Abstract
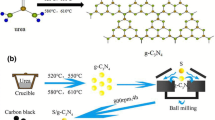
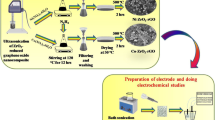
Herein, synthesis of graphitic carbon nitride (g-C3N4) and its nanocomposites with monometallic nickel (Ni-g-C3N4), and bimetallic zirconium-nickel (ZrNi-g-C3N4) is reported. New materials for high storage performance are investigated eagerly in electrochemical energy applications. With this aim, the electrochemical method is used for the evaluation of hydrogen storage capacity (HSC) of the samples in the present research work. Synthesis of samples were carried out using a combination of thermal and hydrothermal methods. The evaluation of electrochemical hydrogen sorption and storage capability of g-C3N4 and its nanocomposites was done after sample characterization. Characterization techniques followed were powder X-ray diffraction (XRD), Nitrogen adsorption and desorption isotherms measurements at 77 K, thermo-gravimetric analysis (TGA), Fourier transform infrared spectroscopy (FTIR), Field-emission scanning electron microscopy (FESEM) and energy dispersive X-ray spectrometry (EDS). Cyclic voltammetric and chronopotentiometry measurements carried out in an alkaline medium helped to calculate the HSC. The discharge capacity was measured to be 82 mAhg−1 for g-C3N4, 182 mAhg−1 for Ni-g-C3N4 and 380 mAhg−1 for ZrNi-g-C3N4 at 1 mAg−1 of current density. Catalytic properties of the metal nanoparticles augment the storage capability of the g-C3N4 nanocomposites. These nanocomposites help to store hydrogen which serves as safe carrier in fuel cell applications.













Similar content being viewed by others
References
D. Huang, X. Yan, M. Yan, G. Zeng, C. Zhou, J. Wan, M. Cheng, W. Xue, ACS Appl. Mater. Interfaces 10, 21035 (2018)
J. Zhu, P. Xiao, H. Li, S.A.C. Carabineiro, ACS Appl. Mater. Interfaces 6, 16449 (2014)
X. Tan, L. Kou, H.A. Tahini, S.C. Smith, Chemsuschem 8, 3626 (2015)
A. Wang, C. Wang, L. Fu, W. Wong-Ng, Y. Lan, Nano-Micro Lett. 9, 47 (2017)
A. Nasri, B. Jaleh, S. Khazalpour, M. Nasrollahzadeh, M. Shokouhimehr, Int. J. Biol. Macromol. 164, 3012 (2020)
X. Guo, Y. Wang, F. Wu, Y. Ni, S. Kokot, Microchim. Acta 183, 773 (2016)
W. Gong, J. Zou, S. Zhang, X. Zhou, J. Jiang, Electroanalysis 28, 227 (2016)
Y. Wang, Y. Wang, Y. Li, H. Shi, Y. Xu, H. Qin, X. Li, Y. Zuo, S. Kang, L. Cui, Catal. Commun. 72, 24 (2015)
M. Chegeni, Z. Mousavi, M. Soleymani, S. Dehdashtian, Diam. Relat. Mater. 101, 107621 (2020)
S. Wojtyła, K. Śpiewak, T. Baran, J. Photochem. Photobiol. A Chem. 391, 112355 (2020)
H. Li, Y. Xu, H. Sitinamaluwa, K. Wasalathilake, D. Galpaya, Chin. J. Catal. 38, 1006 (2017)
N.L. Reddy, M.V. Shankar. (2020)
A.A.S. Nair, R. Sundara, N. Anitha, Int. J. Hydrogen Energy 40, 3259 (2015)
L. Sun, Z. Shi, L. Liang, S. Wei, H. Wang, D. Dastan, K. Sun, R. Fan, J. Mater. Chem. C 8, 10257 (2020)
H.J. Lin, H.W. Li, H. Shao, Y. Lu, K. Asano, Mater. Today Energy 17, 100463 (2020)
M. Zarezadeh Mehrizi, J. Abdi, M. Rezakazemi, E. Salehi, Int. J. Hydrogen Energy 45, 17583 (2020)
G. Koh, Y.-W. Zhang, H. Pan, Int. J. Hydrogen Energy 37, 4170 (2012)
C. Wang, H. Fan, X. Ren, J. Fang, J. Ma, N. Zhao, Mater. Charact. 139, 89 (2018)
M. Ghiyasiyan-Arani, M. Salavati-Niasari, Ind. Eng. Chem. Res. 58, 23057 (2019)
S. Zinatloo-Ajabshir, M.S. Morassaei, O. Amiri, M. Salavati-Niasari, L.K. Foong, Ceram. Int. 46, 17186–17196 (2020)
Y.-J. Han, S.-J. Park, Appl. Surf. Sci. 415, 85 (2017)
H.H. Shen, X.T. Zu, B. Chen, C.Q. Huang, K. Sun, J. Alloys Compd. 659, 23 (2016)
S.K. Singh, A.K. Singh, K. Aranishi, Q. Xu, J. Am. Chem. Soc. 133, 19638 (2011)
C. Shen, W. Zhou, H. Yu, L. Du, Chin. J. Chem. Eng. 26, 322 (2018)
F. Altaf, R. Batool, R. Gill, Z.U. Rehman, H. Majeed, A. Ahmad, M. Shafiq, D. Dastan, G. Abbas, K. Jacob, Renew. Energy 164, 709 (2021)
M.S. Morassaei, A. Salehabadi, O. Amiri, M. Salavati-Niasari, A. Akbari, J. Alloys Compd. 826, 154023 (2020)
S. Tonda, S. Kumar, S. Kandula, V. Shanker, J. Mater. Chem. A 2, 6772 (2014)
T. Gholami, M. Salavati-Niasari, S. Varshoy, Int. J. Hydrogen Energy 41, 9418 (2016)
G.L. Tan, D. Tang, D. Dastan, A. Jafari, J.P.B. Silva, X.T. Yin, Mater. Sci. Semicond. Process. 122, 105506 (2021)
D. Dastan, N. Chaure, M. Kartha, J. Mater. Sci. Mater. Electron. 28, 7784 (2017)
C. Zhou, J.A. Szpunar, X. Cui, ACS Appl. Mater. Interfaces 8, 15232 (2016)
T. Muhmood, A. Uddin, Chem. Phys. Lett. 753, 137604 (2020)
T. Plachy, M. Masar, M. Mrlik, M. Machovsky, Z. Machovska, E. Kutalkova, I. Kuritka, Adv. Powder Technol. 30, 714 (2019)
G. Sharma, A. Kumar, S. Sharma, M. Naushad, R. Prakash-Dwivedi, Z.A. ALOthman, G.T. Mola, J. King Saud Univ. Sci. 31, 257 (2019)
M. Asadzadeh, F. Tajabadi, D. Dastan, P. Sangpour, Z. Shi, N. Taghavinia, Ceram. Int. 47, 5487 (2020)
B. Zhang, X. Ye, W. Hou, Y. Zhao, Y. Xie, J. Phys. Chem. B 110, 8978 (2006)
M. Fathinezhad, M. AbbasiTarighat, D. Dastan, Environ. Nanotechnol. Monit. Manag. 14, 100307 (2020)
L. Sun, L. Liang, Z. Shi, H. Wang, P. Xie, D. Dastan, K. Sun, R. Fan, Eng. Sci. 12, 95 (2020)
W. Zhang, X. Zhu, L. Liang, P. Yin, P. Xie, D. Dastan, K. Sun, R. Fan, Z. Shi, J. Mater. Sci. 56, 4254 (2021)
F. Sadat, M. Salavati-niasari. (2018).
M.H. Choi, Y.J. Min, G.H. Gwak, S.M. Paek, J.M. Oh, J. Alloys Compd. 610, 231 (2014)
N. Liu, L. Yin, L. Kang, X. Zhao, C. Wang, L. Zhang, D. Xiang, R. Gao, Y. Qi, N. Lun, Int. J. Hydrogen Energy 35, 12410 (2010)
X.P. Gao, Y. Lan, G.L. Pan, F. Wu, J.Q. Qu, D.Y. Song, P.W. Shen, Electrochem. Solid-State Lett. 4, 173 (2001)
S.S. Gunasekaran, T.K. Kumaresan, S.A. Masilamani, S.Z. Karazhanov, K. Raman, R. Subashchandrabose, Mater. Lett. 273, 127919 (2020)
D. Qu, X. Xu, L. Zhou, W. Li, J. Wu, D. Liu, Z. Zhong Xie, J. Li, H. Tang, Int. J. Hydrogen Energy 44, 7326 (2019)
M. Kaur, K. Pal, J. Mater. Sci. Mater. Electron. 31, 10903 (2020)
L. Sun, Z. Shi, H. Wang, K. Zhang, D. Dastan, K. Sun, R. Fan, J. Mater. Chem. A 8, 5750 (2020)
Acknowledgements
The authors thank the Commonwealth Scholarship Commission, UK, University of Glasgow, UK, and Indian Institute of Technology Roorkee, India for all the financial and laboratory support for the research work. Authors would like to thank Professor Duncan H Gregory and his group at University of Glasgow, for all the support during the research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no competing interest influence the research in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaur, M., Pal, K. Synthesis, characterization and electrochemical evaluation of hydrogen storage capacity of graphitic carbon nitride and its nanocomposites in an alkaline environment. J Mater Sci: Mater Electron 32, 12475–12489 (2021). https://doi.org/10.1007/s10854-021-05882-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-05882-x