Abstract
Microalgae are emerging as an important renewable and sustainable source of high-value biomolecules having applications in food, cosmetics, pharmaceutical, agrochemicals and fuel industries. Deriving high-value biomolecules from micro-algae however faces numerous process and technological challenges. It is essential to develop innovative ways of intensifying processes used for valorising microalgae. Hydrodynamic cavitation (HC) offers an attractive platform for process intensification relevant to microalgae because of its scalability, ability to handle dense slurries, intense physicochemical effects, and low cost. Here we briefly review the overall processes involved in deriving high-value biomolecules from micro-algae. Opportunities for intensifying these processes and enhancing productivity of processing microalgae via HC are then identified and critically reviewed. The current state of the art and yet unresolved challenges are highlighted. An attempt is made to identify specific suggestions to help direct future research efforts. The review will be useful for researchers and practitioners aiming to harness HC for deriving high-value products from microalgae.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Microalgae are microscopic photosynthetic organisms that account for 40% of global photosynthesis forming the base of food chains. Microalgae are emerging as a renewable and sustainable bio-resource. Bioactive molecules derived from microalgae are finding numerous applications in a variety of industries like food, cosmetics, pharmaceutical, diet supplement, agrochemicals, bio-stimulants, animal feed and fuel (Singh and Kalia 2017; MU et al. 2019; Junior et al. 2020). Residual algal mass after extracting high-value biomolecules can also be further valorised by deriving other relatively low-value biomolecules like carbohydrates and cellulosic material and bioenergy (in the form of biogas) (Chen and Quinn 2021; Goswami et al. 2021; Torres et al. 2021). Microalgae are important photosynthetic organisms due to their high photon-to-biomass conversion efficiency (3%) against 1% in higher plants (Perin et al. 2019). Microalgae grow in various types of habitats and can produce various commercially valuable biomolecules such as proteins, carbohydrates, lipids pigments etc. Microalgae can be cultivated in wastewater on barren waste land, without any significant input in terms of fertilizer or pesticides. Microalgae are also used for wastewater treatment and CO2 sequestration, adding to the advantages of microalgae cultivation and its bioprocessing for a variety of commercially important bioactive biomolecules (Levasseur et al. 2020).
While significant potential exists for valorising microalgae by deriving high-value bioactives and bioenergy, it is essential to develop cost-effective and scalable solutions for all the relevant steps involved in valorisation of microalgae ranging from cultivation to purification. Due to the microscopic size of microalgae (e.g., the diameter of Arthrospira platensis is about 8 μm), the handling of such a small cell size and collective biomass is a challenge during cultivation and harvesting. For the same reason, conventional pre-treatment methods may not be effective in the breakage of cell wall (cell disruption) for opening up the cell and the release of intramolecular content, thus, is a challenge. The small quantity of biomolecules per cell of microalgae warrants a huge quantity for industrial applications. Using inefficient methods results in high production and operation cost. Methods such as ultrasonication, homogenization, pressurized liquid, enzymatic methods, pulse electric method, supercritical fluid extraction, microwave etc., are presently in use for pre-treatment for extraction (Skorupskaite et al. 2019; Lee et al. 2021; Mehariya et al. 2021; Pagels et al. 2021; Vasistha et al. 2021; Timira et al. 2022).
However, the constraints to realise economically feasible commercialization of this nascent bio-industry to realise valorisation of microalgal biomass are summarised in Table 1.
Hydrodynamic cavitation (HC) has the potential to address these limitations and is gaining interest as a pre-treatment method. It is reported to be low cost, energy efficient, minimization of toxic solvent usage, scalable and ability to obtain superior processed products when compared to conventional methods (Ranade and Utikar 2022). Despite its various advantages and potential in microalgal-based industries, it is underutilised and needs the insight to develop an understanding of the HC-based operations to open its scope. Thus, a review is required to show the path for developing the understanding, and new strategies for the efficient processing of microalgae, towards improving the economic feasibility and sustainability. To the best of our knowledge, no review on evaluating the potential of HC in microalgae-based industry is available.
Here, we critically review the potential of harnessing hydrodynamic cavitation for intensifying processes and provide a new perspective for assessing the potential of HC in microalgae valorisation. In the following subsections, we briefly review bioactives derivable from microalgae and various steps involved in the valorisation process. Hydrodynamic cavitation is then discussed. Various HC devices used in practice are briefly reviewed. Later we critically review application of HC for cultivation and harvesting of microalgae, pre-treatment for extraction of bioactives and purification of extracted bioactives.
An attempt is made to critically evaluate the merits of using HC with respect to alternative technologies. Specific comments and suggestions on further research for improving performance are included. Some comments on developing a bio-refinery based on microalgae as feedstock and outlook are included. We believe that it will stimulate further research on harnessing HC for process intensification.
Bioactives from microalgae
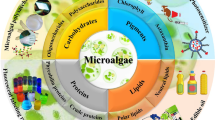
The diversity in family and species of microalgae offers a variety of high-value biomolecules, which can be further diversified by modifying the growing conditions. The diversity in terms of strains of microalgae can be classified under different phyla such as Chlorophyta, Rhodophyta, Haptophyta etc. (Kim and Chojnacka 2015; Ruggiero et al. 2015). Microalgae e.g., Aphanizomenon, Arthrospira, Botryococcus, Chlamydomonas, Chlorella, Dunaliella, and Hemiselmis are natural and rich sources of bioactives (Fernandes and Cordeiro 2020; Levasseur et al. 2020). Some of the variety of bioactives (polyunsaturated fatty acids (PUFA), monounsaturated fatty acids (MUFA), docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), pigments (chlorophylls (a & b), phycoerythrin (PE), phycocyanin (PC), allophycocyanin (APC), carotenoids (β-carotene, astaxanthin, lutein), proteins, vitamins, polyphenols, phytosterols, exopolysaccharides) from microalgae are presented in Fig. 1 (Mourelle et al. 2017; Bhalamurugan et al. 2018; Dixon and Wilken 2018; Khanra et al. 2018; Galasso et al. 2019; Scaglioni et al. 2019). These bioactives find applications in different fields of pharmaceuticals, nutraceuticals (single-cell proteins) and cosmetics (Del Mondo et al. 2020; Santhakumaran et al. 2020; Wali et al. 2020; Reynolds et al. 2021), and can be used as food ingredients in functional foods or in the form of tablets, capsules etc., as dietary supplements summarised in Table 2 (Panda and Manickam 2019; Srivastava et al. 2021; Cazarin et al. 2021; Gupta and Gupta 2020; Saha et al. 2015; Molino et al. 2020; Fernandes and Cordeiro 2020; Gharajeh et al. 2020; Gautam and Mannan 2020). For example, lipids from microalgae can be a potential ingredient in feed and food products and can be a replacement for fish and plant-based polyunsaturated fatty acids (Katiyar and Arora 2020). The global market for phytosterols is expected to reach US$ 935 million by 2022 and plant-based PUFAs may not be able to meet increased demand through 2030 and beyond, whereas microalgae could yield 678–6035 kg ha−1 year−1 of phytosterol which is more than the current productivity of phytosterols from rapeseed plants, highlighting their economic potential (Randhir et al. 2020). The cosmetic industry has recently started the inclusion of algal compounds in innovative formulations. Herein, microalgal pigments such as chlorophyll, β-carotene, astaxanthin, xanthophylls, and phycobiliproteins find applications in innovative cosmetics (Almendinger et al. 2020; Morocho-Jácome et al. 2020; Tabarzad et al. 2020) and lipids find use in skin creams and lotions (Kumar et al. 2021; Fernández et al. 2020; Vieira et al. 2020; De Luca et al. 2021; Miguel et al. 2021). For example, microalgae (Arthrospira platensis and Dunaliella salina) are being introduced into cookies for their superior nutritional profile (Sahin 2020). Microalgal biomass of Arthrospira and Chlorella have been reported to improve the growth performance of broiler chickens (Sugiharto 2020) and have been added to animal (livestock and fish) feed (Ritu et al. 2023).
Schematic representation of different high-value products derived from micro-algae (the images in this figure are taken from www.commons.wikimedia.org)
Steps to extract bioactives
To extract bioactives, which are generally high-value and low-volume biomolecules from microalgae a series of steps needs to be followed. Microalgal cultivation is the starting point followed by harvesting, pre-treatment and extraction, and purification (shown in Fig. 2). Optimised cultivation of microalgae is crucial to achieving desirable productivity of algal biomass and high yield of bioactives. Cultivation modes such as raceway ponds, photobioreactors and their advancements are used at a commercial scale for microalgal production. The cultivated biomass is then harvested by various methods such as flocculation, filtration, settling for further processing and value addition.
Schematic representation of the steps to valorise microalga—from cultivation to products (the images in this figure are taken from www.commons.wikimedia.org; www.pixabay.com)
To obtain bioactives, harvesting is followed by pre-treatment and extraction and involves pre-treatment methods (mechanical methods: bead milling, ultrasonication, microwave, high-speed and high-pressure homogenizer, autoclaving, pulsed electric field, and non-mechanical methods: enzymatic or alkali treatment) intending to maximize the yield of target biomolecule. The cell wall provides mechanical and chemical protection to cells, overcoming which is crucial during extraction. The cell wall structure and composition of some of the commercially important microalgae is given below. The cell wall thickness and composition vary with microalgal species for example, D. salina and similar hypersaline strains only have a plasma membrane and lack any rigid polysaccharide in the cell wall (Torzillo and Masojidek 2008). The cell wall of Crypthecodinium cohnii is composed of several membranes surrounded by thecal plates and exopolysaccharides and is 133 nm thick which provides strength to the microalgae. The cell wall of Chlorella vulgaris is of 100–200 nm thickness (Sydney et al. 2014). The cell walls of C. vulgaris and other green microalgae have rigid wall components embedded within a more plastic polymeric matrix containing uronic acids, rhamnose, arabinose, fucose, xylose, mannose, galactose, and glucose. The predominant amino sugar found in the rigid cell wall of Chlorella is a chitin-like glycan, making it rigid (Gerken et al. 2013). Like chitin, sporopollenins is a tough non-hydrolysable polysaccharide, present in the cell wall of H. pluvialis aplanospores making it difficult to extract the antioxidant, astaxanthin from these cells (Ye et al. 2020) and the Arthrospira cell wall gains its strength from peptidoglycan with an absence of cellulosic compounds in the cell wall (Canelli et al. 2021; Machado et al. 2022). The selection of pre-treatment methods is important for efficient extraction owing to different cell wall structure of biomass, which needs to be disrupted (Alhattab et al. 2019).
After extraction, purification is a mandatory step for value addition. Methods such as electrophoresis, membrane separation, ultracentrifugation, aqueous two-phase extraction (ATPE), adsorption, ammonium sulphate precipitation, chromatography, etc., are gaining a lot of attention. After the extraction and purification of high-value biomolecules, valorisation of spent biomass through the biorefinery approach will lead to value addition (Jacob-Lopes et al. 2019). To make these biomolecules commercially viable and feasible, research efforts are being made to develop fast-growing and high-yielding strains, simple cultivation methods, easy and efficient harvesting techniques, advanced cell disruption, extraction and purification methods for enhancing valorisation potential of microalgae (Quinn and Davis 2015; Milano et al. 2016; Waghmare et al. 2019).
Algae processing involves several energy-intensive operations such as aeration, mixing, harvesting, cell disruption and interphase mass transfer. The low biomass concentration in the production media aggravates these limitations further. Thus, it is essential to develop better ways of intensifying these desired transport processes without incurring significant energy penalties.
In this pursuit, cavitation-based methods have the potential to realise process intensification and enhance productivity. Cavitation acts as an energy concentrator and offers an attractive avenue for intensifying processes like particle size reduction, extraction and interphase mass transfer (Subhedar and Gogate 2013; Abiev 2021). The phenomena of cavitation involve the formation, growth and collapse of microbubbles for a very short duration of time (order of nanoseconds) (Lohani et al. 2016). The sudden collapse of the microbubbles results in a rush of liquid to occupy the void, developing a high-velocity jet of stream in the bulk liquid. Cavitation generates very high local temperatures (∼10,000 K) and pressure (∼1000 atm) at millions of locations, resulting in high shear (Ranade and Bhandari 2014; Bhandari et al. 2016; Suryawanshi et al. 2018; Sarvothaman et al. 2019). This jet stream of liquid can disrupt the cell wall and boundary layers, facilitating the rapid migration of biomolecules. Thus, increasing the rate of heat and mass transfer, and increasing the intrinsic rate of chemical reaction at much lower overall energy inputs, in turn, improves the extraction efficiencies. The micron-size vapour/gas bubbles are generated in a low-pressure region which eventually travel to a region of higher pressure where they collapse. The collapse under certain conditions results in intense shear, hammer pressure and localised hot spots. These localised physio-chemical effects can be harnessed for a variety of process intensification applications (Ranade and Utikar 2022). The collapse of cavities, results in the generation of microbubbles and can be useful in microalgal cultivation through media regeneration, aeration, water disinfection, gas mixing and agitation. It acts by removing competitive bacterial cells, rotifers, oxidation etc. the nanobubbles can be useful in harvesting by flocculation.
Cavitation may be realised primarily in two ways: ultrasonic cavitation and hydrodynamic cavitation (Ranade and Utikar 2022). Hydrodynamic cavitation is a method of introducing discrete energy input, where the energy dissipated per unit volume into the system. During HC, the energy is dissipated simultaneously to extremely small zones, but at millions of locations in the system, making it similar to acoustic cavitation. Unlike ultrasonic cavitation, hydrodynamic cavitation is scalable and offers a promising low-cost option for intensifying processes used in valorisation of algae. Hydrodynamic cavitation is the formation, growth and collapse of vapour/gas bubbles due to pressure fluctuations in the flowing liquid. HC is reported to be a possible alternative to ultrasonication for its energy efficiency and scale-up capability (Lohani et al. 2016).
As indicated in Fig. 3 the processes involved in microalgae-based process intensification, need to be optimised to achieve higher efficiency. The ability to generate nanobubbles or microdroplets by HC can be utilised during harvesting and for efficient mass transfer. There are no reports of employing HC for media regeneration, aeration and harvesting. Thus, it was thought prudent to document the gaps and scopes of HC towards valorising microalgae.
Hydrodynamic cavitation – a promising technology
Hydrodynamic cavitation is gaining popularity as a technique with application in various fields such as pre-treatment, food processing, wastewater treatment, cosmetics etc. HC is gaining interest and importance among pre-treatment technologies for the minimization of toxic solvent usage, cost-effectiveness in operation, and ability to obtain superior processed products when compared to conventional methods. Hydrodynamic cavitation was originally a bane for shipping industries and pumping equipment, as it causes erosion of the inner walls. However, on the brighter side, it intensifies various physical and chemical processes to become a novel technique that has extensive applications. The applications ranges from HC-assisted extraction, emulsification, food processing, biofuel synthesis (Iskalieva et al. 2012; Kim et al. 2015a; Hilares et al. 2017; Zieliński et al. 2019; Hilares et al. 2020; Nalawade et al. 2020; Lauberte et al. 2021; Thaker and Ranade 2021b; Bimestre et al. 2022; Nagarajan and Ranade 2022; Thangavelu et al. 2022), biomolecule degradation (Yi et al. 2021) and wastewater remediation (Ranade and Bhandari 2014; Bhandari et al. 2016; Gaikwad 2016; Jain et al. 2019; Burzio et al. 2020; Mane et al. 2020; Abbas-Shiroodi et al. 2021; Gostiša et al. 2021; Mane et al. 2021; Patil et al. 2021a, b; Ranade et al. 2021c) agricultural waste (Garuti et al. 2022), food industry (Asaithambi et al. 2019; Arya et al. 2020; Panda et al. 2020; Vigneshwaran et al. 2022). HC has been reported as a scalable, energy-efficient technique for the cell disruption and extraction of biomolecules (such as phenolics, enzymes, and flavonoids), delignification of wheat straw, biomass pre-treatment etc. for bioresource utilization (Gogate and Pandit 2008; Iskalieva et al. 2012; Lohani et al. 2016; Hilares et al. 2017).
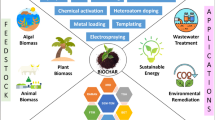
In Fig. 4 the potential steps where HC can be employed in the microalgae industry are represented. Markets are consumer-driven and there is a significant increase in the demand for varieties of consumer products. To meet this demand at lower production costs and to enhance the quality of the product, industries are looking toward greener processing technologies. Here, we present a critical review of potential of HC to realise process intensification for the valorisation of microalgae.
Potential applications of hydrodynamic cavitation in the microalgae sector (the images in this figure are taken from www.commons.wikimedia.org)
To utilize cavitation for beneficial purposes, several devices are used for generating cavitation and enhancing its effectiveness for cell wall disruption at lower energy requirements (Grillo et al. 2019; Wu et al. 2019b).
HC can be categorized based on design of the reactor, which governs the cavitational phenomena. HC devices can be classified into two main types: devices without moving parts and devices with moving parts (Table 3). Devices without moving parts are the ones with, orifice plates (Simpson and Ranade 2018; Thangavelu et al. 2018; Jain et al. 2019; Lee et al. 2019; Burzio et al. 2020; Sarvothaman et al. 2020b; Patil et al. 2021a; Yi et al. 2021), venturi tubes (More and Gogate 2018; Simpson and Ranade 2019c; Yasuda and Ako 2019; Dey et al. 2020), high-pressure nozzles (Thanekar et al. 2021), and vortex diode (Nagarajan and Ranade 2019; Sarvothaman et al. 2019; Mane et al. 2020; Nagarajan and Ranade 2020; Sarvothaman et al. 2020b; Patil et al. 2021a; Ranade et al. 2021c). The advantages of orifice plates, venturi tubes, and high-pressure nozzles HC devices are that they are simple designs and cheap (Sarvothaman et al. 2020b). However, they require powerful feeding pumps to overcome the high-magnitude pressure drop and to generate cavitation. These pumps face issues of frequent clogging, resulting in profit loss, when treating solid–liquid mixtures. To overcome these issues, novel vortex diodes have been designed and developed (Ranade et al. 2016, 2017; Jain et al. 2019; Sarvothaman et al. 2019).
Another type of HC device with moving parts is rotary equipment or dynamic cavitational elements, e.g., rotor–stator devices (RSD) or pinned disc rotating generators. The use of a novel pinned disc rotating hydrodynamic cavitation generator has been reported by Gostiša et al., (2021) for wastewater treatment and various parameters. These were developed to generate HC with less energy input and to overcome issues faced by devices without moving parts (Badve et al. 2015; Cerecedo et al. 2018; Sun et al. 2018). Higher inlet pressure leads to a higher degree of cavitation and is expected to result in better efficiency (Batista et al. 2017), ascribed to higher turbulence (Wu et al. 2012). However, with an increase in inlet pressure, the energy input will increase affecting the efficiency of the process (Batista et al. 2017). The authors also tried to correlate efficiency with the cell concentration cavitation effectiveness and reported mixed conclusions. For instance, Kim et al. (2017) reported that at lower solid loading (cell concentration) the damage to cells is less as the probability of interactions between cells and cavitation bubbles is minimised. On the other hand, Xu et al. (2006) contradicted this observation and reported that a lower initial concentration yielded better results, whereas, Halim et al. (2013) observed an open-down parabolic relationship between cell destruction and initial concentration in the case of high-pressure homogenisation. However, Greenly and Tester (2015) reported that cell concentration does not affect efficiency, however the sample volume may have a role in the efficiency of the process. This is particularly true in the case of extraction by HC, where the processing of higher volume requires more time, thus more time for metabolite release into the extraction medium. Besides this, cell shape and size also have a role to play in deciding cavitation efficiency (Wang et al. 2014a) changing the effectiveness of cavitation with cell type which is governed by the microalgal species. This can be due chemical composition and thickness of the cell wall as mentioned above, making cells more or less susceptible to cavitation.
In any method or technique, process parameters affect the effectiveness and efficiency of operation; thus optimization is an important step. In HC, parameters such as the geometry of the device, flow rate, cavitation number, number of passes etc., influence the degree and efficiency of cavitation (Ranade and Utikar 2022).
Hence, several models such as multi-layer and per-pass degradation models have been used to study the relation between cavitation reactor performance, its design and operation (Pathania et al. 2018; Sarvothaman et al. 2019). Further, reports are also available explaining the application of HC in batch operation of cavitation reactor and its modelling (Simpson and Ranade 2018; Cappa et al. 2020). Furthermore, other versions of HC devices, namely orifice with swirl, and venturi with swirl have been modelled (Sarvothaman et al. 2020b).
Despite the advantages of HC, only limited reports are available on employing HC for deriving bioactives from microalgae. The major strategies reported for cell disruption of algae (micro and macro) and extraction of bioactives involve osmotic shock (Ramola et al. 2019), alkali hydrolysis method (Ogretmen and Duyar 2018; Mensi 2019), pressurized liquid extraction (Otero et al. 2018), maceration in the presence of liquid nitrogen(Munier et al. 2014), freeze grinding, freezing and thawing (Senthilkumar et al. 2013), and homogenization (Harnedy and FitzGerald 2013). There are also reports on the application of process intensification methods such as high-voltage pulsed electric field (Prabhu et al. 2019) and ultrasonication (Le Guillard et al. 2016; Mittal et al. 2017; Devi et al. 2020). However, these methods have limitations being energy-intensive, requiring high operating temperatures and have scalability issues, e.g. ultrasonication has the challenge of wave attenuation due to a decrease in sound wave amplitude with distance and the pressure waves generated fade off soon, creating a low penetration through the media, leaving most of the mixture untreated (Kadam et al. 2015), and high-voltage pulsed electric field has issues of high power dissipation and amplitude during the pulse (Reberšek and Miklavčič 2011). By contrast, HC-based extraction methods are reported to have significantly lower energy consumption and enjoy advantages (such as higher efficacy and ease of scale-up) over US (Gogate and Pandit 2008). However, exposure times also have a direct effect cell disruption (Rajasekhar et al. 2012). Optimisation of duration of exposure is important, as overexposure to reactive ionic entities generated during cavitation may affect the stability of sensitive biomolecules by oxidising the bonds in biomolecules.
Accordingly, a HC based pre-treatment method has tremendous potential for deriving metabolites from microalgae. However, only a few reports are available on employing HC for the extraction of biomolecules from microalgae. For the benefit of researchers and algae-based industries, HC and its promising roles in different steps such as cultivation, harvesting etc., to realise valorisation of algae, HC is discussed in subsequent sections.
Cultivation and harvesting of microalgae
Obtaining good quality bioactives in desirable volume requires high productivity of quality microalgal biomass. Recent developments in algal cultivation and harvesting are discussed in a subsequent section and the limitations are summarised in Fig. 5.
Key issues in production, cultivation and harvesting of microalgae (the images in this figure are taken from www.commons.wikimedia.org)
Raceway ponds
Raceway ponds are the most common cultivation system for microalgae and are equipped with paddlewheels for mixing nutrients, gases and exposure of the algae to sunlight. The scientific community is working on improving the design of raceway ponds for enhanced yield (Sivakaminathan et al. 2020). Enhanced productivity in the range of 20–59% was achieved by altering the depth of pond (Raeisossadati and Moheimani 2020) or the light spectra by orange-coloured polyvinyl chloride sheets (Kumar et al. 2020), light-diffusing systems (red luminescent solar concentrators) (Raeisossadati and Moheimani 2020) for microalgae such as A. platensis and Scenedesmus sp. (Raeisossadati et al. 2020). Productivity was enhanced by introducing baffles in a 1000 L multi-stage raceway pond (MSRP) for the cultivation of C. vulgaris (Matanguihan et al. 2020). Employing rotating algal biofilm (RAB) has reduced electricity and water consumption by up to 83 and 30% per kilogram of biomass (20% DW) of algae meal when comparing open raceways ponds (ORP) for the production of Te. suecica (Morales et al. 2020). Enhanced productivity of Arthrospira cells and Nannochloropsis sp. was achieved by microporous diaphragm aerators (MDA) in raceway pond and flue gas, respectively (Bhadra et al. 2020; Cunha et al. 2020; Kumar et al. 2020). Strategies such as mixotrophic cultivation are employed to improve the cultivation of microalgae, leading to utilization of inorganic and organic carbon in the presence of light (Yu et al. 2020).
For economic advantages, raceway ponds are popular and adopted in major commercial plants. However, the fluid dynamics of the raceway operation are quite complex. Thus, to improve the understanding and to gain insight, computational fluid dynamics (CFD) -based software simulation has been employed to optimize the design (Kusmayadi et al. 2020). CFD tools were used to improve the design by modelling, and evaluating the geometry, specifically raceway length, width, flow depth and operational conditions such as operational velocity of raceways ponds (Lima et al. 2021; Romagnoli et al. 2020). The approach of utilising treated wastewater (after disinfection), media recycling and efficient aeration (CO2 gas exchange) can be used to further improve productivity and reduce the operational cost of raceway ponds. Hydrodynamic cavitation in microalgal cultivation is a novel concept and envisaged the media regeneration, aeration, and harvesting by water disinfection, gas mixing and agitation, and flocculation by nanobubbles, respectively.
Suitable water quality water is a primary requirement for microalgal cultivation, however, fresh water in such huge volumes may not be a feasible approach. Hence, treating wastewater by removing competitive bacterial cells (Kim et al. 2021), rotifers (Kim et al. 2017), and degradation of solvents (Patil et al. 2021b) etc., is required for microalgal cultivation (Fig. 6). Water disinfection employing HC has been achieved (Ranade et al. 2009; Gaikwad 2016; Sarvothaman et al. 2018; Jain et al. 2019; Mule et al. 2021). This degradation of extracellular or metabolic waste produced during cultivation or breaks down complex biomolecules in culture media into monomeric nutrients for media recycling (Kim et al. 2021). HC acts by the formation of different radicals (predominately OH radical and H radical) and consequent oxidation at high local temperatures of 5000 K during collapsing of cavitation bubble (Arrojo et al. 2008), however, oxidation is not a principal method for the eradication of bacteria for their larger sizes. Shock waves, shear flow, supercritical water conditions, and pressure and temperature spikes which accompany the aggressive bubble collapse play an important (sometimes decisive) role in damaging the bacteria (Šarc et al. 2016). Besides disinfection, HC by virtue of the ability to generate nanobubbles can be used to replace spargers and paddles to improve the interfacial area and therefore gas mass transfer (Patel et al. 2021).
Typical results of water dis-infection employing HC with potential for raceway pond water (the image of centrifuge from www.commons.wikimedia.org)
Wastewater from slaughterhouses was treated using HC to reduce chemical oxygen demand (COD) for microalgal cultivation (Terán Hilares et al. 2022). Reduction of COD mainly happens due to oxidation happening during HC. Advanced oxidation processes (AOPs) in synergy with HC are reported to significantly lower the wastewater treatment cost (Badmus et al. 2018; Joshi and Gogate 2019; Fedorov et al. 2021). The reduction of ammoniacal nitrogen in wastewaters employing HC was demonstrated by Patil et al. (2021). Advanced oxidation processes (AOPs) in synergy with HC are reported to significantly lower the wastewater treatment cost (Badmus et al. 2018; Joshi and Gogate 2019; Fedorov et al. 2021). HC at standardised pH (Zampeta et al. 2022) and in combination with hydrogen peroxide (Agarkoti et al. 2022) and ozone (Wang et al. 2020b) for their influence on the degradation of pollutants, has resulted in enhanced efficiency of the treatment demonstrating a synergistic effect. Attempts for artificial neural network (ANN) and CFD modelling the HC-based wastewater treatments showed excellent ability to interpolate and reasonable ability to extrapolate (Ranade et al. 2021a; Sun et al. 2021; Terán Hilares et al. 2022). Thus, it can be summarised that molecular bond breaking, thermal decomposition, and free radical formation during HC (Wang et al. 2021), make it a potential method for wastewater treatment for microalgae cultivation. HC can be used for enhanced gas exchange by nanobubbles generation, resulting in improving the productivity of photobioreactors. This will further advance its edge over raceway ponds and is discussed in the following section.
Photobioreactors
Recent years have witnessed a growth in interest in the cultivation of microalgae. However, industrial production remains a major issue – mainly due to the constraints posed by classical cultivation systems such as raceway ponds. This is due to limitations related to light exposure, shear stress, gas exchange, and biofouling. Productivity of biomass varies with geographical locations and climate factors, including solar irradiance, air temperature, relative humidity, and wind velocity and it was found that the productivity varies from 2000 to 13,000 t km−2 year−1 (Banerjee and Ramaswamy 2019). These issues are addressed by employing photobioreactors and their advanced modifications (Assunção and Malcata 2020).
In this field, a novel pond-tubular hybrid photobioreactor (PTH-PBR) comprising horizontal tubes installed with a flashing-light system was developed by Xu et al., (2020) that enhanced the yield of microalgal biomass and chlorophyll content by 31.2 and 33.6%, respectively. Advances include integration of photovoltaic panels in a thermally insulating photobioreactor (Nwoba et al. 2020; Yustinadiar et al. 2020), membrane photobioreactors (MPBR) (Solmaz and Işık 2020), application of low-frequency intermittent light pulses (Lima et al. 2020), exposure to the different intensities of magnetic field (60 and 30 mT) (Costa et al. 2020), microbubble-assisted CO2 sequestration reactor (Dey et al. 2020) and sequential-flow bubble column photobioreactor system (Dasan et al. 2020).
Mixotrophic cultivation (Nanochloropsis oceanica in association with microbe Halomonas aquamarina) in photobioreactors (Subasankari et al. 2020), airlift photobioreactor comprising a transparent draft tube (Madhubalaji et al. 2020), and presence of a low concentration of Cr (VI) (0.5 and 1.0 mg L−1) in MPBR (Lu et al. 2021) also have been found to increase biomass productivity and metabolites.
The growth of microalgae depends on the microenvironment in the media, which is controlled by the mass transfer of nutrients, inhibitors and gases in cultivation media and thus plays an important role to improve the overall productivity of any bioprocess. Mass transfer of gases such as CO2 and O2 is an important parameter in microalgae cultivation, as the kLaatm (transfer coefficient) value depends mostly on the surface area between culture and atmosphere (Caia et al. 2018; Oliveira et al. 2018; Li et al. 2019a). Balance of these gases in media is crucial for the growth of microalgae and the maintenance of pH. It has been reported that smaller bubble size during cultivation improves the growth of microalgae (Kim et al. 2014), where 26–44% enhancement in microalgae growth by air nanobubbles due to better mass transfer as demonstrated by Patil et al. (2021a) and enhanced lipid productivity (Yang et al. 2018). The gas-to-liquid interfacial area increases with the decrease in bubble size. Bubble size achieved using baffles, orifice or fine-pore diffusers resulted in air bubble size of equivalent diameter (de) in the range of 1.0 to 2.0 mm (Cheng et al. 2016; Yang et al. 2018), whereas bubbles generated by HC can be of the nanoscale levels. Hence, HC will be useful in maintaining the CO2 and O2 levels and temperature in the cultivation media. Reports employing devices such as a cavitation tube and a needle valve to generate the size of the bubbles ranging from 100 to 550 nm in all surfactant solutions, after 30 min of recirculation and pine oil to change the interfacial tension are available (Etchepare et al. 2017; Oliveira et al. 2018). Hence, HC owing to its ability to generate microbubbles can be employed for sparging and generation of microbubbles during microalgae cultivation (Sarvothaman et al. 2020a). Cavitation leads to the generation of nanobubbles via simultaneous generation of microbubbles and nanobubbles and subsequent shrinkage of microbubbles with increasing surface charge density to become nanobubbles. High-shear rotor–stator device was used to generate bulk nanobubbles (BNBs) in pure water (Jadhav et al. 2021). Improved and efficient exchange of gases will reduce the operation cost during microalgal cultivation.
Harvesting
Harvesting is crucial but difficult step, due to the very small cell size and buoyancy of the cells. High volume of growing media in raceway ponds and bioreactors and low biomass concentration ( ≈ 3 g L−1) adds to the cost due to the energy-intensive dewatering and drying step, thus warranting a favourable method to bypass the dewatering and drying steps (Waghmare et al. 2019). This section aims to summarise both conventional and recently developed techniques of harvesting. The reported methods are employed alone or in combinations to concentrate algal suspensions to process them into the final products. At the time of harvesting algal suspension consists of 2–7% w/v biomass. Harvested biomass is dewatered to reach final biomass concentration of 15–25%, which contributes to 3–15% of algae biomass production costs (Fasaei et al. 2018). Harvesting is reported to be carried out employing methods such as centrifugation (Pahl et al. 2013; Barros et al. 2015; Kim et al. 2015b), chemical flocculation (Vandamme et al. 2013; Lama et al. 2016; Roselet et al. 2017), nanoparticle flocculation (Wang et al. 2014b; Zhao et al. 2018; Liu et al. 2019; Liu et al. 2020a; Ma et al. 2020; Wang et al. 2020a), bio-flocculation (Liu et al. 2013; Ummalyma et al. 2016; Úbeda et al. 2017; Pandey et al. 2019), electrocoagulation (Shuman et al. 2016; Fayad et al. 2017), filtration (Monte et al. 2018; Singh and Patidar 2018; Leam et al. 2020), and dewatering-independent methods (Gross et al. 2013; Prajapati et al. 2014; Prochazkova et al. 2015; Peng et al. 2020). Among these, methods such as microwave-assisted flocculation and magnetic nanoparticles-assisted flocculation are recent developments (Liu et al. 2020b). The advantage of these methods is reported to be their higher flocculation efficiency and non-destructive nature on cell walls (Liu et al. 2020b; Vashist et al. 2020).
Bio-flocculant (alum (Al2(SO4)3), eggshells and calcium carbonate have been used for harvesting C. pyrenoidosa and alum resulted in maximum harvesting efficiency (99%) with a few deformities in algal cell surfaces. (Pandey et al. 2019). Membranes (mainly ultrafiltration) are also used as separating tools to harvest the biomass e.g., D. salina (Monte et al. 2018) (Hafiz et al. 2020). Harvesting efficiency of Isochrysis zhanjiangensis was improved by increasing the cross-flow velocity (in turn permeate flux) (Zhang et al. 2019) or by reducing the membrane fouling by reversing the feed flow direction (Yang et al. 2019).
Kim et al. (2017) reported that HC was the lowest energy-consuming method among other physical methods. Kim et al. (2021) have reported the application of HC for bacterial disinfection and media recycling for the cultivation of Ettlia sp. HC also resulted in a slight increase (1.6-fold) in lipid yield (Kim et al. 2021). Work on the application of HC mainly for cell disruption employing HC and US is reported (Zupanc et al. 2019; Liu et al. 2022). However, it has also been reported that nanobubbles improve the flotation of particles (Oliveira et al. 2018; Nazari et al. 2019; Pourkarimi et al. 2021) and the same is demonstrated by Itoh et al. (2019) in their work where HC was employed for the generation of microbubbles for dispersed air flotation of microalgae using venturi tube type microbubble generator to achieve harvesting. Hence, due to the ability of HC to generate nanobubbles (Li et al. 2021; Nazari et al. 2022), it can be used to harvest microalgal biomass by flocculation. Followed by harvesting, pre-treatment of harvested biomass is the next step. Thus, in the subsequent section various pre-treatment methods are discussed and potential role of HC in the intensification of the process.
Pre-treatment methods for extraction of bioactives
Bioactives extraction from the biomass is facilitated by cell disruption, making pre-treatment an important downstream processing step. Pre-treatment becomes crucial in case of high-value and low-volume target biomolecules. The selection of pre-treatment methods for efficient extraction is important due to different cell wall composition, making cell disruption a critical step (Praveenkumar et al. 2015; Mittal et al. 2017; Mittal and Raghavarao 2018; Tavanandi and Raghavarao 2020).
Various methods such as ultrasonication or ultrasound-assisted extraction, high-pressure homogenization, pressurized liquid extraction, enzyme-assisted extraction, pulse electric field assisted extraction, solvent and supercritical fluid extraction, Ionic liquid assisted extraction, ozonation, microwave-assisted extraction and HC are explored for cell breakage and release of biomolecules of interest (Fig. S1). These methods and their advantages are discussed in the following section and are summarised in Table 4.
In recent times, the application of HC for the processing and valorisation of biomass has been of interest. The advancements in the exploitation of cavitation for various applications such as pre-treatment of biomass (cell disruption), droplet size reduction, mixing, hydrolysis etc., are available in the literature. However, reports on application of HC for the pre-treatment of microalgae are very limited (summarized in Table 5).
Lipids extracted from microalgae can be coverted to biofuels, however, the main problem in the production of biodiesel from microalgae is the high cost of extraction which escalates the production cost. Thus, to improve the economic feasibility of biodiesel production, improved cell disruption is important (Setyawan et al. 2018a, 2020). A few process optimization studies of HC for cell disruption to extract lipid and chlorophyll demonstrate that HC has higher product yield HC has resulted in 26–99% lipid yield when compared to autoclave and ultrasonication, which resulted in 16–65 and 5 to 27% yield from N. salina, respectively. In another study, HC was able to process 40 L of T. suecica slurry in 4 min whereas it took 600 min with US (Lee and Han 2015; Lee et al. 2015). It was demonstrated that HC is a more energy-efficient method for microalgal cell disruption (Lee et al. 2015; Waghmare et al. 2019) and the mechanism of action is represented in Fig. 7. HC was employed for cell disruption and simultaneous lipid extraction from N. salina. It resulted in a high lipid yield (25.9–99.0%) when compared with an autoclave (16.2–66.5%) and ultrasonication (5.4–26.9%) in terms of the specific energy input (500–10,000 kJ kg−1). Setyawan et al. (2018a, 2020) using Nannochloropsis sp., demonstrated that maximum lipid yield was obtained at 5 bar driving pressure and the energy extraction requirement of 17.79 kJ g−1 lipids is less than the biodiesel heating value, with value of volumetric mass transfer coefficient almost 20-fold higher than the conventional extraction method. Lee et al. (2015) in a study found that for lipid extraction HC has a disruption energy requirement of 3 MJ kg−1, making it 10 times more energy-efficient than sonication, However, it still accounted for approximately 13% of the total energy in the biomass, which is too high for biofuel production and thus, needs further improvement in terms of design and development and process optimisation.
Key concepts of hydrodynamic cavitation – cavity collapse and its effects on algal cell wall matrix (the images in this figure are adapted from www.commons.wikimedia.org)
A venturi-hydrodynamic cavitation reactor has been used to treat a mixture of Nannochloropsis sp. within methanol/hexane (20.5 mL/47.5 mL) at 34 °C and 6.8 bar of sample chamber pressure. Hydrodynamic cavitation extraction (HCE) is more rapid than the agitation method at 260 and 1000 rpm. A lipid yield of 8.9% was achieved by HCE in 10 min, while 5.44–7.3% yields were achieved in 60 min by stirring, respectively. This translates into a volumetric mass transfer coefficient of 7.37 s−1 and 0.53 to 0.12 s−1 with HCE and stirring, respectively. According to the thermodynamic parameters, ΔH0 (20.72 kJ mol−1), ΔS0 (58.05 J mol−1 K−1) and DG (1.97–3.01 kJ mol−1 at 34–50 °C), the process of lipid extraction was endothermic, non-spontaneous and irreversible, indicating that more energy was consumed compared to the extraction from other oil plant sources (Setyawan et al. 2018b).
As design and process parameters such as geometric configuration, number of passes etc., affect the cavitation and in turn cell disruption, optimization of process parameters is important. Different types of orifice plates with different diameters were used to find the best conditions for lipid extraction from microalgae (46.0 ± 3.7%). It is reported that lower orifice hole areas resulted in high vapour pressure and resulted in a higher degree of cell disruption (Lee et al. 2019). In a study with closed-type HCR with an orifice plate (0.5 mm × 13 holes in radial pattern) for the lipid extraction from wet microalgae N. salina with hexane (1:0.8 v/v). A lipid yield reached 45.5% by using 0.89% sulfuric acid at 0.4 MPa inlet pressure in 25 min by using HCE compared to autoclave and ultrasonication (Lee and Han 2015). Similarly, lipids in Nannochloropsis sp. were extracted with a co-solvent of hexane and methanol within an HCR. Under the optimum conditions, the specific energy consumption reached 16.743 MJ kg−1 lipid (Setyawan et al. 2018a).
Pre-treatment efficiency of any method can be improved by employing combination of two methods, such as HC in combination with alkaline, enzyme or thermal treatment etc., however, literature on HC in such combination is scant. Such an approach must be used with caution for not to degrade sensitive biomolecules. However, a combination of HC with harsh treatments may be useful for biofuels.
Like HC, ultrasound-assisted extraction (UAE) is used as an eco-friendly method for cell disruption. Ultrasonication (US) creates cavitation in the medium that disrupts the cell wall and facilitates the release of biomolecules. The effect of cavitation depends on the frequency of ultrasonication (Yamamoto et al. 2015; Kurokawa et al. 2016; Zupanc et al. 2019; Liu et al. 2022) intensity, and exposure duration. Cell destruction correlated to the intensity and duration of treatment (Keris-Sen et al. 2014; Zupanc et al. 2019). Higher intensities cause more aggressive cavitation leading to better cell disruption, however, to a certain extent. At high intensities, too many cavities can interact among themselves resulting in the cushioning effect (Keris-Sen et al. 2014). Several studies on the extraction of lipids, pigments, carotenoids, polyphenols, proteins etc., and other commercially important biomolecules have been reported (Gong et al. 2017; Jaeschke et al. 2017; Mittal et al. 2017; Rodrigues et al. 2018; Sasikala et al. 2018; Tey et al. 2019; Bachchhav et al. 2020).
Ultrasound-assisted extraction (UAE) of lipids and carotenoids from Heterochlorella luteoviridis (Jaeschke et al. 2017) and C-phycocyanin (C-PC) and allophycocyanin (A-PC) from the dried biomass of A. maxima (Devi et al. 2020). To exploit synergy, ultrasound in combination with ionic liquids (ILs) of phycobiliproteins (Rodrigues et al. 2018), and the presence of surfactant (Triton X-100, Tween-20 and Tween-80) and lysozyme resulted in C-PC extraction efficiency of 77.92% and purity of 1.09 from A. platensis (Tavanandi and Raghavarao 2020). US in combination with freezing and thawing for the C-PC extraction from A. maxima resulted in extraction efficiency of 92% (Tavanandi et al. 2018b). The spent biomass was valorised for the extraction of chlorophyll employing ultrasonication and an increase of 125% in yield and a reduction of 83.3% in extraction time is reported (Tavanandi and Raghavarao 2019). Ultrasonication integrated with ATPE resulted in a higher degree of extraction and purification of astaxanthin from H. pluvialis when compared to ATPE alone (Khoo et al. 2020a).
Pulsed electric field (PEF) is a promising technology that allows the selective extraction of high-added value compounds by electroporation. The electric current is drawn into parallel plates to develop an electrical field that breaks the cell wall to release intracellular content (Nafis et al. 2015). It enhanced carotenoid (73%) and lipid (13%) extraction from Nannochloropsis sp., at a moderate electric field (1 Hz frequency) (Nafis et al. 2015). PEF is reported to be used for energy-efficient isolation of valuable microalgal bioactive substances (i.e., pigments and polyphenols) (Zhang et al. 2020). PEF pre-treatment increases esterase activity resulting in higher yield on pre-treatment followed by incubation in ethanol for 6 h. It resulted in extraction of 96% (18.3 mg car g−1 dw) of the total carotenoid from H. pluvialis, compared to 80% (15.3 mg car g−1 dw) (Martínez et al. 2019b).
Lutein (2.2 ± 0.1-fold) and chlorophyll yields (5.2 ± 3.4-fold) from C. vulgaris also increased when compared to non-treated cells using a single-stage ethanol extraction process (Soto-Sierra et al. 2020a). Extraction of several other biomolecules such as pigments (phycoerythrin) and polyphenols, from fresh biomass of P. cruentum (Martínez et al. 2019a), T. chuii and P. tricornutum (Kokkali et al. 2020) was also enhanced employing PEF at lower energy input. A microwave-assisted pre-treatment method is also reported for effective microalgal lipid extraction (Min et al. 2018).
Like PEF, a photoelectrochemical method for cell disruption is reported, where N-doped TiO2-coated photoanode captures UV–Visible light and generates electrons (e−). The e− is captured by palladium phosphorus-based cathode to generate hydroxyl radicals (•OH). The •OH interacts with cell wall components of microalgal cell walls and membranes causing cell disruption and release of intracellular metabolites (Wu et al. 2021).
High-pressure homogenization (HPH) is another method reported to employ for the extraction of biomolecules microalgae. Navarro-López et al., (2020) reported that HPH pre-treatment (at 600 bar pressure) of Scenedesmus sp., resulted in 72.9% of amino acids in concentrate. High and ultra-high pressure-based extraction (UHPE) processes using ‘Generally Recognized As Safe’ (GRAS) solvents are reported for simultaneous cell disruption and extraction of carotenoids from H. pluvialis, B-phycoerythrin (B-PE), carotenoids, and PUFAs from P. cruentum, Chlorococum and Chlorella thermophila (Gallego et al. 2019; 2020a; Babadi et al. 2020; Bueno et al. 2020; Sarkar et al. 2020). Similarly, high hydrostatic pressure (HHP) treatment for extraction is in trend as a “green” extraction method and is explored for B-PE extraction from P. cruentum without damaging the structural integrity of B-PE, however, it is an energy-intensive method (Bueno et al. 2020; Navarro-López et al. 2020). Besides this, HHP, UHPE technique did not improve carotenoid extraction from N. oceanica compared to pressurized liquid extraction (PLE) (Gallego et al. 2020a). Similarly, pressurized liquid extraction in combination with enzymes did not show a significant increase in lipid yield (Castejón and Señoráns 2019).
Enzyme-assisted extraction acts by weakening the cell wall in turn increasing the permeability. The selection of enzymes is substrate dependent and requires optimization of process parameters. Enzyme-assisted extraction resulted in the release of 73 ± 7% of lipids from C. reinhardtii (Soto-Sierra et al. 2020b). The synergistic effect of the sequential alkaline-enzymatic approach by Callejo-López et al. 2020 resulted in high extraction yields of protein- and/or amino acid peptide-enriched extract from fresh biomass of a consortium of microalgae such as C. vulgaris, N. gaditana and S. obliquus (Callejo-López et al. 2020) and enhanced lipid extraction from Nannochloropsis sp. biomass. Similarly, a combination of an enzyme-assisted method with ultrasonication resulted in the A-PC yield of 44.08 mg g−1 (db) with a purity of 1.09 (Tavanandi et al. 2019). Enzyme-assisted three-phase partitioning (EA-TPP) process by cellulase enhanced extraction yield of lipids from wet Nannochloropsis sp. biomass by (90.40%) at standardised conditions (Qiu et al. 2019). Similar enhancement in extraction in lipid yield is reported employing consortia of enzymes namely cellulase, protease, lysozyme, and pectinase (Wu et al. 2017). However, enzyme (viscozyme and celluclast) in combination with PLE and UAE for extraction of lipids from microalga Isochrysis galbana has no significant effect (Señoráns et al. 2020).
Chemical methods such as solvent extraction, ionic liquid-based methods and their combinations are also reported. Solvent extraction method was employed for the extraction of astaxanthin, fatty acids and lutein from H. pluvialis (Molino et al. 2018; Sanzo et al. 2018). Supercritical CO2 extraction and its combination with deep eutectic solvents (DESs) and microwaves (MWs) resulted in extraction of neutral lipids, chlorophylls, carotenoids lutein and β-carotene from Nannochloropsis and Pseudokirchneriella subcapitata (Chronopoulou et al. 2019; Iovine et al. 2019; Leone et al. 2019; Di Caprio et al. 2020; Mouahid et al. 2020; Saliu et al. 2020) and to improve triglyceride in an extract from P. tricornutum (Tommasi et al. 2017).
Besides conventional solvents, ionic liquids have emerged as an alternative solvent for the extraction of phycobiliproteins (allophycocyanin, phycocyanin, phycoerythrin), lipids, PUFA, lutein etc. from Chlorella sp, Chlorococum sp., A. platensis, Thraustochytrium sp. etc., (Rodrigues et al. 2018, 2019; Zhang et al. 2018; Khoo et al. 2020b, c, 2021; Morandeira et al. 2020). However, these methods have limitations of scalability and high energy consumption to be applied at an industrial scale when compared to the orifice or venturi devices driven cavitation (Waghmare et al. 2019). These limitations thus can be overcome by employing HC and in particular by a vortex-based diode. The extracted high-value bioactives need to be processed and purified for value addition and end-use.
Methods for purification of bioactives
During extraction more than one biomolecule gets extracted depending on solubility, making purification a mandatory step to separate compounds of interest for desired end applications. Purification of the biomolecule adds to its value and fetches higher value in the market and thus gained a lot of attention from researchers evident by several reports. Purification methods such as electrophoresis, membrane separation, ultracentrifugation, ATPE, adsorption, ammonium sulphate precipitation, chromatography, etc., are summarised in Fig. S2. The purification methods along with HC as a potential purification method are discussed in the following section.
Centrifugation is employed for purification mainly by separating molecular components. It works on the principle of differential sedimentation of molecules based on their densities and under the influence of gravity or g-force. Centrifugation can remove impurities (e.g., chlorophyll, cell debris) in an extract of C-phycocyanin (Mittal et al. 2019; Tavanandi and Raghavarao 2019). It is used to separate precipitates during protein purification. However, centrifugation is an energy-intensive procedure and is not suitable for the separation of biomolecules. Electrophoresis is another well-known technique for purification. However, this technique is mostly used for analytical and characterization studies by separating proteins, DNA and other macromolecules (Santos and Brodbelt 2020).
Adsorption is the surface phenomenon where adhesion of atoms/molecules takes place on the surface of adsorbents and has grabbed attention, attributed to its low cost and minimal effects on yield. The adsorption of biomolecule, such as phycoerythrin, depends on various conditions mainly pH and ionic strength (IS) (Mittal et al. 2022). Electrostatic attraction is responsible for adsorption at pH below pI, whereas hydrophobic interaction at pI and pH above pI. Adsorption is the combination of hydrophobic attraction, reaction of water with adsorbent and electrostatic interaction (Biesheuvel et al. 2004; Mittal et al. 2022). Adsorbents like chitosan and activated charcoal have been reported to produce effective results for the purification of phycobiliproteins. Activated carbon was used for the purification of astaxanthin from H. pluvialis (Casella et al. 2020) and fucoxanthin (carotenoid pigment) from Tisochrysis lutea by selective adsorption of chlorophyll (Gallego et al. 2020b). Purity index of 5.26 for C-PC employing chitosan, activated charcoal, ammonium sulfate precipitation and ion-exchange chromatography (Safaei et al. 2019). EBA (expanded bed adsorption) column and streamline DEAE (Diethylaminoethyl) resin in a new vortex flow reactor are used for the purification of B-phycoerythrin from P. cruentum and resulted in 71–78% (Bermejo et al. 2013) and 86.6% of yield (Ibáñez-González et al. 2016). Marennine is a blue-green pigment from the diatom Haslea ostrearia and is purified using a graphitic stationary phase to achieve a yield of 66% (higher compared to 57% from ultrafiltration) (Bélanger et al. 2020).
Cavitation has been reported to create a homogeneous solution to facilitate adsorption on the adsorbent surface. HC in integration with the adsorption process is reported for the removal of recalcitrant organic pollutants like dyes and phenols. HC in combination with polymer hydro gel packed bed adsorption for the removal organic pollutants from wastewater is reported (Bethi et al. 2017). However, HC may not have any direct role during adsorption, however, can be used during desorption. The shear forces generated during HC can detach adsorbed compound or particle from the adsorbent, helping in regeneration. Besides shear forces, oxidation of adsorbent may occur at longer processing duration.
Aqueous two-phase extraction (ATPE) is an environmentally benign method for purification with the ability to concentrate the purified biomolecules, such as natural colours of commercial importance from a variety of sources (Chang et al. 2018; Santos et al. 2018; Chandralekha et al. 2020). Aqueous two-phases are formed by the combination of polymer/polymer, polymer/salt or alcohol/salt as phase-forming components. In ATPE, the target solute selectively partitions to one phase and contaminant biomolecules to the other phase, achieving both purification and concentration of the target biomolecule at a suitable phase volume ratio (Chang et al. 2018; Mittal et al. 2019). Polymer–salt phase systems have the additional advantages of being economical and having lower phase viscosity, thus requiring a shorter time duration for phase separation when compared to the polymer–polymer phase systems. It provides advantages over other methods in terms of low processing time, ease of scale-up, simultaneous enrichment and concentration of the product, and the scope for continuous operation on complex mixtures consisting of undesired biomolecules (Diamond and Hsu 1992; Zaslavsky 1994; Raghavarao et al. 1995; Walter 2012) and thus can be employed in integrated bio-refinery approaches for purifying high-value low-volume biomolecules. Biomolecules such as C-phycocyanin, allophycocyanin, phycoerythrin, chlorophyll, lutein, proteins and carbohydrates were extracted and purified from several microalgae using polymer–polymer and polymer-ionic liquid phases (Chethana et al. 2015; Kaewdam et al. 2020; Porav et al. 2020; Ruiz et al. 2020). Aqueous two-phase extraction is integrated with various cholinium-based ionic liquids for the fractionation of microalgal components such as proteins, pigments, lipids, and carbohydrates (Chang et al. 2018; Suarez Ruiz et al. 2018; Suarez Ruiz et al. 2020). In another very interesting application of ATPE the valuable compounds of Botryococcus braunii were simultaneously extracted into organic solvent phase (limonene, n-decanol and n-decane) without significant negative effects on cell growth (Concha et al. 2018). The ability of aqueous two-phase systems (ATPSs) to fractionate different biomolecules simultaneously, makes ATPE an important method towards establishing the microalgae industry as a multiproduct biorefinery.
The ability of ATPE is improved by employing advanced fluidic devices. Devices namely, straight-tube extractor, helical-coiled extractor and coiled flow inverter extractor were used and coiled flow inverter at a flow rate from 10 to 30 mL min−1 resulted in an increase in partition coefficient by 1.74-fold of C-phycocyanin extraction from A. maxima (Ruiz-Ruiz et al. 2019). The same pigment-algae combination was subjected to vortex fluidic device intensified ATPE (PEG and potassium phosphate) resulted in a 9.3-fold increase in phase demixing efficiency, increasing C-PC purity and yield 1.18-fold and 22%, respectively compared to conventional ATPE (Luo et al. 2016). From this preview, HC can be integrated with ATPE for its ability to generate microdroplets. Stirring during ATPE for achieving equilibrium (for mixing two phases forming components) can be replaced by HC. As mentioned in Sect. 3 and shown in Fig. 8.
Potential applications of hydrodynamic cavitation for purification of biomolecules (the image in this figure is adapted from (www.commons.wikimedia.org))
Membrane processing is gaining attention for its advantage of being functional at ambient temperature, requiring low energy for operation, and easy to scale-up methods (Lamdande et al. 2020; Mittal et al. 2020). Separation of biomolecules with membrane processing occurs mainly based on molecular weight or size of the biomolecule. Membranes act as a barrier for the passage of molecules higher than the molecular weight cut-off (MWCO) of the membrane. Different types of membrane processing namely microfiltration, ultrafiltration (UF), reverse osmosis (RO), and nanofiltration (NF) are available, out of these, ultrafiltration is mainly employed for the separation of biomolecules. UF being a scalable, cost-effective, and energy-efficient method can be used for purification of biomolecules from microalgae, meeting the global demand (Aron et al. 2020). Ultrafiltration resulted in extraction of 48% of polysaccharide-free B-phycoerythrin with a purity index of 2.3 from Porphyridium (Marcati et al. 2014). Recovery of lipids from Parachlorella kessleri by cross-flow filtration employing hydrophilic polyacrylonitrile (PAN) 500 kDa membrane (Rivera et al. 2020) and peptides from Synechococcus sp. by ultrafiltration has been reported (Suttisuwan et al. 2019). UF in different configurations such as conventional flow, tangential flow, and hollow fibre was employed for the recovery of proteins from A. platensis (Menegotto et al. 2020). However, irreversible/reversible fouling can be a factor limiting the performance during membrane processing (Zaouk et al. 2020). Thus, represents a potential method for the downstream processing of natural anti-inflammation food-grade ingredients, drugs, and cosmetic products.
HC pre-treatment of contaminated water stream at low H2O2 concentrations resulted in effective removal of cells from the water column. It also resulted in reduction in contamination by the release of organic compounds in the cells (especially cyanotoxins) without disrupting cells and the cells remain alive. HC in combination with ozonation was employed for the purification of eutrophic water (Zezulka et al. 2020).
As discussed earlier, the ability of HC for generating nanobubbles will enhance mixing and facilitate mass transfer between the two phases of the system. Higher interfacial area provided by nanobubbles will enhance the movement of biomolecules across the interface, resulting in process intensification (Thaker and Ranade 2022; Mittal and Ranade 2023). Hence, HC has the potential to be used indirectly for the purification of biomolecules (Fig. 8). Purification by selective extraction of biomolecules can be achieved by manipulating the operating variables involved, and a wide range of intensity of cavitation can be achieved resulting in a varying extent of disruption. Such observations on the selective release of α-glucosidase (periplasmic) (Mevada et al. 2019), invertase (cell wall-bound) (Balasundaram and Pandit 2001), alcohol dehydrogenase (cytoplasmic) and glucose-6-phosphate dehydrogenase (cytoplasmic) (Balasundaram and Harrison 2006) employing HC are available. HC is reported to assist in the purification of phycobiliproteins, where vortex flow devices for the intensified purification of B-phycoerythrin from P. cruentum by adsorption on Streamline DEAE resin (Ibáñez-González et al. 2016) and C-Phycocyanin from A. maxima by aqueous two-phase extraction (Luo et al. 2016). It is demonstrated that hydrodynamic cavitation can be employed indirectly for the separation and purification of biodiesel and water (Kolhe et al. 2017; Zezulka et al. 2020).
HC is being used for micromixing, formation of microdroplets and emulsions (Thaker and Ranade 2021b, a) and formation of radical ions or high localised temperature had no adverse effect on the stability or properties of biomolecules was reported (Lee and Han 2015; Lee et al. 2019; Setyawan et al. 2020; Thaker and Ranade 2021a, b). The stability of thermolabile biomolecules was not jeopardised during ultrasound-assisted extraction of biomolecules from algae (Mittal et al. 2017; Lamdande et al. 2020; Chandralekha et al. 2021). However, exposure to the radical ions generated at high intensities of cavitation and localised temperatures for a longer duration can affect the stability of metabolites. Hence, the pre-treatment should be performed at standardised conditions, to minimise the generation of reactive ions and exposure duration, without compromising on the performance of HC (Zupanc et al. 2019; Liu et al. 2022). HC as a method has not been directly used for the purification of any biomolecule, except for purification of water by disinfection. However, it can be useful for purification mainly by virtue of selective extraction of biomolecules and by promoting mass transfer for the ability to generate micro and nanodroplets and enabling macro and micromixing. This formation of nanodroplets and micromixing facilitates purification and will help to realise the process intensification of purification, this is particularly true in case of liquid–liquid extraction such as ATPE, three-phase partitioning (Narayan et al. 2008) and reverse-micellar extraction (also known as nanoreactors) (Saran Chaurasiya et al. 2015).
Hydrodynamic cavitation is mainly used for wastewater treatment and transesterification. For achieving these objectives, mainly orifice or venturi-based cavitation devices were used. Modelling studies are reported to investigate the influence of venturi configuration on the inception and extent of cavitation (Simpson and Ranade 2019c) and the influence of different orifice designs (Simpson and Ranade 2018).
Besides reports on orifice and venture-based devices, vortex-based cavitation device is gaining popularity for their higher efficiency. With the vortex flow-based diode coming into existence, advancements are reported in terms of its modelling and flow characterisation employing CFD (Sarvothaman et al. 2019; Simpson and Ranade 2019b; Thaker and Ranade 2021a, b). Artificial neural network-based modelling for biomass pre-treatment and wastewater treatment is the most recent advancement in the development and understanding of HC (Ranade et al. 2021a; b). However, modelling and simulation of the HC-based processing of microalgae are required to understand the functioning of HC at microlevels, as no report on modelling and simulation of HC concerning is available.
Hydrodynamic cavitation for valorisation and biorefinery
Hydrodynamic cavitation, for its advantages and characteristics, can be employed in various processes (Fig. 9). Its application as a pre-treatment method in almost all processes ranging from conversion of lignocellulosic biomass to extraction of metabolites from plant sources makes it a suitable method for the valorisation of biomass and to realise the concept of biorefinery. Though several reports on employing HC as a pre-treatment method are available, most are on the valorisation of lignocellulosic biomass (Nagarajan and Ranade 2019; Hilares et al. 2020; Konde et al. 2021).
In order to understand the hurdles in realising the biorefinery of microalgae, it can be divided into different stages of the process. The concept of biorefinery has been developed to improve the economic feasibility of the process. However, there are various challenges in making process profitable and realising the concept in practice, it is necessary to address the issues and overcome the challenges.
As discussed, algae have gained attention as a potential source of feedstock for commercial applications. Life cycle assessment (LCA) is to evaluate and ensure environmentally sustainable, production/consumption of various goods and services. It is a systematic, rigorous, and standardized approach aimed at quantifying resources consumed/depleted, pollutants released, and the related environmental and health impacts through the course of consumption and production of goods/services (Hosseinzadeh-Bandbafha et al. 2019) and thus has been applied widely to algal biorefineries, and results vary across studies (Sills et al. 2020). It has been noticed that LCA of mainly biofuel-related processes have been performed without giving much importance to other biomolecules (Gnansounou and Raman 2016; Roux et al. 2017; Sharma et al. 2018). Production of biomolecules (be it bioactive molecules or biofuels) from microalgae deals with two major steps that involve upstream processes (cultivation aspects) and downstream processes (aspects related to harvesting, cell disruption, and recovery purification). Assessment of process on these steps indicates the feasibility and acceptability of the process and is vital for the identification and mitigation of limiting steps.
Algal production for biomolecules commonly contains stages of algal growth, harvest and extraction. Which are decided based on different algal species, their biomolecule yield and characteristic growth parameters. The life cycle of biomolecules influences the choice of algae as each algal is rich range of biomolecules. For example, C. protothecoides, C. zofingiensis, Ochromonas danica etc., are high lipid-yielding species (Lin et al. 2019; Rebello et al. 2020). Microalgae are mainly used as lipid sources and the lipid is converted into biodiesel employing a wide array of physical, chemical, mechanical, enzymatic, and hydrothermal methods. LCA analysis indicates that the cultivation of microalgal biomass on wastewater is a promising option, as it will reduce the expenses on photoreactors and fresh water and major portion of the cost for algal by-products incurs towards downstream processing methods (or extraction techniques), however, it is suitable for biofuel application. The results of LCA can be different for the production of biomolecules for pharmaceutical and nutraceutical applications. This can be understood with an example of LCA analysis of algal biofuel production employing USLCI recommended conventional method for dewatering, consuming 3556 kg kg−1 of fossil energy. Alternative methods (such as centrifugation and pressing or solar drying) to thermal dewatering can be used at optimized conditions for dewatering. Besides this, other novel methods such as impinging jet mixers (generate turbulence) for mixing lipids with solvents permitting faster lipid recovery of algae to bypass dewatering step (Tseng et al. 2019), and electroporation of C. reinhardtii algae to facilitate easy recovery of by-products (Bodenes et al. 2019). LCA is focused on identification of different strategies helpful in reducing environmental hazards such as the emission of greenhouse gases against base methods (Shi et al. 2019; Wu et al. 2019a). LCA for HC-based operations in microalgae algae-based industry is identified as a knowledge gap, as such reports are not available.
Improvement in process efficiency can also be achieved by properly understanding the micro-processes happening at micro-scale levels during cavitation. Modelling and simulation tools can help to visualize and get insight (Ranade 2022; Ranade et al. 2022). The understanding can be used to modify the conditions to achieve the desired outcome. Models and equations can be developed and explored to gain insight into various dimensions of chemical reactions or physical changes occurring during processing (Ranade and Utikar 2022). The outcome can be used for a better understanding of process and knowledge generation.
Besides using the tools for understanding, management aspects of the processing by developing an onsite establishment (establishing the HC set-up at the site of harvesting and subjecting the wet biomass to cavitation), with an online approach (sequential approach) can be improved. Onsite establishment will be helpful by minimizing the logistics cost, cost of land, biomass availability etc. Onsite set-up will open the possibility of continuous process from both wet, as well as dry bulk biomass to derive high-value low-volume biomolecules. Processing of wet biomass is desirable, as it omits drying and soaking steps, besides easy disruption of cell wall. It is easy to disrupt fresh (wet biomass) as the biomass undergoes several physiological changes such as hardening during drying, possibly adversely affecting the extraction efficiency. Wet biomass leads to better biomolecules both qualitatively and quantitively. The extraction efficiency may also vary with the change in species of microalgae as cellular strength is the function of microalgae species. The semi-processed or final product then can be supplied for further processing or consumption. Process efficiency can also be improved by integrating two or more techniques to maximize the effect by utilizing a synergistic effect. Integration generally results in the improvement of energy efficiency of the process. However, integration warrants more resources and expertise to perform different unit operations in the same process. On a similar platform, HC can be integrated with other methods such as grinding, to achieve higher energy efficiency. During HC the circulation of feed aids in the dissipation of heat generated during cavitation, thus minimising the requirement of a cooling system. HC can overcompensate for the need for multiple US systems, working in series or parallel mode (Das et al. 2022). Thaker and Ranade (2022) reported the energy efficiency of vortex-based devices to be significantly higher than conventional devices. The processing capacity of the vortex-based HC system can be modified by changing the device scale, for example, vortex-based HC device with a larger throat diameter (dt = 3, 6, 12, 48 mm) can be operated for larger volumes for the same processing duration (Sarvothaman et al. 2018; Ranade et al. 2021c; Thaker and Ranade 2022). However, parameters need to be optimised to achieve the same efficiency. Also, rotor–stator-based cavitation systems can be used for pre-treatment as cavitation in a rotor–stator is a function of RPM (rotation per min) and larger volumes can be processed at various flow rates through the device. The use of soundproof systems and earmuffs is also not necessary in the case of HC for its low decibel operating conditions, resulting in no health hazards to the operator (Wu et al. 2018). Making HC a suitable pre-treatment method for larger scale and scale-up.
Hydrodynamic cavitation has huge potential for the processing of microalgae, due to the small cell size of microalgae and the earlier-mentioned advantages of HC. The scope of employing different HC generators for the extraction process, its modelling and simulation are of huge scope in research and development and commercial applications.
Summary and conclusions
Microalgae are a promising source of high-value biomolecules, for various industries and applications with added advantages of reducing carbon footprint to realise the net negative CO2 goal and ability to grow on wastelands and wastewater. For realising the economics, complete utilization of any resource to its full capacity is mandatory, making it necessary to develop an efficient downstream process including pre-treatment for cell disruption and extraction. In this review various aspects of upstream and downstream processing are discussed with an emphasis on HC, it's design and scale-up. It is realised that very limited reports on microalgal pre-treatment by HC are available, opening the doors for HC in the microalgae industry for various commercially important microalgae. As it is not a much-explored field, standardization of the process parameters and attempting various strategies will be required. We hope that this review provides a useful basis and starting point for harnessing HC for the valorisation of microalgae.
Data Availability
Data sharing not applicable to this article as no datasets were generated during the current study.
References
Abbas-Shiroodi Z, Sadeghi M-T, Baradaran S (2021) Design and optimization of a cavitating device for Congo red decolorization: Experimental investigation and CFD simulation. Ultrason Sonochem 71:105386
Abiev RS (2021) Process intensification in chemical engineering: General trends and Russian contribution. Rev Chem Eng 37:69–97
Agarkoti C, Gogate PR, Pandit AB (2022) Coupling of acoustic/hydrodynamic cavitation with ozone (O3), hydrogen peroxide (H2O2), magnesium oxide (MgO) and manganese dioxide (MnO2) for the effective treatment of CETP effluent. Sep Purif Technol 284:120281
Alhattab M, Kermanshahi-Pour A, Brooks MS-L (2019) Microalgae disruption techniques for product recovery: influence of cell wall composition. J Appl Phycol 31:61–88
Almendinger M, Saalfrank F, Rohn S, Kurth E, Springer M, Pleissner D (2020) Characterization of selected microalgae and cyanobacteria as sources of compounds with antioxidant capacity. Algal Res 53:102168
Arashiro LT, Boto-Ordóñez M, Van Hulle SW, Ferrer I, Garfí M, Rousseau DP (2020) Natural pigments from microalgae grown in industrial wastewater. Bioresour Technol 303:122894
Ardiles P, Cerezal-Mezquita P, Salinas-Fuentes F, Órdenes D, Renato G, Ruiz-Domínguez MC (2020) Biochemical composition and phycoerythrin extraction from red microalgae: A comparative study using green extraction technologies. Processes 8:1628
Armaini A, Dharma A, Salim M (2020) The nutraceutical effect of Scenedesmus dimorphus for obesity and nonalcoholic fatty liver disease–linked metabolic syndrome. J Appl Pharmaceut Sci 10:070–076
Aron NSM, Khoo KS, Chew KW, Veeramuthu A, Chang J-S, Show PL (2020) Microalgae cultivation in wastewater and potential processing strategies using solvent and membrane separation technologies. J Water Process Eng 39:101701
Arrojo S, Benito Y, Tarifa AM (2008) A parametrical study of disinfection with hydrodynamic cavitation. Ultrason Sonochem 15:903–908
Arya S, Sawant O, Sonawane SK, Show P, Waghamare A, Hilares R, Santos JCD (2020) Novel, nonthermal, energy efficient, industrially scalable hydrodynamic cavitation–applications in food processing. Food Rev Int 36:668–691
Asaithambi N, Singha P, Dwivedi M, Singh SK (2019) Hydrodynamic cavitation and its application in food and beverage industry: A review. J Food Process Eng 42:e13144
Assunção J, Malcata FX (2020) Enclosed “non-conventional” photobioreactors for microalga production: A review. Algal Res 52:102107
Babadi FE, Boonnoun P, Nootong K, Powtongsook S, Goto M, Shotipruk A (2020) Identification of carotenoids and chlorophylls from green algae Chlorococcum humicola and extraction by liquefied dimethyl ether. Food Bioprod Process 123:296–303
Bachchhav MB, Kulkarni MV, Ingale AG (2020) Process-intensified extraction of phycocyanin followed by β-carotene from Spirulina platensis using ultrasound-assisted extraction. Sep Sci Technol 55:932–944
Badmus KO, Tijani JO, Massima E, Petrik L (2018) Treatment of persistent organic pollutants in wastewater using hydrodynamic cavitation in synergy with advanced oxidation process. Env Sci Pollut Res 25:7299–7314
Badve MP, Alpar T, Pandit AB, Gogate PR, Csoka L (2015) Modeling the shear rate and pressure drop in a hydrodynamic cavitation reactor with experimental validation based on KI decomposition studies. Ultrason Sonochem 22:272–277
Balasundaram B, Harrison S (2006) Disruption of Brewers’ yeast by hydrodynamic cavitation: Process variables and their influence on selective release. Biotechnol Bioeng 94:303–311
Balasundaram B, Pandit A (2001) Selective release of invertase by hydrodynamic cavitation. Biochem Eng J 8:251–256
Banerjee S, Ramaswamy S (2019) Comparison of productivity and economic analysis of microalgae cultivation in open raceways and flat panel photobioreactor. Bioresour Technol Rep 8:100328
Barros AI, Gonçalves AL, Simões M, Pires JC (2015) Harvesting techniques applied to microalgae: a review. Renew Sust Energy Rev 41:1489–1500
Barta DG, Coman V, Vodnar DC (2021) Microalgae as sources of omega-3 polyunsaturated fatty acids: Biotechnological aspects. Algal Res 58:102410
Batista MD, Anhê ACBM, de Souza Inácio Gonçalves JC (2017) Use of hydrodynamic cavitation for algae removal: effect on the inactivation of microalgae belonging to genus Scenedesmus. Water Air Soil Pollut 228:1-8
Bélanger W, Arnold AA, Turcotte F, Saint-Louis R, Deschênes J-S, Genard B, Marcotte I, Tremblay R (2020) Extraction improvement of the bioactive blue–green pigment “marennine” from diatom Haslea ostrearia’s blue water: A solid-phase method based on graphitic matrices. Mar Drugs 18:653
Bermejo R, Ruiz E, Ramos A, Acién FG (2013) Pilot-scale recovery of phycoerythrin from Porphyridium cruentum using expanded bed adsorption chromatography. Sep Sci Technol 48:1913–1922
Bethi B, Sonawane SH, Potoroko I, Bhanvase BA, Sonawane SS (2017) Novel hybrid system based on hydrodynamic cavitation for treatment of dye waste water: A first report on bench scale study. J Env Chem Eng 5:1874–1884
Bhadra S, Salam PA, Sarker NK (2020) Microalgae-based biodiesel production in open raceway ponds using coal thermal flue gas: A case of West Bengal, India. Environ Qual Manage 29:27–36
Bhalamurugan GL, Valerie O, Mark L (2018) Valuable bioproducts obtained from microalgal biomass and their commercial applications: A review. Environ Eng Res 23:229–241
Bhandari VM, Sorokhaibam LG, Ranade VV (2016) Industrial wastewater treatment for fertilizer industry—A case study. Desal Water Treat 57:27934–27944
Biesheuvel PM, Stroeve P, Barneveld PA (2004) Effect of protein adsorption and ionic strength on the equilibrium partition coefficient of ionizable macromolecules in charged nanopores. J Phys Chem B 108:17660–17665
Bimestre TA, Júnior JAM, Canettieri EV, Tuna CE (2022) Hydrodynamic cavitation for lignocellulosic biomass pretreatment: a review of recent developments and future perspectives. Bioresour Bioprocess 9:7
Bodenes P, Bensalem S, Français O, Pareau D, Le Pioufle B, Lopes F (2019) Inducing reversible or irreversible pores in Chlamydomonas reinhardtii with electroporation: Impact of treatment parameters. Algal Res 37:124–132
Bueno M, Gallego R, Chourio AM, Ibáñez E, Herrero M, Saldaña MD (2020) Green ultra-high pressure extraction of bioactive compounds from Haematococcus pluvialis and Porphyridium cruentum microalgae. Innov Food Sci Emerg Technol 66:102532
Burzio E, Bersani F, Caridi G, Vesipa R, Ridolfi L, Manes C (2020) Water disinfection by orifice-induced hydrodynamic cavitation. Ultrason Sonochem 60:104740
Caia M, Bernard O, Béchet Q (2018) Optimizing CO2 transfer in algal open ponds. Algal Res 35:530–538
Callejo-López J, Ramírez M, Cantero D, Bolívar J (2020) Versatile method to obtain protein-and/or amino acid-enriched extracts from fresh biomass of recalcitrant microalgae without mechanical pretreatment. Algal Res 50:102010
Canelli G, Murciano Martínez P, Austin S, Ambühl ME, Dionisi F, Bolten CJ, Carpine R, Neutsch L, Mathys A (2021) Biochemical and morphological characterization of heterotrophic Crypthecodinium cohnii and Chlorella vulgaris cell walls. J Agric Food Chem 69:2226–2235
Cappa OAP, Soeira TVR, Simões ALA, Junior GBL, Gonçalves JCdSI (2020) Experimental and computational analyses for induced cavitating flows in orifice plates. Braz J Chem Eng 37:89–99
Casella P, Musmarra D, Dimatteo S, Chianese S, Karatza D, Mehariya S, Molino A (2020) Purification of astaxanthin from microalgae by using commercial activated carbon. Chem Eng Transact 79:295–300
Castejón N, Señoráns FJ (2019) Simultaneous extraction and fractionation of omega-3 acylglycerols and glycolipids from wet microalgal biomass of Nannochloropsis gaditana using pressurized liquids. Algal Res 37:74–82
Cazarin CBB, Bicas JL, Pastore GM, Junior MRM (Eds) (2021) Bioactive Food Components Activity in Mechanistic Approach. Elsevier, Amsterdam
Cerecedo LM, Dopazo C, Gomez-Lus R (2018) Water disinfection by hydrodynamic cavitation in a rotor-stator device. Ultrason Sonochem 48:71–78
Chandralekha A, Hamsavi G, Sahota S, Mittal R, Tavanandi HA, Raghavarao K (2021) Recent developments in the downstream processing of phycobiliproteins from algae: A review. Curr Biochem Eng 7:13–25
Chandralekha A, Prashanth H, Tavanandi H, Raghavarao K (2020) A novel method for double encapsulation of C-phycocyanin using aqueous two phase systems for extension of shelf life. J Food Sci Technol 58:1750–1763
Chang Y-K, Show P-L, Lan JC-W, Tsai J-C, Huang C-R (2018) Isolation of C-phycocyanin from Spirulina platensis microalga using Ionic liquid based aqueous two-phase system. Bioresour Technol 270:320–327
Chen PH, Quinn JC (2021) Microalgae to biofuels through hydrothermal liquefaction: Open-source techno-economic analysis and life cycle assessment. Appl Energy 289:116613
Cheng J, Yang Z, Ye Q, Zhou J, Cen K (2016) Improving CO2 fixation with microalgae by bubble breakage in raceway ponds with up–down chute baffles. Bioresour Technol 201:174–181
Chethana S, Nayak CA, Madhusudhan M, Raghavarao K (2015) Single step aqueous two-phase extraction for downstream processing of C-phycocyanin from Spirulina platensis. J Food Sci Technol 52:2415–2421
Chronopoulou L, Dal Bosco C, Di Caprio F, Prosini L, Gentili A, Pagnanelli F, Palocci C (2019) Extraction of carotenoids and fat-soluble vitamins from Tetradesmus obliquus microalgae: an optimized approach by using supercritical CO2. Molecules 24:2581
Concha E, Heipieper HJ, Wick LY, Navia R (2018) Effects of limonene, n-decane and n-decanol on growth and membrane fatty acid composition of the microalga Botryococcus braunii. AMB Express 8:189
Costa SS, Peres BP, Machado BR, Costa JAV, Santos LO (2020) Increased lipid synthesis in the culture of Chlorella homosphaera with magnetic fields application. Bioresour Technol 315:123880
Cunha P, Pereira H, Costa M, Pereira J, Silva JT, Fernandes N, Varela J, Silva J, Simões M (2020) Nannochloropsis oceanica cultivation in pilot-scale raceway ponds—from design to cultivation. Appl Sci 10(5):1725
Das RS, Tiwari BK, Chemat F, Garcia-Vaquero M (2022) Impact of ultrasound processing in alternative protein systems: protein extraction, nutritional effects and associated challenges. Ultrason Sonochem 91:106234
Dasan YK, Lam MK, Yusup S, Lim JW, Show PL, Tan IS, Lee KT (2020) Cultivation of Chlorella vulgaris using sequential-flow bubble column photobioreactor: A stress-inducing strategy for lipid accumulation and carbon dioxide fixation. J CO2 Utiliz 41:101226
De Luca M, Pappalardo I, Limongi AR, Viviano E, Radice RP, Todisco S, Martelli G, Infantino V, Vassallo A (2021) Lipids from microalgae for cosmetic applications. Cosmetics 8:52
Del Mondo A, Smerilli A, Sané E, Sansone C, Brunet C (2020) Challenging microalgal vitamins for human health. Microb Cell Fact 19:201
Devi AC, Tavanandi HA, Govindaraju K, Raghavarao K (2020) An effective method for extraction of high purity phycocyanins (C-PC and A-PC) from dry biomass of Arthrospira maxima. J Appl Phycol 32:1141–1151
Dey S, Bhattacharya A, Kumar P, Malik A (2020) High-rate CO2 sequestration using a novel venturi integrated photobioreactor and subsequent valorization to microalgal lipids. Green Chem 22:7962–7973
Di Caprio F, Altimari P, Pagnanelli F (2020) Sequential extraction of lutein and β‐carotene from wet microalgae biomass. J Chem Tech Biotech 95:3024–3033
Diamond A, Hsu J (1992) Aqueous two-phase systems for biomolecule separation. Adv Biochem Eng Biotechnol 47:89–135
Dixon C, Wilken LR (2018) Green microalgae biomolecule separations and recovery. Bioresour Bioprocess 5:1–24
Etchepare R, Oliveira H, Nicknig M, Azevedo A, Rubio J (2017) Nanobubbles: Generation using a multiphase pump, properties and features in flotation. Miner Eng 112:19–26
Fardinpoor M, Perendeci NA, Yılmaz V, Taştan BE, Yılmaz F (2022) Effects of hydrodynamic cavitation-assisted NaOH pretreatment on biofuel production from cyanobacteria: promising approach. BioEnergy Res 15:289–302
Fasaei F, Bitter J, Slegers P, Van Boxtel A (2018) Techno-economic evaluation of microalgae harvesting and dewatering systems. Algal Res 31:347–362
Fayad N, Yehya T, Audonnet F, Vial C (2017) Harvesting of microalgae Chlorella vulgaris using electro-coagulation-flocculation in the batch mode. Algal Res 25:1–11
Fedorov K, Dinesh K, Sun X, Soltani RDC, Wang Z, Sonawane S, Boczkaj G (2021) Synergistic effects of hybrid advanced oxidation processes (AOPs) based on hydrodynamic cavitation phenomenon–a review. Chem Eng J 432:134191
Fernandes T, Cordeiro N (2020) Hemiselmis andersenii and Chlorella stigmatophora as new sources of high-value compounds: a lipidomic approach. J Phycol 56:1493–1504
Fernández FGA, Reis A, Wijffels RH, Barbosa M, Verdelho V, Llamas B (2020) The role of microalgae in the bioeconomy. New Biotechnol 61:99–107
Gaikwad V, Ranade V (2016) Disinfection of water using vortex diode as hydrodynamic cavitation reactor. Asian J Chem 28(8):1867
Galasso C, Gentile A, Orefice I, Ianora A, Bruno A, Noonan DM, Sansone C, Albini A, Brunet C (2019) Microalgal derivatives as potential nutraceutical and food supplements for human health: A focus on cancer prevention and interception. Nutrients 11:1226
Gallego R, Bueno M, Chourio AM, Ibáñez E, Saldaña MD, Herrero M (2020a) Use of high and ultra-high pressure based-processes for the effective recovery of bioactive compounds from Nannochloropsis oceanica microalgae. J Supercrit Fluids 167:105039
Gallego R, Martínez M, Cifuentes A, Ibáñez E, Herrero M (2019) Development of a green downstream process for the valorization of Porphyridium cruentum biomass. Molecules 24:1564
Gallego R, Tardif C, Parreira C, Guerra T, Alves MJ, Ibáñez E, Herrero M (2020b) Simultaneous extraction and purification of fucoxanthin from Tisochrysis lutea microalgae using compressed fluids. J Sep Sci 43:1967–1977
García AB, Longo E, Murillo M, Bermejo R (2021) Using a B-phycoerythrin extract as a natural colorant: Application in milk-based products. Molecules 26:297
Gargouch N, Karkouch I, Elleuch J, Elkahoui S, Michaud P, Abdelkafi S, Laroche C, Fendri I (2018) Enhanced B-phycoerythrin production by the red microalga Porphyridium marinum: A powerful agent in industrial applications. Int J Biol Macromol 120:2106–2114
Garuti M, Sinisgalli E, Soldano M, Fermoso FG, Rodriguez AJ, Carnevale M, Gallucci F (2022) Mechanical pretreatments of different agri-based feedstock in full-scale biogas plants under real operational conditions. Biomass Bioenergy 158:106352
Gautam S, Mannan MAU (2020) The Role of Algae in Nutraceutical and Pharmaceutical Production. In: Singh J, Meshram V, Gupta M (eds) Bioactive Natural products in Drug Discovery. Springer, Singapore, pp 665–685
Geada P, Moreira C, Silva M, Nunes R, Madureira L, Rocha CM, Pereira RN, Vicente AA, Teixeira JA (2021) Algal proteins: Production strategies and nutritional and functional properties. Bioresour Technol 332:125125
Gerken HG, Donohoe B, Knoshaug EP (2013) Enzymatic cell wall degradation of Chlorella vulgaris and other microalgae for biofuels production. Planta 237:239–253
Gharajeh NH, Valizadeh M, Dorani E, Hejazi MA (2020) Dunaliella sp. ABRIINW-I1 as a cell factory of nutraceutical fatty acid pattern: An optimization approach to improved production of docosahexaenoic acid (DHA). Chem Eng Process-Process Intensif 155:108073
Gnansounou E, Raman JK (2016) Life cycle assessment of algae biodiesel and its co-products. Appl Energy 161:300–308
Gogate PR, Pandit AB (2008) Application of cavitational reactors for cell disruption for recovery of intracellular enzymes. J Chem Technol Biotechnol 83:1083–1093
Gómez-Zorita S, Trepiana J, González-Arceo M, Aguirre L, Milton-Laskibar I, González M, Eseberri I, Fernández-Quintela A, Portillo MP (2020) Anti-obesity effects of microalgae. Int J Mol Sci 21:41
Gong M, Wang Y, Bassi A (2017) Process analysis and modeling of a single-step lutein extraction method for wet microalgae. Appl Microbiol Biotechnol 101:8089–8099
Gostiša J, Širok B, Repinc SK, Levstek M, Stražar M, Bizjan B, Zupanc M (2021) Performance evaluation of a novel pilot-scale pinned disc rotating generator of hydrodynamic cavitation. Ultrason Sonochem 72:105431
Goswami RK, Agrawal K, Verma P (2021) Microalgae-based biofuel-integrated biorefinery approach as sustainable feedstock for resolving energy crisis. In: Srivastava M, Sivastava N, Singh R (eds) Bioenergy research: Commercial opportunities & challenges. Springer, Singapore, pp 267–293
Greenly JM, Tester JW (2015) Ultrasonic cavitation for disruption of microalgae. Bioresour Technol 184:276–279
Grillo G, Boffa L, Binello A, Mantegna S, Cravotto G, Chemat F, Dizhbite T, Lauberte L, Telysheva G (2019) Cocoa bean shell waste valorisation; extraction from lab to pilot-scale cavitational reactors. Food Res Int 115:200–208
Gross M, Henry W, Michael C, Wen Z (2013) Development of a rotating algal biofilm growth system for attached microalgae growth with in situ biomass harvest. Bioresour Technol 150:195–201
Gupta J, Gupta R (2020) Nutraceutical status and scientific strategies for enhancing production of omega-3 fatty acids from microalgae and their role in healthcare. Curr Pharm Biotechnol 21: 1616–1631
Hafiz MA, Hawari AH, Das P, Khan S, Altaee A (2020) Comparison of dual stage ultrafiltration and hybrid ultrafiltration-forward osmosis process for harvesting microalgae (Tetraselmis sp.) biomass. Chem Eng Process-Process Intensif 157:108112
Halim R, Rupasinghe TW, Tull DL, Webley PA (2013) Mechanical cell disruption for lipid extraction from microalgal biomass. Bioresour Technol 140:53–63
Harnedy PA, FitzGerald RJ (2013) Extraction of protein from the macroalga Palmaria palmata. LWT-Food Sci Technol 51:375–382
Hilares RT, de Almeida GF, Ahmed MA, Antunes FA, da Silva SS, Han J-I, Dos Santos JC (2017) Hydrodynamic cavitation as an efficient pretreatment method for lignocellulosic biomass: A parametric study. Bioresour Technol 235:301–308
Hilares RT, Dionízio RM, Muñoz SS, Prado CA, de Sousa JR, da Silva SS, Santos JC (2020) Hydrodynamic cavitation-assisted continuous pre-treatment of sugarcane bagasse for ethanol production: Effects of geometric parameters of the cavitation device. Ultrason Sonochem 63:104931
Hosseinzadeh-Bandbafha H, Tabatabaei M, Aghbashlo M, Sulaiman A, Ghassemi A (2019) Life-Cycle Assessment (LCA) analysis of algal fuels. In: Spilling K (Ed) Biofuels from Algae. Humana, New York, pp 121–151
Ibáñez-González MJ, Mazzuca-Sobczuk T, Redondo-Miranda RM, Molina-Grima E, Cooney CL (2016) A novel vortex flow reactor for the purification of B-phycoerythrin from Porphyridium cruentum. Chem Eng Res Design 111:24–33
Imbimbo P, Bueno M, D’Elia L, Pollio A, Ibañez E, Olivieri G, Monti DM (2020) Green compressed fluid technologies to extract antioxidants and lipids from Galdieria phlegrea in a biorefinery approach. ACS Sust Chem Eng 8:2939–2947
Iovine A, Cerbone A, Mehariya S, Musmarra D, Casella P, Molino A (2019) Effect of mechanical pretreatment on Nannochloropsis gaditana on the extraction of omega-3 by using accelerated solvent extraction technology. Chem Eng Trans 74:943–948
Iskalieva A, Yimmou BM, Gogate PR, Horvath M, Horvath PG, Csoka L (2012) Cavitation assisted delignification of wheat straw: a review. Ultrason Sonochem 19:984–993
Itoh K, Kashino Y, Ifuku K, Kouji M, Yamamoto T, Taguchi S (2019) Dispersed air flotation of microalgae using venturi tube type microbubble generator. Biomass Bioenergy 130:105379
Jacob-Lopes E, Maroneze MM, Deprá MC, Sartori RB, Dias RR, Zepka LQ (2019) Bioactive food compounds from microalgae: an innovative framework on industrial biorefineries. Curr Opin Food Sci 25:1–7
Jadhav AJ, Ferraro G, Barigou M (2021) Generation of bulk nanobubbles using a high-shear rotor–stator device. Indust Eng Chem Res 60:8597–8606
Jaeschke DP, Rech R, Marczak LDF, Mercali GD (2017) Ultrasound as an alternative technology to extract carotenoids and lipids from Heterochlorella luteoviridis. Bioresour Technol 224:753–757
Jain P, Bhandari VM, Balapure K, Jena J, Ranade VV, Killedar DJ (2019) Hydrodynamic cavitation using vortex diode: An efficient approach for elimination of pathogenic bacteria from water. J Environ Manage 242:210–219
Joshi SM, Gogate PR (2019) Intensification of industrial wastewater treatment using hydrodynamic cavitation combined with advanced oxidation at operating capacity of 70 L. Ultrason Sonochem 52:375–381
Junior WGM, Gorgich M, Corrêa PS, Martins AA, Mata TM, Caetano NS (2020) Microalgae for biotechnological applications: Cultivation, harvesting and biomass processing. Aquaculture 528:735562
Kadam SU, Tiwari BK, Smyth TJ, O’Donnell CP (2015) Optimization of ultrasound assisted extraction of bioactive components from brown seaweed Ascophyllum nodosum using response surface methodology. Ultrason Sonochem 23:308–316
Kaewdam S, Jaturonglumlert S, Varith J, Nitatwichit C, Narkprasom K (2020) Effect of isothermal and thermal diffusion on aqueous two-phase extraction for the purification of C-phycocyanin from Spirulina platensis. Int Food Res J 27:280-286
Katiyar R, Arora A (2020) Health promoting functional lipids from microalgae pool: A review. Algal Res 46:101800
Keris-Sen UD, Sen U, Soydemir G, Gurol MD (2014) An investigation of ultrasound effect on microalgal cell integrity and lipid extraction efficiency. Bioresour Technol 152:407–413
Khan TA, Khan TA, Kumar Yadav A (2022) A hydrodynamic cavitation-assisted system for optimization of biodiesel production from green microalgae oil using a genetic algorithm and response surface methodology approach. Env Sci Pollut Res 29:49465–49477
Khanra S, Mondal M, Halder G, Tiwari O, Gayen K, Bhowmick TK (2018) Downstream processing of microalgae for pigments, protein and carbohydrate in industrial application: A review. Food Bioprod Process 110:60–84
Khoo KS, Chew KW, Yew GY, Manickam S, Ooi CW, Show PL (2020a) Integrated ultrasonic assisted liquid biphasic flotation for efficient extraction of astaxanthin from Haematococcus pluvialis. Ultrason Sonochem 67:105052
Khoo KS, Chong YM, Chang WS, Yap JM, Foo SC, Khoiroh I, Lau PL, Chew KW, Ooi CW, Show PL (2020b) Permeabilization of Chlorella sorokiniana and extraction of lutein by distillable CO2-based alkyl carbamate ionic liquids. Sep Purif Technol 256:117471
Khoo KS, Ooi CW, Chew KW, Foo SC, Lim JW, Tao Y, Jiang N, Ho S-H, Show PL (2021) Permeabilization of Haematococcus pluvialis and solid-liquid extraction of astaxanthin by CO2-based alkyl carbamate ionic liquids. Chem Eng J 411:128510
Khoo KS, Ooi CW, Chew KW, Foo SC, Show PL (2020c) Bioprocessing of Chaetoceros calcitrans for the recovery of fucoxanthin using CO2-based alkyl carbamate ionic liquids. Bioresour Technol 322:124520
Kim D, Kim EK, Koh HG, Kim K, Han J-I, Chang YK (2017) Selective removal of rotifers in microalgae cultivation using hydrodynamic cavitation. Algal Res 28:24–29
Kim I, Lee I, Jeon SH, Hwang T, Han J-I (2015a) Hydrodynamic cavitation as a novel pretreatment approach for bioethanol production from reed. Bioresour Technol 192:335–339
Kim K, Choi J, Ji Y, Park S, Do H, Hwang C, Lee B, Holzapfel W (2014) Impact of bubble size on growth and CO2 uptake of Arthrospira (Spirulina) platensis KMMCC CY-007. Bioresour Technol 170:310–315
Kim K, Shin H, Moon M, Ryu B-G, Han J-I, Yang J-W, Chang YK (2015b) Evaluation of various harvesting methods for high-density microalgae, Aurantiochytrium sp. KRS101. Bioresour Technol 198:828–835
Kim M, Kim D, Cho JM, Nam K, Lee H, Nayak M, Han J-I, Oh H-M, Chang YK (2021) Hydrodynamic cavitation for bacterial disinfection and medium recycling for sustainable Ettlia sp. cultivation. J Env Chem Eng 9(4):105411
Kim S-K, Chojnacka K (eds) (2015) Marine algae extracts: processes, products, and applications. John Wiley & Sons
Kokkali M, Martí-Quijal FJ, Taroncher M, Ruiz M-J, Kousoulaki K, Barba FJ (2020) Improved extraction efficiency of antioxidant bioactive compounds from Tetraselmis chuii and Phaedoactylum tricornutum using pulsed electric fields. Molecules 25:3921
Kolhe NS, Gupta AR, Rathod VK (2017) Production and purification of biodiesel produced from used frying oil using hydrodynamic cavitation. Resour-Efficient Technol 3:198–203
Konde KS, Nagarajan S, Kumar V, Patil SV, Ranade VV (2021) Sugarcane bagasse based biorefineries in India: potential and challenges. Sustain Energy Fuels 5:52–78
Kumar BR, Mathimani T, Sudhakar M, Rajendran K, Nizami A-S, Brindhadevi K, Pugazhendhi A (2021) A state of the art review on the cultivation of algae for energy and other valuable products: Application, challenges, and opportunities. Renew Sustain Energy Rev 138:110649
Kumar S, Cheng J, Kubar AA, Guo W, Song Y, Liu S, Chen S, Tian J (2020) Orange light spectra filtered through transparent colored polyvinyl chloride sheet enhanced pigment content and growth of Arthrospira cells. Bioresour Technol 319:124179
Kurokawa M, King PM, Wu X, Joyce EM, Mason TJ, Yamamoto K (2016) Effect of sonication frequency on the disruption of algae. Ultrason Sonochem 31:157–162
Kusmayadi A, Suyono EA, Nagarajan D, Chang JS, Yen HW (2020) Application of computational fluid dynamics (CFD) on the raceway design for the cultivation of microalgae: a review. J Indust Microbiol Biotechnol 47:373–382
Lama S, Muylaert K, Karki TB, Foubert I, Henderson RK, Vandamme D (2016) Flocculation properties of several microalgae and a cyanobacterium species during ferric chloride, chitosan and alkaline flocculation. Bioresour Technol 220:464–470
Lamdande AG, Mittal R, Raghavarao K (2020) Flux evaluation based on fouling mechanism in acoustic field-assisted ultrafiltration for cold sterilization of tender coconut water. Innov Food Sci Emerg Technol 61:102312
Lauberte L, Telysheva G, Cravotto G, Andersone A, Janceva S, Dizhbite T, Arshanitsa A, Jurkjane V, Vevere L, Grillo G (2021) Lignin–derived antioxidants as value-added products obtained under cavitation treatments of the wheat straw processing for sugar production. J Clean Prod 303:126369
Le-Feuvre R, Moraga-Suazo P, Gonzalez J, San Martin S, Henríquez V, Donoso A, Agurto-Muñoz C (2020) Biotechnology applied to Haematococcus pluvialis Fotow: challenges and prospects for the enhancement of astaxanthin accumulation. J Appl Phycol 32:3831–3852
Le Guillard C, Bergé J-P, Donnay-Moreno C, Bruzac S, Ragon J-Y, Baron R, Fleurence J, Dumay J (2016) Soft liquefaction of the red seaweed Grateloupia turuturu Yamada by ultrasound-assisted enzymatic hydrolysis process. J Appl Phycol 28:2575–2585
Leam JJ, Bilad MR, Wibisono Y, Wirzal MDH, Ahmed I (2020) Membrane technology for microalgae harvesting. In: Yousof, A (ed) Microalgae Cultivation for Biofuels Production. Academic Press, Amsterdam pp 97–110
Lee AK, Lewis DM, Ashman PJ (2015) Microalgal cell disruption by hydrodynamic cavitation for the production of biofuels. J Appl Phycol 27:1881–1889
Lee I, Han J-I (2015) Simultaneous treatment (cell disruption and lipid extraction) of wet microalgae using hydrodynamic cavitation for enhancing the lipid yield. Bioresour Technol 186:246–251
Lee I, Oh Y-K, Han J-I (2019) Design optimization of hydrodynamic cavitation for effectual lipid extraction from wet microalgae. J Environ Chem Eng 7:102942
Lee SY, Khoiroh I, Vo D-VN, Senthil Kumar P, Show PL (2021) Techniques of lipid extraction from microalgae for biofuel production: a review. Environ Chem Lett 19:231–251
Leone GP, Balducchi R, Mehariya S, Martino M, Larocca V, Di Sanzo G, Iovine A, Casella P, Marino T, Karatza D (2019) Selective extraction of ω-3 fatty acids from Nannochloropsis sp. using supercritical CO2 extraction. Molecules 24(13):2406
Levasseur W, Perré P, Pozzobon V (2020) A review of high value-added molecules production by microalgae in light of the classification. Biotech Adv 41:107545
Li M, Bussonnière A, Bronson M, Xu Z, Liu Q (2019a) Study of Venturi tube geometry on the hydrodynamic cavitation for the generation of microbubbles. Miner Eng 132:268–274
Li T, Cui Z, Sun J, Jiang C, Li G (2021) Generation of bulk nanobubbles by self-developed venturi-type circulation hydrodynamic cavitation device. Langmuir 37:12952–12960
Li T, Xu J, Wu H, Jiang P, Chen Z, Xiang W (2019b) Growth and biochemical composition of Porphyridium purpureum SCS-02 under different nitrogen concentrations. Mar Drugs 17:124
Lima A, Marinho B, Morais T (2021) Hydrodynamic analysis of flow in raceway ponds for algae cultivation under versatile conditions. Aquacult Int 29:19–35
Lima S, Schulze PS, Schüler LM, Rautenberger R, Morales-Sánchez D, Santos TF, Pereira H, Varela JC, Scargiali F, Wijffels RH (2020) Flashing light emitting diodes (LEDs) induce proteins, polyunsaturated fatty acids and pigments in three microalgae. J Biotech 325:15–24
Lin Z, Li C, Ju LK (2019) Glycerol and acetate additions to maximize lipid content in high-density cultures of phagotrophic alga Ochromonas danica. J Am Oil Chem Soc 96:231–238
Liu J, Zhu Y, Tao Y, Zhang Y, Li A, Li T, Sang M, Zhang C (2013) Freshwater microalgae harvested via flocculation induced by pH decrease. Biotechnol Biofuels 6:98
Liu P, Wang T, Yang Z, Hong Y, Xie X, Hou Y (2020a) Effects of Fe3O4 nanoparticle fabrication and surface modification on Chlorella sp. harvesting efficiency. Sci Total Environ 704:135286
Liu W, Cui Y, Cheng P, Huo S, Ma X, Chen Q, Cobb K, Chen P, Ma J, Gao X (2020) Microwave assisted flocculation for harvesting of Chlorella vulgaris. Biores Technol 314:123770
Liu Y, Jin W, Zhou X, Han S-F, Tu R, Feng X, Jensen PD, Wang Q (2019) Efficient harvesting of Chlorella pyrenoidosa and Scenedesmus obliquus cultivated in urban sewage by magnetic flocculation using nano-Fe3O4 coated with polyethyleneimine. Bioresour Technol 290:121771
Liu Y, Liu X, Cui Y, Yuan W (2022) Ultrasound for microalgal cell disruption and product extraction: A review. Ultrason Sonochem 87:106054
Lohani UC, Muthukumarappan K, Meletharayil GH (2016) Application of hydrodynamic cavitation to improve antioxidant activity in sorghum flour and apple pomace. Food Bioprod Process 100:335–343
Lu M-M, Gao F, Li C, Yang H-L (2021) Response of microalgae Chlorella vulgaris to Cr stress and continuous Cr removal in a membrane photobioreactor. Chemosphere 262:128422
Luo X, Smith P, Raston C, Zhang W (2016) Vortex fluidic device-intensified aqueous two phase extraction of C-phycocyanin from Spirulina maxima. ACS Sust Chem Eng 4:3905–3911
Lupette J, Benning C (2020) Human health benefits of very-long-chain polyunsaturated fatty acids from microalgae. Biochimie 178:15–25
Ma J, Xia W, Fu X, Ding L, Kong Y, Zhang H, Fu K (2020) Magnetic flocculation of algae-laden raw water and removal of extracellular organic matter by using composite flocculant of Fe3O4/cationic polyacrylamide. J Clean Product 248:119276
Machado L, Carvalho G, Pereira RN (2022) Effects of innovative processing methods on microalgae cell wall: prospects towards digestibility of protein-rich biomass. Biomass 2:80–102
Madhubalaji C, Chandra TS, Chauhan V, Sarada R, Mudliar SN (2020) Chlorella vulgaris cultivation in airlift photobioreactor with transparent draft tube: effect of hydrodynamics, light and carbon dioxide on biochemical profile particularly ω-6/ω-3 fatty acid ratio. J Food Sci Technol 57:866–876
Mane MB, Bhandari VM, Balapure K, Ranade VV (2020) Destroying antimicrobial resistant bacteria (AMR) and difficult, opportunistic pathogen using cavitation and natural oils/plant extract. Ultrason Sonochem 69:105272
Mane MB, Bhandari VM, Ranade VV (2021) Safe water and technology initiative for water disinfection: Application of natural plant derived materials. J Water Process Eng 43:102280
Marcati A, Ursu AV, Laroche C, Soanen N, Marchal L, Jubeau S, Djelveh G, Michaud P (2014) Extraction and fractionation of polysaccharides and B-phycoerythrin from the microalga Porphyridium cruentum by membrane technology. Algal Res 5:258–263
Marinoa T, Casellab P, Sangiorgioc P, Verardic A, Ferrarod A, Hristoforoud E, Molinob A, Musmarraa D (2020) Natural beta-carotene: A microalgae derivate for nutraceutical applications. Chem Eng 79:103–108
Martínez JM, Delso C, Álvarez I, Raso J (2019a) Pulsed electric field permeabilization and extraction of phycoerythrin from Porphyridium cruentum. Algal Res 37:51–56
Martínez JM, Gojkovic Z, Ferro L, Maza M, Álvarez I, Raso J, Funk C (2019b) Use of pulsed electric field permeabilization to extract astaxanthin from the Nordic microalga Haematococcus pluvialis. Bioresour Technol 289:121694
Matanguihan AED, Demafelis RB, Martinez-Goss M, Nacorda JOO, Torreta NK, Sanchez DES, Cuneta LF, Eleazar EMP, Dizon LSH (2020) Design, fabrication, and performance evaluation of open raceway ponds for the cultivation of Chlorella vulgaris Beijerinck in the Philippines. Philippine J Sci 149:383–392
Matman N, Oo YM, Amnuaikit T, Somnuk K (2022) Continuous production of nanoemulsion for skincare product using a 3D-printed rotor-stator hydrodynamic cavitation reactor. Ultrason Sonochem 83:105926
Mehariya S, Fratini F, Lavecchia R, Zuorro A (2021) Green extraction of value-added compounds form microalgae: A short review on natural deep eutectic solvents (NaDES) and related pre-treatments. J Environ Chem Eng 9:105989
Menegotto ALL, Fernandes IA, Colla LM, Duarte J, Andrade MZ, Abirached C, Franceschi E, Steffens J, Valduga E (2020) Thermic and techno-functional properties of Arthrospira platensis protein fractions obtained by membrane separation process. J Appl Phycol 32:3885–3900
Mensi F (2019) Agar yield from R-phycoerythrin extraction by-product of the red alga Gracilaria verrucosa. J Appl Phycol 31:74–751
Mevada J, Devi S, Pandit A (2019) Large scale microbial cell disruption using hydrodynamic cavitation: Energy saving options. Biochem Eng J 143:151–160
Miguel SP, Ribeiro MP, Otero A, Coutinho P (2021) Application of microalgae and microalgal bioactive compounds in skin regeneration. Algal Res 58:102395
Milano J, Ong HC, Masjuki H, Chong W, Lam MK, Loh PK, Vellayan V (2016) Microalgae biofuels as an alternative to fossil fuel for power generation. Renew Sust Energy Revs 58:180–197
Min B, Kim SK, Kim JW (2018) Optimization of microwave-assisted pretreatment conditions for enzyme-free hydrolysis of lipid extracted microalgae. Kor Chem Eng Res 56:229–239
Mittal R, Lamdande AG, Sharma R, Raghavarao K (2020) Membrane processing for purification of R-Phycoerythrin from marine macro-alga, Gelidium pusillum and process integration. Separat Purif Technol 252:117470
Mittal R, Raghavarao K (2018) Extraction of R-Phycoerythrin from marine macro-algae, Gelidium pusillum, employing consortia of enzymes. Algal Res 34:1–11
Mittal R, Ranade VV (2023) Intensifying extraction of biomolecules from macroalgae using vortex based hydrodynamic cavitation device. Ultrason Sonochem 94:106347
Mittal R, Sharma R, Raghavarao K (2019) Aqueous two-phase extraction of R-Phycoerythrin from marine macro-algae, Gelidium pusillum. Bioresour Technol 280:277–286
Mittal R, Sharma R, Raghavarao K (2022) Novel adsorption approach for the enrichment of R-phycoerythrin from marine macroalga Gelidium pusillum. Algal Res 62:102605
Mittal R, Tavanandi HA, Mantri VA, Raghavarao K (2017) Ultrasound assisted methods for enhanced extraction of phycobiliproteins from marine macro-algae, Gelidium pusillum (Rhodophyta). Ultrason Sonochem 38:92–103
Molino A, Iovine A, Leone GP, Di Sanzo G, Palazzo S, Martino M, Sangiorgio P, Marino T, Musmarra D (2020) Microalgae as alternative source. Chem Eng Trans 79:277–282
Molino A, Rimauro J, Casella P, Cerbone A, Larocca V, Chianese S, Karatza D, Mehariya S, Ferraro A, Hristoforou E (2018) Extraction of astaxanthin from microalga Haematococcus pluvialis in red phase by using generally recognized as safe solvents and accelerated extraction. J Biotech 283:51–61
Monte J, Ribeiro C, Parreira C, Costa L, Brive L, Casal S, Brazinha C, Crespo JG (2020) Biorefinery of Dunaliella salina: Sustainable recovery of carotenoids, polar lipids and glycerol. Bioresour Technol 297:122509
Monte J, Sá M, Galinha CF, Costa L, Hoekstra H, Brazinha C, Crespo JG (2018) Harvesting of Dunaliella salina by membrane filtration at pilot scale. Sep Purif Technol 190:252–260
Morales M, Bonnefond H, Bernard O (2020) Rotating algal biofilm versus planktonic cultivation: LCA perspective. J Clean Prod 257:120547
Morandeira L, Martínez-Baltasar A, Sanromán MÁ, Rodríguez A, Deive FJ (2020) Designing novel biocompatible oligopeptide-based ionic liquids for greener downstream processes. J Clean Prod 279:123356
More NS, Gogate PR (2018) Intensified degumming of crude soybean oil using cavitational reactors. J Food Eng 218:33–43
Morocho-Jácome AL, Ruscinc N, Martinez RM, de Carvalho JCM, de Almeida TS, Rosado C, Costa JG, Velasco MVR, Baby AR (2020) (Bio) Technological aspects of microalgae pigments for cosmetics. Appl Microbiol Biotechnol 104:9513–9522
Mouahid A, Seengeon K, Martino M, Crampon C, Kramer A, Badens E (2020) Selective extraction of neutral lipids and pigments from Nannochloropsis salina and Nannochloropsis maritima using supercritical CO2 extraction: Effects of process parameters and pre-treatment. J Supercrit Fluids 165:104934
Mourelle ML, Gómez CP, Legido JL (2017) The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics 4:46
Mu N, Mehar JG, Mudliar SN, Shekh AY (2019) Recent advances in microalgal bioactives for food, feed, and healthcare products: commercial potential, market space, and sustainability. Compr Rev Food Sci Food Saf 18:1882–1897
Mule CM, Doltade SB, Pandit AB (2021) A review on pesticide degradation from irrigation water and techno-economic feasibility of treatment technologies. Water Environ Res 93:2391–2413
Munier M, Jubeau S, Wijaya A, Morançais M, Dumay J, Marchal L, Jaouen P, Fleurence J (2014) Physicochemical factors affecting the stability of two pigments: R-phycoerythrin of Grateloupia turuturu and B-phycoerythrin of Porphyridium cruentum. Food Chem 150:400–407
Nafis GA, Mumpuni PY, Indarto, Budiman (2015) A Combination pulsed electric field with ethanol solvent for Nannochloropsis sp. extraction In: AIP Conference Proceedings 1699:030021
Nagarajan S, Ranade VV (2019) Pretreatment of lignocellulosic biomass using vortex-based devices for cavitation: influence on biomethane potential. Ind Eng Chem Res 58:15975–15988
Nagarajan S, Ranade VV (2020) Pre-treatment of distillery spent wash (vinasse) with vortex based cavitation and its influence on biogas generation. Bioresour Technol Rep 11:100480
Nagarajan S, Ranade VV (2022) Pretreatment of milled and unchopped sugarcane bagasse with vortex based hydrodynamic cavitation for enhanced biogas production. Bioresour Technol 361:127663
Nalawade K, Saikia P, Behera S, Konde K, Patil S (2020) Assessment of multiple pretreatment strategies for 2G L-lactic acid production from sugarcane bagasse. Biomass Conversion Biorefinery 13:647–660
Narayan A, Madhusudhan M, Raghavarao K (2008) Extraction and purification of Ipomoea peroxidase employing three-phase partitioning. Appl Biochem Biotechnol 151:263–272
Navarro-López E, del Carmen Cerón-García M, López-Rodríguez M, Acién-Fernández FG, Molina-Grima E (2020) Biostimulants obtained after pilot-scale high-pressure homogenization of Scenedesmus sp. grown in pig manure. Algal Res 52:102123
Nazari S, Chelgani SC, Shafaei S, Shahbazi B, Matin S, Gharabaghi M (2019) Flotation of coarse particles by hydrodynamic cavitation generated in the presence of conventional reagents. Sep Purif Technol 220:61–68
Nazari S, Hassanzadeh A, He Y, Khoshdast H, Kowalczuk PB (2022) Recent developments in generation, detection and application of nanobubbles in flotation. Minerals 12:462
Nwoba EG, Parlevliet DA, Laird DW, Alameh K, Moheimani NR (2020) Outdoor phycocyanin production in a standalone thermally-insulated photobioreactor. Bioresour Technol 315:123865
Ogretmen ÖY, Duyar HA (2018) The effect of different extraction methods and pre-treatments on agar yield and physico-chemical properties of Gelidium latifolium (Gelidiaceae, Rhodophyta) from Sinop Peninsula Coast of Black Sea, Turkey. J Appl Phycol 30:1355–1360
Oliveira H, Azevedo A, Rubio J (2018) Nanobubbles generation in a high-rate hydrodynamic cavitation tube. Miner Eng 116:32–34
Omelyanyuk M, Ukolov A, Pakhlyan I, Bukharin N, El Hassan M (2022) Experimental and numerical study of cavitation number limitations for hydrodynamic cavitation inception prediction. Fluids 7:198
Otero P, Quintana SE, Reglero G, Fornari T, García-Risco MR (2018) Pressurized liquid extraction (ple) as an innovative green technology for the effective enrichment of Galician Algae extracts with high quality fatty acids and antimicrobial and antioxidant properties. Mar Drugs 16:156
Pagels F, Pereira RN, Vicente AA, Guedes AC (2021) Extraction of pigments from microalgae and cyanobacteria—A review on current methodologies. Appl Sci 11:5187
Pahl S, Lee A, Kalaitzidis T, Ashman P, Sathe S, Lewis D (2013) Harvesting, Thickening and dewatering microalgae biomass. In: Borowitzka MA, Moheimani NR (eds) Algae for Biofuels and Energy. Springer, Dordrecht, pp 165–185
Panda D, Manickam S (2019) Cavitation technology—The future of greener extraction method: A review on the extraction of natural products and process intensification mechanism and perspectives. Appl Sci 9:766
Panda D, Saharan VK, Manickam S (2020) Controlled hydrodynamic cavitation: a review of recent advances and perspectives for greener processing. Processes 8:220
Pandey A, Pathak VV, Kothari R, Black PN, Tyagi V (2019) Experimental studies on zeta potential of flocculants for harvesting of algae. J Environ Manage 231:562–569
Patel AK, Singhania RR, Chen C-W, Tseng Y-S, Kuo C-H, Wu C-H, Di Dong C (2021) Advances in micro-and nano bubbles technology for application in biochemical processes. Environ Technol Innov 23:101729
Pathania S, Ho QT, Hogan SA, McCarthy N, Tobin JT (2018) Applications of hydrodynamic cavitation for instant rehydration of high protein milk powders. J Food Eng 225:18–25
Patil PB, Bhandari VM, Ranade VV (2021a) Improving efficiency for removal of ammoniacal nitrogen from wastewaters using hydrodynamic cavitation. Ultrason Sonochem 70:105306
Patil PB, Bhandari VM, Ranade VV (2021b) Wastewater treatment and process intensification for degradation of solvents using hydrodynamic cavitation. Chem Eng Process-Process Intensif 166:108485
Peng J, Kumar K, Gross M, Kunetz T, Wen Z (2020) Removal of total dissolved solids from wastewater using a revolving algal biofilm reactor. Water Environ Res 92:766–778
Perin G, Bellan A, Bernardi A, Bezzo F, Morosinotto T (2019) The potential of quantitative models to improve microalgae photosynthetic efficiency. Physiol Plant 166:380–391
Porav AS, Bocăneală M, Fălămaş A, Bogdan DF, Barbu-Tudoran L, Hegeduş A, Dragoş N (2020) Sequential aqueous two-phase system for simultaneous purification of cyanobacterial phycobiliproteins. Bioresour Technol 315:123794
Pourkarimi Z, Rezai B, Noaparast M, Nguyen A, Chelgani SC (2021) Proving the existence of nanobubbles produced by hydrodynamic cavitation and their significant effects in powder flotation. Adv Powder Technol 32:1810–1818
Prabhu MS, Levkov K, Livney YD, Israel A, Golberg A (2019) High-voltage pulsed electric field preprocessing enhances extraction of starch, proteins, and ash from marine macroalgae Ulva ohnoi. ACS Sustain Chem Eng 7:17453–17463
Prajapati SK, Kumar P, Malik A, Choudhary P (2014) Exploring pellet forming filamentous fungi as tool for harvesting non-flocculating unicellular microalgae. BioEnergy Res 7:1430–1440
Praveenkumar R, Lee K, Lee J, Oh Y-K (2015) Breaking dormancy: an energy-efficient means of recovering astaxanthin from microalgae. Green Chem 17:1226–1234
Prochazkova G, Kastanek P, Branyik T (2015) Harvesting freshwater Chlorella vulgaris with flocculant derived from spent brewer’s yeast. Bioresour Technol 177:28–33
Qiu C, He Y, Huang Z, Li S, Huang J, Wang M, Chen B (2019) Lipid extraction from wet Nannochloropsis biomass via enzyme-assisted three phase partitioning. Bioresour Technol 284:381–390
Quinn JC, Davis R (2015) The potentials and challenges of algae based biofuels: a review of the techno-economic, life cycle, and resource assessment modeling. Bioresour Technol 184:444–452
Raeisossadati M, Moheimani NR (2020) Can luminescent solar concentrators increase microalgal growth on anaerobically digested food effluent? J Appl Phycol 32:3703–3710
Raeisossadati M, Moheimani NR, Parlevliet D (2020) Red luminescent solar concentrators to enhance Scenedesmus sp. biomass productivity. Algal Res 45:101771
Raghavarao K, Rastogi N, Gowthaman M, Karanth N (1995) Aqueous two-phase extraction for downstream processing of enzymes/proteins. Adv Appl Microbiol 41:97–171
Rajasekhar P, Fan L, Nguyen T, Roddick FA (2012) Impact of sonication at 20 kHz on Microcystis aeruginosa, Anabaena circinalis and Chlorella sp. Water Res 46:1473–1481
Ramola B, Kumar V, Nanda M, Mishra Y, Tyagi T, Gupta A, Sharma N (2019) Evaluation, comparison of different solvent extraction, cell disruption methods and hydrothermal liquefaction of Oedogonium macroalgae for biofuel production. Biotechnol Rep 22:e00340
Ranade NV, Nagarajan S, Sarvothaman V, Ranade VV (2021a) ANN based modelling of hydrodynamic cavitation processes: Biomass pre-treatment and wastewater treatment. Ultrason Sonochem 72:105428
Ranade NV, Sarvothaman V, Ranade VV (2021b) Acoustic analysis of vortex-based cavitation devices: Inception and extent of cavitation. Indust Eng Chem Res 60:8255–8268
Ranade VV (2022) Modeling of hydrodynamic cavitation reactors: Reflections on present status and path forward. ACS Eng Au 2:461–476
Ranade VV, Bhalchandra A, Anil AC, Sawant SS, Ilangovan D, Madhan R, Venkat KP (2009) Apparatus for filtration and disinfection of sea water/ship's ballast water and a method of same. USA Patent 2008/0017591
Ranade VV, Bhandari VM (2014) Industrial wastewater treatment, recycling and reuse. Elsevier, Amsterdam
Ranade VV, Bhandari VM, Nagarajan S, Sarvothaman VP, Simpson AT (2022) Hydrodynamic Cavitation: Devices, Design and Applications. Wiley-VCH, Weinheim
Ranade VV, Kulkarni AA, Bhandari VM (2016) Vortex diodes as effluent treatment devices. USA Patent 2016/9422952
Ranade VV, Kulkarni AA, Bhandari VM (2017) Apparatus and method for reduction in ammoniacal nitrogen from waste waters. USA Patent 2017/9725338
Ranade VV, Sarvothaman VP, Simpson A, Nagarajan S, Group I (2021c) Scale-up of vortex based hydrodynamic cavitation devices: A case of degradation of di-chloro aniline in water. Ultrason Sonochem 70:105295
Ranade VV, Utikar RP (2022) Multiphase Flows for Process Industries: Fundamentals and Applications. Wiley-VCH, Weinheim
Randhir A, Laird DW, Maker G, Trengove R, Moheimani NR (2020) Microalgae: a potential sustainable commercial source of sterols. Algal Res 46:101772
Rebello S, Anoopkumar A, Aneesh EM, Sindhu R, Binod P, Pandey A (2020) Sustainability and life cycle assessments of lignocellulosic and algal pretreatments. Bioresour Technol 301:122678
Reberšek M, Miklavčič D (2011) Advantages and disadvantages of different concepts of electroporation pulse generation. Automatika 52:12–19
Reynolds D, Huesemann M, Edmundson S, Sims A, Hurst B, Cady S, Beirne N, Freeman J, Berger A, Gao S (2021) Viral inhibitors derived from macroalgae, microalgae, and cyanobacteria: A review of antiviral potential throughout pathogenesis. Algal Res 57:102331
Ritu JR, Ambati RR, Ravishankar GA, Shahjahan M, Khan S (2023) Utilization of astaxanthin from microalgae and carotenoid rich algal biomass as a feed supplement in aquaculture and poultry industry: An overview. J Appl Phycol 35:145–171
Rivera EC, Villafaña-López L, Liu S, Kumar RV, Viau M, Bourseau P, Monteux C, Frappart M, Couallier E (2020) Cross-flow filtration for the recovery of lipids from microalgae aqueous extracts: membrane selection and performances. Process Biochem 89:199–207
Rodrigues RDP, de Castro FC, de Santiago-Aguiar RS, Rocha MVP (2018) Ultrasound-assisted extraction of phycobiliproteins from Spirulina (Arthrospira) platensis using protic ionic liquids as solvent. Algal Res 31:454–462
Rodrigues RDP, de Lima PF, de Santiago-Aguiar RS, Rocha MVP (2019) Evaluation of protic ionic liquids as potential solvents for the heating extraction of phycobiliproteins from Spirulina (Arthrospira) platensis. Algal Res 38:101391
Romagnoli F, Ievina B, Perera WAARP, Ferrari D (2020) Novel stacked modular open raceway ponds for microalgae biomass cultivation in biogas plants: Preliminary design and modelling. Environ Clim Technol 24:1–19
Roselet F, Vandamme D, Roselet M, Muylaert K, Abreu PC (2017) Effects of pH, salinity, biomass concentration, and algal organic matter on flocculant efficiency of synthetic versus natural polymers for harvesting microalgae biomass. BioEnergy Res 10:427–437
Roux J-M, Lamotte H, Achard J-L (2017) An overview of microalgae lipid extraction in a biorefinery framework. Energy Procedia 112:680–688
Ruggiero MA, Gordon DP, Orrell TM, Bailly N, Bourgoin T, Brusca RC, Cavalier-Smith T, Guiry MD, Kirk PM (2015) A higher level classification of all living organisms. PLoS One 10:e0119248
Ruiz-Ruiz F, López-Guajardo E, Vázquez-Villegas P, del Angel-Chong ME, Nigam K, Willson RC, Rito-Palomares M (2019) Continuous aqueous two-phase extraction of microalgal C-phycocyanin using a coiled flow inverter. Chem Eng Process-Process Intensif 142:107554
Ruiz CS, Martins M, Coutinho J, Wijffels R, Eppink M, Van den Berg C, Ventura S (2020) Neochloris oleoabundans biorefinery: Integration of cell disruption and purification steps using aqueous biphasic systems-based in surface-active ionic liquids. Chem Eng J 399:125683
Sá M, Monte J, Brazinha C, Galinha CF, Crespo JG (2019) Fluorescence coupled with chemometrics for simultaneous monitoring of cell concentration, cell viability and medium nitrate during production of carotenoid-rich Dunaliella salina. Algal Res 44:101720
Safaei M, Maleki H, Soleimanpour H, Norouzy A, Zahiri HS, Vali H, Noghabi KA (2019) Development of a novel method for the purification of C-phycocyanin pigment from a local cyanobacterial strain Limnothrix sp. NS01 and evaluation of its anticancer properties. Sci Rep 9(1):9474
Saha SK, McHugh E, Murray P, Walsh DJ (2015) Microalgae as a source of nutraceuticals. In: Botana LM, Alfonso A (eds) Phycotoxins: Chemistry and Biochemistry, 2nd edn. Wiley-Blackwell, Oxford, pp 255–291
Sahin OI (2020) Functional and sensorial properties of cookies enriched with SPIRULINA and DUNALIELLA biomass. J Food Sci Technol 57:3639–3646
Saliu F, Magoni C, Torelli A, Cozza R, Lasagni M, Labra M (2020) Omega-3 rich oils from microalgae: A chitosan mediated in situ transesterification method. Food Chem 337:127745
Santhakumaran P, Ayyappan SM, Ray JG (2020) Nutraceutical applications of twenty-five species of rapid-growing green-microalgae as indicated by their antibacterial, antioxidant and mineral content. Algal Res 47:101878
Santos IC, Brodbelt JS (2020) Recent developments in the characterization of nucleic acids by liquid chromatography, capillary electrophoresis, ion mobility, and mass spectrometry (2010–2020). J Sep Sci 44:340–372
Santos JH, Capela EV, Boal-Palheiros I, Coutinho JA, Freire MG, Ventura SP (2018) Aqueous biphasic systems in the separation of food colorants. Biochem Mol Biol Educ 46:390–397
Sanzo GD, Mehariya S, Martino M, Larocca V, Casella P, Chianese S, Musmarra D, Balducchi R, Molino A (2018) Supercritical carbon dioxide extraction of astaxanthin, lutein, and fatty acids from Haematococcus pluvialis microalgae. Mar Drugs 16:334
Saran Chaurasiya R, Umesh Hebbar H, Raghavarao K (2015) Recent developments in nanoparticulate based reverse micellar extraction for downstream processing of biomolecules. Curr Biochem Eng 2:118–134
Šarc A, Oder M, Dular M (2016) Can rapid pressure decrease induced by supercavitation efficiently eradicate Legionella pneumophila bacteria? Desal Water Treat 57:2184–2194
Sarkar S, Manna MS, Bhowmick TK, Gayen K (2020) Extraction of chlorophylls and carotenoids from dry and wet biomass of isolated Chlorella thermophila: Optimization of process parameters and modelling by artificial neural network. Process Biochem 96:58–72
Sarvothaman VP, Nagarajan S, Ranade VV (2018) Treatment of solvent-contaminated water using vortex-based cavitation: influence of operating pressure drop, temperature, aeration, and reactor scale. Ind Eng Chem Res 57:9292–9304
Sarvothaman VP (2020) Hydrodynamic Cavitation for Effluent Treatment: Using Vortex-based Cavitation Devices. Thesis, Queen's University Belfast
Sarvothaman VP, Simpson A, Ranade VV (2020) Comparison of hydrodynamic cavitation devices based on linear and swirling flows: Degradation of dichloroaniline in water. Ind Eng Chem Res 59:13841–13847
Sarvothaman VP, Simpson AT, Ranade VV (2019) Modelling of vortex based hydrodynamic cavitation reactors. Chem Eng J 377:119639
Sasikala P, Chandralekha A, Chaurasiya RS, Chandrasekhar J, Raghavarao K (2018) Ultrasound-assisted extraction and adsorption of polyphenols from ginger rhizome (Zingiber officinale). Sep Sci Technol 53:439–448
Scaglioni PT, de Oliveira GS, Badiale-Furlong E (2019) Inhibition of in vitro trichothecenes production by microalgae phenolic extracts. Food Res Int 124:175–180
Señoráns M, Castejón N, Señoráns FJ (2020) Advanced extraction of lipids with DHA from Isochrysis galbana with enzymatic pre-treatment combined with pressurized liquids and ultrasound assisted extractions. Molecules 25:3310
Senthilkumar N, Kurinjimalar C, Thangam R, Suresh V, Kavitha G, Gunasekaran P, Rengasamy R (2013) Further studies and biological activities of macromolecular protein R-Phycoerythrin from Portieria hornemannii. Int J Biol Macromol 62:107–116
Setyawan M, Budiman A, Mulyono P (2018) Optimum extraction of algae-oil from microalgae using hydrodynamic cavitation. Int J Renew Energy Res 8:451–458
Setyawan M, Mulyono P, Budiman A (2018b) Comparison of Nannochloropsis sp. cells disruption between hydrodynamic cavitation and conventional extraction. MATEC Web Conf 154:01023
Setyawan M, Mulyono P, Sutijan S, Pradana YS, Prasakti L, Budiman A (2020) Effect of devices and driving pressures on energy requirements and mass transfer coefficient on microalgae lipid extraction assisted by hydrodynamic cavitation. Int J Renew Energy Develop 9:467–473
Sharma PK, Saharia M, Srivstava R, Kumar S, Sahoo L (2018) Tailoring microalgae for efficient biofuel production. Front Mar Sci 5:382
Shi H, Nikrityuk P (2020) The influence of inflow swirl on cavitating and mixing processes in a venturi tube. Fluids 5:170
Shi R, Handler RM, Shonnard DR (2019) Life cycle assessment of novel technologies for algae harvesting and oil extraction in the renewable diesel pathway. Algal Res 37:248–259
Shiels K, Tsoupras A, Lordan R, Nasopoulou C, Zabetakis I, Murray P, Saha SK (2021) Bioactive lipids of marine microalga Chlorococcum sp. SABC 012504 with anti-inflammatory and anti-thrombotic activities. Mar Drugs 19(1):28
Shuman TR, Mason G, Reeve D, Schacht A, Goodrich A, Napan K, Quinn J (2016) Low-energy input continuous flow rapid pre-concentration of microalgae through electro-coagulation–flocculation. Chem Eng J 297:97–105
Sills DL, Van Doren LG, Beal C, Raynor E (2020) The effect of functional unit and co-product handling methods on life cycle assessment of an algal biorefinery. Algal Res 46:101770
Simpson A, Ranade VV (2018) Modelling of hydrodynamic cavitation with orifice: Influence of different orifice designs. Chem Eng Res Des 136:698–711
Simpson A, Ranade VV (2019a) 110th Anniversary: comparison of cavitation devices based on linear and swirling flows: hydrodynamic characteristics. Indust Eng Chem Res 58:14488–14509
Simpson A, Ranade VV (2019b) Flow characteristics of vortex based cavitation devices: Computational investigation on influence of operating parameters and scale. AIChE J 65:e16675
Simpson A, Ranade VV (2019c) Modeling hydrodynamic cavitation in venturi: Influence of venturi configuration on inception and extent of cavitation. AIChE J 65:421–433
Singh G, Patidar S (2018) Microalgae harvesting techniques: A review. J Environ Manag 217:499–508
Singh L, Kalia VC (2017) Waste biomass management-A holistic approach. Springer, Cham
Singh SK, Kaur R, Bansal A, Kapur S, Sundaram S (2020) Biotechnological exploitation of cyanobacteria and microalgae for bioactive compounds. In: Verma ML, Chandel AK (eds) Biotechnological Production of Bioactive Compounds. Elsevier, Amsterdam, pp 221–259
Sivakaminathan S, Wolf J, Yarnold J, Roles J, Ross IL, Stephens E, Henderson G, Hankamer B (2020) Light guide systems enhance microalgae production efficiency in outdoor high rate ponds. Algal Res 47:101846
Skorupskaite V, Makareviciene V, Sendzikiene E, Gumbyte M (2019) Microalgae Chlorella sp. cell disruption efficiency utilising ultrasonication and ultrahomogenisation methods. J Appl Phycol 31:2349–2354
Solmaz A, Işık M (2020) Optimization of membrane photobioreactor; the effect of hydraulic retention time on biomass production and nutrient removal by mixed microalgae culture. Biomass Bioenergy 142:105809
Soto-Sierra L, Kulkarni S, Woodard S, Nikolov Z (2020a) Processing of permeabilized Chlorella vulgaris biomass into lutein and protein-rich products. J Appl Phycol 32:1697–1707
Soto-Sierra L, Wilken LR, Dixon CK (2020b) Aqueous enzymatic protein and lipid release from the microalgae Chlamydomonas reinhardtii. Bioresour Bioprocess 7:46
Spinola MV, Díaz-Santos E (2020) Microalgae nutraceuticals: the role of lutein in human health. In: Alam MA, Xu J-L, Wang Z (eds) Microalgae Biotechnology for Food, Health and High Value Products. Springer, Singapore, pp 243–263
Srivastava N, Singh A, Kumari P, Nishad JH, Gautam VS, Yadav M, Bharti R, Kumar D, Kharwar RN (2021) Advances in extraction technologies: Isolation and purification of bioactive compounds from biological materials. In: Sinha RP, Häder D-P (eds) Natural Bioactive Compounds. Academic Press, London, pp 409–433
Suarez Ruiz CA, Emmery DP, Wijffels RH, Eppink MH, van den Berg C (2018) Selective and mild fractionation of microalgal proteins and pigments using aqueous two-phase systems. J Chem Technol Biotechnol 93:2774–2783
Suarez Ruiz CA, Kwaijtaal J, Peinado OC, Van Den Berg C, Wijffels RH, Eppink MH (2020) Multistep fractionation of microalgal biomolecules using selective aqueous two-phase systems. ACS Sustain Chem Eng 8:2441–2452
Subasankari K, Thanappan V, Jeyapragash D, Anantharaman P, Sarangi R (2020) Growth promoting studies on co-culturing Nannochloropsis oceanica with Halomonas aquamarina actively enhance the algal biomass and lipid production. Biocatal Agric Biotech 29:101790
Subhedar PB, Gogate PR (2013) Intensification of enzymatic hydrolysis of lignocellulose using ultrasound for efficient bioethanol production: a review. Ind Eng Chem Res 52:11816–11828
Sugiharto S (2020) Nutraceutical aspects of microalgae Spirulina and Chlorella on broiler chickens. Livest Res Rural Dev 32(6):84
Sun X, Park JJ, Kim HS, Lee SH, Seong SJ, Om AS, Yoon JY (2018) Experimental investigation of the thermal and disinfection performances of a novel hydrodynamic cavitation reactor. Ultrason Sonochem 49:13–23
Sun X, Xuan X, Song Y, Jia X, Ji L, Zhao S, Yoon JY, Chen S, Liu J, Wang G (2021) Experimental and numerical studies on the cavitation in an advanced rotational hydrodynamic cavitation reactor for water treatment. Ultrason Sonochem 70:105311
Suryawanshi PG, Bhandari VM, Sorokhaibam LG, Ruparelia JP, Ranade VV (2018) Solvent degradation studies using hydrodynamic cavitation. Environ Progress Suste Energy 37:295–304
Suttisuwan R, Phunpruch S, Saisavoey T, Sangtanoo P, Thongchul N, Karnchanatat A (2019) Isolation and characterization of anti-inflammatory peptides derived from trypsin hydrolysis of microalgae protein (Synechococcus sp. VDW). Food Biotech 33(4):303–324
Sydney EB, Novak AC, de Carvalho JC, Soccol CR (2014) Respirometric balance and carbon fixation of industrially important algae. In: Pandey A, Lee D-J, Chisti Y, Soccol CR (eds) Biofuels from algae. Elsevier, Amsterdam, pp 67–84
Tabarzad M, Atabaki V, Hosseinabadi T (2020) Anti-inflammatory activity of bioactive compounds from microalgae and cyanobacteria by focusing on the mechanisms of action. Mol Biol Rep 47:6193–6205
Tavanandi HA, Devi AC, Raghavarao K (2018a) A newer approach for the primary extraction of allophycocyanin with high purity and yield from dry biomass of Arthrospira platensis. Sep Purif Technol 204:162–174
Tavanandi HA, Mittal R, Chandrasekhar J, Raghavarao K (2018b) Simple and efficient method for extraction of C-Phycocyanin from dry biomass of Arthospira platensis. Algal Res 31:239–251
Tavanandi HA, Raghavarao K (2019) Recovery of chlorophylls from spent biomass of Arthrospira platensis obtained after extraction of phycobiliproteins. Bioresour Technol 271:391–401
Tavanandi HA, Raghavarao K (2020) Ultrasound-assisted enzymatic extraction of natural food colorant C-Phycocyanin from dry biomass of Arthrospira platensis. LWT 118:108802
Tavanandi HA, Vanjari P, Raghavarao K (2019) Synergistic method for extraction of high purity Allophycocyanin from dry biomass of Arthrospira platensis and utilization of spent biomass for recovery of carotenoids. Sep Purif Technol 225:97–111
Terán Hilares R, Sánchez Vera FP, Colina Andrade GJ, Tejada Meza K, García JC, Pacheco Tanaka DA (2022) Continuous cultivation of microalgae in cattle slaughterhouse wastewater treated with hydrodynamic cavitation. Water 14:1288
Tey WY, Lee KM, Sidik NAC, Asako Y (2019) Delfim-Soares explicit time marching method for modelling of ultrasonic wave in microalgae pre-treatment. IOP Conf Ser: Earth Environ Sci 268:012106
Thaker AH, Ranade VV (2021a) Drop breakage in a single‐pass through vortex‐based cavitation device: Experiments and modeling. AIChE J 69(1):e17512
Thaker AH, Ranade VV (2021b) Towards harnessing hydrodynamic cavitation for producing emulsions: Breakage of an oil drop in a vortex based cavitation device. Chem Eng Processing-Process Intensif 180:108753
Thaker AH, Ranade VV (2022) Emulsions using a vortex-based cavitation device: influence of number of passes, pressure drop, and device scale on droplet size distributions. Ind Eng Chem Res. https://doi.org/10.1021/acs.iecr.2c03714
Thanekar P, Gogate PR, Znak Z, Sukhatskiy Y, Mnykh R (2021) Degradation of benzene present in wastewater using hydrodynamic cavitation in combination with air. Ultrason Sonochem 70:105296
Thangavelu K, Desikan R, Taran OP, Uthandi S (2018) Delignification of corncob via combined hydrodynamic cavitation and enzymatic pretreatment: process optimization by response surface methodology. Biotechnol Biofuels Bioprod 11:203
Thangavelu K, Desikan R, Uthandi S (2022) Optimization of combined lime and hydrodynamic cavitation for pretreatment of corncob biomass using response surface methodology for lignin removal. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-02728-2
Timira V, Meki K, Li Z, Lin H, Xu M, Pramod SN (2022) A comprehensive review on the application of novel disruption techniques for proteins release from microalgae. Crit Rev Food Sci Nutr 62:4309–4325
Tommasi E, Cravotto G, Galletti P, Grillo G, Mazzotti M, Sacchetti G, Samorì C, Tabasso S, Tacchini M, Tagliavini E (2017) Enhanced and selective lipid extraction from the microalga P. tricornutum by dimethyl carbonate and supercritical CO2 using deep eutectic solvents and microwaves as pretreatment. ACS Sust Chem Eng 5:8316–8322
Torres A, Padrino S, Brito A, Díaz L (2021) Biogas production from anaerobic digestion of solid microalgae residues generated on different processes of microalgae-to-biofuel production. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-021-01898-9
Torzillo G, Masojidek J (2008) Mass cultivation of freshwater microalgae. Encyclopedia of Ecology. Elsevier, Oxford
Tseng Y-H, Mohanty SK, McLennan JD, Pease LF III (2019) Algal lipid extraction using confined impinging jet mixers. Chem Eng Sci: X 1:100002
Úbeda B, Gálvez JÁ, Michel M, Bartual A (2017) Microalgae cultivation in urban wastewater: Coelastrum cf. pseudomicroporum as a novel carotenoid source and a potential microalgae harvesting tool. Bioresour Technol 228:210–217
Ummalyma SB, Mathew AK, Pandey A, Sukumaran RK (2016) Harvesting of microalgal biomass: Efficient method for flocculation through pH modulation. Bioresour Technol 213:216–221
Vandamme D, Foubert I, Muylaert K (2013) Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotech 31:233–239
Vashist V, Chauhan D, Bhattacharya A, Rai MP (2020) Role of silica coated magnetic nanoparticle on cell flocculation, lipid extraction and linoleic acid production from Chlorella pyrenoidosa. Nat Prod Res 34:2852–2856
Vasistha S, Khanra A, Clifford M, Rai MP (2021) Current advances in microalgae harvesting and lipid extraction processes for improved biodiesel production: A review. Rene Sust Energy Rev 137:110498
Vieira MV, Pastrana LM, Fuciños P (2020) Microalgae encapsulation systems for food, pharmaceutical and cosmetics applications. Mar Drugs 18:644
Vigneshwaran G, More PR, Arya SS (2022) Non-thermal hydrodynamic cavitation processing of tomato juice for physicochemical, bioactive, and enzyme stability: Effect of process conditions, kinetics, and shelf-life extension. Curr Res Food Sci 5:313–324
Waghmare A, Nagula K, Pandit A, Arya S (2019) Hydrodynamic cavitation for energy efficient and scalable process of microalgae cell disruption. Algal Res 40:101496
Wali AF, Al Dhaheri Y, Ramakrishna Pillai J, Mushtaq A, Rao PG, Rabbani SA, Firdous A, Elshikh MS, Farraj DAA (2020) Lc-ms phytochemical screening, in vitro antioxidant, antimicrobial and anticancer activity of microalgae Nannochloropsis oculata extract. Separations 7:54
Walter H (2012) Partitioning in aqueous two–phase system: theory, methods, uses, and applications to biotechnology. Elsevier, New York
Wang B, Su H, Zhang B (2021) Hydrodynamic cavitation as a promising route for wastewater treatment–A review. Chem Eng J 412:128685
Wang C, Yang Y, Hou J, Wang P, Miao L, Wang X, Guo L (2020a) Optimization of cyanobacterial harvesting and extracellular organic matter removal utilizing magnetic nanoparticles and response surface methodology: A comparative study. Algal Res 45:101756
Wang J, Chen H, Yuan R, Wang F, Ma F, Zhou B (2020b) Intensified degradation of textile wastewater using a novel treatment of hydrodynamic cavitation with the combination of ozone. J Environ Chem Eng 8:103959
Wang M, Yuan W, Jiang X, Jing Y, Wang Z (2014a) Disruption of microalgal cells using high-frequency focused ultrasound. Bioresour Technol 153:315–321
Wang S-K, Wang F, Hu Y-R, Stiles AR, Guo C, Liu C-Z (2014b) Magnetic flocculant for high efficiency harvesting of microalgal cells. ACS Appl Mat Interf 6:109–115
Wu C, Xiao Y, Lin W, Li J, Zhang S, Zhu J, Rong J (2017) Aqueous enzymatic process for cell wall degradation and lipid extraction from Nannochloropsis sp. Bioresour Technol 223:312–316
Wu P, Bai L, Lin W, Wang X (2018) Mechanism and dynamics of hydrodynamic-acoustic cavitation (HAC). Ultrason Sonochem 49:89–96
Wu W, Lei Y-C, Chang J-S (2019a) Life cycle assessment of upgraded microalgae-to-biofuel chains. Bioresour Technol 288:121492
Wu Y, Xiang W, Li L, Liu H, Zhong N, Chang H, Rittmann BE (2021) A novel photoelectrochemical system to disrupt microalgae for maximizing lipid-extraction efficiency. Chem Eng J 420:130517
Wu Z, Ferreira DF, Crudo D, Bosco V, Stevanato L, Costale A, Cravotto G (2019b) Plant and biomass extraction and valorisation under hydrodynamic cavitation. Processes 7:965
Wu Z, Shen H, Ondruschka B, Zhang Y, Wang W, Bremner DH (2012) Removal of blue-green algae using the hybrid method of hydrodynamic cavitation and ozonation. J Hazard Mat 235:152–158
Xu J, Cheng J, Xin K, Xu J, Yang W (2020) Strengthening flash light effect with a pond-tubular hybrid photobioreactor to improve microalgal biomass yield. Bioresour Technol 318:124079
Xu Y, Yang J, Wang Y, Liu F, Jia J (2006) The effects of jet cavitation on the growth of Microcystis aeruginosa. J Env Sci Health A 41:2345–2358
Yamamoto K, King PM, Wu X, Mason TJ, Joyce EM (2015) Effect of ultrasonic frequency and power on the disruption of algal cells. Ultrason Sonochem 24:165–171
Yang L, Wang L, Ren S, Pan B, Li J, Zhang X, Chen Y, Hu Q (2019) Harvesting of Scenedesmus acuminatus using ultrafiltration membranes operated in alternative feed directions. J Biosci Bioeng 128:103–109
Yang Y, Du L, Hosokawa M, Miyashita K (2020) Total lipids content, lipid class and fatty acid composition of ten species of microalgae. J Oleo Sci 69:1181–1189
Yang Z, Pei H, Han F, Wang Y, Hou Q, Chen Y (2018) Effects of air bubble size on algal growth rate and lipid accumulation using fine-pore diffuser photobioreactors. Algal Res 32:293–299
Yasuda K, Ako D (2019) Effect of Venturi tube shape on reaction performance by hydrodynamic cavitation. J Chem Eng Jpn 52:280–282
Ye Z, Tan XH, Liu ZW, Aadil RM, Tan YC, Inam-ur-Raheem M (2020) Mechanisms of breakdown of Haematococcus pluvialis cell wall by ionic liquids, hydrochloric acid and multi-enzyme treatment. Int J Food Sci Technol 55:3182–3189
Yi L, Li B, Sun Y, Li S, Qi Q, Qin J, Sun H, Wang X, Wang J, Fang D (2021) Degradation of norfloxacin in aqueous solution using hydrodynamic cavitation: Optimization of geometric and operation parameters and investigations on mechanism. Sep Purif Technol 259:118166
Yu Q, Wang H, Li X, Yin Y, Qin S, Ge B (2020) Enhanced biomass and CO2 sequestration of Chlorella vulgaris using a new mixotrophic cultivation method. Process Biochem 90:168–176
Yustinadiar N, Manurung R, Suantika G (2020) Enhanced biomass productivity of microalgae Nannochloropsis sp. in an airlift photobioreactor using low-frequency flashing light with blue LED. Bioresour Bioprocess 7(1):43
Zampeta C, Arvanitaki F, Frontistis Z, Charalampous N, Dailianis S, Koutsoukos P, Vayenas DV (2022) Printing ink wastewater treatment using combined hydrodynamic cavitation and pH fixation. J Env Manage 317:115404
Zaouk L, Massé A, Bourseau P, Taha S, Rabiller-Baudry M, Jubeau S, Teychené B, Pruvost J, Jaouen P (2020) Filterability of exopolysaccharides solutions from the red microalga Porphyridium cruentum by tangential filtration on a polymeric membrane. Environ Technol 41:1167–1184
Zaslavsky BY (1994) Aqueous two-phase partitioning: physical chemistry and bioanalytical applications. CRC Press, Florida
Zezulka Š, Maršálková E, Pochylý F, Rudolf P, Hudec M, Maršálek B (2020) High-pressure jet-induced hydrodynamic cavitation as a pre-treatment step for avoiding cyanobacterial contamination during water purification. J Environ Manage 255:109862
Zhang R, Lebovka N, Marchal L, Vorobiev E, Grimi N (2020) Pulsed electric energy and ultrasonication assisted green solvent extraction of bio-molecules from different microalgal species. Innov Food Sci Emerg Technol 62:102358
Zhang S, Gao Y, Liu Q, Ye J, Hu Q, Zhang X (2019) Harvesting of Isochrysis zhanjiangensis using ultrafiltration: Changes in the contribution ratios of cells and algogenic organic matter to membrane fouling under different cross-flow velocities. Algal Res 41:101567
Zhang Y, Ward V, Dennis D, Plechkova NV, Armenta R, Rehmann L (2018) Efficient extraction of a docosahexaenoic acid (DHA)-rich lipid fraction from Thraustochytrium sp. using ionic liquids. Materials 11(10):1986
Zhao Y, Wang X, Jiang X, Fan Q, Li X, Jiao L, Liang W (2018) Harvesting of Chlorella vulgaris using Fe3O4 coated with modified plant polyphenol. Environ Sci Pollut Res 25:26246–26258
Zieliński M, Dębowski M, Kisielewska M, Nowicka A, Rokicka M, Szwarc K (2019) Cavitation-based pretreatment strategies to enhance biogas production in a small-scale agricultural biogas plant. Energy Sustain Dev 49:21–26
Zupanc M, Pandur Ž, Perdih TS, Stopar D, Petkovšek M, Dular M (2019) Effects of cavitation on different microorganisms: The current understanding of the mechanisms taking place behind the phenomenon. A review and proposals for further research. Ultrason Sonochem 57:147–165
Acknowledgements
Authors thank the Bernal Institute, University of Limerick, for providing the infrastructure and facilities.
Funding
Open Access funding provided by the IReL Consortium Authors thank the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 801165 and Science Foundation Ireland for providing the funds under PROCESS Fellowship programme.
Author information
Authors and Affiliations
Contributions
Rochak Mittal: Conceptualization; Data curation; Investigation; Methodology; Visualization; Writing – original draft; Writing – review & editing.
Vivek Ranade: Conceptualization; Funding acquisition; Supervision; Visualization; Writing – review; Project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mittal, R., Ranade, V. Bioactives from microalgae: A review on process intensification using hydrodynamic cavitation. J Appl Phycol 35, 1129–1161 (2023). https://doi.org/10.1007/s10811-023-02945-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-02945-w