Abstract
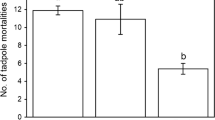
In a field survey the distribution of pond-breeding anuran species and their potential large predators was investigated along a freshwater habitat gradient, ranging from ephemeral pools to permanent ponds. In a laboratory experiment predator-induced plasticity was examined for all tadpole species to test whether the plastic response of ephemeral and temporary pond species differs from that of permanent pond species. Desiccation and predation pose conflicting demands; reduced activity lowers the risk of death by predation but increases the risk of death by desiccation. It was expected that species from time-constrained habitats would display a morphotype that would reduce vulnerability to invertebrate predators, thus allowing these species to maintain a high level of activity, whereas species from permanent ponds would avoid predation both morphologically and behaviourally. Species distribution and predator composition along the hydroperiod gradient differed. Variations between ephemeral and temporary ponds can be attributed to hydroperiod differences and the presence of large invertebrate predators in temporary ponds, whereas the contrasts between temporary and permanent ponds can only be attributed to the hydroperiod, since the presence and abundance of top predators are similar in both habitat types. With the exception of bufonids, all species showed predator-induced plasticity in agreement with previous studies. Tadpole species differed in the integration of the phenotypic traits measured, but differences observed between species could not be attributed only to habitat. Species from temporary habitats showed an expected response, with a low reduction of activity in comparison with the rest of the species. The lack of general patterns in the morphological changes suggests that species within the same habitat type did not converge on similar phenotypes, perhaps due to functional constraints on differences in microhabitat use in the water column.




Similar content being viewed by others
References
Altwegg, R., 2002. Predator-induced life-history plasticity under time constraints in pool frogs. Ecology 83: 2542–2551.
Anholt, B. R. & E. E. Werner, 1995. Interaction between food availability and predation mortality mediated by activity. Ecology 76: 2235–2239.
Anholt, B. R., D. K. Skelly & E. E. Werner, 1996. Factors modifying antipredator behaviour in larval toads. Herpetologica 52: 301–313.
Anholt, B. R., E. E. Werner & D. K. Skelly, 2000. Effect of food and predators on the activity of four larval ranid frogs. Ecology 81: 3509–3521.
Babbitt, K. J., J. B. Matthew & T. L. Tarr, 2003. Patterns of larval amphibian distribution along a wetland hydroperiod gradient. Canadian Journal of Zoology 81: 1539–1552.
Bookstein, F. L., 1991. Morphometrics Tool for Landmark Data: Geometry and Biology. Cambridge University Press, Cambridge.
Bridges, C. M., 2002. Tadpoles balance foraging and predator avoidance: effects of predation, pond drying, and hunger. Journal of Herpetology 36: 627–634.
Chovanec, A., 1992. The influence of tadpole swimming behaviour on predation by dragonfly nymphs. Amphibia-Reptilia 13: 341–349.
Cronin, J. T. & J. Travis, 1986. Size-limited predation on larval Rana aerolata (Anura: Ranidae) by two species of backswimmers (Heteroptera: Notonectidae). Herpetologica 42: 171–174 .
DeWitt, T. J. & S. M. Scheiner, 2004. Phenotypic Plasticity: Functional and Conceptual Approaches. Oxford University Press, New York, USA.
Díaz-Paniagua, C., 1987a. Estudio en cautividad de la actividad alimenticia de las larvas de siete especies de anuros. Revista Española de Herpetología 2: 189–197.
Díaz-Paniagua, C., 1987b. Tadpole distribution in relation to vegetal heterogeneity in temporary ponds. Herpetological Journal 1: 167–169.
Eklöv, P. & C. Halvarsson, 2000. The trade-off between foraging activity and predation risk for Rana temporaria in different food environments. Canadian Journal of Zoology 78: 734–739.
Felsenstein, J., 1985. Phylogenies and the comparative method. American Naturalist 125: 1–13.
Gunzburger, M. S. & J. Travis, 2004. Evaluating predation pressure on green treefrog larvae across a habitat gradient. Oecologia 140: 422–429.
Laurila, A. & J. Kujasalo, 1999. Habitat duration, predation risk and phenotypic plasticity in common frog (Rana temporaria) tadpoles. Journal of Animal Ecology 68: 1123–1132.
Lardner, B., 2000. Morphological and life history responses to predators in larvae of seven anurans. Oikos 88: 169–180.
Loy, A., M. Corti & L. F. Marcus, 1993. Landmarks data: size and shape analysis in systematics. A case study on old world talpidae (Mammalia, Insectivora). In Marcus, L. F., E. Bello & A. García-Valdecasas (eds), Contributions to Morphometrics. Monografías del Museo Nacional de Ciencias Naturales, Madrid, 215–239.
McCollum, S. A. & J. D. Leimberger, 1997. Predator-induced morphological changes in an amphibian: predation by dragonflies affects tadpole shape and color. Oecologia 109: 612–615.
Reeve, J. & E. Abouheif, 2003. Phylogenetic Independence. Version 2.0, Department of Biology, McGill University. Distributed freely by the authors on request.
Relyea, R.A., 2002. Cost of phenotypic plasticity. American Naturalist 159: 272–282.
Relyea, R. A. 2004. Integrating phenotypic plasticity when death is on the line: insights from predator–prey systems. In Pigliucci, M. & K. Preston (eds), Phenotypic Integration: Studying the Ecology and Evolution of Complex Phenotypes. Oxford University Press, New York, USA.
Relyea, R. A. & E. E. Werner, 1999. Quantifying the relation between predator-induced behavior and growth performance in larval anurans. Ecology 80: 2117–2124.
Relyea, R. A. & E. E. Werner, 2000. Morphological plasticity in four larval anurans distributed along an environmental gradient. Copeia 2000: 178–190.
Richardson, J. M. L., 2001a. The relative roles of adaptation and phylogeny in determination of larval traits in diversifying anuran lineages. American Naturalist 157: 282–299.
Richardson, J. M. L., 2001b. A comparative study of activity levels in larval anurans and response to the presence of different predators. Behavioural Ecology 12: 51–58.
Richardson, J. M. L., 2002a. A comparative study of phenotypic traits related to resource utilization in anuran communities. Evolutionary Ecology 16: 101–122.
Richardson, J. M. L., 2002b. Burst swim speed in tadpoles inhabiting ponds with different top predators. Evolutionary Ecology Research 4: 627–642.
Richter-Boix, A., G. A. Llorente & A. Montori, 2006a. A comparative analysis of the adaptive developmental plasticity hypothesis in six Mediterranean anuran species along a pond permanency gradient. Evolutionary Ecology Research 8: 1139–1154.
Richter-Boix, A., G. A. Llorente & A. Montori, 2006b. Breeding phenology of an amphibian community in a Mediterranean area. Amphibia-Reptilia 27: 544–559.
Richter-Boix, A., G. A. Llorente & A. Montori, 2007. Hierarchical competition in pond-breeding anuran larvae in a Mediterranean area. Amphibia-Reptilia 28: in press.
Rohlf, F. J., 2001. Thin-Plate Spline (TPS) Software. Free available on:http://life.bio.sunysb.edu/morph/ (last modified October 31, 2005).
Schaffer, H. B., R. A. Alford, B. D. Woodward, S. J. Richards, R. G. Altig & C. Gascon, 1994. Quantitative sampling of amphibian larvae. In Heyer, W. R., M. A. Donnelly, R. W. McDiarmid, L.C. Hayek & M. S. Foster (eds), Measuring and Monitoring Biological Diversity Standard Methods for Amphibians. Smithsonian Institution Press, Washington, 130–141.
Schlichting, C. & M. Pigliucci, 1998. Phenotypic Evolution: A Reaction Norm Perspective. Sinauer, Sunderland, 400 pp.
Schneider, D. W. & T. M. Frost, 1996. Habitat duration and community structure in temporary ponds. Journal of the North American Benthological Society 15: 64–86.
Skelly, D. K., 1994. Activity level and the susceptibility of anuran larvae to predation. Animal Behaviour 47: 465–468.
Skelly, D. K., 1995. A behavioural trade-off and its consequences for the distribution of Pseudacris treefrog larvae. Ecology 76: 150–164.
Skelly, D. K., 1996. Pond drying, predators, and the distribution of Pseudacris treefrog tadpoles. Copeia 1996: 599–605.
Skelly, D. K. & E. E. Werner, 1990. Behavioral and life-historical responses of larval American toads to an odonate predator. Ecology 71: 2313–2322.
Snodgrass, J. W., A. L. Bryan & J. Burger, 2000. Development of expectations of larval amphibian assemblage structure in southeastern depression wetlands. Ecological Applications 10: 1219–1229.
Stocks, R. & M. A. McPeek, 2003. Predators and life histories shape Lestes damselfly assemblages along a freshwater habitat gradient. Ecology 84: 1576–1587.
Teplitsky, C., S. Plénet & P. Joly, 2005. Cost and limits of dosage response to predation risk: to what extent can tadpoles invest in anti-predator morphology? Oecologia 145: 364–370.
Travis, J., W. H. Keen & J. Juilianna, 1985. The role of relative body size in a predator–prey relationship between dragonfly naiads and larval anurans. Oikos 45: 59–65.
Van Buskirk, J., 2000. The cost of an inducible defense in anuran larvae. Evolution 81: 2813–2821.
Van Buskirk, J., 2002. A comparative test of the adaptive plasticity hypothesis: relationships between habitat and phenotype in anuran larvae. American Naturalist 160: 87–102.
Van Buskirk, J., 2003. Habitat partitioning in European and North American pond-breeding frogs and toads. Diversity and Distributions 9: 399–410.
Van Buskirk, J. & M. Arioli, 2005. Habitat specialization and adaptive phenotypic divergence of anuran populations. Journal of Evolutionary Biology 18: 596–608.
Van Buskirk, J. & R. A. Relyea, 1998. Natural selection for phenotypic plasticity: predator-induced responses in tadpoles. Biological Journal of the Linnean Society 65: 301–328.
Van Buskirk, J., S. A. McCollum & E. E. Werner, 1997. Natural selection for environmentally-induced phenotypes in tadpoles. Evolution 52: 1983–1992.
Via, S., R. Gomulkiewicz, G. De Jong, S. Scheiner, C. D. Schlichting & P. H. Van Tienderen, 1995. Adaptive phenotypic plasticity: consensus and controversy. Trends in Ecology and Evolution 10: 212–217.
Watt, P. J., S. F. Nottingham & S. Young, 1997. Toad tadpole aggregation behaviour: evidence for a predator avoidance function. Animal Behaviour 54: 865–872.
Wellborn, G. A., D. K. Skelly & E. E. Werner, 1996. Mechanism creating community structure across a freshwater habitat gradient. Annual Review in Ecology and Systematics 27: 337–363.
Werner, E. E. & M. A. McPeek, 1994. Direct and indirect effects of predators on two anuran species along an environmental gradient. Ecology 75: 1368–1382.
Wilbur, H. M., 1997. Experimental ecology of food webs: complex systems in temporary ponds. Ecology 78: 2279–2302.
Woodward, B. D., 1983. Predator–prey interactions and breeding-pond use of temporary-pond species in a desert anuran community. Ecology 64: 1549–1555.
Acknowledgements
We sincerely thank Nuria Garriga for help during laboratory experiments and for useful comments. We thank Laura Pérez and Santi Escartín for assisting with field surveys, and S. Llacuna and F. Llimona for providing the corresponding permits and fieldwork facilities in the Natural Parks. We also thank S. Carranza for their assistance in the phylogenetic topology reconstruction of the species object of study and providing personal data for this purpose. We extend our gratitude to two anonymous referees for providing useful comments and suggestions. Permission to capture was granted by the Departament de Medi Ambient de la Generalitat de Catalunya.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: K. Martens
Rights and permissions
About this article
Cite this article
Richter-Boix, A., Llorente, G.A. & Montori, A. A comparative study of predator-induced phenotype in tadpoles across a pond permanency gradient. Hydrobiologia 583, 43–56 (2007). https://doi.org/10.1007/s10750-006-0475-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-006-0475-7