetti CM, Phillips GN, Howe GA (2011) Adaptive evolution of threonine deaminase in plant defense against insect herbivores. Proc Natl Acad Sci U S A 108:5897–5902
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Grafen A, Hails R (2002) Modern statistics for the life sciences. In: Oxford University press. Oxford, New York
Gunzburger MS, Travis J (2005) Critical literature review of the evidence for unpalatability of amphibian eggs and larvae. J Herpetol 39:547–557
Hagman M, Hayes RA, Capon RJ, Shine R (2009) Alarm cues experienced by cane toad tadpoles affect post-metamorphic morphology and chemical defenses. Funct Ecol 23:126–132
Harvell CD (1990) The ecology and evolution of inducible defenses. Q Rev Biol 65:323–340
Hayes RA, Piggott AM, Dalle K, Capon RJ (2009) Microbial biotransformation as a source of chemical diversity in cane toad steroid toxins. Bioorg Med Chem Lett 19:1790–1792
Hettyey A, Tóth Z, Van Buskirk J (2014) Inducible chemical defences in animals. Oikos 123:1025–1028
Jared C, Antoniazzi MM, Jordao AE, Silva JRM, Greven H, Rodrigues MT (2009) Parotoid macroglands in toad (Rhinella jimi): their structure and functioning in passive defence. Toxicon 54:197–207
Jared SGS, Jared C, Egami MI, Mailho-Fontana PL, Rodrigues MT, Antoniazzi MM (2014) Functional assessment of toad parotoid macroglands: a study based on poison replacement after mechanical compression. Toxicon 87:92–103
Kamalakkannan V, Salim AA, Capon RJ (2017) Microbiome-mediated biotransformation of cane toad bufagenins. J Nat Prod 80:2012–2017
Kamano Y, Kotake A, Hashima H, Inoue M, Morita H, Takeya K, Hideji I, Nandachi N, Segawa T, Yukita A, Saitou K, Katsuyama M, Pettit GR (1998) Structure-cytotoxic activity relationship for the toad poison bufadienolides. Bioorg Med Chem 6:1103–1115
Kempken F, Rohlfs M (2010) Fungal secondary metabolite biosynthesis–a chemical defence strategy against antagonistic animals? Fungal Ecol 3:107–114
Kicklighter C (2012) Chemical defences against predators. In: Brönmark C, Hansson L-A (eds) Chemical ecology in aquatic systems. Oxford University Press, Oxford, pp 236–249
Kulzer E (1954) Untersuchungen über die Schreckreaktion der Erdkrötenkaulquappen (Bufo bufo L.). Z Vgl Physiol 36:443–463
Kurali A, Pásztor K, Hettyey A, Tóth Z (2016) Toxin depletion has no effect on antipredator responses in common toad (Bufo bufo) tadpoles. Biol J Linn Soc 119:1000–1010
Lardner B (2000) Morphological and life history responses to predators in larvae of seven anurans. Oikos 88:169–180
Laurila A, Kujasalo J, Ranta E (1998) Predator-induced changes in life history in two anuran tadpoles: effects of predator diet. Oikos 83:307–317
Le Quang Trong Y (1973) Structure et dévelopment de la peau et des glandes cutanées de Bufo regularis Reuss. Bull Soc Zool Fr 98:449–485
Lee SS, Derguini F, Bruening RC, Nakanishi K, Wallick ET, Akizawa T, Rosenbaum CS, Butler VP (1994) Digitalis-like compounds of toad bile: sulfation and reduction of bufadienolides decrease potency of Na+, K+-ATPase inhibition. Heterocycles (2):669–686
Lenth RV (2016) Least-squares means: the R package lsmeans. J Stat Softw 69:1–33
Maag N, Gehrer L, Woodhams DC (2012) Sink or swim: a test of tadpole behavioral responses to predator cues and potential alarm pheromones from skin secretions. J Comp Physiol A 198:841–846
Magarlamov TY, Melnikova DI, Chernyshev AV (2017) Tetrodotoxin producing bacteria: detection, distribution and migration of the toxin in aquatic systems. Toxins 9(5):166
Mailho-Fontana PL, Antoniazzi MM, Toledo LF, Verdade VK, Sciani JM, Rodrigues MT, Jared C (2014) Passive and active defence in toads: the parotoid macroglands in Rhinella marina and Rhaebo guttatus. J Exp Zool 321:65e77
Mangoni ML, Miele R, Renda TG, Barra D, Simmaco M (2001) The synthesis of antimicrobial peptides in the skin of Rana esculenta is stimulated by microorganisms. FASEB J 15:1431–1432
Marquis O, Saglio P, Neveu A (2004) Effects of predators and conspecific chemical cues on the swimming activity of Rana temporaria and Bufo bufo tadpoles. Arch Hydrobiol 160:153–170
Matsukawa M, Akizawa T, Morris JF, Butler J, Yoshioka M (1996) Marinoic acid, a novel bufadienolide-related substance in the skin of the giant toad, Bufo marinus. Chem Pharm Bull 44:255–257
McGugan JR, Byrd GD, Roland AB, Caty SN, Kabir N, Tapia EE, Trauger SA, Coloma LA, O’Connell LA (2016) Ant and mite diversity drives toxin variation in the little devil poison frog. J Chem Ecol 42:537–551
Mebs D (2001) Toxicity in animals. Trends in evolution? Toxicon 39:87–96
Mebs D, Wagner MG, Pogoda W, Maneyro R, Kwet A, Kauert G (2007) Lack of bufadienolides in the skin secretion of red bellied toads, Melanophryniscus spp. (Anura, Bufonidae), from Uruguay. Comp Biochem Physiol 144:398–402
Meng Q, Yau LF, Lu JG, Wu ZZ, Zhang BX, Wang JR, Jiang ZH (2016) Chemical profiling and cytotoxicity assay of bufadienolides in toad venom and toad skin. J Ethnopharmacol 187:74–82
Meyer M (1962) Kegel-und andere Sonderzellen der larvalen Epidermis von Froschlurchen. Z Mikrosk Anat Forsch 68:79–131
Mithöfer A, Boland W (2012) Plant defense against herbivores: chemical aspects. Annu Rev Plant Biol 63:431–450
Nunes AL, Richter-Boix A, Laurila A, Rebelo R (2013) Do anuran larvae respond behaviourally to chemical cues from an invasive crayfish predator? A community-wide study. Oecologia 171:115–127
Nunes AL, Orizaola G, Laurila A, Rebelo R (2014) Rapid evolution of constitutive and inducible defenses against an invasive predator. Ecology 95:1520–1530
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2017) nlme: linear and nonlinear mixed effects models. R Package Version 3:1–121 http://CRAN.R-project.org/package=nlme. Accessed 01 Aug 2017
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna URL https://www.R-project.org. Accessed 01 July 2017
Richelle-Maurer E, De Kluijver MJ, Feio S, Gaudencio S, Gaspar H, Gomez R, Tavares R, Van de Vyver G, Van Soest RWM (2003) Localization and ecological significance of oroidin and sceptrin in the Caribbean sponge Agelas conifera. Biochem Syst Ecol 31:1073–1091
Richter-Boix A, Llorente GA, Montori A (2007) A comparative study of predator-induced phenotype in tadpoles across a pond permanency gradient. Hydrobiologia 583:43–56
Rodríguez C, Rollins-Smith L, Ibanez R, Durant-Archibold AA, Gutierrez M (2017) Toxins and pharmacologically active compounds from species of the family Bufonidae (Amphibia, Anura). J Ethnopharmacol 198:235–254
Sciani JM, Angeli CB, Antoniazzi MM, Jared C, Carvalho Pimenta D (2013) Differences and similarities among parotoid macrogland secretions in south American toads: a preliminary biochemical delineation. Sci World J 2013:1–9
Shimada K, Ohishi K, Fukunaga H, Ro JS, Nambara T (1985) Structure-activity relationship of bufotoxins and related compounds for the inhibition of Na+, K+−adenosine triphosphatase. Aust J Pharm 8:1054–1059
Shimada K, Ishii N, Nambara T (1986) Occurrence of bufadienolides in the skin of Bufo viridis Laur. Chem Pharm Bull 34:3454–3457
Shimada K, Ro J, Kanno C, Nambara T (1987a) Occurrence of bufogenin conjugates in the skin of Korean toad. Chem Pharm Bull 35:4996–4999
Shimada K, Sato Y, Nambara T (1987b) Occurrence of marinobufotoxin and telocinobufotoxin homologs in the skin of Bufo bankorensis, BORBOUR. Chem Pharm Bull 35:2300–2304
Steyn PS, van Heerden FR (1998) Bufadienolides of plant and animal origin. Nat Prod Rep 15:397–413
Toledo RC, Jared C (1995) Cutaneous granular glands and amphibian venoms. Comp Biochem Physiol A Mol Integr Physiol 111:1–29
Toledo RC, Jared C, Brunner A (1992) Morphology of the large granular alveoli of toad (Bufo ictericus) parotoid glands before and after compression. Toxicon 30:745–753
Ujszegi J, Móricz ÁM, Krüzselyi D, Hettyey A (2017) Skin toxin production of toads changes during early ontogeny but is not adjusted to the microbiota of the aquatic environment. Evol Ecol 31:925–936
Üveges B, Fera G, Móricz ÁM, Krüzselyi D, Bókony V, Hettyey A (2017) Age- and environment-dependent changes in chemical defences of larval and post-metamorphic toads. BMC Evol Biol 17:137
Van Buskirk J (2009) Natural variation in morphology of larval amphibians: phenotypic plasticity in nature? Ecol Monogr 79:681–705
Venables WN, Ripley BD (2002) Modern applied statistics with S, Fourth edn. Springer, New York
Wang DL, Qi FH, Tang W, Wang FS (2011) Chemical constituents and bioactivities of the skin of Bufo bufo gargarizans Cantor. Chem Biodivers 8:559–567
Wang DZ, Zhang SF, Zhang Y, Lin L (2016) Paralytic shellfish toxin biosynthesis in cyanobacteria and dinoflagellates: a molecular overview. J Proteome 135:132–140
War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S, Sharma HC (2012) Mechanisms of plant defense against insect herbivores. Plant Signal Behav 7:1306–1320
West-Eberhard MJ (1989) Phenotypic plasticity and the origins of diversity. Annu Rev Ecol Evol Syst 20:249–278
Zvereva EL, Kozlov MV (2016) The costs and effectiveness of chemical defenses in herbivorous insects: a meta-analysis. Ecol Monogr 86:107–124
Acknowledgements
We thank Katalin Pásztor for her assistance in the laboratory during the experiment and Dániel Krüzselyi for his help in the chemical analysis. We are also grateful to Prof. Rob J. Capon for making the marinobufotoxin standard available and for his thoughtful comments on a previous version of the manuscript. Financial support was provided by a research grant from the Hungarian Scientific Research Fund to ZT (OTKA, PD108938), a ‘Lendület’ grant of the Hungarian Academy of Sciences to AH (MTA, LP2012-24/2012), and an FP7 Marie Curie Career Integration Grant to AH (PCIG13-GA-2013-631722).
Funding
Open access funding provided by MTA Centre for Agricultural Research (MTA ATK).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 196 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Tóth, Z., Kurali, A., Móricz, Á.M. et al. Changes in Toxin Quantities Following Experimental Manipulation of Toxin Reserves in Bufo bufo Tadpoles. J Chem Ecol 45, 253–263 (2019). https://doi.org/10.1007/s10886-019-01045-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-019-01045-9