Abstract
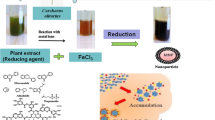
In this work, iron oxide nanoparticles (Fe3O4 NPs) were modified by chitosan (CS). Fe3O4 NPs were synthesized by co-precipitation method and their antimicrobial potential and photo-catalytic degradation of Chloramine T (CT) were investigated. The free Fe3O4 NPs and chitosan-coated Fe3O4 NPs (CS-Fe3O4 NPs) were characterized by XRD, FTIR, SEM, and HRTEM. Fe3O4 NPs have spherical shape and their diameter varied from 18.0 nm to 25.0 nm with average particle size at 21.0 nm. Antimicrobial activity was tested towards some pathogenic bacteria and Candida cells as zone of inhibition (ZOI) and minimum inhibitory concentration (MIC). UV-assisted photocatalytic degradation of CT was investigated. Various parameters affecting the photocatalytic efficiency such as (pH on CT removal, CT initial concentration, and adsorbent dose) were studied. Antimicrobial results showed that CS-Fe3O4 NPs possesses a maximum potential against Escherichia coli, Bacillus subtilis and Candida albicans, by 18.0, 17.0, 14.2 mm ZOI, respectively. Results obtained from the photocatalytic activity indicated that CS-Fe3O4 NPs (2.0 gm/l) possessed a promising removal potential, achieving 86.0% removal of CT in the neutral solution (pH = 7.0). The synthesized CS-Fe3O4 NPs are effective for the removal of CT and potent disinfectant agent for pathogenic microbes with possible application in the wastewater treatment.












Similar content being viewed by others
References
Patra, J.K., et al., Nano based drug delivery systems: recent developments and future prospects. Journal of nanobiotechnology, 2018. 16(1): p. 71.
El-Batal, A., et al., Synthesis of silver nanoparticles and incorporation with certain antibiotic using gamma irradiation. Journal of Pharmaceutical Research International, 2014: p. 1341-1363.
Singh, A.P., et al., Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal transduction and targeted therapy, 2019. 4(1): p. 1-21.
Díaz-García, D., et al., Preparation and study of the antibacterial applications and oxidative stress induction of copper maleamate-functionalized mesoporous silica nanoparticles. Pharmaceutics, 2019. 11(1): p. 30.
Wong, C.W., et al., Response surface methodology optimization of mono-dispersed MgO nanoparticles fabricated by ultrasonic-assisted sol–gel method for outstanding antimicrobial and antibiofilm activities. Journal of Cluster Science, 2020. 31(2): p. 367-389.
Elkhenany, H., et al., Comparison of different uncoated and starch-coated superparamagnetic iron oxide nanoparticles: implications for stem cell tracking. International journal of biological macromolecules, 2020. 143: p. 763-774.
Abd Elkodous, M., et al., Fabrication of Ultra-Pure Anisotropic Zinc Oxide Nanoparticles via Simple and Cost-Effective Route: Implications for UTI and EAC Medications. Biological trace element research, 2020. 196: p. 297-317.
Abd Elkodous, M., et al., Engineered nanomaterials as potential candidates for HIV treatment: Between opportunities and challenges. Journal of Cluster Science, 2019. 30(3): p. 531-540.
Kluchova, K., et al., Superparamagnetic maghemite nanoparticles from solid-state synthesis–Their functionalization towards peroral MRI contrast agent and magnetic carrier for trypsin immobilization. Biomaterials, 2009. 30(15): p. 2855-2863.
Nehra, P., et al., Antibacterial and antifungal activity of chitosan coated iron oxide nanoparticles. British journal of biomedical science, 2018. 75(1): p. 13-18.
Su, C., Environmental implications and applications of engineered nanoscale magnetite and its hybrid nanocomposites: A review of recent literature. Journal of hazardous materials, 2017. 322: p. 48-84.
Park, Y.-H., et al., Effect of the size and surface charge of silica nanoparticles on cutaneous toxicity. Molecular & Cellular Toxicology, 2013. 9(1): p. 67-74.
Li, Q., et al., Correlation between particle size/domain structure and magnetic properties of highly crystalline Fe 3 O 4 nanoparticles. Scientific reports, 2017. 7(1): p. 1-7.
Nejadshafiee, V., et al., Magnetic bio-metal–organic framework nanocomposites decorated with folic acid conjugated chitosan as a promising biocompatible targeted theranostic system for cancer treatment. Materials Science and Engineering: C, 2019. 99: p. 805-815.
Frimpong, R.A. and J.Z. Hilt, Magnetic nanoparticles in biomedicine: synthesis, functionalization and applications. Nanomedicine, 2010. 5(9): p. 1401-1414.
Gupta, A.K., et al., Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. 2007.
Gupta, A.K. and M. Gupta, Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. biomaterials, 2005. 26(18): p. 3995-4021.
Unsoy, G., et al., Synthesis optimization and characterization of chitosan-coated iron oxide nanoparticles produced for biomedical applications. Journal of Nanoparticle Research, 2012. 14(11): p. 964.
Zhang, Y., H.F. Chan, and K.W. Leong, Advanced materials and processing for drug delivery: the past and the future. Advanced drug delivery reviews, 2013. 65(1): p. 104-120.
Verma, M., K. Singh, and M.S. Bakshi, Surface active magnetic iron oxide nanoparticles for extracting metal nanoparticles across an aqueous–organic interface. Journal of Materials Chemistry C, 2019. 7(34): p. 10623-10634.
Nam, J., et al., Surface engineering of inorganic nanoparticles for imaging and therapy. Advanced drug delivery reviews, 2013. 65(5): p. 622-648.
Hotze, E.M., T. Phenrat, and G.V. Lowry, Nanoparticle aggregation: challenges to understanding transport and reactivity in the environment. Journal of environmental quality, 2010. 39(6): p. 1909-1924.
Schladt, T.D., et al., Synthesis and bio-functionalization of magnetic nanoparticles for medical diagnosis and treatment. Dalton Transactions, 2011. 40(24): p. 6315-6343.
Bakshi, P.S., et al., Chitosan as an environment friendly biomaterial–a review on recent modifications and applications. International journal of biological macromolecules, 2020. 150: p. 1072-1083.
Kumar, S., et al., Chitosan nanocomposite coatings for food, paints, and water treatment applications. Applied Sciences, 2019. 9(12): p. 2409.
Ngah, W.W., L. Teong, and M. Hanafiah, Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydrate polymers, 2011. 83(4): p. 1446-1456.
Shrifian-Esfahni, A., et al., Chitosan-modified superparamgnetic iron oxide nanoparticles: design, fabrication, characterization and antibacterial activity. Chemik, 2015. 69(1): p. 19-32.
Bradbury, R.S., et al., Antimicrobial susceptibility testing of cystic fibrosis and non-cystic fibrosis clinical isolates of Pseudomonas aeruginosa: a comparison of three methods. British journal of biomedical science, 2011. 68(1): p. 1-4.
Saxena, S. and C. Gomber, Comparative in vitro antimicrobial procedural efficacy for susceptibility of Staphylococcus aureus, Escherichia coli and Pseudomonas species to chloramphenicol, ciprofloxacin and cefaclor. British journal of biomedical science, 2008. 65(4): p. 178-183.
Nobile, C.J. and A.D. Johnson, Candida albicans biofilms and human disease. Annual review of microbiology, 2015. 69: p. 71-92.
Manikandan, C. and A. Amsath, Antibiotic susceptibility pattern of Escherichia coli isolated from urine samples in Pattukkottai, Tamilnadu. Int. J. Curr. Microbiol. App. Sci, 2014. 3(10): p. 449-457.
Yoon, J.E., et al., Antibiotic susceptibility and imaging findings of the causative microorganisms responsible for acute urinary tract infection in children: a five-year single center study. Korean journal of pediatrics, 2011. 54(2): p. 79.
Habib, S., Highlights for management of a child with a urinary tract infection. International journal of pediatrics, 2012. 2012.
Campbell, M.M. and G. Johnson, Chloramine T and related N-halogeno-N-metallo reagents. Chemical Reviews, 1978. 78(1): p. 65-79.
Goehring, R.R., Chloramine. Encyclopedia of Reagents for Organic Synthesis, 2001.
Roorda, B.M., H.L. Nienhuis, and M.L. Schuttelaar, Anaphylactic reaction caused by skin contact with the disinfectant chloramine‐T. Contact dermatitis, 2019. 80(5): p. 321-322.
Kanerva, L., et al., Occupational allergic contact urticaria from chloramine-T solution. Contact Dermatitis, 1997. 37(4): p. 180-181.
Blomqvist, A., et al., Atopic allergy to chloramine-T and the demonstration of specific IgE antibodies by the radioallergosorbent test. International archives of occupational and environmental health, 1991. 63(5): p. 363-365.
Pham, X.N., et al., Synthesis and characterization of chitosan-coated magnetite nanoparticles and their application in curcumin drug delivery. Advances in Natural Sciences: Nanoscience and Nanotechnology, 2016. 7(4): p. 045010.
Zavvar Mousavi, H. and S. Seyedi, Kinetic and equilibrium studies on the removal of Pb (II) from aqueous solution using nettle ash. Journal of the Chilean Chemical Society, 2010. 55(3): p. 307-311.
El-Batal, A.I., et al., Response surface methodology optimization of melanin production by Streptomyces cyaneus and synthesis of copper oxide nanoparticles using gamma radiation. Journal of Cluster Science, 2017. 28(3): p. 1083-1112.
Baraka, A., et al., Synthesis of silver nanoparticles using natural pigments extracted from Alfalfa leaves and its use for antimicrobial activity. Chemical Papers, 2017. 71(11): p. 2271-2281.
El-Batal, A.I., et al., Biogenic synthesis of copper nanoparticles by natural polysaccharides and Pleurotus ostreatus fermented fenugreek using gamma rays with antioxidant and antimicrobial potential towards some wound pathogens. Microbial pathogenesis, 2018. 118: p. 159-169.
El-Batal, A.I., et al., Antimicrobial, antioxidant and anticancer activities of zinc nanoparticles prepared by natural polysaccharides and gamma radiation. International journal of biological macromolecules, 2018. 107: p. 2298-2311.
Balouiri, M., M. Sadiki, and S.K. Ibnsouda, Methods for in vitro evaluating antimicrobial activity: A review. Journal of pharmaceutical analysis, 2016. 6(2): p. 71-79.
El-Batal, A.I., et al., Antibiofilm and antimicrobial activities of silver boron nanoparticles synthesized by PVP polymer and gamma rays against urinary tract pathogens. Journal of Cluster Science, 2019. 30(4): p. 947-964.
Mosallam, F.M., et al., Biomolecules-mediated synthesis of selenium nanoparticles using Aspergillus oryzae fermented Lupin extract and gamma radiation for hindering the growth of some multidrug-resistant bacteria and pathogenic fungi. Microbial pathogenesis, 2018. 122: p. 108-116.
Attia, M.S., et al., Spirulina platensis-Polysaccharides Promoted Green Silver Nanoparticles Production Using Gamma Radiation to Suppress the Expansion of Pear Fire Blight-Producing Erwinia amylovora. Journal of Cluster Science, 2019. 30(4): p. 919-935.
El-Sayyad, G.S., et al., Facile biosynthesis of tellurium dioxide nanoparticles by Streptomyces cyaneus melanin pigment and gamma radiation for repressing some Aspergillus pathogens and bacterial wound cultures. Journal of Cluster Science, 2020. 31(1): p. 147-159.
Abd Elkodous, M., et al., Therapeutic and diagnostic potential of nanomaterials for enhanced biomedical applications. Colloids and Surfaces B: Biointerfaces, 2019. 180: p. 411-428.
Brownlee, K., Probit Analysis: A Statistical Treatment of the Sigmoid Response Curve. 1952, JSTOR.
Amoli Diva, M. and K. Pourghazi, Magnetic nanoparticles grafted pH-responsive poly (methacrylic acid-co-acrylic acid)-grafted polyvinylpyrrolidone as a nano-carrier for oral controlled delivery of atorvastatin. Nanomedicine Research Journal, 2017. 2(1): p. 18-27.
Stoia, M., R. Istratie, and C. Păcurariu, Investigation of magnetite nanoparticles stability in air by thermal analysis and FTIR spectroscopy. Journal of Thermal Analysis and Calorimetry, 2016. 125(3): p. 1185-1198.
Kataria, N. and V. Garg, Green synthesis of Fe3O4 nanoparticles loaded sawdust carbon for cadmium (II) removal from water: regeneration and mechanism. Chemosphere, 2018. 208: p. 818-828.
Tang, Z.-X. and B.-F. Lv, MgO nanoparticles as antibacterial agent: preparation and activity. Brazilian Journal of Chemical Engineering, 2014. 31(3): p. 591-601.
Ashour, A., et al., Antimicrobial activity of metal-substituted cobalt ferrite nanoparticles synthesized by sol–gel technique. Particuology, 2018. 40: p. 141-151.
Maksoud, M.A., et al., Antibacterial, antibiofilm, and photocatalytic activities of metals-substituted spinel cobalt ferrite nanoparticles. Microbial pathogenesis, 2019. 127: p. 144-158.
El-Sayyad, G.S., et al., Merits of photocatalytic and antimicrobial applications of gamma-irradiated CoxNi1-xFe2O4/SiO2/TiO2; x= 0.9 nanocomposite for pyridine removal and pathogenic bacteria/fungi disinfection: implication for wastewater treatment. RSC Advances, 2020. 10(9): p. 5241-5259.
Pal, S., Y.K. Tak, and J.M. Song, Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Applied and environmental microbiology, 2007. 73(6): p. 1712-1720.
Ma, S., et al., Responses of the Microbial Community Structure in Fe (II)-Bearing Sediments to Oxygenation: The Role of Reactive Oxygen Species. ACS Earth and Space Chemistry, 2019. 3(5): p. 738-747.
El-Batal, A.I., et al., Nystatin-mediated bismuth oxide nano-drug synthesis using gamma rays for increasing the antimicrobial and antibiofilm activities against some pathogenic bacteria and Candida species. RSC Advances, 2020. 10(16): p. 9274-9289.
Hezma, A., A. Rajeh, and M.A. Mannaa, An insight into the effect of zinc oxide nanoparticles on the structural, thermal, mechanical properties and antimicrobial activity of Cs/PVA composite. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2019. 581: p. 123821.
El-Sayyad, G.S., et al., Gentamicin-Assisted Mycogenic Selenium Nanoparticles Synthesized Under Gamma Irradiation for Robust Reluctance of Resistant Urinary Tract Infection-Causing Pathogens. Biological Trace Element Research, 2020. 195: p. 323-342.
El-Batal, A.I., F.M. Mosallam, and G.S. El-Sayyad, Synthesis of Metallic Silver Nanoparticles by Fluconazole Drug and Gamma Rays to Inhibit the Growth of Multidrug-Resistant Microbes. Journal of Cluster Science, 2018. 29(6): p. 1003-1015.
Matei, E., et al., Leaching tests for synthesized magnetite nanoparticles used as adsorbent for metal ions from liquid solutions. Digest Journal of Nanomaterials and Biostructures, 2011. 6(4): p. 1701-1708.
Chiou, M.-S., P.-Y. Ho, and H.-Y. Li, Adsorption of anionic dyes in acid solutions using chemically cross-linked chitosan beads. Dyes and pigments, 2004. 60(1): p. 69-84.
Wahab, H.S. and A.A. Hussain, Photocatalytic oxidation of phenol red onto nanocrystalline TiO 2 particles. Journal of Nanostructure in Chemistry, 2016. 6(3): p. 261-274.
Ollis, D.F., Kinetics of photocatalyzed reactions: Five lessons learned. Frontiers in chemistry, 2018. 6: p. 378.
Abd Elkodous, M., et al., Carbon-dot-loaded Cox Ni1–x Fe2 O4; x= 0.9/SiO2/TiO2 nanocomposite with enhanced photocatalytic and antimicrobial potential: An engineered nanocomposite for wastewater treatment. Scientific Reports, 2020. 10(1): p. 11534.
Abdel Maksoud, M.I.A., et al., Nanostructured Mg substituted Mn-Zn ferrites: A magnetic recyclable catalyst for outstanding photocatalytic and antimicrobial potentials. Journal of Hazardous Materials, 2020. 399: p. 123000.
Acknowledgments
The authors would like to thank the Nanotechnology Research Unit (P.I. Prof. Dr. Ahmed I. El-Batal), Drug Microbiology Lab., Drug Radiation Research Department, NCRRT, Egypt, for financing and supporting this study under the project “Nutraceuticals and Functional Foods Production by using Nano/Biotechnological and Irradiation Processes”. Figures 6 and 12 in this paper were created with BioRender.com.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research Involving Human Participation and/or Animals
Not applicable.
Informed Consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El-Khawaga, A.M., Farrag, A.A., Elsayed, M.A. et al. Antimicrobial and Photocatalytic Degradation Activities of Chitosan-coated Magnetite Nanocomposite. J Clust Sci 32, 1107–1119 (2021). https://doi.org/10.1007/s10876-020-01869-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-020-01869-6