Abstract
Ochratoxin A (OTA) is one of the most dangerous and that pollute agricultural products, inducing a variety of toxic effects in humans and animals. The current study explored the protective effect of different concentrations of Aspergillus awamori (A. awamori) against OTA (0.3 mg/kg diet) induced renal and cardiac damage by exploring its mechanism of action in 60 New Zealand white male rabbits. Dietary supplementation of A. awamori at the selected doses of 50, 100, and 150 mg/kg diet, respectively, for 2 months significantly improved the rabbit’s growth performance; modulated the suppressed immune response and restored the altered hematological parameters; reduced the elevated levels of renal injury biomarkers such as urea, creatinine, and alkaline phosphatase; and increased serum total proteins concentrations. Moreover, it also declined enzymatic activities of cardiac injury biomarkers, including AST, LDH, and CK-MB. A. awamori alleviated OTA-induced degenerative and necrotic changes in the kidney and heart of rabbits. Interestingly, A. awamori upregulated Nrf2/OH-1 signaling pathway. Therefore enhanced TAC, CAT, and SOD enzyme activities and reduced OTA-induced oxidative and nitrosative stress by declining iNOS gene expression and consequently lowered MDA and NO levels. In addition to attenuating renal and cardiac inflammation via reducing IL-1β, TNF-α gene expressions in a dose-dependent response. In conclusion,this is the first report to pinpoint that dietary incorporation of A. awamori counteracted OTA-induced renal and cardiac damage by potentiating the rabbit’s antioxidant defense system through its potent antioxidant, free radical scavenging, and anti-inflammatory properties in a dose-dependent response. Based on our observations, A. awamori could be utilized as a natural protective agent against ochratoxicosis in rabbits.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ochratoxins are secondary toxic metabolites produced by various fungi of the genus Aspergillus and Penicillium (Ostry et al. 2013) that cause several harmful influences on multiple animals (Pfohl-Leszkowicz and Manderville 2007; Battacone et al. 2010). Rabbits are one of the applied alternative sources to face the scarcity of meat resources in developing countries (Dalle Zotte and Szendrő, 2011). Rabbit meat consumption is a rising economic industry routinely consumed in Egypt (Baviera-Puig et al., 2017). Rabbits are comparatively more vulnerable to Ochratoxin A (OTA) than mice, rats, and guinea pigs (Ponnuchamy, 2000). OTA has gained a special consideration among the recorded mycotoxins due to its nephrotoxic, teratogenic, embryotoxic, immunosuppressive, genotoxic, neurotoxic, and carcinogenic properties (O'Brien et al.2001). OTA has good thermal stability, making its eradication from the food chain impossible (Malir et al. 2016). Based on OTA nature, humans and domestic animals that are chronically exposed to low doses of OTA (50–250 μg OTA/kg b.m.) are potentially at risk for renal diseases or may even exhibit carcinogenic possibilities (Abdel-Wahhab et al. 2005; Ringot et al. 2006; Brown et al. 2007; Zain, 2011; Gruber-Dorninger et al. 2019). The most relevant impacts of OTA are the nephrotoxicity and nephron-carcinogenicity in rodents (Benford et al., 2001). The high sensitivity of kidneys to OTA can be attributed to the kidney's critical importance as an exclusive excretory organ for OTA elimination (Marquardt and Frohlich, 1992).
Moreover, the exact mechanisms of its toxicity are not fully understood. Among these mechanisms, oxidative stress appears to be of particular interest as it is common for many toxic effects of OTA (Klauning and Kamendulis, 2004). Reactive oxygen species (ROS) have a significant role in mycotoxicosis, mediating cellular damage (Surai et al. 2008). ROS’s excessive formation leads to oxidative stress, which can trigger cell damage by oxidizing macromolecular structures and modifying their biological functions, ultimately causing cell cycle arrest and cell apoptosis (Ting et al. 2010). The nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor responsible for regulating cellular redox balance in eukaryotic organisms (Habtemariam, 2019). Once Nrf2 is activated, it binds to the antioxidant responsive elements (ARE) in the promoter region of target genes. Hence, activation of Nrf2 leads to the induction of HO-1 expression and other antioxidant proteins (Itoh et al. 2003). Antioxidant treatment is a medical approach for protection against the disturbed oxidant-antioxidant status and has been considered a hopeful remedy for the prevention and treatment of many diseases (Abd El Latif et al., 2021). For that, compounds that ameliorate OTA-induced disorders must be identified to be offered in animal diets and as protective agents for human health. Therefore, substantial efforts have been directed toward identifying natural antioxidants with free radical scavenging action to combat oxidative stress-mediated toxicity.
Feed supplements with immunostimulating properties as medicinal plants or probiotics have been widely used within the last years (Abdelhady and El-Abasy 2015; Abdelhady et al. 2017; Markowiak and Śliżewska 2018; Dawood et al. 2020 Moustafa et al. 2020; Assar et al. 2021). Aspergillus awamori has been known as a safe and efficient probiotic microorganism (Lee et al. 2008; Saleh et al. 2015). A. awamori supplementation in animal diet modulates digestive enzymes, therefore enhancing the nutrients digestibility (Tamang et al. 2016), reducing skeletal muscle lipid peroxidation (Saleh et al. 2012 and El-Deep et al. 2014), and improving the immune response of growing rabbits (El-Deep et al. 2021).
There are no previous reports on the modulating effects of A. awamori against induced renal and cardiac damage in rabbits. Therefore, the present study aimed to evaluate the protective effects of dietary supplementation with A. awamori against OTA-induced nephrotoxicity and cardiotoxicity in growing New Zealand rabbits by assessing the growth performance, hematological parameters, immune response, oxidative and antioxidant status, serum organ dysfunction biomarkers, histopathology with exploring the possible underlying regulatory molecular mechanism through gene expression profiles of Nrf2, HO-1, iNOs, IL-1β, and TNF-α.
Materials and methods
Chemicals
All chemicals used in this study were of analytical grade. Ochratoxin was purchased from Sigma Chemical Co. (St. Luis, MO, USA). Asp. Awamori powder was kindly supplied by Prof. K. Hayashi, Department of Biochemical Science and Technology, Faculty of Agriculture, Kagoshima University, Kagoshima, Japan. The kits of aspartate aminotransferase (AST), alkaline phosphatase (ALP), total protein, albumin, urea, creatinine, malondialdehyde (MDA), catalase (CAT), and total antioxidant capacity (TAC) were obtained from Biodiagnostics Co. (Cairo, Egypt). While LDH kit was purchased from Randox Laboratories Ltd. (Crumlin, UK), and CK-MB was obtained from Stanbio™ (CK-NAC [UV-Rate] kit; Boerne, TX, USA).
Experimental animals and design
Sixty 6-week-old New Zealand male rabbits with an average body weight of 990 ± 5.05 g were obtained from Animal Production Research Center, Sakha, Kafr Elsheikh. Rabbits were acclimatized to a 16 h light/8 h dark cycle for 3 weeks before the experiment. They were individually housed in galvanized metal wire cages (60 × 50 × 35 cm) equipped with feeding and water troughs. Feed was formulated to cover all essential nutrient requirements for growing rabbits, as shown in Table 1 (De Blas and Mateos 1998; NRC 1997). Feed and water were offered ad libitum.
After the acclimatization period, rabbits were randomly divided into five groups (n = 12): the control group received a basal diet free from mycotoxins in the form of pellets; the OTA group received a basal diet contaminated with 0.3 mg/kg diet OTA following the dosage of (Prabu et al. 2013); OTA + A. awamori (group 3–5) fed basal diet contaminated with 0.3 mg/kg diet OTA and supplemented with 50, 100, and 150 mg/kg diet A. awamori, respectively following the dosage and formulation of Abdelhady et al. (2017). The treatments were administered for 2 months. The experimental protocol is described in (Fig. 1). Live body weight, feed intake, and several dead rabbits were recorded. The experimental protocol was approved and carried out following the Ethics Committee of Kafrelshiekh University.
Formulation of OTA feed pellets
Fifteen milligrams OTA was dissolved in 100 ml ethanol and mixed thoroughly with a 50 kg diet to get 0.3 mg/kg OTA. The ethanol was then removed by vaporization under lowered pressure. Quantification of OTA in the diet was determined by reverse-phase liquid chromatography with post-column derivatization with iodine, as previously described by. The obtained analytical data were confirmed with solution fluorometry with bromine, and the deviations found ranged from 3.5 to 7.5%, with the best results obtained from the first quantification method. To validate this method in rabbit feed matrix, blank rabbit feed samples were provided for recovery determination. The mean recoveries for OTA was 85.5%, with mean relative standard deviations (RSD) of 16.3% as determined by reverse-phase liquid chromatography with post-column derivatization with iodine. The detection limits (LD) and quantification (LQ) were 0.3 µg/kg and 1.0 µg/kg, respectively.
Sampling
At the end of the experimental period, rabbits were anesthetized (isoflurane, 5 mg/kg) (Parasuraman et al.2010). Blood samples were collected from the marginal ear vein of each animal. The 1st part was on EDITA-containing tubes for hematological assay, and the 2nd one was on the heparinized tube for estimating phagocytic activity. The 3rd part was collected on plain tubes left to clot at room temperature, then centrifuged at 3000 rpm for 15 min for serum separation and kept at – 20 °C for antioxidant assay and biochemical analysis. Then the rabbits were sacrificed, and kidney and heart samples were rapidly excised and collected from each animal for histopathological examination and real-time PCR.
Growth performance and immunological assessment
Rabbits were weighed at the 6th and the 14th week of age, and the live body weights (LBW) in grams were recorded. Daily weight gain, feed intake, and feed conversion ratio (FCR) were calculated.
The performance index was calculated according to (Ayyat et al. 2018). The average body weight gain (BWG) and feed conversion ratio (FCR) were calculated according to Alagawany et al. (2016). Phagocytic activity (PA) and phagocytic index (PI) using were performed according to the methods of Rudkin et al. (2013).
Phagocytic activity = macrophages containing yeast/total number of macrophages × 100; Phagocytic index = number of cells phagocytized/Number of phagocytic cells.
Hematological investigation
Red blood cells count (RBCs), hemoglobin (Hb) concentration and hematocrit value (PCV%), total leukocyte counts (WBCs), and differential leukocyte count were analyzed by using Orphee Mythic 22 CT Hematology Analyzer (Orphee SA Co., Plan-les-Ouates, Switzerland). Erythrocytic indices such as mean cell volume (MCV), mean cell hemoglobin (MCH), and mean cell hemoglobin concentration (MCHC) were determined as described by Feldman et al. (2000).
Serum biochemical assay
Serum biomarkers for renal damage, including (urea, creatinine, sodium, potassium, and ALP), cardiac injury biomarkers such as (AST, LDH, and CK-MB) together with total proteins, albumin, and glucose, were determined. According to Larsen (1972) and Coulombe and Favreau (1963), creatinine and urea were determined. Total proteins (TP) were estimated according to Lowry et al. (1951), and albumin (Alb) was measured according to Henry et al. (1974). Alkaline phosphatase (ALP) was measured according to Tietz et al. (1983). According to Trinder (1951) and Terri and Sesin (1958), sodium and potassium were determined. Aspartate amino transferase (AST) was evaluated according to Reitman and Frankel (1957). Serum LDH activity was detected according to (Buhl and Jackson 1978). Creatine kinase MB (CK-MB) activity was measured according to the method developed by Szasz et al. (1979). Glucose was determined according to Trinder (1969).
Estimation of lipid peroxidation and antioxidants biomarkers
Serum contents of lipid peroxidation biomarkers malondialdehyde (MDA) and nitric oxide (NO) were determined according to Esterbauer et al. (1982) and Montgomery and Dymock (1961), respectively. The enzymatic antioxidant biomarkers, including catalase (CAT), was estimated according to Aebi (1984); total antioxidant capacity (TAC) was determined according to Koracevic et al. (2001), and SOD according to Packer and Glazer (1990) following the manufacturers’ instructions (Bio-diagnostic Co., Giza, Egypt).
Histopathological examination
The collected tissue samples from the kidney and heart of each animal were rapidly fixed in a 10% neutral-buffered formalin solution. Fixed specimens were processed through the standard paraffin embedding technique, including dehydration in ascending grades of ethanol, clearing in xylene, and embedded in paraffin wax. Then, 4-µm-thick paraffin tissue sections were stained with hematoxylin and eosin (H&E) (Bancroft and Layton 2013).
Real-time PCR for gene expression of Nrf2, HO-1, IL-1β, TNF-α and iNOs
The real-time PCR (qPCR) was used to determine nuclear factor E2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1), and inducible nitric oxide synthase (iNOs) in addition to inflammation-related genes; interleukin one beta (IL-1β), tumor necrosis factor-alpha (TNF-α). Total RNA was extracted from kidney and heart tissues of rabbits using Gene JET RNA Purification Kit (Thermo Scientific, # K0731, USA). RNA samples were reverse transcribed using Revert Aid H Minus Reverse Transcriptase (Thermo Scientific, USA, Cat no. EP0451) as previously described (Awad et al., 2020). The sequence of the used primers is presented in Table 2. In addition to primers and cDNA samples, 2X Maxima SYBR Green/ROX qPCR Master Mix (Thermo Scientific, USA, cat no. K0221) and StepOnePlus thermal cycler (Applied Biosystem, USA) was used to perform qPCR. The relative gene expression differences were normalized with the house-keeping gene β actin. The thermal cycling conditions included 10 min at 95 °C followed by 45 cycles (95 °C for 15 s, 60 °C for 30 s, and 72 for 30 s). Calculation of all fold changes in gene expression of treated groups (G2–G5) relative to expression in the control group (G1) was performed as previously depicted (El-Magd et al., 2016). Samples in each group were analyzed in triplicates and non-template control and negative RT controls in each plate.
Statistical analysis
All data were examined for the normality and homogeneity by the Shapiro–Wilks test. The obtained data were analyzed using one-way ANOVA with Duncan’s post hoc test using SPSS version 17.0 (SPSS Inc, Chicago, USA) to determine the experimental groups. All data are presented as mean ± SEM. P value ≤ 0.05 was considered statistically significant.
Results
Clinical signs and post-mortem lesions
The control group was clinically normal, with no recorded mortalities. In contrast, OTA-treated rabbits showed loss of appetite, lethargy, and emaciation. Mortalities were recorded in 2 out of 12 rabbits (16.67%), which started from the 18th day until the 25th day. The central gross pathological lesion in dead and sacrificed rabbits who received OTA was the enlargement of their hearts and kidneys. Surprisingly, A. awamori supplemented rabbits were greatly improved clinically without mortalities in a dose-dependent response in all groups compared to OTA-treated rabbits. The best recovery was noticed in rabbits who received A. awamori at150 mg/kg diet.
Growth performance and immune responses
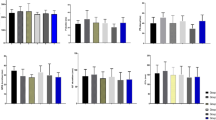
A significant reduction in feed intake, final body weight (FBW), and body weight gain (BWG), with a substantial increase in the food conversion ratio (FCR), was observed in rabbits fed 0.3 mg of OTA/kg diet compared to the control group (Fig. 2). However, rabbits fed an OTA-containing diet supplemented with A. awamori (50, 100, or 150 mg/kg diet) showed a significant improvement in feed intake, FBW, and BWG and a significant decline in FCR compared to the OTA treated rabbits. The improvement in growth performance was related to the increase in A. awamori concentration (Fig. 2).
Effect of OTA and/or A. awamori supplementation on rabbits IBW initial body weight, FBW final body weight, BWG body weight gain, FCR feed conversion ratio. Data were presented as mean ± SEM (n = 7 per group). a–dMeans column carrying different superscript letters are significantly different at P ≤ 0.05
Moreover, OTA-treated rabbits showed a significant reduction in the phagocytic activity (PA) and the phagocytic index (PI) compared to the control group. However, the dietary incorporation of A. awamori significantly enhanced the PA and the PI in a dose-dependent manner, with the best improvement by A. awamori’s highest concentration compared with the OTA treated group, as shown in Fig. 3.
Hematological parameters
The OTA-intoxicated group displayed normocytic normochromic anemia as a decline in RBCs count, Hb concentration, and PCV% without changes in RBCs indices. In addition to stress, picture of leukogram is represented by a significant leukocytosis, heterophilia, monocytosis, lymphopenia, and eosinopenia. In addition to raised heterophils/lymphocytes ratio as compared to the control rabbits fed the basal diet (Table 3). In contrast, dietary A. awamori addition markedly restored the altered hematological parameters to the comparable references of the control rabbits. The best improvement was detected in rabbits who received A. awamori at a 150 mg/kg diet.
Biochemical parameters
Rabbits fed OTA-contaminated diets showed significantly elevated levels of urea, creatinine, and ALP with a significant reduction in sodium, potassium, total proteins, and albumin levels compared to rabbits fed the basal diet only (Table 4). The levels of these biomarkers were markedly modulated and normalized in all rabbits fed a diet containing OTA and A. awamori, with the best-documented impact in rabbits who received A. awamori at 150 mg/kg diet.
Regarding serum biomarkers concerning cardiac injury, the rabbits fed OTA-contaminated diet exhibited higher enzymatic activities of AST, LDH, CK-MB, and elevated glucose concentration than the control group. Interestingly, the dietary addition of A. awamori ameliorated nearly all OTA adverse effects with the most significant influence in rabbits who gained A. awamori at 150 mg/kg diet. Changes in biochemical parameters are illustrated in Table 4.
Oxidative stress and antioxidant biomarkers
Rabbits intoxicated with OTA exhibited significantly higher serum MDA and NO levels. However, serum TAC, CAT, and Gxp activities were depleted considerably compared with the control rabbit group. A. awamori dietary supplementation reversed these altered parameters to a comparable level of the control group, with the best effect for A. awamori at a 150 mg/kg diet. Changes in lipid peroxidation and antioxidant enzymes are portrayed in Fig. 4.
Effect of OTA and/or A. awamori supplementation on anti-oxidant/oxidant balance. Data were presented as mean ± SEM (n = 7 per group). a–d Means column carrying different superscript letters are significantly different at P ≤ 0.05.CAT = Catalase; SOD = Super oxide dismutase; TAC = Total anti-oxidant capacity; NO = Nitric oxide and MDA = Malonalaldehyde
Histopathological findings of the kidney and heart
Kidney
The kidneys of control rabbits showed the normal histological appearance of the glomeruli and renal tubules in both cortical and medullary regions (Fig. 5A). In contrast, rabbits provided with OTA exhibited various histological alterations; hyperemia in glomerular capillaries and interstitial vessels and marked vacuolar degeneration of tubular epithelium (Fig. 5B). Other renal tubules showed cystic dilatation with pressure atrophy on the surrounding tubules (Fig. 5C). In addition, multiple focal mononuclear cell infiltrations, mainly of macrophages and lymphocytes with some heterophils, are either periglomerular (Fig. 5D) or interstitial (Fig. 5E). In addition, focal areas of coagulative tubular necrosis were noticed either cortically or medullary with a condensed eosinophilic cytoplasm and pyknotic nucleus. Also, hyaline casts in some of the tubular lumens were seen. The severity of these tubular lesions was improved by supplementation of A. awamori in a dose-dependent response with the highest restoration of renal parenchyma at a concentration of 150 mg/kg of A. awamori (Fig. 5F) with minimal degenerative changes. A moderate alleviation of renal histological changes was observed at a concentration of 0.1℅ with a modest degree of renal tubular vacuolation, necrosis, and cellular infiltrations (Fig. 5G). However, in rabbits supplied with a 50 mg/kg diet of A. awamori, a mild defending effect against the OTA renal damaging effect (Fig. 5H) with continuity of tubular degenerative changes and inflammatory cells infiltration.
Histopathological changes of the rabbit’s kidney in different experimental groups. A Control group shows the normal histological appearance of the kidney. B In the OTA-treated group, the kidney shows marked tubular cytoplasmic vacuolation with pyknotic nuclei of renal epithelial cells (arrow). C OTA-treated group, kidney showing cystic dilatation of some tubules inducing pressure atrophy of the surrounding parenchyma. D OTA-treated group, kidney showing periglomerular mononuclear cells infiltrations (arrow) predominantly lymphocytes and some heterophils with the presence of a necrotic area of a dark condensed cytoplasm and pyknotic nucleus on the periphery. E OTA-treated group, kidney showing focal interstitial mononuclear cells infiltrations (arrow) mainly of macrophages and lymphocytes accompanied with variable degenerative changes of the surrounding tubules. F OTA + 150 mg/kg diet of A. awamori-treated group, kidney showing marked restoring of normal renal architecture with mild inter-tubular vessels dilation and few renal casts. G OTA + 100 mg/kg diet of A. awamori-treated group, kidney showing a moderate degree of renal damage with vacuolar tubular degeneration (arrow) and some necrotic tubules. H OTA + 50 mg/kg diet of A. awamori-treated group, kidney showing marked interstitial inflammatory cells infiltration (arrow) and an area of tubular necrosis. All are H&E stained, bar = 50 µ
Heart
The heart of the control group showed a normal histological appearance of regularly distributed myocardial fibers (Fig. 6A). In contrast, hearts of the OTA-exposed group showed marked swelling of myocardial fibers, marked fatty vacuolation of myocardial cells (Fig. 6B) in some parts of the myocardium, and others showed complete myolysis with rarefied cytoplasm and karyolysis (Fig. 6C). The degenerative changes were exaggerated to necrosis and destruction of the muscle fibers (Fig. 6D) and loss of their regular distribution pattern. Focal infiltration of mononuclear cells between the muscle fibers (Fig. 6E) was detected in addition to multiple areas of vascular congestion and hemorrhages. Amazingly, in rabbits exposed to combine administration of OTA and A. awamori, there was a notable improvement in heart structure which was more distinct in rabbits supplemented with a 150 mg/kg diet of A. awamori, where no cytoplasmic vacuolation or swelling of myocardial cells was seen (Fig. 6F). In rabbits supplied with a 100 mg/kg diet of A. awamori, the degree of congestion, myocardial cytoplasmic vacuolation, necrosis, and inflammatory cell infiltration was moderately decreased (Fig. 6G). Unfortunately, the hearts of rabbits that received a 50 mg/kg diet of A. awamori were weakly restored the normal histology showed degenerative and necrotic changes (Fig. 6H).
Histopathological changes of the rabbit’s heart in different experimental groups. A Control group, normal histological structure of the heart. B OTA-treated group, heart showing fatty vacuolation (arrow) of cardiac myocytes with variably sized vacuoles (arrow). C OTA-treated group, heart showing massive degenerative changes of myofibers with cytoplasmic rarefaction and loss of nuclei (arrow) and others showing cell necrosis with eosinophilic cytoplasm and pyknotic nuclei. D OTA-treated group, heart showing muscle degeneration and loss of myocardial bundles arrangement (arrow) with slight infiltration of mononuclear cells and atrophy of the surrounding myocytes. E OTA-treated group, heart showing the focal area of mononuclear cells infiltration (arrow) in between muscle fibers with disorganization of the surrounding myofibers. F OTA + 0.15% A. awamori-treated group, heart showing marked improvement of the heart histological appearance where no cytoplasmic vacuolation or swelling. G OTA + 0.1% A. awamori-treated group, heart showing moderate degenerative changes of myocardial cells accompanied with slight inflammatory cells infiltration (arrow) and areas of hemorrhage. H OTA + 0.05% A. awamori-treated group, heart showing marked myolysis with rarefied cytoplasm and karyolysis. All are H&E stained, bar = 50 µ
Gene expression of oxidative stress and inflammation-related genes
In OTA fed rabbits, significant downregulation of mRNA expression level of Nrf2 and HO-1 (cytoprotective factor regulating the expression of genes coding for antioxidant) genes was seen while marked upregulation of iNOs (nitrosative stress) level (Fig. 7) along with TNF-α and IL-1β expression levels (Fig. 8). The dietary addition of A. awamori modulated these gene expression profiles. The best regulatory effect was correlated with A. awamori at a 150 mg/kg diet.
Influence of OTA and/or A. awamori administration on Nrf2, HO-1, and iNOS mRNA expression in renal and cardiac tissues of rabbits. Data were presented as mean ± SEM (n = 7 per group). a−d Means coulmncarrying different superscript letters are significantly different at P ≤ 0.05. Nrf2 nuclear factor E2-related factor 2, HO-1 heme oxygenase-1, iNOS inducible nitric oxide synthase
Influence of OTA and/or A. awamori administration on IL-1β and TNF-α mRNA expression in renal and cardiac tissues of rabbits. Data were presented as mean ± SEM (n = 7 per group). Transforming growth factor alpha = TNF-α and Interleukin 1 beta = IL-1β. a–d Means coulmncarrying different superscript letters are significantly different at P ≤ 0.05
Discussion
Many reports demonstrated the adverse toxicological influences of OTA on rabbits (Mézes 2008; Sun et al. 2018; Jedziniak et al. 2019). Here, the rabbits fed a diet contaminated with OTA showed a mortality rate of about 16.67%, similar to data reported by other researchers (Kumar et al. 2003; Elaroussi et al.2006). Moreover, OTA reduced feed intake, body weight gain, and elevated FCR, indicating poor growth performance (Zhang et al.2016 and Gan et al. 2017). The reduction in weight gain may be due to the harmful effect of OTA on the intestinal tract by reducing feed absorption (Raju and Devegowda, 2000). Unlike rabbits fed a diet contaminated with OTA alone, those cotreated with A. Awamori showed improvement in growth performance following (El-Deep et al.2020). This improvement may be attributed to the endogenous release of digestive enzymes in the animal’s gut, which accelerates the digestion and absorption of ingested diets (Phuoc and Jamikorn, 2017). In addition to increasing the metabolizable energy of the feed along with enhancement of cellulose digestion, volatile fatty acid formation or due to enhancing the survival and implantation of live microbial content in the gastrointestinal tract (Saleh et al. 2012) or duo the improvement of lipid and fiber digestibility (El-Deep et al. 2020). Broilers fed on diets containing 0.05% and 1% A. Awamori increased the carcass weights (Yamamoto et al. 2007). Cellulase and xylanase enzymes are required for the digestion of soluble non-starch polysaccharides that were found to be produced by A. awamori (Bhat and Hazlewood 2001). Aspergillus is known to improve the nutritional quality of soybean due to its enzymes that degrade trypsin inhibitors (Hong et al. 2004). A growth-promoting effect was found in fermented products processed by A. awamori (Kamizono et al. 2013); A. awamori was also involved in the appetite regulation in the central nervous system (Kamisoyama et al. 2009).
OTA harms the immune response and antioxidant status, which ultimately increases the susceptibility of animals to metabolic diseases and tissue damage (Kumar et al. 2014). OTA has an immunosuppressive effect as a potent inhibitor of protein synthesis, which delays the division of the rapidly dividing cells of the immune system (Harvey et al. 1992). OTA impairs lymphocytes production, activation, differentiation, and proliferation (Haubeck et al. 1981; Lea et al. 1989). Several in vitro studies focused on OTA impacts on neutrophils and macrophages, including oxidative stress, apoptosis, phosphorylation of the ERK1/2, and release of TNF-α via NF-kB pathways (Liu et al., 2012; Giromini et al. 2016; Brennan et al. 2017). OTA also decreases the phagocytic activity of the macrophages, probably due to the reduction of basal interferon (Harvey et al. 1992). In the present study, dietary A. awamori improved the suppressed immune response induced by OTA. A more robust immune response was indicated by the increased total leukocyte and lymphocyte counts and increased phagocytic activity and phagocytic index of heterophils with elevated complete proteins concentrations. Our results agreed with Falcao-e-Cunha et al. (2007), who reported that prebiotics stimulates the immune responses in rabbits.
Additionally, Sakai et al. (2001) recorded enhancement of phagocytic activity following the addition of yeast to OTA-intoxicated Nile tilapia. Similarly, Mateos et al. (2010) detected a stimulatory effect on rabbits’ immune response when fed a diet with specific oligosaccharides. Yang et al. (2009) stated that probiotics could enhance the immune system by adhering to the intestinal mucosa. Further, probiotic stimulates the immune response by activating macrophages and lymphocytes and higher production of immunoglobulins and γ-interferon. Similarly, Wintrobe (1983) who recorded increase in the total leukocyte count by probiotic supplementation duo to polymorphonuclear cell proliferation in the white pulp with activated B-lymphocytes in the red pulp of the spleen.
Moreover, probiotic plays a pivotal role in inducing a healthy intestinal structure and enhancing immunity Rajput and Li (2012). Additionally, Glick (2000) stated that gut microflora is essential for the early stimulation and maturation of the cellular component of the intestinal immune system. Therefore, an improved intestinal microenvironment stimulates pluripotent hemopoietic precursors into clones of lymphocytes, which could be one factor in the increase in total leukocyte count.
Oxidative stress is an important mechanism associated with the OTA-induced nephrotoxicity. Therefore, we measured the oxidative and nitrosative stress markers MDA and NO together with antioxidant biomarkers TAC, CAT, and SOD activities. A. awamori at the studied concentrations exerted antioxidant power in a dose-dependent response. In our study, the protective effect of A. awamori against OTA-induced oxidative stress could be either directly by scavenging free radicals and inhibiting lipid peroxidation by reducing MDA and NO levels or indirectly via enhancing the antioxidant activity of SOD, CAT, and TAC. A. awamori owed higher antioxidant active constituents such as (citric acid, citric acid isomers, octadecenoic, octadecadienoic, and octadecanoic acid's derivatives), polyphenols, and flavonoids that potentiate A. awamori biological activity as antioxidant and anticancer agents (Assar et al. 2021). Lin et al. (2012) stated that A. awamori increased antioxidant activity and DNA protecting effect concerning the conversion of phenolics of litchi pericarp, a readily accessible source of the natural antioxidants in the food industry. Similarly, Lee et al. (2008) stated that A. awamori was superior for exhibiting the highest antioxidant activity during the fermentation process due to polyphenol content enhancement of the β-glucosidase enzyme. El-Deep et al. (2014; 2021) and Abdelhady et al. (2017) provided an additional explanation for A. awamori antioxidant activity through increasing mRNA expression of genes encoding antioxidant enzymes such as GPx, SOD, and CAT in broiler chickens and rabbits fed a diet with A. awamori. Saleh et al. (2012) and El-Deep et al. (2014) reported that the dietary addition of A. awamori (0.1%) or A. niger (0.05%) or feeding A. awamori (0.05%) declined the thiobarbituric acid reactive substances (TBARS) value while enhanced α-tocopherol content in the broiler breast muscle referring to A. awamori antioxidative properties.
The current work showed that rabbits who received OTA suffered from normocytic normochromic anemia and leukocytosis, neutrophilia, monocytosis, lymphopenia, eosinopenia, and increased H/L, indicating a stress picture of leukogram. These results supported the findings of Elaroussi et al. (2006) and Sawale et al. (2009), who detected a significant reduction in the total number of erythrocytes in broiler chickens fed an OTA-contaminated diet. The high number of WBCs despite the declined RBCs, Hb, and PCV indicates a partial depressive effect of OTA on the bone marrow duo metabolic disturbance (Janaczyk et al. 2006). H/L is disturbed by stressors, and it can be used as a sensitive hematological sign of stress response (Janaczyk et al. 2006; Kowalski et al. 2006 and Salamano et al. 2010). Jelkmann (2011) stated that multiple mechanisms might contribute to anemia in renal diseases; tubular atrophy during chronic kidney disease generates tubule-interstitial fibrosis, leading to a reduction of erythropoietin synthetic capacity contributes to the associated anemia. In addition, the increase of serum alkaline phosphatase and hyperphosphataemia may also play a role in chronic renal disease-associated anemia and EPO hypo-responsiveness and reduction in erythropoietin hormone (Epo) expression (Stachurska et al., 2013). Simultaneous dietary supplementation with A. awamori can reverse the OTA-induced reduction in total RBCs, PCV%, and Hb concentration. In harmony with Fathi et al. (2017), who detected that A. awamori as a probiotic enhanced the RBCs count and hemoglobin concentration in rabbits via its anti-inflammatory and immune-modulatory properties (Group 2014). This may pinpoint the free radical-scavenging properties and the antioxidant activities of A. awamori (Assar et al., 2021) that protect against lipid peroxidation of the erythrocyte membrane.
OTA induces oxidative stress and releases reactive oxygen and nitrogen species (Tao et al., 2018) therefore mediating cellular damage with the release of intracellular enzymes (Surai et al., 2008). In the current study, we found a remarkable increase in levels of creatinine, urea, and ALP activities with the marked decline of sodium, potassium, total proteins, and albumin levels in OTA-intoxicated rabbits as compared to the control rabbits. Although exact mechanisms involved in OTA-induced renal toxicity are not fully known, Glahn et al. (1989) suggested that OTA may cause an osmotic diuresis in pullets by inhibiting the tubular reabsorption of electrolytes. Anzai et al. (2010) stated that OTA mainly impairs proximal tubular functions and causes glucosuria. The elevated urea and creatinine levels could be attributed to the inhibiting effect of OTA metabolites on glomerular filtration and catabolic protein rates, which reduced the renal capacity to get rid of urea (Akturk et al., 2006). Serum total proteins concentrations are commonly used as humoral immunity indicators. The reduced level of complete serum proteins can be due to vascular leaking and failure of their synthesis, and elevated rates of proteolysis. The reduced albumin concentration can be explained by increased renal excretion and loss of protein synthesis by OTA-induced liver malfunction in rabbits (Kumar et al., 2007). The noted hypoproteinemia may also be attributed to insufficient digestion; malabsorption preceded by damage to the gastrointestinal tract. Mycotoxins can also exert their effects on the hepatic tissue causing hepatocytes lipid infiltration, necrosis, or hepatic cell death (Cagauan et al. 2004). ALP can be drastically increased in patients with renal insufficiency and duo hepatic injuries. OTA mediated kidney damage through induction of oxidative stress that arises from the excessive generation of ROS, which has been reported to attack various biological molecules, including lipids, and causes lipid peroxidation (Zheng et al. 2013). Kamp et al. (2005) reported that OTA induces oxidative stress, which may lead to subsequent damage or initiation of the apoptotic process. Increased apoptosis rates in the kidney may lead to polycystic kidney disease, glomerular sclerosis, or interstitial fibrosis. Klaric et al. (2008) showed that OTA induced cytotoxicity and apoptosis in porcine kidney 15 (PK15) cells, suggesting that OTA-induced nephrotoxicity is related to oxidative damage. However, renal dysfunction biomarkers were significantly modulated by A. awamori supplementation, suggesting its improving effects on rabbits (El-Katcha et al. 2011).
Cardiac injury biomarkers such as CK-MB and LDH are markedly released into the extracellular fluid during cardiac damage. In this experiment, OTA-treated rabbits exhibited markedly elevated enzymatic activities of AST, LDH, and CK-MB together with increased glucose concentration as compared with the control group. OTA-treated group revealed elevation of all myocardial enzymes (Cui et al. 2020) due to mitochondrial dysfunction. Similarly, reported biochemical changes in chickens’ heart injury induced by OTA. Moreover, OTA hyperglycemic effect in the exposed rabbits may be due to a disturbance in carbohydrate metabolism Verma and Shalini (1998). Verma and Shalini (1998) supported our results, reporting that OTA could induce hyperglycemia in rabbits. Simultaneous dietary A. awamori counteracts the OTA hyperglycaemic effect. Consistent with this, Takemoto et al. (2014) found that A. awamori-fermented diet ameliorated the alloxan-induced hyperglycemia in mice.Similarly, Doi et al. (2015) confirmed that Burdock Root fermentation with A. awamori inhibited the induced hyperglycemia.
Here, the observed renal histopathological alterations due to OTA exposure were consistent with previous reports by Battacone et al. (2010). OTA can induce degenerative and necrotic lesions in the kidneys (Francisco and Maria, 2010), and these lesions could be correlated to oxidative damage of OTA. Likewise, retardation of growth performance with renal histopathological changes was also observed in piglets after OTA exposure (Zhang et al. 2016 and Gan et al. 2017), rabbits (Prabu et al. 2013), rats (Aydin et al. 2003; Biro et al. 2002 and Malekinejad et al. 2011), and porcine through inducing renal cytotoxicity and apoptosis (Klaric et al. 2008). The observed cystic dilation in OTA-treated rabbits may be attributed to the direct toxic effect of OTA on the tubule epithelium impairing their absorption and secretion, or may result from the lower urinary tract obstruction, deposition of tubular crystals, interstitial inflammation and/or fibrosis, and chronic progressive nephropathy (Greaves 2012). Moreover, cystic dilatation was also observed in nephropathic mice exposed to dietary OTA at 40 μg/g (Wanda et al. 2002). The observed cast formation may be owed to the tubular epithelium detachment, deposition, and interaction with the tubular lumen proteins. Secondly, impaired sodium reabsorption because of damaged tubular epithelium, which increases sodium concentration in the tubular lumen, producing protein polymerization (Abuelo 2007). Rising renal injury biomarkers supported these findings as urea, creatinine, and ALP and elevation of oxidative stress markers in line (Jan et al., 2017). In this work, OTA-intoxicated rabbits exhibited marked myocardial histopathological changes confirmed by the remarkable increase in serum cardiac injury biomarkers and the notable elevation of serum oxidative stress biomarkers such as MDA and NO with significant inhibition of TAC, CAT, and GPX activities. In harmony with, who reported that OTA induced chickens’ heart injury biomarkers elevation. noted that OTA induced myocardial congestion, swelling and necrosis, and ultrastructural changes in the myocardium of rats. Similar histopathological changes were reported in OTA-treated rats (Hussein et al. 1997). In the same line, Cui et al.(2020) reported that OTA-induced mouse myocardial tissue damage was represented by massive cytoplasmic vacuolar degeneration, myocardial swelling, and necrosis due to mitochondrial dysfunction. Stoev et al. (2021) detected some lytic changes and irregular staining of the myofibrils of OTA exposed chicks. OTA strongly affects the performance of the myocardium and the cardiovascular system of the rat. The OTA-induced myocardial injury may be due to the elevated calcium level within the myocardium (Hussein et al., 1997). Khan et al. (1989) suggested a possible direct effect of OTA on the integrity of myocardial cell membrane through leakage of calcium-loaded microsomes resulting in an influx of extracellular calcium ions. Interestingly, dietary A. awamori-supplied rabbits restored the altered renal and cardiac histological architecture to normal in a dose-dependent response with the best improvement at the highest concentration of A. awamori.
NO production is increased in inflammation and has pro-inflammatory and regulatory effects. Cavin et al. (2009) reported that OTA stimulated NO’s formation through an NF-kB-dependent induction of iNOS. Here, we also found higher expression levels of IL1b, TNFa, and iNOS in the kidney and hearts of OTA intoxicated rabbits. The mechanism of NO stress and genotoxicity may be explained by its reaction with superoxide radical to form the pro-oxidant peroxynitrite, which rapidly decomposed to the nitro radical, causing nitrosative stress and DNA damage (Heikal et al., 2009). All these findings suggest the involvement of oxidative stress in the OTA-mediated renal and cardiac toxicity and point out the role of ROS in OTA’s adverse effects. In the present experiment, OTA-intoxicated rabbits exhibited a significant reduction in renal and cardiac mRNA expression of Nrf2 and HO-1 with a marked elevation in renal and cardiac mRNA expression of iNOS compared with the control group. Boesch-Saadatmandi et al. ( 2008) reported that OTA nephrotoxic effect was mediated by the downregulation of Nrf2 and HO-1 genes. Similar findings for reducing Nrf2 and HO-1 were detected in the kidneys of piglets (Marin et al., 2018) and the myocardial tissue of mice treated with OTA (Cui et al., 2020). HO-1 is recognized for its cytoprotective activities, chiefly in the cardiovascular system, even though its positive impacts on kidney diseases have been described in several diverse in vitro and animal models (Jarmi and Agarwal 2009). Surprisingly, stated that complicated mechanisms of OTA nephrotoxicity might be partially overcome by HO-1 activation through upregulation of MAPK kinases activity via inducing ERK1/2 and activation of major Nrf2-regulated antioxidants enzymes. Nrf2 participates in both the basal expression and induction of several genes, including genes encoding for detoxification, cytoprotection, and antioxidant enzyme activation (Kensler et al., 2007). OTA also inhibited SOD increased ROS, and weakened the expression of glutathione S-transferase (GST) with reduced activation of Nrf2 (Bösch-Saadatmandi et al., 2009). Herein, inhibition of Nrf2 is strongly linked with the inhibition of HO-1; lowered levels of serum TAC, CAT, and GPX activities; and the observed renal and cardiac histopathological alterations of OTA-exposed rabbits. Therefore, inhibition of antioxidative defense is the probable mechanism that could contribute to OTA nephrotoxicity and cardiotoxicity. Interestingly, OTA-intoxicated rabbits supplied with dietary A. awamori upregulated Nrf2 and HO-1 in a dose-dependent response. Thus, A. awamori reversed the oxidative stress by achieving the balance between liberating (MDA and NO) and scavenging ROS by (TAC, CAT, and SOD) through inducing (Nrf2 and HO-1) expressions. It is well-known that Nrf2 activators potentially hinder carcinogenesis in animal models and humans (Cavin et al., 2007). Nrf2 plays a role in protecting cells from oxidative stress and inflammatory insults (Chen et al. 2006). Similarly, found that selenium-rich yeast attenuated OTA-induced small intestinal injury in broilers by activating the Nrf2 pathway and inhibiting NF-KB. The inflammatory response is organized by pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α. Furthermore, IL-lβ triggered the expression of iNOS mRNA, which stimulated NO production. Our results have shown that OTA induces a significant increase of IL-1β and TNF-α mRNA in the kidney and heart of intoxicated rabbits, referring to an OTA-induced inflammatory response. Marin et al. (2017 and 2018) observed higher levels of IL-1β and TNF-α in piglets’ kidneys sub-chronically intoxicated with OTA. In contrast, the dietary addition of A. awamori to OTA-intoxicated rabbits has an anti-inflammatory effect via downregulation of IL-1β and TNF-α. The hepatoprotective effect of A. awamori against aflatoxin B1 induced hepatic oxidative stress and inflammation in rabbits was previously investigated by Abdelhady et al. (2017) and against the initiation process of liver carcinogenesis caused by diethylnitrosamine (DEN) in a rat model (Assar et al., 2021).
Conclusions
This study revealed that the exposure of growing rabbits to an OTA-contaminated diet retarded their growth, altered immune-hematological and biochemical parameters, and induced histopathological changes in the kidney and heart of the exposed rabbits. To the best of our knowledge, this is the first report which pinpoints the protective effect of simultaneous dietary supplementation of A. awamori against OTA-induced renal and cardiac injuries through potentiating the rabbit’s anti-oxidant defense system via inducing the inducing Nrf2 signaling pathway, which activates heme oxygenate-1 (HO-1) enzyme, therefore enhancing TAC, CAT, and SOD activities and reducing oxidative and nitrosative stress by downregulating of iNOS expression thus reducing MDA and NO levels. In addition to its anti-inflammatory properties via downregulating of IL-1β and TNF-α expression levels, the best improvement at the highest concentration of the supplied A. awamori. Based on our observations, A. awamori could be utilized as a natural nephron and cardio protective agent against ochratoxicosis in rabbits.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Abbreviations
- A. awamori :
-
Aspergillus awamori
- AST:
-
Aspartate aminotransferase
- ALP:
-
Alkaline phosphatase
- CAT:
-
Catalase
- CK-MB:
-
Creatine kinase-MB
- TNF-α:
-
Tumor necrosis factor-alpha
- IL-1β:
-
Interleukin 1 beta
- HO-1:
-
Haemoxygenase-1
- iNOS:
-
Nitric oxide synthases
- LDH:
-
Lactate dehydrogenase
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- MDA:
-
Malondialdehyde
- NO:
-
Nitric oxide
- OTA:
-
Ochratoxin A
- SOD:
-
Superoxide dismutase
- TAC:
-
Total antioxidant capacity
References
Abd El Latif A, Assar DH, Elkaw EM, Hamza HA, Alkhalifah DHM, Hozzein WN, Hamouda RA (2021) Protective role of Chlorella vulgaris with Thiamine against Paracetamol induced toxic effects on haematological, biochemical, oxidative stress parameters and histopathological changes in Wistar rats. Sci Rep 11(1):3911. https://doi.org/10.1038/s41598-021-83316-8
Abdelhady DH, El-Abasy MA, Elsayed SS, Elbialy ZI, Shukry M, Hussein AH, Saleh AA, El-Magd MA (2017) The ameliorative effect of Aspergillus awamori on aflatoxin B1-induced hepatic damage in rabbits. World Mycotoxin J 10(4):363–373
Abdelhady DH, El-Abasy MA (2015) Effect of prebiotic and probiotic on growth, immuno-hematological responses, and biochemical parameters of infected rabbits with Pasteurella multocida. Benha Veterinary Med J 28(2):40–51
Abdel-Wahhab MA, Abdel-Galil MM, El-Lithey M (2005) Melatonin counteracts oxidative stress in rats fed an ochratoxin A contaminated diet. J Pineal Res 38:130–135
Abuelo JG (2007) Normotensive ischemic acute renal failure. N Engl J Med 357(8):797–805
Aebi H (1984) Catalase in Vitro, Methods in Enzymology. Elsevier, pp. 121–126
Akturk O, Demirin H, Sutcu R, Yilmaz N, Koylu H, Altuntas I (2006) The effects of diazinon on lipid peroxidation and anti-oxidant enzymes in rat heart and ameliorating role of vitamin E and vitamin C. Cell Biol Toxicol 22:455–461
Alagawany M, Ashour EA, Reda FM (2016) Effect of dietary supplementation of garlic (Allium sativum) and turmeric (Curcuma longa) on growth performance, carcass traits, blood profile and oxidative status in growing rabbits. Ann Anim Sci 16:489–505
Anzai N, Jutabha P, Endou H (2010) Molecular mechanism of ochratoxin A transport in kidney. Toxins 2:1381–1398
Assar DH, Mokhbatly AA, Ghazy E, Ragab AE, Abou Asa S, Abdo W, Elbialy ZI, Elbialy NM, El-Far AH (2021) Ameliorative effects of Aspergillus awamori against the initiation of hepatocarcinogenesis induced by diethylnitrosamine in a rat model: regulation of Cyp19 and p53 gene expression. Anti-oxidants 10(6):922. https://doi.org/10.3390/antiox10060922
Attar M, Ling KHJ, Tang-Liu DDS, Neamati N, Lee VHL (2005) Cytochrome P450 3A expression and activity in the rabbit lacrimal gland: glucocorticoid modulation and the impact on androgen metabolism. Invest Ophthalmol Vis Sci 46(12)
Awad MG, Ali RA, Abd El-Monem DD, El-Magd MA (2020) Graviola leaves extract enhances the anticancer effect of cisplatin on various cancer cell lines. Mol Cell Toxicol. https://doi.org/10.1007/s13273-020-
Ayyat MS, Al-Sagheer AA, Abd El-Latif KM, Khalil BA (2018) Organic selenium, probiotics, and prebiotics effects on growth, blood biochemistry, and carcass traits of growing rabbits during summer and winter seasons. Biol Trace Elem Res 186:162–173
Badawy AA, El-Magd MA, AlSadrah SA (2018) Therapeutic effect of camel milk and its exosomes on MCF7 cells in vitro and in vivo. Integr Cancer Ther 17(4):1235–1246
Bancroft JD, Layton C (2013) The Hematoxylin and eosin. In: Suvarna S. K, Layton C, Bancroft J. D, editors. Theory Practice of histological techniques., 7th ed. Ch. Philadelphia: Churchill Livingstone of El Sevier
Battacone G, Nudda A, Pulina G (2010) Effects of ochratoxin A on livestock production. Toxins 2:1796–1824
Baviera-Puig A, Buitrago-Vera J, Escriba-Perez C, Montero-Vicente L (2017) Rabbit meat sector value chain. World Rabbit Sci 25:95–108
Benford D, Boyle C, Dekant W, Fuchs R, Gaylor DW, Hard G, Mc Gregor, DB, Pitt JJ, Plestina R, Sheppard G, et al (2001) Ochratoxin A, safety evaluation of certain mycotoxins in food WHO Food Additives, Series 47, Vol. 74, pp. 281–415. WHO, Gene've
Bhat MK, Hazlewood GP (2001) Enzymology and other characteristics of cellulases and xylanases, in: BEDFORD, MR & PARTRIDGE, GG (Eds) Enzymes in Farm Animal Nutrition, pp. 11–60 (CAB International, Wallingford, UK)
Bösch-Saadatmandi C, Wagner AE, Graeser AC, Hundhausen C, Wolffram S, Rimbach G (2009) Ochratoxin A impairs Nrf2-dependent gene expression in porcine kidney tubulus cells. J Anim Physiol Anim Nutr 93:547–554. https://doi.org/10.1111/j.1439-0396.2008.00838
Brennan KM, Oh SY, Yiannikouris A, Graugnard DE, Karrow NA (2017) Differential gene expression analysis of bovine macrophages after exposure to the penicillium mycotoxins citrinin and/or ochratoxin A. Toxins (basel) 9:366. https://doi.org/10.3390/toxins9110366
Brown AL, Odell EW, Mantle PG (2007) DNA ploidy distribution in renal tumours induced in male rats by dietary ochratoxin A. Exp Toxicol Pathol 59:85–95
Buhl SN, Jackson KY (1978) Optimal conditions and comparison of lactate dehydrogenase catalysis of the lactate-to-pyruvate and pyruvate-to-lactate ions in human serum at 25, 30, and 37 degrees C. Clin Chem 24:828–831
Cagauan AG, Tayaban RH, Somga J, Bartolome RM (2004) Effect of aflatoxin-contaminated feeds in Nile tilapia (Oreochromis niloticus L) abstract of the 6th international symposium on tilapia in aquaculture (ISTA 6) section: health management and diseases. Manila, Philippines, pp 16
Cavin C, Delatour T, Marin-Kuan M, Fenaille F, Holzhauser D, Guignard G, Bezençon C, Piguet D, Parisod V, Richoz-Payot J, Schilter B (2009) Ochratoxin A-mediated DNA and protein damage: roles of nitrosative and oxidative stresses. Toxicol Sci 110:84–94
Cavin C, Delatour T, Marin-Kuan M, Holzhauser D, Higgins L, Bezencon C, Guignard G, Junod S, Richoz-Payot J, Gremaud E et al (2007) Reduction in anti-oxidant defenses may contribute to ochratoxin a toxicity and carcinogenicity. Toxicol Sci 96:30–39
Chen XL et al (2006) Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am J Physiol Heart Circ Physiol 290(5):H1862–H1870
Coulombe JJ, Favreau L (1963) A new simple semimicro method for colorimetric determination of urea. Clin Chem 9:102–108
Cui G, Li L, Xu W, Wang M, Jiao D, Yao B, Xu K, Chen Y, Yang S, Long M, Li P, Guo Y (2020) Astaxanthin protects ochratoxin A-induced oxidative Cyprinus carpioL. J Fish Dis 24:433–438
Dalle Zotte A, Szendrő Z (2011) The role of rabbit meat as functional food. Meat Sci 88:319–331
Dawood MAO, Abo-Al-Ela HG, Hasan MT (2020) Modulation of transcriptomic profile in aquatic animals: probiotics, prebiotics and synbiotics scenarios. Fish Shellf Immunol 97:268–282
De Blas JC, Mateos GG (1998) Feed formulation. In: de Blas C, Wiseman J (eds) The Nutrition of the Rabbit. CABI Publishing, Wallingford, UK, pp 241–253
Elaroussi MA, Mohamed FR, El Barkouky EM, Atta AM, Abdou M, Hatab MH (2006) Experimental ochratoxicosis in broiler chickens. Avian Pathol 35:263–269
El-Deep MH, Amber KA, Elgendy S, Dawood MAO, Elwakeel EM, Paray BA (2020) Oxidative stress, hemato-immunological, and intestinal morphometry changes induced by ochratoxin A in APRI rabbits and the protective role of probiotics. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-020-09837-3
El-Deep MH, Dawood MAO, Assar MH, Ahamad Paray B (2021) Aspergillus awamori positively impacts the growth performance, nutrient digestibility, antioxidative activity and immune responses of growing rabbits. Veterinary Medicine and Science 7:226–235
El-Deep MH, Ijiri D, Eid EZ, Yamanaka H, Ohtsuka A (2014) Effects of dietary supplementation with Aspergillus awamorion growth performance and antioxidative status of broiler chickens exposed to high ambient temperature. J Poult Sci 51:281–288
El-Katcha M, Ismail E, Soltan M, El-Naggar MJAJVS (2011) Effect of dietary probiotics supplementation on growth performance, immune response, some blood parameters and carcass quality of growing rabbits. Alexandria J Vet Sci 34:153–169
El-Magd MA, Saleh AA, Abdel-Hamid TM, Saleh RM, Afifi MA (2016) Is really endogenous ghrelin a hunger signal in chickens?: Association of GHSR SNPs with increase appetite, growth traits, expression and serum level of GHRL, and GH. Gen Comp Endocrinol 237:131–139
Moustafa Eman M, Dawood Mahmoud AO, Assar Doaa H, Omar Amira A, Elbialy Zizy I, Farrag Foad A, Shukry Mustafa, Zayed Mohamed M (2020) Modulatory effects of fenugreek seeds powder on the histopathology, oxidative status, and immune related gene expression in Nile tilapia (Oreochromis niloticus) infected with Aeromonas hydrophila. Aquaculture 515:734589
Esterbauer HKH, Cheeseman MU, Poli Dianzani G, Slater TF (1982) Separation and characterization of the aldehydic products of lipid peroxidation stimulated by ADP-Fe2+ in rat liver microsomes. Biochem. J 208:129–140
Falcao-e-Cunha L, Castro-Solla L, Maertens L, Marounek M, Pinheiro V, Freire J, Mourao JL (2007) Alternatives to antibiotic growth promoters in rabbit feeding: a review. World Rabbit Science 15:127–140
Fathi M, Abdelsalam M, Al-Homidan I, Ebeid T, El-Zarei M, AbouEmera O (2017) Effect of probiotic supplementation and genotype on growth performance, carcass traits, hematological parameters and immunity of growin rabbits under hot environmental conditions. Anim Sci J 88:1644–1650
Feldman BF, Zinkl JG, Jain NC (2000) Schalms veterinary haematolog, 5th edn. Williams Tand Wilkins, Philadelphia, pp 21–100
Francisco A, Maria CR (2010) Ochratoxin A producing species in the genus Penicillium. Toxins 2 1111–1120 h Pharmacol, 391(10):1147–1156
Giromini C, Rebucci R, Fusi E, Rossi L, Saccone F, Bald A (2016) Cytotoxicity, apoptosis, DNA damage and methylation in mammary and kidney epithelial cell lines exposed to ochratoxin A. Cell Biol Toxicol 32:249–258
Glahn R, Shapiro R, Vena V, Wideman R, Huff W (1989) Effects of chronic ochratoxin A and citrinin toxicosis on kidneyfunction of single combwhiteLeghorn pullets. Poult. Sci 68(9):1205
Gan F, Hou LL, Zhou YJ, Liu YH, Huang D, Chen, XX, Huang KH (2017) Effects of ochratoxin A on ER stress, mapk signaling pathway and autophagy of kidney and spleen in pigs. Environ Toxicol 32:2277–2286
Glick B (2000) Immunophysiology. In: Whittow GC (ed) Sturkie's avian physiology, 5th edn. Academic Press, New York p 657–670
Greaves P (2012) Histopathology of preclinical toxicity studies. 4th edn. Amsterdam Elsevier
Gruber-Dorninger C, Jenkins T, Schatzmayr G (2019) Global mycotoxin occurrence in feed: a ten-year survey," Toxins 11(7):375
Habtemariam S (2019) The Nrf2/HO-1 axis as targets for flavanones: neuroprotection by Pinocembrin, Naringenin, and Eriodictyol. Oxid Med Cell Longev 2019:1–15. https://doi.org/10.1155/2019/4724920
Harvey RB, Elissalde MH, Kubena LF, Weaver EA, Corrier DE, Clement BA (1992) Immunotoxicity of ochratoxin A to growing gilts. Am J Vet Res 53:1966–1970
Heikal L, Martin GP, Dailey LA (2009) Characterisation of the decomposition behaviour of S-nitrosoglutathione and a new class of analogues: SNitroso phyto chelatins. Nitric Oxide 20:157–165
Henry RJ, Canmon DC, Winkelman JW (1974) Principles and techniques, harper, and row. Clin Chem pp 415
Hong KL, Lee CH, Kim SW (2004) Aspergillus oryzaeGB-107 fermentation improves nutritional quality of food soybeans and feed soybean meals. J Med Food 7:430–435
Hussein AA, Omran AA, Nabil ZI, Arbid MS (1997) Effect of acute administration of ochratoxin A on the heartrate, arterialblood pressure and ECG of the rat. J Natural Toxins 6(1):85
Itoh K, Wakabayashi N, Katoh Y, Ishii T, O’Connor T, Yamamoto M (2003) Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells 8(4):379–391
Jan R, Sadique U, Ul HZ, Farid K, Ahmad S, Khan S, Khan H (2017) Toxico-pathological and reproductive effects of concurrent oral administration of ochratoxin A and endosulfan in pregnant rabbits (Oryctolagus cuniculus). Pak Vet J 37:19–24
Janaczyk B, Pliszak-Krol A, Graczyk S, Houszka M, Rouibah K (2006) Morphological and functional evaluation of chicken blood leukocytes in chronic ochratoxicosis. Int J Poult Sci 5:191–194
Jarmi T, Agarwal A (2009) Heme oxygenase and renal disease. Curr Hypertens Rep 11(1):56–62
Jedziniak P, Panasiuk L, Pietruszka K, Posyniak A (2019) Multiple mycotoxins analysis in animal feed with LC-MS/MS: comparison of extract dilution and immunoaffinity clean-up. J Sep Sci 42:1240–1247
Jelkmann W (2011) Regulation of erythropoietin production. J Physiol 589(Pt 6):1251–8. https://doi.org/10.1113/jphysiol.2010.195057
Kamisoyama H, Honda K, Saneyasua T, Sugahara K, Hasegawa S (2009) (2009): Corticotropin-releasing factor is a downstream mediator of the beta-melanocyte-stimulating hormone-induced anorexigenic pathway in chicks. Neurosci Lett 458:102–105
Kamizono T, Ohtsuka A, Hashimoto F, Hayashi K (2013) (2013): Dibutoxybutane suppresses protein degradation and promotes growth in cultured chicken muscle cells. J Poult Sci 50:37–43
Kamp HG, Eisenbrand G, Janzowski C, Kiossev J (2005) Ochratoxin A induces oxidative DNA damage in liver and kidney after oral dosing to rats. Mol Nutr Food Res 49(12):1160-7. https://doi.org/10.1002/mnfr.200500124
Kensler TW, Wakabayashi N, Biswal S (2007) Cell survival response to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47:6.1-6.28
Khan S, Martin M, Bartsch H, Rahimtula A (1989) Perturbation of liver microsomal calcium homeostasis by ochratoxin A. Biochem Pharmacol 38:67
Klarić MS, Rumora L, Ljubanović D, Pepeljnjak S (2008) Cytotoxicity and apoptosis induced by fumonisin B(1), beauvericin and ochratoxin A in porcine kidney PK15 cells: effects of individual and combined treatment. Arch Toxicol. 82(4):247–55
Klauning JE, Kamendulis LM (2004) The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol 44:239–267
Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V (2001) Method for the measurement of anti-oxidant activity in human fluids. J Clin Pathol 54:356–361
Kumar A, Jindal N, Shukla CL, Yash, P, Ledoux DR, Rottinghaus GE (2003). Effect of ochratoxin a on escherichia coli-challenged broiler chicks. Avian Dis 47:415–424
Kumar M, Dwivedi P, Sharma A, Telang A, Patil R, Singh N, Sankar M (2007) Ochratoxin A and citrinin induced biochemical changes in New Zealand white rabbits. Indian J Vet Pathol 31:135–139
Kumar M, Dwivedi P, Sharma AK, Sankar M, Patil RD, Singh ND (2014) Apoptosis and lipid peroxidation in ochratoxin A- and citrinin-induced nephrotoxicity in rabbits. Toxicol Ind Health 30(1):90–98
Larsen K (1972) Creatinine assay in the presence of protein with LKB 8600 reaction rate analyser. Clin Chim Acta 38:475–476
Lea T, Steien K, Stormer FC (1989) Mechanism of ochratoxin A induced immunosuppression. Mycopathologia 107:153–159
Lee IH, Hung YH, Chou CC (2008) Solid-state fermentation with fungi to enhance the antioxidative activity, total phenolic and anthocyanin contents of black bean. Int J Food Microbiol. 121:150–156
Levonen AL, Inkala M, Heikura T, Jauhiainen S, Jyrkkanen HK, Kansanen E, Maatta K, Romppanen E, Turunen P, Rutanen J, Yla-Herttuala S (2007) Nrf2 gene transfer induces anti-oxidant enzymes and suppresses smooth muscle cell growth in vitro and reduces oxidative stress in rabbit aorta in vivo. Arterioscler Thromb Vasc Biol 27:741–747. https://doi.org/10.1161/01.ATV.0000258868.80079.4d
Lin A, Wang Y, Tang J, Xue P, Li C, Liu L et al (2012) Nitric oxide and protein S-nitrosylation are integral to hydrogen peroxide-induced leaf cell death in rice. Plant Physiol 158:451–464. https://doi.org/10.1104/pp.111.184531
Liu J, Wang Y, Cui J, Xing L, Shen H, Wu S, Lian H, Wang J, Yan X and Zhang X, (2012) Ochratoxin A induces oxidative DNA damage and G1 phase arrest in human peripheral blood mononuclear cells in vitro. Toxicology Lett. 1;211(2):164–71.https://doi.org/10.1016/j.toxlet.2012.03.800
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Malekinejad H, Farshid AA, Mirzakhani N (2011) Liquorice plant extract reduces ochratoxin A-induced nephrotoxicity in rats. Exp Toxicol Pathol 63(1–2):125–30. https://doi.org/10.1016/j.etp.2009.10.006
Malir F, Ostry V, Pfohl-Leszkowicz A, Malir J, Toman J (2016) Ochratoxin a: 50 Years of research. Toxins (Basel) 04:8
Marin DE, Pistol GC, Gras M, Palade M, Taranu I (2018) A comparison between the effects of ochratoxin A and aristolochic acid on the inflammation and oxidative stress in the liver and kidney of weanling piglets. Naunyn Schmiedebergs Arch Pharmacol 391(10):1147–1156. https://doi.org/10.1007/s00210-018-1538-9
Markowiak P, Śliżewska K (2018) The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog 10:21
Marquardt RR, Frohlich AA (1992) A review of recent advances in understanding ochratoxicosis. J Anim Sci 70:3968–3988
Mateos GG, Rebollar PG, de Blas C (2010) Minerals, vitamins and additives. In: the nutrition of the rabbit. (Edit. De Blas J.C. and Wiseman J.), 2nd Ed. CABI, Wallingford, pp.119–150
Mézes M (2008) Mycotoxins and other contaminants in rabbit feeds, proceedings of the 9th world rabbit congress, Verona, Italy, 10–13 June 2008. World rabbit science association, pp 491–506
Montgomery H, Dymock F (1961) Determination of nitrite in water mycotoxin journal. 10(4):363–373
NRC (1997) National Research Council. In: Nutrient requirements of rabbits. 6th Ed., National Academy Press, Washington
O’Brien E, Heussner AH, Dietrich DR (2001) Species-, sex-, and cell type-specific effects of ochratoxin A and B. Toxicol Sci 63:256–264
Ofitserova M, Nerkar S, Pickering M, Torma L, Thiex N (2009) Multiresidue mycotoxin analysis in corn grain by column high-performance liquid chromatography with postcolumn photochemical and chemical derivatization: single-laboratory validation. J AOAC Int 92(1):15–25. https://doi.org/10.1093/jaoac/92.1.15
Ostry V, Mair F, Ruprich J (2013) Producers and important dietary sources of ochratoxin A and citrinin. Toxins 5:1574–1586
Packer L, Glazer AN (1990) Methods in enzymology, oxygen radicals in biological systems part B: oxygen radicals and antioxidants. Academic Press, New York 186:251
Parasuraman S, Raveendran R, Kesavan R (2010) Blood sample collection in small laboratory animals. Journal of Pharmacology & Pharmaco - Therapeutics 1:87–93. https://doi.org/10.4103/0976-500X.72350
Pfohl-Leszkowicz A, Manderville RA (2007) Ochratoxin A: an overview on toxicity and carcinogenicity in animals and humans. Mol Nutr Food Res 51:1189–1192
Phuoc TL, Jamikorn U (2017) Effects of probiotic supplement (Bacillus subtilis and Lactobacillus acidophilus) on feed efficiency, growth performance, and microbial population of weaning rabbits. Asian Australas J Anim Sci 30:198
Ponnuchamy V (2000) Studies on pathology and immunology of ochratoxin-A induced toxicosis and its interaction with Salmonella typhimurium infection in guinea pigs. MS Thesis. Veterinary Research Institute, Izatnagar, India
Prabu PC, Dwivedi P, Sharma AK (2013) Toxicopathological studies on the effects of aflatoxin B1, ochratoxin A and their interaction in New Zealand white rabbits. Exp Toxicol Pathol 65:277–286
Rajput IR, Li WF (2012) Potential role of probiotics in mechanism of intestinal immunity. Pak Vet J 32:303–308
Raju M, Devegowda G (2000) Influence of glucomannan on performance and organ morphology, serum biochemistry and hematology in broilers exposed to ochratoxin British. Poultry Sci. 41:640–650
Reitman S, Frankel S (1957) J Clin Path 28:56
Ringot D, Chango A, Schneider YJ, Larondelle Y (2006) Toxicokinetics and toxicodynamics of Ochratoxin A, an update. Chem Biol Interact 2006(159):18–46
Rudkin FM, Bain JM, Walls C, Lewis LE, Gow NA, Erwig LP (2013) Altered dynamics of Candida albicans phagocytosis by macrophages and PMNs when both phagocyte subsets are present. MBio 4:e00810–e00813
Sakai M, Taniguchi K, Mamoto K, Ogawa H, Tabata M (2001) Immunostimulant effects of nucleotide isolated from yeast RNA on carp, Cyprinus carpio L. J Fish Dis 24:433–438
Salamano G, Mellia E, Tarantola M, Gennero MS, Doglione L, Schiavone A (2010) Acute phaseproteins and heterophil:lymphocyte ratio in laying hens in different housing systems. Vet Rec 167:749–751
Saleh AA, Eid YZ, Ebeid TA, Ohtsuka A, Hayashi K (2012) Feeding Aspergillus awamori reduces skeletal muscle protein breakdown and stimulates growth in broilers. Anim Sci J 83(594–598):2012
Saleh AA, Hayashi K, Ijiri D, Ohtsuka A (2015) Effect of feeding Aspergillus awamori and canola seed on the growth performance and muscle fatty acid profile in broiler chicken. Anim Sci J 86:305–311
Sawale GK, Gosh RC, Ravikanth K, Maini S, Rekhe DS (2009) Experimental mycotoxicosis in layer induced by ochratoxin A and its amelioration with herbomineral toxin binder 'Toxiroal' Int J Poult Sci 8:798–803
Schnupf P, Sansonetti PJ (2012) Quantitative RT-PCR profiling of the rabbit immune response: assessment of acute shigella flexneri infection. PLoS ONE 7(6):e36446. https://doi.org/10.1371/journal.pone.0036446
Stachurska A, Ciesla M, Kozakowska M, Wolffram S, Saadatmandi CB, Rimbach G, Jozkowicz A, Dulak J, Loboda A (2013) Cross-talk between microRNAs, nuclear factor E2-related factor 2, and heme oxygenase-1 in ochratoxin A-induced toxic effects in renal proximal tubular epithelial cells. Mol Nutr Food Res 2013(57):504–515
Sun Y, Dong G, Guangxin E, Liao M, Tao L, Lv J (2018) The effects of low levels of aflatoxin B1 on health, growth performance and reproductivity in male rabbits. World Rabbit Sci 26:123–133
Surai PF, Mezes M, Melnichuk SD, Fotina TI (2008) Mycotoxins and animal health: from oxidative stress to gene expression. Krmiva 50:35–43
Szasz G, Waldenstrom J, Gruber W (1979) Creatine kinase in serum: Inhibition by endogenous polyvalent cations, and effect of chelators on the activity and stability of some assay components. Clin Chem 25:446–545
Tamang JP, Shin D-H, Jung S-J, Chae S-W (2016) Functional properties of microorganisms in fermented foods. Front Microbiol 7:578
Tao Y, Xie S, Xu F, Liu A, Wang Y, Chen D, PanaY Huang L, Penga D, Wanga X, Yuan Z (2018) Ochratoxin A: toxicity, oxidative stress and metabolism. Food Chem Toxicol 112:320–331. https://doi.org/10.1016/j.fct.2018.01.002
Trinder P (1969) Enzymatic determination of glucose in blood serum. Ann Clin Biochem 6:24
Terri AE, Sesin PG (1958) Determination of serum potassium by using sodium tetraphenylboron. Am J Clin Pathol 29:86–90
Tietz NW, Burtis CA, Duncan P, Ervin K, Petitclerc CJ, Rinker AD, Shuey D (1983) A reference method for measurement of alkaline phosphatase activity in human serum. Clin Chem 29(5):751–761
Ting CM, Lee YM, Wong CK, Wong AS, Lung HL, Lung ML, Lo KW, Wong RN, Mak NK (2010) 2-Methoxyestradiol induces endoreduplication through the induction of mitochondrial oxidative stress and the activation of MAPK signaling pathways. Biochem. Pharmacol 79:825–841
Trinder P (1951) Photometric determination of serum sodium. Analyst 76:596
Verma RJ, Shalini M (1998) Hyperglycemia induced in rabbits exposed to ochratoxin. Bull Environ Contam Toxicol 60:626–631
Wanda M, Haschek KAV, Val RB (2002) Selected mycotoxins affecting animal and human health in Handbook of Toxicologic Pathology. 2nd ed. p. 645–699
Wintrobe NM (1983) The size of haemoglobin content of erythrocytes. Method of determination and clinical application. J Lab Clin Med 17:87
Yang Y, Iji PA, Choct M (2009) Dietary modulation of gut microflora in broiler chickens: a review of the role of six kinds of alternatives to in-feed antibiotics. World’s Poult Sci J 65:97–114
Yamamoto M, Saleh F, Tahir M, Ohtsuka A, Hayashi K (2007) Effect of Koji-feed (fermented distillery by-product) on the growth performance and nutrient metabolizability in broiler. J Poult Sci 44:291–296
Yamashita Y, Ueyama T, Nishi T, Yamamoto Y, Kawakoshi A et al (2014) Nrf2-Inducing anti-oxidation stress response in the rat liver—new beneficial effect of lansoprazole. PLoS ONE 9(5):e97419. https://doi.org/10.1371/journal.pone.0097419
Zain ME (2011) Impact of mycotoxins on humans and animals," J Saudi Chem Soc 15(2):129–144
Zhang Z, Gan F, Xue H, Liu Y, Huang D, Khan AZ, Chen X, Huang K (2016) Nephropathy and hepatopathy in weaned piglets provoked by natural ochratoxin A and involved mechanisms. Exp Toxicol Pathol 68:205–213
Zheng J, Zhang Y, Xu W, Luo Y, Hao J, Shen XL, Yang X, Li X, Huang K (2013) (2013): Zinc protects hepg2 cells against the oxidative damage and DNA damage induced by ochratoxin A. Toxicol Appl Pharmacol 268:123–131
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Doaa H. Assar: formal analysis, methodology, writing—original draft. Mustafa Shukry: conceptualization, validation. Mona N. BinMowyna: conceptualization, methodology. Zizy I. Elbialy: validation, writing—review and editing, publication. Amera Abd El Latif: formal analysis, investigation, methodology. Norah A. Althobaiti: Data curation, resources. Moshira A. El-Abasy: investigation, resources. Samah Abou Asa: formal analysis, validation, writing-reviewing and editing. Mohammed A. El-Magd: formal analysis, methodology.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Research Ethical Committee of Faculty of Veterinary Medicine, Kafrelsheikh University, Egypt. All precautions were followed to minimize animal suffering during the experiment.
Institutional review board statement
The study was conducted based on the recommended NIH Guide for the care and use of laboratory animals by the Faculty of Veterinary Medicine Ethics Committee, Kafrelsheik University, Egypt. All precautions were followed to diminish animal suffering during the experiment.
Informed consent
Not applicable.
Consent to participate
This work does not involve human participants.
Consent for publication
This work does not involve human participants.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Assar, D.H., Asa, S.A., El-Abasy, M.A. et al. Aspergillus awamori attenuates ochratoxin A-induced renal and cardiac injuries in rabbits by activating the Nrf2/HO-1 signaling pathway and downregulating IL1β, TNFα, and iNOS gene expressions. Environ Sci Pollut Res 29, 69798–69817 (2022). https://doi.org/10.1007/s11356-022-20599-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20599-y