Abstract
Ulcerative colitis (UC) is a chronic colonic inflammation with a significant health hazard. Aspergillus awamori (A. awamori) is a microorganism with various bioactive compounds with natural antioxidant and anti-inflammatory properties. The present work aimed to elucidate the protective and therapeutic effects of varying concentrations of A. awamori against acetic acid (AA)-induced ulcerative colitis (UC) in rats. Nine groups of albino male rats were established: a control negative group (G1), a control positive group (G2,AA), and preventive protocol groups (including G3A, G4A, and G5A) that received 100 mg, 50 mg, and 25 mg/kg b.w, respectively, of A. awamori orally and daily from the 1st day of the experiment and for 7 consecutive days. Then, they were subjected to one dose of AA intrarectally on day 8th. G3B, G4B, and G5B were termed as curative protocol groups that received one dose of AA on day 8th and then administered 100 mg, 50 mg, and 25 mg/kg b.w. of A. awamori, respectively, on day 9th and continued receiving these doses daily until day 16th. Rats in the AA group exhibited marked histopathological alterations of the distal colon, with an exaggeration of the DAI. In addition, a remarkable increase in oxidative stress was represented by the elevation of MDA and NO levels with a decline in SOD and GPx activities. In addition, upregulation of TNF-α, IL-6, and IL-1β mRNA expressions and downregulation of Muc2 and Nrf2 levels were detected. Unambiguously, a remarkable anti-inflammatory effect was noticed either in A. awamori prevented or treated groups expounded by reducing and regulating TNF-α, IL-6, and IL-1β with improved pathological lesion scoring. The Muc2, Nrf2, and bcl-2 gene levels were upregulated and restored also. In summary, the findings in this work reveal that A. awamori supplementation successfully alleviated the UC induced by AA, which had a better effect when administered before colitis induction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ulcerative colitis (UC) and Crohn’s disease (CD) are chronic diseases of inflammatory bowel disease (IBD) that affect life quality significantly (Khor et al. 2011). Persistent ulcerative inflammation of the distal colon and rectal mucosa is known medically as UC (Podolsky 2002), which has increased gradually in recent years all over the world (Ananthakrishnan et al. 2022; Iskandar et al. 2015). UC is specified by ulceration, edema, bleeding, and infiltration of the intestinal mucosa by inflammatory cells with abscess formation in the mucosal crypts (Danese and Fiocchi 2011.). However, the causative agent of IBD has been widely studied during the last several decades, and the exact etiology of IBD occurrence and pathogenesis remains unclear. Genetic, environmental, and immune factors may be incorporated (Fiocchi 1998; Loftus Jr 2004).
Elevation of oxidative stress with over production of reactive oxygen species (ROS) and pro-inflammatory chemokines are essential factors implicated in the IBD pathogenesis and intestinal illness (Ardizzone and Bianchi Porro 2005; Nakamura et al. 2006). Tissue injury arises due to the uncontrolled and excessive activation of the immune response and elevated levels of nitrogen and oxygen metabolites (Pavlick et al. 2002). The reduction in the level of antioxidant enzymes with upregulation of oxidative stress leads to loss of mucosal barrier integrity, increased intestinal permeability, apoptosis, activation of NF-kB, and eventually colonic inflammation (Elblehi et al. 2021; Molodecky and Kaplan 2010). Crucially, apoptosis and extensive inflammatory cytokines, besides inflammatory cell infiltration, have been incorporated into IBD pathophysiology (Becker et al. 2013; Raish et al. 2021). INOS and COX-2 increase when inflammatory cytokines or microorganisms stimulate host cells, indicating their role in UC (Nussler and Billiar 1993; Simon 1999).
Until now, there is no straightforward remedy for UC and IBD treatment, as there is difficulty in controlling the exaggerated response of the immune system and oxidative stress (Aleisa et al. 2014). However, corticosteroids, anti‑TNF‑α antibodies, and immunosuppressive antibiotics, such as adalimumab, certolizumab, and infliximab, are drugs used in IBD treatment (Ardizzone et al. 2010). Unfortunately, the available therapies for IBD are characterized by their high costs, numerous side effects, and lack of effectiveness (Sartor 2004). Therefore, growing interest in finding safe, natural products with potent anti-inflammatory and antioxidant properties to treat UC has been developed recently (Shahid et al. 2022; Wang et al. 2019).
The antioxidant treatment approach has been used recently as a hopeful remedy for treating various diseases resulting from the imbalance between oxidant and antioxidant mechanisms (Baiseitova et al. 2023; Habotta et al. 2023).
A. awamori is one of the microorganisms that have various bioactive compounds of natural antioxidant and anti-inflammatory properties such as p-coumaric acid, gallic acid, and ascorbic acid (Salar et al. 2017), nigragillin, dyramide, and 5-caboxybenzofuran (Lotfy et al. 2019). It is considered a robust probiotic microorganism that has been utilized in food processing (Bigelis and Lasure 1987; Yokoyama et al. 2001), as it is used in citric acid production (Kadooka et al. 2019, 2020). In addition, A. awamori has many enzymes that enhance molecule digestion (Arora and Chandra 2010; Gracia et al. 2003). In addition, (Kanauchi et al. 2008)) reported that A. awamori produces a natural substance named feruloyl esterase, which is considered an antioxidative substance. Moreover, A. awamori alleviated lactose intolerance, hypocholesterolemia, and gastrointestinal illness (Delcenserie et al. 2008; Ljungh and Wadstrom 2006). Additionally, A. awamori has been reported to be effective against hepatic carcinoma (Assar et al. 2021) and cardiac and renal damage (Assar et al. 2022), with the enhancement of growth efficiency, immune response, nutrient digestibility, and reduction of lipid peroxidation in skeletal muscle (El‐Deep et al. 2021; Saleh et al. 2011, 2012).
All of the benefits mentioned above of A. awamori prove the fungi’s antioxidant, anti-inflammatory, and anti-microbial properties. Therefore, the current study was outlined to evaluate the preventive and curative action of A. awamori extract against AA-induced UC in male albino rats via assessing the hematological parameters, histopathological lesion scoring, apoptosis, and oxidative and inflammatory biomarkers.
Materials and methods
A. awamori extract preparation
A. awamori powder was brought from Kagoshima University, Faculty of Agriculture, Kagoshima, Japan. The preparations and administration of A. awamori were mentioned before (Assar et al. 2021). A. awamori powder was dissolved in saline at a concentration of 0.5 mg/mL as a stock solution, and the same concentration was used each time. The A. awamori extract was analyzed phytochemically using Ultra-Performance Liquid Chromatography (UPLC), as reported previously (Assar et al. 2021; Salar et al. 2017).
Biological activity of A. awamori extract
Anti-oxidant activity
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay
The free-radical scavenging potential activity (FRSP) of A. awamori extract was evaluated using the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging assay as outlined earlier by (Salar et al. 2016) with a few modifications. A microtiter plate reader (BioTek Elx808, USA) was used according to the manufacturer’s guidelines to determine the changes in the absorbance at 517 nm for 0 to 30 min. The FRSP (%) was calculated using the equation
Animal management and experimental design
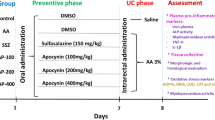
Eighty (n = 80) male albino rats, 8–10 weeks old and weighing 150–170 g, were purchased from the Laboratory Animal Research Center, Dokki, Egypt. Fresh and clean water was provided ad libitum to rats in separate metal cages. Rats were kept in the same habitat throughout the trial and fed the same diet. Before the experiment began, the animals were given 7 days of acclimatization. Animal pain and suffering were kept to a minimum as possible. The rats were randomly divided into nine groups, as seen in supplementary Fig. 1. The first group (G1, which served as a normal negative control, NC), comprised of 5 rats, received saline orally for 16 days (the duration of the experiment), with a single rectal instillation of saline on the 8th day of the experiment. The second group (G2, positive control group, acetic acid group = AA group) included ten rats receiving a single dose of 5% acetic acid (1 mL/rat) intrarectally on the 8th day. G3A, G4A, and G5A, termed prophylactic (preventive groups), and G3B, G4B, and G5B, termed treated or curative groups, have ten rats in each group. Groups 3A, 4A, and 5A received 100 mg, 50 mg, and 25 mg/kg b.w. orally of A. awamori aqueous solution (dissolved in saline)/daily by stomach tube for 7 consecutive days and then subjected to one dose of AA intrarectally at day 8th of the experiment. However, the curative groups received one dose of AA intrarectally on day 8th of the experiment. They then orally administered 100 mg, 50 mg, and 25 mg/kg b.w. of A. awamori, respectively, on the 9th day and continued receiving A. awamori extract until the 16th day (the end of the experiment). Group 6, comprised of 5 rats, received a single dose of acetic acid intrarectally on day 8th and then received 500 mg/kg of sulphasalazine (SSZ) orally on the 9th day.
Colitis induction
Intrarectal infusion of 5% AA (1 ml) in saline solution was used to induce UC in rats. Soft 6F polypropylene catheters were used to enter the rectum at a depth of 4 cm inside. Rats were put in an inverted Trendelenburg position for 2 min during rectal installation to prevent leakage of intracolonic solution (Bezerra et al. 2017; Shahid et al. 2022). Xylazine and ketamine were used for rat anesthesia (5 mg/kg, i.p.).
Assessments of colitis
Disease activity index (DAI)
Body weight loss scoring, stool consistency, and bloody stool percentage were calculated according to (Cooper et al. 1993), taking the score from 0 to 4. However, 10 cm of the distal part of the colon was excised, washed, opened and the feces were cleared by saline. Ratios of colon length to weight were evaluated.
Gross pathology and macroscopic lesion scoring
On the 16th day, a necropsy was executed immediately after euthanasia. The lower third of the colon was cleaned, weighed, and split longitudinally. Macroscopic lesion scoring for colitis was performed as reported previously (Morris et al. 1989). The macroscopic colon lesion was rated (0–5). 0 = negative visible changes; 1 = no ulcers with focal hyperemia; 2 = ulcers without noteworthy inflammation; 3 = liner ulcer with low inflammation; 4 = focal ulceration and inflammation; and 5 = diffuse inflammation and ulcerations.
Colitis histopathological lesion scoring
Colonic tissue specimens were fixed in 10% neutral buffered formalin. Samples were rinsed, dehydrated in ethyl alcohol, cleared in xylene, and then embedded in paraffin. The paraffin Sects. (4 μm) were stained using hematoxylin and eosin (H&E) (Bancroft and Gamble 2008). As previously stated, the microscopic colon lesions were scored (Galvez et al. 2001; Gonzalez–Rey et al. 2006) on a 0–5 scale as follows: 0 = normal tissue; 1 = low ulceration with few leukocyte infiltration; 2 = mucosal and submucosal inflammation as well as mild leukocyte infiltration with focal or diffuse ulceration; 3 = mucosal, submucosal, and muscular inflammation with focal or diffuse ulceration along with moderate leukocyte infiltration; 4 = mucosal, submucosal, muscular, serosa inflammation, and high leukocyte infiltration with focal or diffuse ulceration; and 5 = inflammation included all layers of mucosa, submucosa, muscular, serosa, and transmural with diffuse extensive ulceration and transmural leukocyte infiltration.
Blood indices
Blood samples were obtained in dry, clean EDTA-coated vacutainer tubes via ocular vein puncture. Exigo®, a wholly automated hematology analyzer (Boule Medical AB, Sweden), was employed in the hematological analysis.
Tissue oxidative stress and antioxidant activity assay
The malondialdehyde (MDA) and nitric oxide (NO) levels were evaluated using a colorimetric assay kit (Biodiagnostic, Co., Dokki, Egypt) according to (Buege and Aust 1978) and (Miranda et al. 2001), respectively. The antioxidant enzymatic biomarkers, including superoxide dismutase (SOD) and glutathione peroxidase (GPx), were evaluated in colon tissues as described earlier (Marklund and Marklund 1974; Paglia and Valentine 1967) using colorimetric kits (Biodiagnostic, Co, Dokki, Egypt) in regarding to the manufacturer’s instructions.
Apoptosis assay
According to the manufacturer’s instructions, caspase-3, bax, caspase-9, and bcl-2 levels were estimated using ELISA kits (CUBIO, Houston, TX, USA). Each sample was analyzed in triplicate.
Quantitative real-time polymerase chain reaction (qRT-PCR) for gene expression
Colon total RNA was extracted using the AllPrep DNA/RNA/Protein Mini Kit, Qiagen. The cDNA was obtained using the QuantiTectR Reverse Transcription Kit, Qiagen. qRT-PCR was done using (SYBR Premix Ex Taq II (Tli RNaseH Plus, Takara Bio) using the primer sequences listed in supplementary Table 1 for estimation of interleukin-1 Beta (IL-1β), interleukin-6 (IL-6), tumor necrosis alpha (TNF-α), mucin (Muc2), and the nuclear factor (erythroid-derived 2)-like 2 (Nrf2). The housekeeping β-actin was used to normalize the value of each examined sample. The relative changes between samples were conducted using the 2−∆Ct method (Livak and Schmittgen 2001).
Data analysis
The normality and homogeneity between all the data were determined using the Shapiro–Wilk test. A one-way ANOVA followed by Duncan’s post hoc test was used to compare the significance between groups using SPSS v.17 statistics. Values that are P < 0.05 are statistically significant between groups. Data were expressed as mean ± standard error (SEM).
Results
Biological activity of A. awamori extract
Anti-oxidant activity (DPPH assay)
A. awamori displayed robust radical scavenging activity using the DPPH assay with 69.32% as compared to 54.75%, and 29.65% for ascorbic acid and catechin respectively. This value potentially determining the effectiveness of A. awamori in neutralizing free radicals in regard to the commonly known antioxidants such as ascorbic acid and catechin.
Bioactive compounds of A. awamori extract using UPLC analysis
The bioactive compounds identified are p′-Coumaric acid, citric acid, ascorbic acid, gallic acid, and gentisic acid and there are many compounds that of unknown details and need further analysis. These compounds were shown in Fig. 1 and supplementary Table 2. The presence of such compounds reveal the biological action potential of A. awamori in our work.
Clinical signs and DAI expressed by experimental animals
Rats inoculated with AA in G2 exhibited severe clinical signs of severe loss in body weight, diarrhea, and bloody stool with mucus compared to the NG group (G1). On the contrary, rats received A. awamori extract (100 mg, 50 mg, and 25 mg/kg b.w.) either before induction of colitis (G3A, G4A, and G5A) or after colitis induction (G3B, G4B, and G5B), appeared clinically normal, and exhibited mild clinical signs of abdominal pain and diarrhea. No clinical signs were seen either in the NC or the sulphasalazine-treated (SSZ) groups (Table 1).
DAI is used to evaluate colonic injury based on the symptoms exhibited by the experimental animal. As displayed in Table 1, a significant increase in DAI, expressed by a reduction in final body weight, bloody diarrhea, mucosal erosion, and rectal bleeding in AA-administered rats (G2) (scoring 4) in relevant to the NC group (G1) (p < 0.05). Moreover, the preventive (G3A, G4A, and G5A) and curative (G3B, G4B, and G5B) groups displayed a lower DAI score (Table 1) compared to the G2. Remarkably, the preventive (4A) group that received (50 mg/kg) showed a lower DAI score (p < 0.01) than the AA group (G2). The NG (G1) and sulphasalazine (G6) did not exhibit any signs of inflammation clinically, and the DAI score was less than ≺ 1 (p < 0.05), (Fig. 5e).
Gross pathology
Grossly, AA-inoculated rats in G2 showed high macroscopic lesion scoring in the form of increased colon weight, colon hemorrhage, and ulceration. However, mild-to-moderate colon swelling, hemorrhage, and ulcerations were observed in the preventive and curative groups (G3A, G4A, G5A, G3B, G4B, and G5B). No visible damage was detected either in G1 or G6.
Blood indices
The hematological parameters of all experimental groups are summarized in supplementary Table 3. An apparent decrease in hemoglobin (Hb) content, red blood cell (RBC) count, hematocrit (HCT), and mean corpuscular hemoglobin concentration (MCHC) were documented in the AA group (G2) in relation to the NC group. On the other hand, as shown in supplementary Table 4, a significant increase in the total count of leucocytes (WBCs), platelets, and mean platelet volume (MPV) was observed in the AA group (G2). The neutrophil count was the highest, while the lymphocyte count was the lowest, with no significant change in eosinophil, basophil, and monocyte counts. In contrast, rats treated with A. awamori (G3A, G4A,, G5A, G3B, G4B, and G5B), as well as those that received the standard sulphasalazine drug (G6), explained a significant decrease in total leucocyte (WBCs), platelets, MPV, and neutrophil count, with a significant increase in lymphocyte count. The best values were seen in rats who received the moderate dose of A. awamori (50 mg/kg bw), especially before induction of colitis.
Oxidative and antioxidant marker assay
As illustrated in Fig. 2, there was a significant upregulation in MDA and NO levels in the AA group with an apparent decrease in GPx and SOD levels, along with a downregulation of the Nrf2 mRNA levels in the AA group (G2) relevant to the NC group (G1), revealing the enhancement of oxidative stress damage and lipid peroxidation. In contrast, a noticeable decrease in MDA and NO levels with a notable increase in GPx, SOD, and Nrf2 levels were observed in the preventive (G3A, G4A, and G5A) and curative (G3B, G4B, and G5B) groups as compared to the AA group (G2). The administration of A. awamori before AA administration gave better-enhanced results, as the protective and boosting effect of A. awamori was shown to have a dose-dependent effect (Fig. 2).
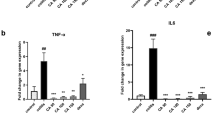
Inflammatory marker assay
The rats in the AA (G2) exhibited severe UC expressed by a dramatic increase in IL-β, TNF-α, IL-6, and iNOS compared to the NC, revealing the triggering of inflammation. Upon administration of A. awamori, a significant decline (P < 0.001) in the expression of inflammatory cytokine genes was detected as compared to the AA (Fig. 3). Remarkably, the preventive groups displayed a better amelioration of UC.
Effect of A. awamori on Muc2
A significant reduction (p < 0.001) in the Muc2 gene was detected in AA (G2) as compared to NC (G1). Administration of A. awamori at dose-dependent values significantly upregulated and restored the levels of the Muc2 gene in comparison to the AA. G4A represents the best-improved group (Fig. 3).
Pro-apoptotic and anti-apoptotic profile pathways
As shown in Table 2, AA exhibited marked apoptosis in the colonic tissues, indicated by a substantial increase in the pro-apoptotic genes (bax, caspase-3, caspase-9), with a considerable reduction in the level of the anti-apoptotic protein (bcl-2) in comparison to the NC. In the preventive groups (G3A, G4A, and G5A) and curative groups (G3B, G4B, and G5B), the anti-apoptotic (bcl-2) gene was significantly upregulated (p ≤ 0.05), with a marked decline in the bax, caspase-3, and caspase-9 pro-apoptotic genes concerning AA (G2). Noteworthy, the preventive groups (G3A, G4A, and G5A) substantially modulated and restored the expression of the anti-apoptotic (bcl-2) gene, revealing low cell death. There were no significant alterations either in the sulphasalazine-treated group or the NC.
A. awamori ameliorated the histopathological lesions induced by AA in rats
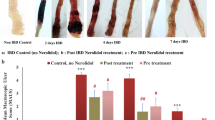
As illustrated in Fig. 4, the AA group (G2) revealed serious microscopic damage scoring (score 5) compared to the NC group. Rats in the AA group exhibited inflammatory reactions in the form of moderate-to-severe mucosal and submucosal erosion, inflammatory cell infiltrations, distortion of cryptic architecture, ulcerations, and epithelial desquamation and necrosis (Fig. 4b). A. awamori, when given as a protective dose before induction of colitis in (G3A, G4A, and G5A) significantly attenuated the severity of the histopathological alterations and restored the colonic mucosa architecture. A very mild degree of colitis (score 2), low inflammatory cell infiltrations with few erosions, and no ulcers were detected in the examined tissues of the rats in that group (Figs. 4c–e). Few or no pathological alterations were detected in the sulphasalazine group (Fig. 4f), as the epithelial mucosa is intact with goblet cell proliferation. However, the A. awamori post-treated groups (G3B, G4B, and G5B) showed moderate improvement in colitis as compared to the acetic acid group. In contrast, the mucosal and submucosal layers showed mild-to-moderate colitis with few-to-moderate inflammatory cell infiltration, mild edema, slight erosions, and ulceration of mucosal columnar epithelial cells (Figs. 5a–d). The histopathological lesion scoring and DAI affirmed the role of A. awamori in protecting the colonic tissues from damage and their ameliorating effect against UC induced by AA (Fig. 5e and d).
Histopathological photos stained by H&E. a) Control rats who received saline rectally showed normal architecture of mucosa, submucosa, and muscularis layer, with the intact epithelial surface. b) Rats received AA rectally characterized by severe colitis described in heavy infiltration of mucosal and submucosal layer by leukocytes (arrows) with sloughing of epithelial mucosa (arrowhead). c–e) preventive groups received (100, 50, and 25 mg/kg A.awamori) respectively. c) A.awamori preventive group received (100 mg/ kg) characterized by moderate colitis with moderate inflammatory cell infiltrations of mucosal and submucosal layers (arrows), goblet cell hyperplasia (arrowhead), and mild epithelial erosions. d) A.awamori preventive group received (50 mg/ kg) displayed mild colitis with few inflammatory cells (arrows) infiltration of mucosal and submucosal layer, intact epithelial mucosa (arrowhead), and mild goblet cell loss (black star). e) A.awamori preventive group received (25 mg/ kg) showed mild-to-moderate colitis with leukocytic cell infiltration (arrows), almost intact epithelial mucosa, and goblet cell hyperplasia (arrowhead). f) The sulphasalazine-treated group showed mild colitis with few inflammatory cells (arrows) infiltration (arrowhead) and intact epithelial mucosa (arrows)
a-d) rats received AA rectally and then post-treated by (100, 50, and 25 mg/kg A.awamori) respectively. a) A.awamori curative group received (100 mg/ kg) characterized by (moderate +) colitis with inflammatory cell infiltrations of mucosal and submucosal layers (arrows), and mild erosion of epithelial mucosa (arrowhead). b) A. awamori curative group received (50 mg/ kg) displayed moderate colitis of leukocytic cell infiltrations (arrows), mild edema (arrowhead), with mild erosion of epithelial mucosa (black star). c), d) A. awamori curative group received (25 mg/ kg) showed moderate + colitis, infiltration of inflammatory cells (arrows) in the mucosal and submucosal layer, and goblet cell hyperplasia (arrowhead), with erosion of epithelial mucosa (black star). d) Higher magnification of previous photo showed moderate + colitis with leukocytic cell infiltration (arrows), erosion of epithelial mucosa (arrowhead), with epithelial desquamation (black star), H&E. e) DAI graph in experimented groups. Data are presented as (Mean ± SEM), SEM = Standard error of the mean. f) Histopathological lesion scoring between different experimented groups
Discussion
IBD is a chronic inflammatory disease that negatively affects the patient’s health, causing severe gastrointestinal disturbances and may lead to colorectal cancer (Pandurangan and Esa 2014). Various factors and mechanisms, such as immunological, environmental, sex, and age, were involved in the IBD pathogenesis, but oxidative stress, with augmentation of free radicals and ROS generation, are the essential contributory factors for IBD occurrence (Bhattacharyya et al. 2014).
AA-induced UC resembles human UC concerning pathological sequences and histopathological alterations; thus, it is considered an effective tool for searching for and screening suitable safe drugs of anti-colitic activity (Randhawa et al. 2014; Shahid et al. 2022).
Herein, the consequences of the AA on the induction of UC and toxic damage of colonic tissues are displayed by a noteworthy upregulation of MDA and NO levels with a reduction of SOD and GPx antioxidant activity. Acute lipid peroxidation (LPO) and ROS overproduction were evidenced here by a substantial increase in MDA levels, disrupting the cell membrane permeability and integrity (Medicherla et al. 2015). Furthermore, NO, the nitrosative stress marker, enhances the formation of the pro-oxidant peroxynitrite after its reaction with the superoxide radical. The pro-oxidant peroxynitrite is further decomposed into the nitro radical, resulting in excessive nitrosative stress and DNA damage (Heikal et al. 2009). GPx is an antioxidant with a central role in ROS scavenging (Shahraki et al. 2023). Additionally, the SOD is a crucial and essential antioxidant defense enzyme against oxidative stress in the cell (Younus 2018). These findings are supported by previous related studies (Arab et al. 2014; Ju et al. 2018; Minaiyan et al. 2014; Nagib et al. 2013; Raish et al. 2021; Ran et al. 2008), which corroborated the role of increased MDA and LPO levels in the induction of extensive cellular damage and elevation of pathological lesion scoring in UC (Nagib et al. 2013; Soliman et al. 2022a; Witaicenis et al. 2012).
Oxidative stress results in the excess production of ROS, which further upregulates the release of pro-inflammatory and inflammatory cytokines (Owumi et al. 2020). Based on the investigation in the current study, AA considerably enhanced the levels of the pro-inflammatory cytokines (IL-1β, Il-6, and TNF-α) in the colonic tissue, which further promotes chemotaxis, chemokine release and triggers the inflammatory mediator’s cascade (Abd-Ellatieff et al. 2020; Monteleone et al. 2002; Shahraki et al. 2023; Shapiro et al. 2007; Stallmach et al. 1992). Moreover, the Nrf2 mRNA level was upregulated. It is a transcription factor, typically present in the cytoplasm of cells, and promotes the reduction of oxidative stress bursts (Zhang and Wang 2018). Nrf2 activates antioxidant enzymes such as SOD and GSH-Px (de Vries et al. 2008), which react with free radicals, minimize oxidative stress, and protect vital cells from damage by disrupting radicals’ activity. By binding to the promoter’s antioxidant gene response element (ARE), Nrf2 stimulates the expression of antioxidant genes. According to the findings in this study, AA considerably declines the expression of the Nrf2 gene, causing disruption of the Nrf2/ARE pathway and thus exaggerating oxidative stress and further tissue damage (de Vries et al. 2008; Itoh et al. 2004). Consequently, pathological outcomes vividly confirmed the UC by severe tissue damage in mucosal erosions, ulcerations, epithelial cell necrosis, neutrophil infiltration, and edema (Mizushima et al. 2009; Siegmund 2002). Furthermore, UC induced by AA was characterized by several clinicopathological alterations in experimental rats, such as bleeding, weight loss, colonic shortening with ulceration, edema, and erosions; however, these results agree with the previous studies (Randhawa et al. 2014; Shahid et al. 2022). In addition, the increase of the DAI in the colitic group was in line with the results of (Abdel-Daim et al. 2015; Akgun et al. 2005; Shahid et al. 2022).
Mitochondrial oxidative stress enhances and triggers apoptosis and other cellular signaling pathways. Apoptosis means the eradication of undesired cells by the death of those cells, which is achieved by intrinsic mitochondrial and extrinsic death receptor pathways (Venkatadri et al. 2016). Inflammatory responses induce alterations in the intestinal integrity and mucosal barrier function leading to apoptosis; therefore, it is a crucial factor in the pathophysiology of IBD (Ali et al. 2017). Apoptosis has been regulated by the anti-apoptotic (bcl-2) and pro-apoptotic (bax, caspase-3, and caspase-9) proteins. The ratio of pro-apoptotic to anti-apoptotic is one of the key factors that regulate apoptosis (Zhao et al. 2019). Bax regulates cytochrome c liberation inside the cytosol and enhances the mitochondrial-permeability transition (MPT) (Shalini et al. 2015), leading to caspase-9 activation, which cleaves caspase-3 (Kuida 2000) and eventually apoptosis occurs (Kaur et al. 2020). However, bcl-2 suppresses the release of bax proteins and stabilizes the MPT, thus restricting the activation of apoptotic pathways. In addition, bcl-2 prevents the discharge of cytochrome c into the cytosol, thus protecting the cell and increasing its longevity. Following these studies, investigations in this work proved the enhancement of apoptosis following AA administration by the significant increase of the pro-apoptotic (caspase-3, caspase-9, and bax) and downregulation of the anti-apoptotic (bcl-2) proteins.
A. awamori is enriched by various bioactive compounds of natural antioxidant and anti-inflammatory properties. A. awamori’s antioxidant mechanism demonstrates a beneficial effect against UC. Interestingly, results in this study revealed that A. awamori, either before or after induction of UC by AA in the preventive and curative protocol, respectively, could stretch a sturdy antioxidant action against the oxidative stress induced by AA. A. awamori supplementation can withstand the oxidative injury via LPO mitigation by reducing of MDA and NO levels. Additionally, A. awamori protected the colonic tissue from damage by restoring and boosting the antioxidant activity of SOD, GPx, and Nrf2 to their normal grades as compared to the AA group. The preventive and curative aptitude of A. awamori in free-radical scavenging is attributed to its richness in flavonoids and polyphenolic compounds, such as p-coumaric acid, gallic acid, cinnamic acid, ascorbic acid (Salar et al. 2017), nigragillin, dyramide, 5-caboxybenzofuran (Lotfy et al. 2019), and feruloyl esterase (Kanauchi et al. 2008). Furthermore, A. awamori contains many enzymes that enhance molecule digestion (Arora and Chandra 2010; Gracia et al. 2003). Additionally, many studies have reported the effectiveness of A. awamori against hepatic carcinoma (Assar et al. 2021), cardiac and renal damage (Assar et al. 2022), and gastrointestinal illness (Delcenserie et al. 2008; Ljungh and Wadstrom 2006), depending upon the antioxidant activity of the A. awamori. Receiving A. awamori (especially the dose of 50 mg/kg) either before or after UC induction improved and ameliorated the pathological alterations and the DAI concerning the NC group. These results align with the previous studies where bioactive polyphenol compounds ameliorate AA-induced UC (Ju et al. 2018; Shapiro et al. 2007; Tahan et al. 2011).
The inflammatory cytokines (TNF-α, IL-β, and iNOS) are considered the earlier signs of inflammation, and controlling their levels and expressions is the target and goal for the proper treatment of any disease (Gillberg et al. 2013; Kitabatake et al. 2021; Liu and Wang 2011; Soliman et al. 2022b). In the current work, the elevated levels of IL-1β, IL-6, and TNF-α in the AA group were reduced and regulated upon A. awamori administration refers to the potent anti-inflammatory activity of A. awamori and its role in inhibiting the transcription of these cytokines, thus decreasing tissue damage (Islam et al. 2008; Shahid et al. 2022). Besides, the anti-apoptotic effect of A. awamori was elucidated by the increased levels of the anti-apoptotic bcl-2 protein, either in preventive or curative groups, compared to the AA group, which prompted upregulation of pro-apoptotic proteins (caspase-3, bax, and caspase-9).
The gastrointestinal mucosa was protected from chemical, microbial, and mechanical damage via mucus, secreted by goblet cells as mucin and encoded by the Muc2 gene (Willemsen et al. 2003). A significantly higher expression of the Muc2 gene in colonic tissues in A. awamori treated (especially G4A) and NC groups compared to the AA group indicates that A. awamori maintains mucus production and protects colonic tissues from damage via suppressing inflammation-associated genes. The lower expression of Muc2 in the colonic tissue, as in the AA, leads to abnormalities in tissue morphology, including increased gut mucosa thickness, inflammatory cell infiltration, and aggravation of UC pathogenesis (Islam et al. 2017).
Nevertheless, the administration of A. awamori (either as a preventive or curative dose) significantly reduced UC features and protected rats from severe colitis. The protection mechanism of A. awamori against UC could be attributed to the anti-inflammatory, antioxidant, and anti-apoptotic properties of A. awamori.
Conclusion
The results of this study elucidated the preventive and curative impact of A. awamori against AA-induced UC in rats. However, this is likely attributed to the anti-inflammatory, antioxidant, and anti-apoptotic properties of A. awamori. Administration of A. awamori in rats either before or after colitis induction maintained the colonic tissue’s architecture, function, and integrity. A notable improvement in the antioxidant defense was achieved by the upregulation of SOD, GPx, and Nrf2 and the decline of MDA and NO levels. Besides, a marked reduction in the pro-inflammatory cytokines and increased anti-apoptotic (bcl-2) protein were noticed. Furthermore, A. awamori supplementation significantly ameliorates DAI and the tissue damage of AA-induced UC. Therefore, further investigations for developing a strategy using A. awamori as a therapeutic and prophylactic product against IBD should be considered.
Data availability
All data sets obtained and analyzed during the current studyare available in the manuscript. Further inquiries can be directed to the corresponding author.
Abbreviations
- A. awamori :
-
Aspergillus awamori
- DAI:
-
Disease Activity Index
- GPx:
-
Glutathione peroxidase
- IL1β:
-
Interleukin-1bet
- IL6:
-
Interleukin 6
- NO:
-
Nitric oxide
- MDA:
-
Malondialdehyde
- Muc2:
-
Mucin
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- PG:
-
Prostaglandins
- SOD:
-
Superoxide dismutase
- TNF-α:
-
Tumor necrosis alpha
References
Abdel-Daim MM, Farouk SM, Madkour FF, Azab SS (2015) Anti-inflammatory and immunomodulatory effects of Spirulina platensis in comparison to Dunaliella salina in acetic acid-induced rat experimental colitis. Immunopharmacol Immunotoxicol 37:126–139
Abd-Ellatieff H, Anwar S, Abas O, Abou-Rawash A-R, Fukushi H, Yanai T (2020) Correlation of immunomodulatory cytokine expression with histopathological changes and viral antigen in a hamster model of equine herpesvirus-9 encephalitis. J Comp Pathol 180:46–54
Akgun E, Caliskan C, Celik H, Ozutemiz A, Tuncyurek M, Aydin H (2005) Effects of N-acetylcysteine treatment on oxidative stress in acetic acid-induced experimental colitis in rats. J Int Med Res 33:196–206
Aleisa AM, Al-Rejaie SS, Abuohashish HM, Ola MS, Parmar MY, Ahmed MM (2014) Pretreatment of Gymnema sylvestre revealed the protection against acetic acid-induced ulcerative colitis in rats. BMC Complement Altern Med 14:1–11
Ali AA, Abd Al Haleem EN, Khaleel SA-H, Sallam AS (2017) Protective effect of cardamonin against acetic acid-induced ulcerative colitis in rats. Pharmacol Rep 69:268–275
Ananthakrishnan AN, Kaplan GG, Bernstein CN, Burke KE, Lochhead PJ, Sasson AN, Agrawal M, Tiong JHT, Steinberg J, Kruis W (2022) Lifestyle, behaviour, and environmental modification for the management of patients with inflammatory bowel diseases: an International Organization for Study of Inflammatory Bowel Diseases consensus. The Lancet Gastroenterology & Hepatology 7(7):666–678. https://doi.org/10.1016/S2468-1253(22)00021-8
Arab HH, Al-Shorbagy MY, Abdallah DM, Nassar NN (2014) Telmisartan attenuates colon inflammation, oxidative perturbations and apoptosis in a rat model of experimental inflammatory bowel disease. PLoS ONE 9:e97193
Ardizzone S, Bianchi PG (2005) Biologic therapy for inflammatory bowel disease. Drugs 65:2253–2286
Ardizzone S, Cassinotti A, Manes G, Bianchi Porro G (2010) Immunomodulators for all patients with inflammatory bowel disease? Ther Adv Gastroenterol 3:31–42
Arora DS, Chandra P (2010) Assay of antioxidant potential of two Aspergillus isolates by different methods under various physio-chemical conditions. Braz J Microbiol 41:765–777
Assar DH, Mokhbatly A-AA, Ghazy EW, Ragab AE, Abou Asa S, Abdo W, Elbialy ZI, Mohamed NE, El-Far AH (2021) Ameliorative effects of Aspergillus awamori against the initiation of hepatocarcinogenesis induced by diethylnitrosamine in a rat model: regulation of Cyp19 and p53 gene expression. Antioxidants 10:922
Assar DH, Asa SA, El-Abasy MA, Elbialy ZI, Shukry M, Latif AAE, BinMowyna MN, Althobaiti NA, El-Magd MA (2022) Aspergillus awamori attenuates ochratoxin A-induced renal and cardiac injuries in rabbits by activating the Nrf2/HO-1 signaling pathway and downregulating IL1β, TNFα, and iNOS gene expressions. Environ Sci Pollut Res 29:69798–69817
Baiseitova A, Shah AB, Khan AM, Idrees M, Kim JH, Lee YH, Kong I-K, Park KH (2023) Antioxidant potentials of furanodihydrobenzoxanthones from Artocarpus elasticus and their protection against oxLDL induced injury in SH-SY5Y cells. Biomed Pharmacother 165:115278
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques. Elsevier health sciences, China
Becker C, Watson AJ, Neurath MF (2013) Complex roles of caspases in the pathogenesis of inflammatory bowel disease. Gastroenterology 144:283–293
Bezerra GB, de Souza LdM, Dos Santos AS, de Almeida GKM, Souza MTS, Santos SL, Camargo EA, dos Santos LB, de Souza Araújo AA, Cardoso JC (2017) Hydroalcoholic extract of Brazilian red propolis exerts protective effects on acetic acid-induced ulcerative colitis in a rodent model. Biomed Pharmacother 85:687–696
Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE (2014) Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev 94:329–354
Bigelis R, Lasure L (1987) Fungal enzymes and primary metabolites used in food processing. Westport Conn, USA, pp 473–516
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Cooper HS, Murthy S, Shah R, Sedergran D (1993) Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 69(2):238–249
Danese S, Fiocchi C (2011) Ulcerative colitis. N Engl J Med 365:1713–1725
de Vries HE, Witte M, Hondius D, Rozemuller AJ, Drukarch B, Hoozemans J, van Horssen J (2008) Nrf2-induced antioxidant protection: a promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radical Biol Med 45:1375–1383
Delcenserie V, Martel D, Lamoureux M, Amiot J, Boutin Y, Roy D (2008) Immunomodulatory effects of probiotics in the intestinal tract. Curr Issues Mol Biol 10:37
Elblehi SS, El-Sayed YS, Soliman MM, Shukry M (2021) Date palm pollen extract avert doxorubicin-induced cardiomyopathy fibrosis and associated oxidative/nitrosative stress, inflammatory cascade, and apoptosis-targeting bax/bcl-2 and caspase-3 signaling pathways. Animals 11:886
El-Deep MH, Dawood MA, Assar MH, Ahamad Paray B (2021) Aspergillus awamori positively impacts the growth performance, nutrient digestibility, antioxidative activity and immune responses of growing rabbits. Veterinary Medicine and Science 7:226–235
Fiocchi C (1998) Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 115:182–205
Galvez J, Coelho G, Crespo M, Cruz T, Rodríguez-Cabezas M, Concha A, Gonzalez M, Zarzuelo A (2001) Intestinal anti-inflammatory activity of morin on chronic experimental colitis in the rat. Aliment Pharmacol Ther 15:2027–2039
Gillberg L, Berg S, de Verdier P, Lindbom L, Werr J, Hellström PM (2013) Effective treatment of mouse experimental colitis by alpha 2 integrin antibody: comparison with alpha 4 antibody and conventional therapy. Acta Physiol 207:326–336
Gonzalez-Rey E, Chorny A, Delgado M (2006) Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology 130:1707–1720
Gracia M, Aranibar MJ, Lazaro R, Medel P, Mateos G (2003) Alpha-amylase supplementation of broiler diets based on corn. Poult Sci 82:436–442
Habotta OA, Abdeen A, El-Hanafy AA, Yassin N, Elgameel D, Ibrahim SF, Abdelrahaman D, Hasan T, Imbrea F, Ghamry HI (2023) Sesquiterpene nootkatone counteracted the melamine-induced neurotoxicity via repressing of oxidative stress, inflammatory, and apoptotic trajectories. Biomed Pharmacother 165:115133
Heikal L, Martin GP, Dailey LA (2009) Characterisation of the decomposition behaviour of S-nitrosoglutathione and a new class of analogues: S-Nitrosophytochelatins. Nitric Oxide 20:157–165
Iskandar HN, Dhere T, Farraye FA (2015) Ulcerative colitis: update on medical management. Curr Gastroenterol Rep 17:1–11
Islam M, Murata T, Fujisawa M, Nagasaka R, Ushio H, Bari A, Hori M, Ozaki H (2008) Anti-inflammatory effects of phytosteryl ferulates in colitis induced by dextran sulphate sodium in mice. Br J Pharmacol 154:812–824
Islam J, Koseki T, Watanabe K, Budijanto S, Oikawa A, Alauddin M, Goto T, Aso H, Komai M, Shirakawa H (2017) Dietary supplementation of fermented rice bran effectively alleviates dextran sodium sulfate-induced colitis in mice. Nutrients 9:747
Itoh K, Tong KI, Yamamoto M (2004) Molecular mechanism activating Nrf2–Keap1 pathway in regulation of adaptive response to electrophiles. Free Radical Biol Med 36:1208–1213
Ju S, Ge Y, Li P, Tian X, Wang H, Zheng X, Ju S (2018) Dietary quercetin ameliorates experimental colitis in mouse by remodeling the function of colonic macrophages via a heme oxygenase-1-dependent pathway. Cell Cycle 17:53–63
Kadooka C, Izumitsu K, Onoue M, Okutsu K, Yoshizaki Y, Takamine K, Goto M, Tamaki H, Futagami T (2019) Mitochondrial citrate transporters CtpA and YhmA are required for extracellular citric acid accumulation and contribute to cytosolic acetyl coenzyme A generation in Aspergillus luchuensis mut. kawachii. Appl Environ Microbiol 85:e03136-e3218
Kadooka C, Nakamura E, Mori K, Okutsu K, Yoshizaki Y, Takamine K, Goto M, Tamaki H, Futagami T (2020) LaeA controls citric acid production through regulation of the citrate exporter-encoding cexA gene in Aspergillus luchuensis mut. kawachii. Appl Environ Microbiol 86:e01950-e2019
Kanauchi M, Watanabe S, Tsukada T, Atta K, Kakuta T, Koizumi T (2008) Purification and characteristics of feruloyl esterase from Aspergillus awamori G-2 strain. J Food Sci 73:C458–C463
Kaur S, Singh G, Sadwal S, Aniqa A (2020) Alleviating impact of hydroethanolic Murraya koenigii leaves extract on bisphenol A instigated testicular lethality and apoptosis in mice. Andrologia 52:e13504
Khor B, Gardet A, Xavier R (2011) Genetics and pathogenesis of inflammatory bowel disease. Nature 474:307–317
Kitabatake M, Matsumura Y, Ouji-Sageshima N, Nishioka T, Hara A, Kayano S-i, Ito T (2021) Persimmon-derived tannin ameliorates the pathogenesis of ulcerative colitis in a murine model through inhibition of the inflammatory response and alteration of microbiota. Sci Rep 11:1–13
Kuida K (2000) Caspase-9. Int J Biochem Cell Biol 32:121–124
Liu X, Wang JM (2011) Iridoid glycosides fraction of Folium syringae leaves modulates NF-κB signal pathway and intestinal epithelial cells apoptosis in experimental colitis. PLoS ONE 6:e24740
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2—ΔΔCT method. Methods 25:402–408
Ljungh A, Wadstrom T (2006) Lactic acid bacteria as probiotics. Curr Issues Intest Microbiol 7:73–90
Loftus EV Jr (2004) Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology 126:1504–1517
Lotfy MM, Hassan HM, Mohammed R, Hetta M, El-Gendy AO, Rateb ME, Zaki MA, Gamaleldin NM (2019) Chemical profiling and biological screening of some river Nile derived-microorganisms. Front Microbiol 10:787
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Medicherla K, Sahu BD, Kuncha M, Kumar JM, Sudhakar G, Sistla R (2015) Oral administration of geraniol ameliorates acute experimental murine colitis by inhibiting pro-inflammatory cytokines and NF-κB signaling. Food Funct 6:2984–2995
Minaiyan M, Asghari G, Taheri D, Saeidi M, Nasr-Esfahani S (2014) Anti-inflammatory effect of Moringa oleifera Lam. seeds on acetic acid-induced acute colitis in rats. Avicenna Journal of Phytomedicine 4(20):127–136
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5:62–71
Mizushima T, Sasaki M, Ando T, Wada T, Tanaka M, Okamoto Y, Ebi M, Hirata Y, Murakami K, Mizoshita T (2009) Blockage of Angiotensin II type 1 receptor regulates TNF-α induced MAdCAM-1 expression via inhibition of NF-κB translocation to nucleus and ameliorates colitis. Am J Physiol Gastrointest Liver Physiol 298(2):G255–G266
Molodecky NA, Kaplan GG (2010) Environmental risk factors for inflammatory bowel disease. Gastroenterology & Hepatology 6:339
Monteleone I, Vavassori P, Biancone L, Monteleone G, Pallone F (2002) Immunoregulation in the gut: Success and failures in human disease. Gut 50(Suppl 3):60–64. https://doi.org/10.1136/gut.50.suppl_3.iii60
Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL (1989) Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 96:795–803
Nagib MM, Tadros MG, ELSayed MI, Khalifa AE (2013) Anti-inflammatory and anti-oxidant activities of olmesartan medoxomil ameliorate experimental colitis in rats. Toxicol Appl Pharmacol 271:106–113
Nakamura K, Honda K, Mizutani T, Akiho H, Harada N (2006) Novel strategies for the treatment of inflammatory bowel disease: selective inhibition of cytokines and adhesion molecules. World J Gastroenterol: WJG 12:4628
Nussler AK, Billiar TR (1993) Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukoc Biol 54:171–178
Owumi SE, Ijadele AO, Arunsi UO, Odunola OA (2020) Luteolin abates reproductive toxicity mediated by the oxido-inflammatory response in Doxorubicin-treated rats. Toxicology Research and Application 4:2397847320972040
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Pandurangan AK, Esa NM (2014) Signal transducer and activator of transcription 3-a promising target in colitis-associated cancer. Asian Pac J Cancer Prev 15:551–560
Pavlick KP, Laroux FS, Fuseler J, Wolf RE, Gray L, Hoffman J, Grisham MB (2002) Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radical Biol Med 33:311–322
Podolsky D (2002) Inflammatory bowel disease. N Engl J Med 347:417–429
Raish M, Shahid M, Bin Jardan YA, Ansari MA, Alkharfy KM, Ahad A, Abdelrahman IA, Ahmad A, Al-Jenoobi FI (2021) Gastroprotective effect of sinapic acid on ethanol-induced gastric ulcers in rats: involvement of Nrf2/HO-1 and NF-κB signaling and antiapoptotic role. Front Pharmacol 12:622815
Ran ZH, Chen C, Xiao SD (2008) Epigallocatechin-3-gallate ameliorates rats colitis induced by acetic acid. Biomed Pharmacother 62:189–196
Randhawa PK, Singh K, Singh N, Jaggi AS (2014) A review on chemical-induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol 18:279
Salar RK, Purewal SS, Bhatti MS (2016) Optimization of extraction conditions and enhancement of phenolic content and antioxidant activity of pearl millet fermented with Aspergillus awamori MTCC-548. Resource-Efficient Technologies 2:148–157
Salar RK, Purewal SS, Sandhu KS (2017) Bioactive profile, free-radical scavenging potential, DNA damage protection activity, and mycochemicals in Aspergillus awamori (MTCC 548) extracts: a novel report on filamentous fungi. 3 Biotech 7(3):164
Saleh A, Eid Y, Ebeid T, Kamizono T, Ohtsuka A, Hayashi K (2011) Effects of feeding aspergillus awamori and aspergillus niger on growth performance and meat quality in broiler chickens. J Poult Sci 48:201–206
Saleh AA, Eid YZ, Ebeid TA, Ohtsuka A, Hioki K, Yamamoto M, Hayashi K (2012) The modification of the muscle fatty acid profile by dietary supplementation with Aspergillus awamori in broiler chickens. Br J Nutr 108:1596–1602
Sartor RB (2004) Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology 126:1620–1633
Shahid M, Raish M, Ahmad A, Bin Jardan YA, Ansari MA, Ahad A, Alkharfy KM, Alaofi AL, Al-Jenoobi FI (2022) Sinapic acid ameliorates acetic acid-induced ulcerative colitis in rats by suppressing inflammation, oxidative stress, and apoptosis. Molecules 27:4139
Shahraki FN, Momtaz S, Baeeri M, Khayatan D, Lashgari N-A, Roudsari NM, Abdollahi AR, Dehpour AR, Abdolghaffari AH (2023) Licofelone attenuates acetic acid-induced colitis in rats through suppression of the inflammatory mediators. Inflammation 46(5):1709–1724
Shalini S, Dorstyn L, Dawar S, Kumar S (2015) Old, new and emerging functions of caspases. Cell Death Differ 22:526–539
Shapiro H, Singer P, Halpern Z, Bruck R (2007) Polyphenols in the treatment of inflammatory bowel disease and acute pancreatitis. Gut 56:426–436
Siegmund B (2002) Interleukin-1β converting enzyme (caspase-1) in intestinal inflammation. Biochem Pharmacol 64:1–8
Simon LS (1999) Role and regulation of cyclooxygenase-2 during inflammation. Am J Med 106:37S-42S
Soliman MM, Aldhahrani A, Elshazly SA, Shukry M, Abouzed TK (2022a) Borate ameliorates sodium nitrite-induced oxidative stress through regulation of oxidant/antioxidant status: involvement of the Nrf2/HO-1 and NF-κB pathways. Biol Trace Elem Res 200:197–205
Soliman MM, Gaber A, Alsanie WF, Mohamed WA, Metwally MM, Abdelhadi AA, Albedawy M (2022b) Shukry M (2022b): Gibberellic acid-induced hepatorenal dysfunction and oxidative stress: Mitigation by quercetin through modulation of antioxidant, anti-inflammatory, and antiapoptotic activities. J Food Biochem 46(2):e14069. https://doi.org/10.1111/jfbc.14069
Stallmach A, Schuppan D, Riese HH, Matthes H, Riecken EO (1992) Increased collagen type III synthesis by fibroblasts isolated from strictures of patients with Crohn’s disease. Gastroenterology 102:1920–1929
Tahan G, Aytac E, Aytekin H, Gunduz F, Dogusoy G, Aydin S, Tahan V, Uzun H (2011) Vitamin E has a dual effect of anti-inflammatory and antioxidant activities in acetic acid–induced ulcerative colitis in rats. Can J Surg 54:333
Venkatadri R, Muni T, Iyer A, Yakisich J, Azad N (2016) Role of apoptosis-related miRNAs in resveratrol-induced breast cancer cell death. Cell Death Dis 7:e2104–e2104
Wang G, Xu B, Shi F, Du M, Li Y, Yu T, Chen L (2019) Protective effect of methane-rich saline on acetic acid-induced ulcerative colitis via blocking the TLR4/NF-κB/MAPK pathway and promoting IL-10/JAK1/STAT3-mediated anti-inflammatory response. Oxid Med Cell Longev 2019:7850324. https://doi.org/10.1155/2019/7850324
Willemsen L, Koetsier M, Van Deventer S, Van Tol E (2003) Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut 52:1442–1447
Witaicenis A, Luchini AC, Hiruma-Lima CA, Felisbino SL, Garrido-Mesa N, Utrilla P, Gálvez J, Di Stasi LC (2012) Suppression of TNBS-induced colitis in rats by 4-methylesculetin, a natural coumarin: comparison with prednisolone and sulphasalazine. Chem Biol Interact 195:76–85
Yokoyama K, Wang L, Miyaji M, Nishimura K (2001) Identification, classification and phylogeny of the Aspergillus section Nigri inferred from mitochondrial cytochrome b gene. FEMS Microbiol Lett 200:241–246
Younus H (2018) Therapeutic potentials of superoxide dismutase. Int J Health Sci 12:88
Zhang L, Wang H (2018) Targeting the NF-E2-related factor 2 pathway: a novel strategy for traumatic brain injury. Mol Neurobiol 55:1773–1785
Zhao Q, Liu Y, Zhong J, Bi Y, Liu Y, Ren Z, Li X, Jia J, Yu M, Yu X (2019) Pristimerin induces apoptosis and autophagy via activation of ROS/ASK1/JNK pathway in human breast cancer in vitro and in vivo. Cell Death Discovery 5:125
Acknowledgements
For all authors.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funding for acheiving this protocol. Open access funding provided by The Science, Technology& Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis are as follows: (Hoda. A. Abd-Ellatieff), conducting the research work, formal analysis, writing the manuscript, review editing, and interpretation. (Abdel-Rahman. A. Abourawash): conceptualization of the research idea, validation, and supervision. (Wael. M. Goda, Kristen Georg): methodology and data analysis. (Emad W. Ghazy, Dalia. H. Samak): data analysis and revision. All authors agreed with the content and all gave explicit consent to submit and they obtained consent from the responsible authorities at (the Faculty of Veterinary Medicine, Damanhur University) where the work was carried out before the work was submitted. All authors have participated in that work, read, and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest;
The authors declared no conflict of interest.
Ethical approval
Ethical approval for this study was approved by the Animal Ethics Committee of the Faculty of Veterinary Medicine at Damanhour University/Egypt, number (DMU-VETMED-PATH-2019-/O144). Taking samples from live and dead animals was carried out in strict accordance with the recommendations of the Committee concerning animal welfare during the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abd-Ellatieff, H., Georg, K., Abourawash, AR.A. et al. Aspergillus awamori: potential antioxidant, anti-inflammatory, and anti-apoptotic activities in acetic acid-induced ulcerative colitis in rats. Inflammopharmacol (2024). https://doi.org/10.1007/s10787-024-01489-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10787-024-01489-w