Abstract
Gastrointestinal (GI) diseases have become a global health issue and an economic burden due to their wide distribution, late prognosis, and the inefficacy of recent available medications. Therefore, it is crucial to search for new strategies for their management. In the recent decades, mesenchymal stem cells (MSCs) therapy has attracted attention as a viable option for treating a myriad of GI disorders such as hepatic fibrosis (HF), ulcerative colitis (UC), acute liver injury (ALI), and non-alcoholic fatty liver disease (NAFLD) due to their regenerative and paracrine properties. Importantly, recent studies have shown that MSC-derived extracellular vesicles (MSC-EVs) are responsible for most of the therapeutic effects of MSCs. In addition, EVs have revealed several benefits over their parent MSCs, such as being less immunogenic, having a lower risk of tumour formation, being able to cross biological barriers, and being easier to store. MSC-EVs exhibited regenerative, anti-oxidant, anti-inflammatory, anti-apoptotic, and anti-fibrotic effects in different experimental models of GI diseases. However, a key issue with their clinical application is the maintenance of their stability and efficacy following in vivo transplantation. Preconditioning of MSC-EVs or their parent cells is one of the novel methods used to improve their effectiveness and stability. Herein, we discuss the application of MSC-EVs in several GI disorders taking into account their mechanism of action. We also summarise the challenges and restrictions that need to be overcome to promote their clinical application in the treatment of various GI diseases as well as the recent developments to improve their effectiveness.
Graphical abstract

A representation of the innovative preconditioning techniques that have been suggested for improving the therapeutic efficacy of MSC-EVs in GI diseases. The pathological conditions in various GI disorders (ALI, UC, HF and NAFLD) create a harsh environment for EVs and their parents, increasing the risk of apoptosis and senescence of MSCs and thereby diminishing MSC-EVs yield and restricting their large-scale applications. Preconditioning with pharmacological agents or biological mediators can improve the therapeutic efficacy of MSC-EVs through their adaption to the lethal environment to which they are subjected. This can result in establishment of a more conducive environment and activation of numerous vital trajectories that act to improve the immunomodulatory, reparative and regenerative activities of the derived EVs, as a part of MSCs paracrine system. ALI, acute liver injury; GI diseases, gastrointestinal diseases; HF, hepatic fibrosis; HSP, heat shock protein; miRNA, microRNA; mRNA, messenger RNA; MSC-EVs, mesenchymal stem cell-derived extracellular vesicles; NAFLD, non-alcoholic fatty liver disease; UC, ulcerative colitis.
Similar content being viewed by others
Introduction
Gastrointestinal (GI) diseases are a series of inflammatory conditions which affect any section of the GI tract, from the oesophagus to the rectum, in addition to the accessory digestive organs—liver, gall bladder and pancreas. Motility problems, visceral hypersensitivity, altered mucosal and immunological function, and altered intestinal microbiota are all hallmarks of these conditions (Oshima and Miwa 2015; Drossman 2016). Irritable bowel diseases (IBD), gastroesophageal reflux disease, liver diseases, peptic ulcer, pancreatitis, and GI malignancy are just a few of many problems that fall under the umbrella of GI disorders, which affect patients worldwide (De Filippis et al. 2020). Many of these diseases negatively impact patients’ quality of life and productivity (Wang et al. 2023). Moreover, their incidence is high, and oftentimes, there are no obvious symptoms in the early stages; hence, most GI diseases are first noted in the middle and late stages where the prognosis is poor (Chen et al. 2022a) and are not effectively managed using current medications (Greenwood-Van Meerveld et al. 2017). As a result, it is crucial to create new and efficient strategies for treating GI disorders.
Over the past few decades, stem cell therapy has attracted attention as a viable option for treating a wide range of pathological conditions. Mesenchymal stem cells (MSCs) have been of particular importance because of their ability to self-renew and differentiate into a wide variety of cell types (Kou et al. 2022). They are commonly extracted from bone marrow (BM), amniotic fluid (AM), adipose tissues (AD), dental pulp, and umbilical cord (Wagner et al. 2005; Hass et al. 2011; Musiał-Wysocka et al. 2019). Notably, numerous preclinical and clinical studies have proved the potential role of MSCs in GI protection and repair (Kubo et al. 2015; Onishi et al. 2015; Ono et al. 2015; Trounson and McDonald 2015). It was once thought that MSCs’ therapeutic efficacy arises from their ability to migrate to and engraft in the target tissues. However, it was shown afterwards that the biological effects observed following MSCs administration are likely the result of their soluble secreted factors such as cytokines, chemokines, growth factors, and extracellular vesicles (EVs) (Keating 2012; Aghajani Nargesi et al. 2017; Gowen et al. 2020). These biological factors act either on MSCs themselves (autocrine functions) to maintain self-renewal capacity, differentiation, and proliferation or on neighbouring cells (the predominant paracrine functions) to modulate the immune system, inflammatory response, and apoptosis and to stimulate neo-angiogenesis (Razavi et al. 2020; Rahimi et al. 2021). Besides, EVs are the main component of paracrine actions of MSCs (Han et al. 2016).
EVs are membrane-bound nanovesicles (with a size range of 30–1000 nm) that transport vital biomolecules such as cytokines, growth factors, signalling lipids, messenger RNAs (mRNA), and micro-RNAs (miRs) between cells and regulate a wide range of cellular processes under both normal as well as pathological circumstances (Gowen et al. 2020; Heydari et al. 2021; Ahmed and Al-Massri 2022). MSC-derived EVs (MSC-EVs) are mainly made up of exosomes (EXOs), microvesicles (MVs), and apoptotic bodies (ABs). It is worth noting that MSC-EVs revealed regenerative, anti-oxidant, anti-inflammatory, anti-apoptotic, and anti-fibrotic effects in different experimental models of GI diseases such as IBD, severe acute and chronic pancreatitis, hepatic fibrosis (HF), acute liver injury (ALI), and non-alcoholic fatty liver disease (NAFLD) (Zhou et al. 2013; Yang et al. 2015; Mao et al. 2017; Xie et al. 2019a; Dong et al. 2020; Ren et al. 2021; Wu et al. 2021; Niu et al. 2022).
Increasing evidence suggested that MSC-EVs, rather than MSCs themselves, are responsible for the majority of the therapeutic actions of MSCs (Zhao et al. 2020a). Therefore, MSC-EVs can be used since they have a better safety profile, are less immunogenic, and can traverse biological barriers (Yeo et al. 2013; Natasha et al. 2014). In addition, problems of MSCs such as risk of ectopic tumour growth, entrapment in lung microvasculature, and immunological rejection could be avoided when MSC-EVs are used (Badillo et al. 2007; Jeong et al. 2011; Wang et al. 2015; Fennema et al. 2018). Despite these obvious potentials, the clinical application of MSC-EVs faces a number of obstacles, such as the inability of EVs to retain their efficacy and stability over time following in vivo transplantation (Wiklander et al. 2015). Therefore, new strategies, like preconditioning of MSC-EVs or their parent cells, could be explored to improve their effectiveness and stability upon application (Lee and Kang 2020).
In this review, we first summarised different approaches used for the isolation, characterisation, purification, and storage of MSC-EVs. We also illustrated their applications in different models of GI diseases and the underlying mechanisms of their bioefficacy. Besides, we discussed recent studies and methods aiming at the improvement of the therapeutic efficacy of MSC-EVs using different biological or pharmacological preconditioning approaches. Finally, we enumerated challenges and restrictions that should be overcome to promote the clinical application of MSC-EVs in various GI diseases.
Biological properties of EVs
Definition and origin of EVs
EVs are heterogenous nanoparticles circumscribed by a phospholipid membrane carrying transmembrane proteins, cytosolic proteins, organelles, transcription factors, mRNAs, miRs and various signal transduction molecules and are generally detected in MSCs, tumour cells, fibroblasts, neurons, endothelial cells, and epithelial cells, serving as versatile messengers between adjacent or distant cells in numerous pathological and physiological processes (Raposo and Stoorvogel 2013; Kalluri 2016; Keshtkar et al. 2018; Kou et al. 2022). Initially, EVs were noticed in the reticulocytes of sheep in the 1980s (Raposo and Stoorvogel 2013) and were thought to be secreted in order to eliminate unwanted compounds from the cell (Johnstone et al. 1987). To mention a few, secreted EVs act primarily on target cells to transfer intercellular information via various modes of action such as internalisation, ligand-receptor interaction, secreted factors, and fusion-mediated transfer of surface receptors (Shah et al. 2019; Rezaie et al. 2022). Commonly, EVs can be classified according to their mechanism of release and size into 3 major categories; EXOs, MVs) and ABs (He et al. 2018; Hessvik and Llorente 2018). There are two distinct secretory processes concerning EXOs and MVs; EXOs are produced via the endocytic pathway and then fuse with either lysosomes or the plasma membrane (Raposo and Stoorvogel 2013; Tetta et al. 2020) in response to elevated intracellular calcium or additional downstream effects of stimuli like stress (Wei et al. 2021). MVs, on the other hand, are generated when the cell membrane protrudes outward from the cell, generating a closed sphere containing cytoplasm (Cocucci et al. 2009; Van Niel et al. 2018), and their release can be induced by various conditions, including oxidative or shear stress, hypoxia, or injury. As for ABs, they are released during apoptosis. The increased hydrostatic pressure after cell contraction causes the plasma membrane to separate from the cytoskeleton, giving rise to these entities (Doyle and Wang 2019). ABs contain materials about to be phagocytosed, such as organelles and DNA segments (Monsel et al. 2016).
Preparation and characterisation methods of EVs
Currently, there is no universally recognised standard for isolation, characterisation, or absolute purification of EVs for large-scale clinical practice; protocols vary according to the source material, the size of sample, and the intended use of EVs (Lötvall et al. 2014; Doyle and Wang 2019). Several technologies for EVs separation are available such as ultracentrifugation, ultrafiltration, density gradient centrifugation, immunoaffinity capture, size-exclusion chromatography (SEC), and polymeric precipitation approaches. Each approach has differential advantages and drawbacks, and a combination of them may be recommended for optimum EVs enrichment (Weng et al. 2021).
EVs isolation methods
Differential centrifugation (ultracentrifugation)
Differential centrifugation is a traditional technique in term of separating EVs and it implicates utilising sequential centrifugation operations of increasing pressures to isolate EVs from impurities-containing samples with respect to their volume and physical characteristics (Revenfeld et al. 2014; De Sousa et al. 2023). This technique encompasses a preliminary phase of low-speed centrifugation to remove cell debris, followed by isolation of vesicles at 19,000–100,000 g (Xu et al. 2017). Different subsets of EVs can be enriched or concentrated, but they will not be fully isolated (Talebjedi et al. 2021). A common problem that is encountered when utilising differential ultracentrifugation is the use of large centrifugal forces which may produce clusters of vesicles as a result of rapid protein aggregation (Linares et al. 2015). Consequently, this method is better suited to laboratory settings than to clinical ones, but discrepancies might arise from several factors such as centrifugation time, speed, rotor type, and temperature, with the possibility of affecting the yield, sedimentation, and purity (Van Deun et al. 2017). In addition, this method is not suitable for isolating EVs from fluids with high viscosity, as in the case of isolating EVs from plasma (Momen-Heravi et al. 2012). Importantly, although it is still used in many studies, ultracentrifugation has been reported to significantly damage EXOs and alter their cargo (Livshts et al. 2015; Sidhom et al. 2020).
Density gradient centrifugation
Density gradient centrifugation (isopycnic separation and zone centrifugation) is an improved method for ultracentrifugation in which EVs are isolated according to their specific density (1.13–1.19 g/ml) in either sucrose or iodixanol solutions (Szatanek et al. 2015; Konoshenko et al. 2018). Although zone centrifugation has been demonstrated to provide greater purity and require no additional chemicals, this approach is labour-intensive, time-consuming (250 min–2 day), and suitable for high sample volumes than for clinical sample processing (De Sousa et al. 2023).
Ultrafiltration
Ultrafiltration, also known as microfiltration, is one of the most common approaches for isolating EVs, depending on size. It involves the use of simple membrane filters to separate EVs quickly and cheaply from larger elements in a suspension (Grant et al. 2011; Konoshenko et al. 2018). Ultrafiltration has many significant benefits: its procedures are easy to carry out, it allows for the processing of multiple samples, there are no constraints on sample volume, and the risk of EVs rupture is also considerably reduced since the vesicles are not subjected to the same pressures and pressure necessary for ultracentrifugation approach (Konoshenko et al. 2018; De Sousa et al. 2023). In spite of this, sample loss due to clogged filters and the contamination of EVS-samples with unwanted proteins are among the downsides of this approach (Carnino et al. 2019), making this technique less suitable for use in subsequent proteomic analysis if used alone (De Sousa et al. 2023).
SEC
SEC is a most commonly used method for isolating EVs via the fractionation or filtration of a sample through a column of porous beads, resulting in a highly pure preparation (Lozano-Ramos et al. 2015; Benedikter et al. 2017). SEC has revealed many benefits, including its high sensitivity, recoverability, reproducibility, adaptability to most laboratories, and insensitivity to highly viscous samples. SEC can also maintain vesicle integrity and contents, does not require additional chemicals, and does not cause EVs aggregation (Konoshenko et al. 2018; Varderidou-Minasian and Lorenowicz 2020; Sidhom et al. 2020; Clos-Sansalvador et al. 2022). Although SEC shows many advantages over other conventional isolation techniques, it has a few limitations such as its relatively high cost, intricate procedures, reliance on specialised apparatus and inability to discriminate between EXOs and MVs of the same size (Štulík et al. 2003; Konoshenko et al. 2018; Sidhom et al. 2020; Liangsupree et al. 2021).
Precipitation polymerisation
This method relies on the ability of the hydrophilic polymers such as polyethylene glycol (PEG) to diminish the solubility of the sample’s components by competing for the solvent, where it forms a mesh-like polymeric web which traps EVs in the 60–180 nm size range before pelletising and precipitating upon centrifugation at low speed (Konoshenko et al. 2018; Clos-Sansalvador et al. 2022). In order to make a two-phase method for isolation, dextran and PEG have been utilised, which has resulted in a great reduction in protein contamination (Zarovni et al. 2015). Despite its low cost, lack of specialised equipment, and the comparability between low and high sample volumes, PEG precipitation concentrates EVs, which makes it inappropriate for detecting EVs biomarker, resulting in false negatives (Coumans et al. 2017; Clos-Sansalvador et al. 2022).
Immunoaffinity capture (immunoaffinity purification or immunoprecipitation)
In this technique, EVs are captured by treating the sample with immunomagnetic beads that have been coated with antibodies specific to EV surface molecules (Liangsupree et al. 2021). Submicron-sized antibodies-coated magnetic beads can improve specificity, sensitivity, and yield of immunoaffinity experiments designed to isolate EVs. This method guarantees the integrity of isolated EVs regardless of vesicle size and ensures quick isolation with little effort. However, this method shows a number of limitations, including the difficulty to elute from EVs the magnetic beads and the negative impact of non-neutral pH and non-physiological salt concentrations on the biological activity of EVs (Nakai et al. 2016; Lane et al. 2017; Yoshida et al. 2017). Moreover, immunoaffinity isolation technologies remain costly, which may limit the scalability for their future clinical use (Clos-Sansalvador et al. 2022).
To date, there is no single isolation technique that can achieve high purity and yield of EVs. Therefore, coupling a good isolation method like SEC with other methods like ultracentrifugation or ultrafiltration or PEG-based retrieval can be the best solution to obtain optimal performance (Sidhom et al. 2020).
Purification methods of EVs
Several methods are used for EVs purification, including differential ultracentrifugation, zone ultracentrifugation, SEC, and affinity capture (Wang et al. 2021). Differential ultracentrifugation is an early, well-established, dependable method and one of the most extensively used methodologies due to its simplicity and relatively high yield (Gardiner et al. 2016). This method, however, is incapable of distinguishing particles with overlapping ranges, such as EXOs and MVs (Böing et al. 2014; Talebjedi et al. 2021; Weng et al. 2021). Zone ultracentrifugation, SEC, and filtration all face similar challenges. Unlike these previous physical separation approaches, affinity capture can separate highly pure EVs, but poor yield is obtained because of the interaction of EVs surface parameters with capture molecules linked to different carriers (e.g. magnetic beads) (Zhu et al. 2020a; Weng et al. 2021).
Characterisation of EVs
It is essential to perfectly characterise EVs, according to the International Society for EV`s minimal specification report, to confirm the validity of their isolation procedures and demonstrate their molecular and biological properties (Casado-Díaz et al. 2020; Weng et al. 2021). A complete EVs characterisation includes the determination of their size, shape, contents, and surface markers (Casado-Díaz et al. 2020). The general characterisation can be achieved by western blot or enzyme-linked immunosorbent assay to identify at least three positive and one negative EV protein marker, where positive protein markers should include at least one transmembrane/lipid-bound protein (e.g. CD63, CD9, CD81) and one cytosolic protein (e.g. TSG101, ALIX) (Abraham and Krasnodembskaya 2020; Weng et al. 2021). Furthermore, the single-vesicle characterisation utilises imaging techniques such as atomic force microscopy, transmission electron microscopy, and scanning electron microscopy to capture high-resolution pictures of EVs morphology. Biophysical characterisation can be also used for single-vesicle characterisation such as nanoparticle tracking analysis (NTA), dynamic light scattering, and flow cytometry (Shao et al. 2018). Although electron microscopy is currently used as the most effective way for analysing EVs’ structure, there is no single technology that could simultaneously evaluate both structural and biological features of EVs (Gurunathan et al. 2019). Other quantification and characterisation methods have been developed to analyse EVs like NTA and several optical flow-based approaches that may quantify EVs to an appropriate level, but these methods are unable to discriminate between particulate and membrane-bound vesicles, a problem which can be solved using electron microscopy (Gimona et al. 2017).
EVs storage and stability
Several investigations have been conducted to assess the impact of different storage temperatures (4 °C, 20 °C, and – 80 °C) and freeze–thaw cycles on the size, content, and function of isolated EVs (Jeyaram and Jay 2017). Overall, it was proved that – 80 °C is the optimal temperature for maintaining EVs’ stability and contents for downstream molecular profiling (Pinky et al. 2021; Sun et al. 2022). Freeze–thaw cycles, on the other hand, lead to the aggregation or lysis of EVs, as well as cargo loss upon their use (Kusuma et al. 2018; Gandham et al. 2020).
Applications of MSC-EVs in different models of GI diseases
There is an increasing evidence that EVs alone are responsible for the therapeutic actions of MSCs in different GI diseases, including ALI, HF, NAFLD, and UC (Jiang et al. 2019; Du et al. 2021, 2022; Cai et al. 2021). Besides, previous studies showed that MSC-EVs can accumulate in the injured tissues and impede inflammation, apoptosis, and fibrogenesis, while modulating immune cells (Li et al. 2013; Zhao et al. 2019; Cheng et al. 2021; Shi et al. 2022). Consequently, recent researches have focussed on the use of MSC-EVs as an alternative to MSCs in the management of GI disorders (Zhao et al. 2021; Du et al. 2022; Didamoony et al. 2023).
ALI
ALI is considered as one of the well-known life-threatening diseases that is characterised by sudden deterioration of normal liver functions, poor clinical prognosis, and high mortality (Didamoony et al. 2022). The escalation of the disease usually initiates a series of clinical syndromes, such as jaundice, coagulation disorders, hepatic encephalopathy, and ascites (Wendon et al. 2017). In ALI, multiple mechanisms work simultaneously to cause hepatic injury through inducing oxidative stress, inflammation, and apoptosis in response to infections, drugs, and chemical toxins (Basir et al. 2022; Didamoony et al. 2022). Growing evidence has indicated the successful application of MSC-EXOs in the management of ALI owing to their anti-inflammatory, anti-oxidant and anti-apoptotic features, as summarised in Table 1 (Sun et al. 2017; Zhao et al. 2019; Wu et al. 2021). Remarkably, BM-MSC-EXOs attenuated concanavalin A-induced liver injury through the improvement of tissue regeneration and the expression of anti-inflammatory cytokines and regulatory T cell (Treg) activity (Tamura et al. 2016). BM-MSC-EXOs may also reverse ALI through hindering apoptosis. The anti-apoptotic effect arises from diminishing the proapoptotic proteins B-cell lymphoma-2 (Bcl2)-associated X protein (Bax) and cleaving caspase-3, while increasing the expression of the autophagy markers, LC3-II and Beclin1, alongside with the anti-apoptotic marker; Bcl2 (Zhao et al. 2019). Interestingly, human umbilical cord MSC-EXOs was also found to exhibit desirable therapeutic effects on acetaminophen-induced ALI in vivo and in vitro via dwindling oxidative stress-induced inflammation and apoptosis after activating extracellular regulated protein kinases 1/2 and phosphoinositide 3-kinase/protein kinase B (PI3K/ AKT) trajectories (Wu et al. 2021). In this respect, human umbilical cord MSCs-EXOs enriched in glutathione peroxidase1 (GPX1) reduced oxidative stress and apoptosis in the hepatocyte and boosted protective effects of EXOs both in vivo and in vitro (Yan et al. 2017). Further studies revealed that human umbilical cord MSC-EXOs ameliorated hepatic inflammation and apoptosis in ischaemia/reperfusion (I/R) model via shuttling miR-1246 to decrease the glycogen synthase kinase 3β-mediated Wnt/β-catenin signalling (Xie et al. 2019a). Another hepatic I/R study indicated the ability of exosomal miR-1246 derived from human umbilical cord MSCs to inhibit inflammation and modulate Treg and T-helper 17 (Th17) cells balance via interleukin-6/glycoprotein130/signal transducer and activator of transcription 3 (IL-6/GP130/STAT3) axis (Xie et al. 2019b). The ability of human umbilical cord MSC-EXOs to lower aminotransferases enzymes in ALI could be also mediated via downregulating the expression of NOD-like receptor pyrin domain containing 3 (NLRP3), caspase-1, IL-1β, and IL-6 (Jiang et al. 2019). Of note, manganese superoxide dismutase in human umbilical cord MSC-EVs could dwindle the infiltration of neutrophils and mitigate apoptosis and oxidative stress (Yao et al. 2019). In case of AD-MSC-EXOs, Liu et al., (2018) found that they ameliorated lipopolysaccharide (LPS) and D-galactosamine-induced ALI in miR-17-dependent manner which reduced thioredoxin-interacting protein/ NLRP3 inflammasome activation in macrophages. More specifically, long-chain non-coding RNA H19 in AD-MSC-EVs curbed hepatic necrosis, inflammation-related cytokines, inflammatory cells infiltration and hepatocyte proliferation via hepatocyte growth factor/ hepatocyte growth factor receptor trajectory in ALI in rats (Jin et al. 2018). In term of BM-MSC-EXOs, it was documented that miR-223 prohibited NLRP3/caspase-1 signalling and suppressed inflammation-related cytokines in antigen S100-induced liver injury with consequent alleviation of hepatitis (Chen et al. 2018).
HF
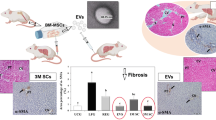
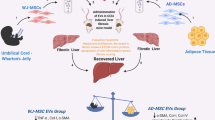
HF is a frequent pathological condition dominated by the energization of immune cells and inflammatory-related cytokines, which leads to hepatic stellate cells (HSCs) activation and consequent extracellular matrix proteins accumulation (Acharya et al. 2021). The progression of HF results in irreversible cirrhosis, hepatocellular carcinoma (HCC), and ultimately liver failure (Doumas et al. 1971; Zhu et al. 2020b). MSC-EVs ameliorated HF in many experimental models (Table 1) by inhibiting hepatic oxidative damage, inflammatory cytokines, collagen deposition, and HSC activation as in the case of AD-MSC-EVs that curbed HSCs activation through the transfer of miR-150-5p resulting in CXC motif chemokine-ligand 1 (CXCL1) underexpression (Du et al. 2021). In addition, AD-MSC-EXOs expressing miR-122 prevented HSCs activation in HF model (Lou et al. 2017). Furthermore, Rong et al. (2019) evidenced that rat BM-MSC-EXOs can mitigate carbon tetrachloride (CCl4)-induced HF by impeding HSCs activation via Wnt/β-catenin pathway both in vivo and in vitro. Moreover, AD-MSC-EXOs-secreted miR-181-5p was shown to block STAT3/Bcl-2/Beclin1 pathway and increase autophagy, hence decreasing transforming growth factor-beta1 (TGF-β1)-induced HSCs activation with consequent hindrance of HF (Qu et al. 2017). In the same pattern, circular RNA mmu_circ_0000623-modified AD-MSC-EXOs was shown to activate autophagy and suppress HF by controlling miR-125 (Zhu et al. 2020b). Interestingly, MSC-EXOs derived from human umbilical cord alleviated HF through obstructing TGF-β1/Smad axis and epithelial-to-mesenchymal transition (Li et al. 2013). Specifically, miR-148a released from human umbilical cord MSC-EXOs was shown to regulate intrahepatic macrophage and control Kruppel-like factor 6/STAT3 activity and, therefore, inhibited HF progression (Tian et al. 2022). Tan et al. (2022) revealed that Beclin1 supplied by human umbilical cord MSC-EXOs led to the stimulation of HSCs ferroptosis as well as the reduction of GPX4. Furthermore, human umbilical cord MSC-EVs revealed their inhibitory effect on HF caused by Schistosoma japonicum via the downregulation of alpha-smooth muscle actin (α-SMA), collagen I, and collagen III as well as inflammatory events including interferon-gamma (IFN-γ), tumour necrosis factor-alpha (TNF-α), and IL-β1 (Dong et al. 2020). Likewise, MSC-EXOs containing miR-125b- and miR-486-5p were found to effectively prevent CCl4-induced HF by inhibiting smoothened expression and consequently hedgehog pathway activation (Hyun et al. 2015; Kim et al. 2021). In addition, Ohara et al. (2018) stated that AM-MSC-EVs improved inflammation and HF by suppressing the activation of HSCs and Kupffer cells (KCs). Recently, Ma et al. (2023) revealed that exosomal circular RNA circCDK13 from BM-MSCs inhibited HF by modulating milk fat globulin-EGF factor 8 expression via miR-17-5p/ lysine Acetyltransferase 2B axis.
NAFLD
NAFLD is distinguished by intra-hepatocyte triglyceride buildup and concurrent immune system involvement, with consequent histological alterations, tissue destruction, and clinical symptoms due to a sedentary lifestyle and high-calorie diets (Zhao et al. 2020b; Moayedfard et al. 2022). It encompasses a cluster of disorders that range from slight steatosis (pure NAFLD) to non-alcoholic steatohepatitis (NASH), ending with cirrhosis, and HCC (Abenavoli et al. 2021; Mahmoudi et al. 2022). In the case of NASH, the development of the disease is frequently relevant to metabolic abnormalities (obesity, insulin resistance, and dysregulations of glucose and lipid metabolism). In addition, cellular and molecular changes may occur such as oxidative stress, inflammation altered immune function, and microvascular and energy dysfunction (Pouwels et al. 2022; Du et al. 2022). Generally, MSC-EVs have shown protective effects in NAFLD through controlling fat deposition-induced insulin resistance, dysregulated lipid metabolism, associated oxidative stress, and inflammatory responses, as shown in Table 2 (Niu et al. 2022; Kang et al. 2022; Du et al. 2022). Niu et al. (2022) revealed that miR-223-3p enriched in AD-MSC-EVs alleviated NAFLD by suppressing the expression of E2F transcription factor 1, hence reducing lipid buildup and HF. More importantly, human umbilical cord MSCs-derived exosomal miR-627-5p relieved liver damage in NAFLD by enhancing glucose and lipid metabolism and curbing fat mass. These metabolic outcomes emerge from the mitigation of fatty acid oxidation, mediated by the obesity-associated gene and peroxisome proliferator-activated receptor alpha (PPARα) (Cheng et al. 2021). Another study has revealed that miR-96-5p-shuttled BM-MSC-EXOs activated mitochondrial autophagy through suppressing its downstream caspase-2 (the governing player in high-fat diet-induced NASH) (El-Derany and AbdelHamid 2021). Kang et al. (2022) and Du et al. (2022) demonstrated that NASH was dramatically mitigated after using human umbilical cord MSCs-EXOs with the involvement of miR-24-3p/ Kelch-like ECH-associated protein 1(Keap1)/PPARα and nuclear factor erythroid 2-related factor 2 (Nrf2)/NADPH quinone oxidoreductase1 pathways. Besides, human umbilical cord MSC-EXOs alleviated methionine and choline-deficient diet-induced NASH in mice by upsurging the anti-inflammatory phenotype of macrophages and augmenting PPARα expression (Shi et al. 2022). Furthermore, AM-MSC-EVs significantly prevented HSCs and KCs activation and amended the degree of hepatocyte inflammation and fibrogenesis in NASH through affecting LPS/ Toll-like receptor 4 (TLR4) pathway (Ohara et al. 2018). Importantly, in a recent study, Yang et al. (2023) reported that calcium/calmodulin-dependent protein kinase 1-enriched human umbilical cord MSC-EXOs eventually prevented NAFLD in vivo and in vitro through stimulating fatty acid oxidation and inhibiting fatty acid synthesis through activation of AMP-activated protein kinase-mediated PPARα/Carnitine palmitoyltransferase 1A and sterol regulatory element-binding protein-1/fatty acid synthase pathways.
Ulcerative colitis (UC)
UC is one of the common forms of IBD which is manifested by recurrent inflammation and ulceration of the colonic mucosa with varying extension from the rectum towards the cecum (Owusu et al. 2020; Guo et al. 2022). Untreated UC may give rise to increased risk of developing colorectal cancer (Olén et al. 2020). Inflammation and oxidative stress are vital factors in the pathogenesis of UC (Soubh et al. 2015; Arafa et al. 2020; De Oliveira et al. 2021) and are considered the key targets of MSC-EVs therapy (Yang et al. 2015; Xia et al. 2021; Zhu et al. 2022). The therapeutic efficacy of MSC-EVs was related to EVs autonomous targeting capabilities to reach the injured colon tissues, reduce inflammatory cell infiltration, and, hence, maintain the integrity of colonic mucosa and mitigate the severity of UC symptoms (Table 2) (Yang et al. 2015; Heidari et al. 2021; Cai et al. 2021). For instance, Mao et al. (2017) reported the amelioration of dextran sulfate sodium (DSS)-induced UC by human umbilical cord MSC-EXOs through profound decline in the recruitment of inflammatory M1 macrophages to the damaged colon and diminished pro-inflammatory cytokines release such as TNF-α, IL-1β, and IL-6. Similarly, the study of Wu et al. (2018) stated that human umbilical cord MSC-EXOs amended UC and inhibited ubiquitin-associated molecules (K48, K63, and FK2)-mediated inflammation through turning off nuclear factor-kappa B (NF-κB) and mammalian target of rapamycin trajectories. Human umbilical cord MSC-EXOs enriched in miR-326 also relieved DSS-induced IBD in mice by preventing the binding of NEDD8 to cullin 1 (neddylation process) as well as NF‐κB signalling (Wang et al. 2020). Noteworthy, human umbilical cord MSC-EXOs mitigated DSS-induced IBD by lessening macrophage pyroptosis via modulation of miR-378a-5p/NLRP3 pathway (Cai et al. 2021). Moreover, Yang et al. (2021) pointed to the anti-inflammatory effect of TNF-α-stimulated gene 6 in human umbilical cord MSC-EXOs that succeeded to mitigate UC in addition to intestinal function and immune homeostasis. In addition, olfactory ecto-MSCs-EXOs alleviated experimental colitis via suppressing differentiation of proinflammatory Th1/Th17 cells and inducing differentiation of anti-inflammatory Treg cells (Tian et al. 2020). Furthermore, BM-MSCs-EXOs were reported to alleviate DSS-induced UC by endorsing M2 macrophage polarisation and modulating Janus kinase 1/STAT1/STAT6 axis (Cao et al. 2019).
In summary, researches that compared EVs to their parent cells demonstrated a more pronounced or a comparable efficacy for the derived EVs with a better safety profile. Obviously, Fattore et al. (2015) verified that MSC-EVs reveal more immunomodulatory effects compared to their parent cells through enhancement of CD4+, CD25+ and CD127low Tregs and anti-inflammatory cytokines. As well, another study proved that MSC-EVs surpassed the lung vasculature and improved HF and restored its function with comparable efficacy to their parent MSCs through targeting various cell types in liver (Rostom et al. 2020; Gupta et al. 2022). Notably, the absence of major histocompatibility complex class I-II on allogeneic and autologous MSC-EVs makes them more safely applied as evidenced by Sengupta et al. (2020) using allogeneic BM-MSC-EVs in COVID-19 patients in a prospective non-randomised cohort study with no demonstrated adverse effects and satisfied safety endpoints. On the other hand, MSCs show many safety concerns like their potential for aberrant differentiation or spontaneous malignancy which have encouraged the replacement of MSCs by EVs, although some clinical results still support the safety of MSCs application (Karussis et al. 2010; Lalu et al. 2012; Kim et al. 2017; Hosseini et al. 2022). Maji et al. (2017) and Sun et al. (2016) also revealed no genotoxic effects or detrimental effects of MSC-EVs on liver and kidneys both in vitro and in vivo, respectively.
Strategies to enhance the therapeutic potential of MSC-EVs
Utilising MSC-EVs in various diseases is limited by accumulating drawbacks that restricted their large-scale applications. These drawbacks include the low yield of MSC-EVs under conventional culture media (Madrigal et al. 2014) and the marked decrease in the therapeutic effect of secreted EVs from MSCs senescence following multiple generations of cultures in vitro (Joo et al. 2020). In addition, the poor targeting characteristics to the site of injury after i.v. administration is regarded as inherent properties of native or unmodified EXOs (Borrelli et al. 2018; Xu et al. 2020). Besides, the diminished efficacy of EVs may arise from their degradation in response to increased oxidative stress under pathological conditions and its consequent cellular autophagy activation (Zhang et al. 2022). Accordingly, it is highly recommended to broaden the clinical applications of MSC-EVs and improve their therapeutic efficacy (Lopez-Santalla and Garin 2021; Didamoony et al. 2023).
Preconditioning approaches of MSC-EVs
Preconditioning is a process encompassing enhancement of the therapeutic efficacy and regenerative abilities of the administered stem cells or their derivatives and can be accomplished by two cytoprotective strategies; the first involves augmenting particular valuable cell trajectory, and the second one is achieved by providing sublethal environment to adapt cells to harsh environment to which they are subjected during pathological conditions (Tilkorn et al. 2012; Touani et al. 2021; Moeinabadi-Bidgoli et al. 2021). Since the characteristics of MSC-EVs are mainly dependent on MSCs status, the preconditioning of MSCs with chemical agents, biomolecules, or cytokines could improve the immunomodulatory activities as well as the reparative and regenerative effects of their derived EVs, a part of MSCs paracrine system (Fig. 1) (Noronha Nc et al. 2019). Importantly, pharmacological preconditioning appears to be a reasonably affordable and a valuable technique that can be applied clinically without the use of sophisticated protocols or specific instrumentations (Chen et al. 2022b).
Pharmacological preconditioning
Preconditioning of MSCs in vitro with drugs or natural medications was documented to enhance the MSCs-EVs therapeutic effects as shown in multiple diseases by modifying various pathways and restoring the lost functions (Hu and Li 2018; Harrell et al. 2019a, b). Optimising MSC-EVs composition is one of the important outcomes of in vitro MSCs preconditioning that result in developing disease-specific, MSC-based, and cell-free products (Harrell et al. 2019a, b). For example, the natural yellow agent obtained from the spice turmeric, curcumin (Cur), provided EXOs with superior effects for NASH treatment using Cur-pre-treated MSCs via amendments of hepatic fibrogenesis, inflammation, oxidative stress in vivo (Motterlini et al. 2000). In addition, Cur-EXOs repressed lipid synthesis genes such as PPAR-α and inverted the lipotoxic effect of palmitic acid-treated HepG2 cells and mitochondrial-dependent apoptosis in vitro, as compared to native MSC-EXOs (Tawfeek and Kasem 2023). In the same manner, the preconditioning of MSCs with baicalin, a flavonoid isolated from roots of Scutellaria baicalensis, produced a remarkable enhancement in the function of their derived EXOs in comparison with unmodified EXOs. This was justified by improving liver functions in ALI through activating p62/Keap1/Nrf2 signalling and inhibiting oxidative burst, inflammation, and lipid peroxidation-induced ferroptosis (Zhao et al. 2022).
Preconditioning with pharmacological agents in vivo robustly urges the survival and therapeutic efficacy of MSCs and their derivatives (Mortezaee et al. 2017; Feng et al. 2018; Yousefi-Ahmadipour et al. 2019). This was evident by using rupatadine, an antihistaminic drug which enhanced the therapeutic effects of MSC-EXOs in vivo against HF in rats as compared to conventional MSC-EXOs. Rupatadine provided a more favourable environment by elevating miR-200a level and hampering oxidative stress, inflammation (platelet activating factor/TNF-α), necroptosis (receptor-interacting protein kinase 3/mixed lineage kinase domain-like protein), and hedgehog pathway with consequent anti-fibrogenic action (Didamoony et al. 2023). Similarly, Wei et al. (2020) demonstrated that combining MSC-EXOs with glycyrrhetinic acid (a triterpenoid saponin isolated from the root and rhizome extracts of liquorice) significantly reinforced the expression of proteins with anti-inflammatory activities and restored the expression of dysregulated proteins associated with inflammation and oxidative stress, resulting in further improvement of MSC-EXOs therapeutic potential in liver injury both in vivo and in vitro. Moreover, utilising nilotinib, a second-generation tyrosine kinase inhibitor, with MSC-EXOs therapy improved the anti-fibrotic effect of EXOs in CCl4-induced HF through inhibiting oxidative stress, inflammation, and apoptosis in comparison with MSC-EXOs therapy alone (Shiha et al. 2020). Furthermore, Chang et al. (2019) proved that combining MSC-EXOs with melatonin, a mitochondrial hormone secreted by the pineal gland (Lopez-Santalla and Garin 2021), alleviated the inflammatory status, apoptosis, and colon injury in rats subjected to DSS, an effect that was better than that obtained using unmodified EXOs. Besides, combining green tea with MSC-EXOs produced better EXOs tolerance to lethal oxidative stress and inflammation (CXC receptor 2 and TLR4), and hence, more pronounced therapeutic potential against UC in rats (El-Desoky Mohamady et al. 2022).
Preconditioning with other mediators
Improving the paracrine efficiency of MSCs results in a consequent enhancement of their derived EXOs therapeutic activity which can be attained by the aid of biological molecules or mediators being one of the preconditioning strategies. Hydrogen sulphide is one of the metabolites produced by the cells during pathological conditions such as ischaemia and oxidative stress. Surprisingly, this mediator possesses ROS scavenging role leading to enhanced cell resistance against hypoxia and oxidative stress (Zhang et al. 2016; Scammahorn et al. 2021). Accordingly, transplantation of the derived EXOs resulted from preconditioning of MSCs with sodium hydrosulfide revealed superior hepatoprotective and immunosuppressive effects as compared to unmodified EXOs via upregulation of the expression of long non-coding RNA metastasis-associated lung adenocarcinoma transcript 1 and anti-apoptotic factor Bcl2 in addition to downregulation of the expression of apoptotic proteins (cleaved caspase-3, Bax and Bcl-2 homologous antagonist/killer1) (Sameri et al. 2022). Growth factors, a vital group of biological mediators, were also found to modulate signal transduction involved in cell growth, proliferation, survival, and other regenerative-related capacities (Hu and Li 2018). In comparison with unmodified MSCs-EXOs, preconditioning of Wharton’s jelly-MSCs with TGF-β1 produced EXOs with maximum repressive effect on TGF-β1/Smad3 axis and fibrotic markers (α-SMA, type I collagen-alpha 1, E-cadherin) in activated LX-2 cells (Bavarsad et al. 2022).
Furthermore, cytokines such as TNF-α, IL-6 and IFN-γ are mediators that improve the regenerative capacity and therapeutic potential of MSC-EXOs. This was observed utilising MSC-EXOs preconditioned with IFN-γ in a murine model with liver cirrhosis which revealed alleviation of both inflammation and fibrosis (Takeuchi et al. 2021). Likewise, EXOs derived from TNF-α-treated MSCs afforded improved therapeutic potential in a mouse model of ALI as compared to untreated EXOs. These outcomes were related to more pronounced overexpression of miR-299-3p which in turn inhibited the recruitment and activation of NLRP3-related inflammatory pathway (Zhang et al. 2020). Similarly, Shao et al. (2020) demonstrated experimentally the ability of IL-6 pre-treated human umbilical cord MSC-EXOs to diminish the generation of inflammatory cytokines via miR-455-3p which targeted the IL-6-related signalling cascades in ALI. In addition, LPS-preconditioned MSC-EXOs mitigated inflammation and the severity of UC compared to ordinary/unmodified MSC-EXOs (Gu et al. 2021). Another study reported that IFN-γ enhanced the therapeutic efficacy of MSC-EXOs for management of colitis in mice through overexpressing miR-125a and miR-125b in MSC-EXOs which directly acted on STAT3 and repressed Th17 cell differentiation as well as inflammation (Yang et al. 2020).
Current challenges of clinical applications of MSC-EVs
MSC-EVs are characterised by similar or even better function in comparison with their parent cells because of their higher biocompatibility, greater trajectory in intercellular communication, and higher efficiency in drug delivery (Cheng et al. 2020; Racchetti and Meldolesi 2021; Yin et al. 2023). Furthermore, MSC-EVs showed no evidence of spontaneous oncogenic potential or any negative immune responses (Cheng et al. 2020; Hou et al. 2021; Yin et al. 2023). On the other hand, MSCs can promote and aggravate tumour growth as demonstrated experimentally in several types of cancer such as breast and colorectal cancer in addition to gastric carcinoma (Karnoub et al. 2007; Quante et al. 2011; De Boeck et al. 2013; Musiał-Wysocka et al. 2019). More importantly, MSC-EVs are easy to store with extreme stability and without using harmful cryopreservatives (Cheng et al. 2020). MSC-EVs also exhibit good penetration of biological barriers and revealed minimal risk of microvascular embolism as compared to their parent MSCs which caused instant blood-mediated inflammatory reaction upon intravenous administration in different experimental studies (Fiedler et al. 2018; Musiał-Wysocka et al. 2019; Han et al. 2020; Sun et al. 2022), leading to pulmonary embolism (Tatsumi et al. 2013). Thus, MSC-EVs show a superior safety profile making them a promising therapeutic approach for a wide range of diseases or disorders (Zhu et al. 2017; Psaraki et al. 2022).
Despite all these advantages, there are numerous challenges that should be overcome before the clinical application of MSC-EVs in GI diseases. These concerns stem from: (a) the inability to choose the optimal EVs source due to the lack of clear comparison among different MSCs sources (Bruno et al. 2020); (b) the molecular heterogeneity in EVs preparations because of the difference in methods of EVs isolation, purification, and characterisation—which contradicts with the homogeneity required for clinical practice (Abraham and Krasnodembskaya 2020; Guo et al. 2021); (c) the difficulty to ascertain the optimal route of delivery and the therapeutic dosage required for each GI condition, which remains a mystery to clinicians due to the lack of well-recognised and standardised techniques for EVs isolation and characterisation (Guo et al. 2021; Ahmed and Al-Massri 2022); (d) the contamination of EVs preparations with apoptotic cells fragments, lipoproteins, or proteins (Choi et al. 2015; Hou et al. 2021); (e) a dearth of techniques for large-scale EVs production and extraction (Guo et al. 2021; Williams et al. 2023); (f) a scarcity of information about the exact content within MSC-EVs which can vary greatly due to different sources and conditions (Cheng et al. 2020); (g) the low temperatures during handling and transplantation, and the freeze–thaw cycles which can induce EVs clumping and cargo degradation (Pinky et al. 2021).
Therefore, from a practical standpoint, the apparent insignificant results of MSC-EVs in clinical trials could be related to the disease stage, the timing of their injection, the dose used, and the source of the MSC-EVs either from healthy or diseased cells. Further investigations are needed to scale up and optimise specific and standardised methodologies for MSC-EVs production, isolation, purification, and characterisation. Besides, it is important to validate the dosage and half-life of MSC-EVs and evaluate alternative approaches for EVs storage to enhance their stability. It is also necessary to examine the potential impacts of EVs derived from different sources of MSCs in various GI disorders and to investigate new techniques for modulating MSC-EVs composition and their biological activity. Furthermore, specific and effective markers for analysing EVs at a single-vesicle level should be identified to distinguish EVs source, ensure their purity, and preclude unknown harmful impacts of their use.
Conclusion
Because of the alarming rise in incidence and prevalence of GI diseases, researchers have been working to identify new approaches for the management of these diseases. MSC-EVs represent an attractive therapeutic paradigm for treating various GI diseases through maintaining the therapeutic advantages of their parents MSCs, but with reduced risks of iatrogenic tumour formation, immunogenicity, and microvascular obstructions. MSC-EVs restore homeostasis and enable the injured cells to recover through their anti-oxidant, anti-apoptotic, anti-inflammatory, anti-fibrotic, and immunomodulatory actions. Besides, the therapeutic efficacy of MSC-EVs can be improved by the preconditioning approach which utilises pharmacological agents or biological mediators to adapt them to the lethal environment to which they are subjected during pathological conditions. Notably, there have been tremendous efforts to improve the separation and production yield of MSC-EVs as well as their efficacy and stability over time following in vivo transplantation. Despite all these efforts, additional studies and methodologies are still needed to overcome the challenges and difficulties of their clinical applications.
Data availability
All data are available in the manuscript.
References
Abenavoli L, Larussa T, Corea A et al (2021) Dietary polyphenols and non-alcoholic fatty liver disease. Nutr 13:494. https://doi.org/10.3390/NU13020494
Abraham A, Krasnodembskaya A (2020) Mesenchymal stem cell-derived extracellular vesicles for the treatment of acute respiratory distress syndrome. Stem Cells Transl Med 9:28. https://doi.org/10.1002/SCTM.19-0205
Acharya P, Chouhan K, Weiskirchen S, Weiskirchen R (2021) Cellular mechanisms of liver fibrosis. Front Pharmacol 12:1072. https://doi.org/10.3389/FPHAR.2021.671640
Aghajani Nargesi A, Lerman LO, Eirin A (2017) Mesenchymal stem cell-derived extracellular vesicles for kidney repair: current status and looming challenges. Stem Cell Res Ther 8:273. https://doi.org/10.1186/S13287-017-0727-7
Ahmed L, Al-Massri K (2022) New approaches for enhancement of the efficacy of mesenchymal stem cell-derived exosomes in cardiovascular diseases. Tissue Eng Regen Med 19:1129–1146. https://doi.org/10.1007/S13770-022-00469-X
Arafa ESA, Mohamed WR, Zaher DM, Omar HA (2020) Gliclazide attenuates acetic acid-induced colitis via the modulation of PPARγ, NF-κB and MAPK signaling pathways. Toxicol Appl Pharmacol 391:114919. https://doi.org/10.1016/J.TAAP.2020.114919
Badillo AT, Beggs KJ, Javazon EH et al (2007) Murine bone marrow stromal progenitor cells elicit an in vivo cellular and humoral alloimmune response. Biol Blood Marrow Transplant 13:412–422. https://doi.org/10.1016/J.BBMT.2006.12.447
Basir HRG, Karbasi A, Ravan AP et al (2022) Is human umbilical cord mesenchymal stem cell-derived conditioned medium effective against oxidative and inflammatory status in CCl4-induced acute liver injury? Life Sci 305:120730. https://doi.org/10.1016/J.LFS.2022.120730
Bavarsad SS, Jalali MT, Nejad DB et al (2022) TGFβ1-pretreated exosomes of wharton jelly mesenchymal stem cell as a therapeutic strategy for improving liver fibrosis. Hepat Mon 221(22):e123416. https://doi.org/10.5812/HEPATMON-123416
Benedikter BJ, Bouwman FG, Vajen T et al (2017) Ultrafiltration combined with size exclusion chromatography efficiently isolates extracellular vesicles from cell culture media for compositional and functional studies. Sci Rep 7:15297. https://doi.org/10.1038/S41598-017-15717-7
Böing AN, van der Pol E, Grootemaat AE et al (2014) Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles. https://doi.org/10.3402/JEV.V3.23430
Borrelli DA, Yankson K, Shukla N et al (2018) Extracellular vesicle therapeutics for liver disease. J Control Release 273:86–98. https://doi.org/10.1016/J.JCONREL.2018.01.022
Bruno S, Chiabotto G, Camussi G (2020) Extracellular vesicles: a therapeutic option for liver fibrosis. Int J Mol Sci 21:1–18. https://doi.org/10.3390/IJMS21124255
Cai X, Zhang Z, Yuan J et al (2021) hucMSC-derived exosomes attenuate colitis by regulating macrophage pyroptosis via the miR-378a-5p/NLRP3 axis. Stem Cell Res Ther 12:416. https://doi.org/10.1186/S13287-021-02492-6
Cao L, Xu H, Wang G et al (2019) Extracellular vesicles derived from bone marrow mesenchymal stem cells attenuate dextran sodium sulfate-induced ulcerative colitis by promoting M2 macrophage polarization. Int Immunopharmacol 72:264–274. https://doi.org/10.1016/J.INTIMP.2019.04.020
Carnino JM, Lee H, Jin Y (2019) Isolation and characterization of extracellular vesicles from Broncho-Alveolar lavage fluid: a review and comparison of different methods. Respir Res 20:240. https://doi.org/10.1186/S12931-019-1210-Z
Casado-Díaz A, Quesada-Gómez JM, Dorado G (2020) Extracellular vesicles derived from mesenchymal stem cells (MSC) in regenerative medicine: applications in skin wound healing. Front Bioeng Biotechnol 8:146. https://doi.org/10.3389/FBIOE.2020.00146
Chang CL, Chen CH, Chiang JY et al (2019) Synergistic effect of combined melatonin and adipose-derived mesenchymal stem cell (ADMSC)-derived exosomes on amelioration of dextran sulfate sodium (DSS)-induced acute colitis. Am J Transl Res 11:2706-2724
Chen L, Lu F et al (2018) BMSCs-derived miR-223-containing exosomes contribute to liver protection in experimental autoimmune hepatitis. Mol Immunol 93:38–46. https://doi.org/10.1016/J.MOLIMM.2017.11.008
Chen HY, Ge P, Liu JY et al (2022a) Artificial intelligence: emerging player in the diagnosis and treatment of digestive disease. World J Gastroenterol 28:2152–2162. https://doi.org/10.3748/WJG.V28.I20.2152
Chen S, Sun F, Qian H et al (2022b) Preconditioning and engineering strategies for improving the efficacy of mesenchymal stem cell-derived exosomes in cell-free therapy. Stem Cells Int 2022:1779346. https://doi.org/10.1155/2022/1779346
Cheng Y, Cao X, Qin L (2020) Mesenchymal stem cell-derived extracellular vesicles: a novel cell-free therapy for sepsis. Front Immunol 11:647. https://doi.org/10.3389/FIMMU.2020.00647
Cheng L, Yu P, Li F et al (2021) Human umbilical cord-derived mesenchymal stem cell-exosomal miR-627-5p ameliorates non-alcoholic fatty liver disease by repressing FTO expression. Hum Cell 34:1697–1708. https://doi.org/10.1007/S13577-021-00593-1
Choi DS, Kim DK, Kim YK, Gho YS (2015) Proteomics of extracellular vesicles: exosomes and ectosomes. Mass Spectrom Rev 34:474–490. https://doi.org/10.1002/MAS.21420
Clos-Sansalvador M, Monguió-Tortajada M, Roura S et al (2022) Commonly used methods for extracellular vesicles’ enrichment: implications in downstream analyses and use. Eur J Cell Biol 101:151227. https://doi.org/10.1016/J.EJCB.2022.151227
Cocucci E, Racchetti G, Meldolesi J (2009) Shedding microvesicles: artefacts no more. Trends Cell Biol 19:43–51. https://doi.org/10.1016/J.TCB.2008.11.003
Coumans FAW, Brisson AR, Buzas EI et al (2017) Methodological guidelines to study extracellular vesicles. Circ Res 120:1632–1648. https://doi.org/10.1161/CIRCRESAHA.117.309417
De Boeck A, Pauwels P, Hensen K et al (2013) Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression through paracrine neuregulin 1/HER3 signalling. Gut 62:550–560. https://doi.org/10.1136/GUTJNL-2011-301393
De Filippis A, Ullah H, Baldi A et al (2020) Gastrointestinal disorders and metabolic syndrome: dysbiosis as a key link and common bioactive dietary components useful for their treatment. Int J Mol Sci 21:4929. https://doi.org/10.3390/IJMS21144929
De Sousa KP, Rossi I, Abdullahi M et al (2023) Isolation and characterization of extracellular vesicles and future directions in diagnosis and therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol 15:e1835. https://doi.org/10.1002/WNAN.1835
De Oliveira RG, Damazo AS, Antonielli LF et al (2021) Dilodendron bipinnatum Radlk. extract alleviates ulcerative colitis induced by TNBS in rats by reducing inflammatory cell infiltration, TNF-α and IL-1β concentrations, IL-17 and COX-2 expressions, supporting mucus production and promotes an antioxidant effect. J Ethnopharmacol 269:113735. https://doi.org/10.1016/J.JEP.2020.113735
Didamoony MA, Atwa AM, Abd El-Haleim EA, Ahmed LA (2022) Bromelain ameliorates D-galactosamine-induced acute liver injury: role of SIRT1/LKB1/AMPK, GSK3β/Nrf2 and NF-κB p65/TNF-α/caspase-8, -9 signalling pathways. J Pharm Pharmacol 74:1765–1775. https://doi.org/10.1093/JPP/RGAC071
Didamoony MA, Atwa AM, Ahmed LA (2023) Modulatory effect of rupatadine on mesenchymal stem cell-derived exosomes in hepatic fibrosis in rats: a potential role for miR-200a. Life Sci 324:121710. https://doi.org/10.1016/J.LFS.2023.121710
Dong L, Pu Y, Chen X et al (2020) HUCMSC-extracellular vesicles downregulated hepatic stellate cell activation and reduced liver injury in S. japonicum-infected mice. Stem Cell Res Ther 11:21. https://doi.org/10.1186/S13287-019-1539-8
Doumas BT, Watson WA, Biggs HG (1971) Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta 31:87–96
Doyle LM, Wang MZ (2019) Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 8:727. https://doi.org/10.3390/CELLS8070727
Drossman DA (2016) Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology 150:1262-1279.e2. https://doi.org/10.1053/J.GASTRO.2016.02.032
Du Z, Wu T, Liu L et al (2021) Extracellular vesicles-derived miR-150-5p secreted by adipose-derived mesenchymal stem cells inhibits CXCL1 expression to attenuate hepatic fibrosis. J Cell Mol Med 25:701–715. https://doi.org/10.1111/JCMM.16119
Du X, Li H, Han X, Ma W (2022) Mesenchymal stem cells-derived exosomal miR-24-3p ameliorates non-alcohol fatty liver disease by targeting Keap-1. Biochem Biophys Res Commun 637:331–340. https://doi.org/10.1016/J.BBRC.2022.11.012
El-Derany MO, AbdelHamid SG (2021) Upregulation of miR-96-5p by bone marrow mesenchymal stem cells and their exosomes alleviate non-alcoholic steatohepatitis: Emphasis on caspase-2 signaling inhibition. Biochem Pharmacol 190:114624. https://doi.org/10.1016/J.BCP.2021.114624
El-Desoky Mohamady RE, Elwia SK, Abo El Wafa SM, Mohamed MA (2022) Effect of mesenchymal stem cells derived exosomes and green tea polyphenols on acetic acid induced ulcerative colitis in adult male albino rats. Ultrastruct Pathol 46:147–163. https://doi.org/10.1080/01913123.2022.2039825
Fattore AD, Luciano R, Pascucci L et al (2015) Immunoregulatory effects of mesenchymal stem cell-derived extracellular vesicles on T-lymphocytes. Cell Transplant 24:2615–2627. https://doi.org/10.3727/096368915X687543
Feng J, Yao W, Zhang Y et al (2018) Intravenous anesthetics enhance the ability of human bone marrow-derived mesenchymal stem cells to alleviate hepatic ischemia-reperfusion injury in a receptor-dependent manner. Cell Physiol Biochem 47:556–566. https://doi.org/10.1159/000489989
Fennema EM, Tchang LAH, Yuan H et al (2018) Ectopic bone formation by aggregated mesenchymal stem cells from bone marrow and adipose tissue: a comparative study. J Tissue Eng Regen Med 12:e150–e158. https://doi.org/10.1002/TERM.2453
Fiedler T, Rabe M, Mundkowski RG et al (2018) Adipose-derived mesenchymal stem cells release microvesicles with procoagulant activity. Int J Biochem Cell Biol 100:49–53. https://doi.org/10.1016/J.BIOCEL.2018.05.008
Gandham S, Su X, Wood J et al (2020) Technologies and standardization in research on extracellular vesicles. Trends Biotechnol 38:1066–1098. https://doi.org/10.1016/J.TIBTECH.2020.05.012
Gardiner C, Di VD, Sahoo S et al (2016) Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J Extracell Vesicles 5:32945. https://doi.org/10.3402/JEV.V5.32945
Gimona M, Pachler K, Laner-Plamberger S et al (2017) Manufacturing of human extracellular vesicle-based therapeutics for clinical use. Int J Mol Sci 18:1190. https://doi.org/10.3390/IJMS18061190
Gowen A, Shahjin F, Chand S et al (2020) Mesenchymal stem cell-derived extracellular vesicles: challenges in clinical applications. Front Cell Dev Biol 8:149. https://doi.org/10.3389/FCELL.2020.00149
Grant R, Ansa-Addo E, Stratton D et al (2011) A filtration-based protocol to isolate human plasma membrane-derived vesicles and exosomes from blood plasma. J Immunol Methods 371:143–151. https://doi.org/10.1016/J.JIM.2011.06.024
Greenwood-Van Meerveld B, Johnson AC, Grundy D (2017) Gastrointestinal physiology and function. Handb Exp Pharmacol 239:1–16. https://doi.org/10.1007/164_2016_118
Gu L, Ren F, Fang X et al (2021) Exosomal MicroRNA-181a derived from mesenchymal stem cells improves gut microbiota composition, barrier function, and inflammatory status in an experimental colitis model. Front Med 8:898. https://doi.org/10.3389/FMED.2021.660614
Guo H, Su Y, Deng F (2021) Effects of mesenchymal stromal cell-derived extracellular vesicles in lung diseases: current status and future perspectives. Stem Cell Rev Reports 17:440–458. https://doi.org/10.1007/S12015-020-10085-8
Guo G, Tan Z, Liu Y et al (2022) The therapeutic potential of stem cell-derived exosomes in the ulcerative colitis and colorectal cancer. Stem Cell Res Ther 13:1–18. https://doi.org/10.1186/S13287-022-02811-5
Gupta S, Pinky V et al (2022) Comparative evaluation of anti-fibrotic effect of tissue specific mesenchymal stem cells derived extracellular vesicles for the amelioration of CCl4 induced chronic liver injury. Stem Cell Rev Reports 18:1097–1112. https://doi.org/10.1007/S12015-021-10313-9
Gurunathan S, Kang MH, Jeyaraj M et al (2019) Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells 8:307. https://doi.org/10.3390/CELLS8040307
Han C, Sun X, Liu L et al (2016) Exosomes and their therapeutic potentials of stem cells. Stem Cells Int 2016:7653489. https://doi.org/10.1155/2016/7653489
Han M, Cao Y, Xue H et al (2020) Neuroprotective effect of mesenchymal stromal cell-derived extracellular vesicles against cerebral ischemia-reperfusion-induced neural functional injury: A pivotal role for ampk and jak2/stat3/nf-κb signaling pathway modulation. Drug Des Devel Ther 14:2865–2876. https://doi.org/10.2147/DDDT.S248892
Harrell CR, Jankovic MG, Fellabaum C et al (2019a) Molecular mechanisms responsible for anti-inflammatory and immunosuppressive effects of mesenchymal stem cell-derived factors. Adv Exp Med Biol 1084:187–206. https://doi.org/10.1007/5584_2018_306
Harrell CR, Jovicic N, Djonov V et al (2019b) Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells 8:1605. https://doi.org/10.3390/CELLS8121605
Hass R, Kasper C, Böhm S, Jacobs R (2011) Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 9:12. https://doi.org/10.1186/1478-811X-9-12
He C, Zheng S, Luo Y, Wang B (2018) Exosome theranostics: biology and translational medicine. Theranostics 8:237–255. https://doi.org/10.7150/THNO.21945
Heidari N, Abbasi-Kenarsari H, Namaki S et al (2021) Adipose-derived mesenchymal stem cell-secreted exosome alleviates dextran sulfate sodium-induced acute colitis by Treg cell induction and inflammatory cytokine reduction. J Cell Physiol 236:5906–5920. https://doi.org/10.1002/JCP.30275
Hessvik NP, Llorente A (2018) Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 75:193. https://doi.org/10.1007/S00018-017-2595-9
Heydari R, Abdollahpour-Alitappeh M, Shekari F, Meyfour A (2021) Emerging role of extracellular vesicles in biomarking the gastrointestinal diseases. Expert Rev Mol Diagnost 21:939–962. https://doi.org/10.1080/14737159.2021.1954909
Hosseini NF, Dalirfardouei R, Aliramaei MR, Najafi R (2022) Stem cells or their exosomes: which is preferred in COVID-19 treatment? Biotechnol Lett 44:159–177. https://doi.org/10.1007/S10529-021-03209-8
Hou Y, Li J, Guan S, Witte F (2021) The therapeutic potential of MSC-EVs as a bioactive material for wound healing. Eng Regen 2:182–194. https://doi.org/10.1016/J.ENGREG.2021.11.003
Hu C, Li L (2018) Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J Cell Mol Med 22:1428. https://doi.org/10.1111/JCMM.13492
Hyun J, Wang S, Kim J et al (2015) MicroRNA125b-mediated Hedgehog signaling influences liver regeneration by chorionic plate-derived mesenchymal stem cells. Sci Rep 51(5):1–15. https://doi.org/10.1038/srep14135
Jeong JO, Han JW, Kim JM et al (2011) Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res 108:1340–1347. https://doi.org/10.1161/CIRCRESAHA.110.239848
Jeyaram A, Jay SM (2017) Preservation and storage stability of extracellular vesicles for therapeutic applications. AAPS J 20:1. https://doi.org/10.1208/S12248-017-0160-Y
Jiang L, Zhang S, Hu H et al (2019) Exosomes derived from human umbilical cord mesenchymal stem cells alleviate acute liver failure by reducing the activity of the NLRP3 inflammasome in macrophages. Biochem Biophys Res Commun 508:735–741. https://doi.org/10.1016/J.BBRC.2018.11.189
Jin Y, Wang J, Li H et al (2018) Extracellular vesicles secreted by human adipose-derived stem cells (hASCs) improve survival rate of rats with acute liver failure by releasing lncRNA H19. EBioMedicine 34:231–242. https://doi.org/10.1016/J.EBIOM.2018.07.015
Johnstone RM, Adam M, Hammond JR et al (1987) Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 262:9412–9420. https://doi.org/10.1016/S0021-9258(18)48095-7
Joo HS, Suh JH, Lee HJ et al (2020) Current knowledge and future perspectives on mesenchymal stem cell-derived exosomes as a new therapeutic agent. Int J Mol Sci 21:727. https://doi.org/10.3390/IJMS21030727
Kalluri R (2016) The biology and function of exosomes in cancer. J Clin Invest 126:1208–1215. https://doi.org/10.1172/JCI81135
Kang Y, Song Y, Luo Y et al (2022) Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate experimental non-alcoholic steatohepatitis via Nrf2/NQO-1 pathway. Free Radic Biol Med 192:25–36. https://doi.org/10.1016/J.FREERADBIOMED.2022.08.037
Karnoub AE, Dash AB, Vo AP et al (2007) Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449:557–563. https://doi.org/10.1038/NATURE06188
Karussis D, Karageorgiou C, Vaknin-Dembinsky A et al (2010) Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol 67:1187–1194. https://doi.org/10.1001/ARCHNEUROL.2010.248
Keating A (2012) Mesenchymal stromal cells: new directions. Cell Stem Cell 10:709–716. https://doi.org/10.1016/J.STEM.2012.05.015
Keshtkar S, Azarpira N, Ghahremani MH (2018) Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther 9:63. https://doi.org/10.1186/S13287-018-0791-7
Kim J, Lee C, Shin Y et al (2021) sEVs from tonsil-derived mesenchymal stromal cells alleviate activation of hepatic stellate cells and liver fibrosis through miR-486-5p. Mol Ther 29:1471–1486. https://doi.org/10.1016/J.YMTHE.2020.12.025
Kim JS, Lee JH, Kwon O, et al (2017) Rapid deterioration of preexisting renal insufficiency after autologous mesenchymal stem cell therapy. Kidney Res Clin Pract 36:200–204. https://doi.org/10.23876/j.krcp.2017.36.2.200
Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP (2018) Isolation of extracellular vesicles: general methodologies and latest trends. Biomed Res Int 2018:8545347. https://doi.org/10.1155/2018/8545347
Kou M, Huang L, Yang J et al (2022) Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: a next generation therapeutic tool? Cell Death Dis 137(13):1–16. https://doi.org/10.1038/s41419-022-05034-x
Kubo K, Ohnishi S, Hosono H et al (2015) Human amnion-derived mesenchymal stem cell transplantation ameliorates liver fibrosis in rats. Transplant Direct 1:e16. https://doi.org/10.1097/TXD.0000000000000525
Kusuma GD, Barabadi M, Tan JL et al (2018) To protect and to preserve: novel preservation strategies for extracellular vesicles. Front Pharmacol 9:1199. https://doi.org/10.3389/FPHAR.2018.01199
Lalu MM, McIntyre L, Pugliese C et al (2012) Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS ONE 7:e47559. https://doi.org/10.1371/JOURNAL.PONE.0047559
Lane RE, Korbie D, Trau M, Hill MM (2017) Purification protocols for extracellular vesicles. Methods Mol Biol 1660:111–130. https://doi.org/10.1007/978-1-4939-7253-1_10
Lee BC, Kang KS (2020) Functional enhancement strategies for immunomodulation of mesenchymal stem cells and their therapeutic application. Stem Cell Res Ther 11:397. https://doi.org/10.1186/S13287-020-01920-3
Li T, Yan Y, Wang B et al (2013) Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev 22:845–854. https://doi.org/10.1089/SCD.2012.0395
Liangsupree T, Multia E, Riekkola ML (2021) Modern isolation and separation techniques for extracellular vesicles. J Chromatogr A 1636:461773. https://doi.org/10.1016/J.CHROMA.2020.461773
Linares R, Tan S, Gounou C et al (2015) High-speed centrifugation induces aggregation of extracellular vesicles. J Extracell Vesicles 4:29509. https://doi.org/10.3402/JEV.V4.29509
Liu Y, Lou G, Li A et al (2018) AMSC-derived exosomes alleviate lipopolysaccharide/d-galactosamine-induced acute liver failure by miR-17-mediated reduction of TXNIP/NLRP3 inflammasome activation in macrophages. EBioMedicine 36:140–150. https://doi.org/10.1016/J.EBIOM.2018.08.054
Livshts MA, Khomyakova E, Evtushenko EG et al (2015) Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci Rep 5:17319. https://doi.org/10.1038/SREP17319
Lopez-Santalla M, Garin MI (2021) Improving the efficacy of mesenchymal stem/stromal-based therapy for treatment of inflammatory bowel diseases. Biomedicines 9:1507. https://doi.org/10.3390/BIOMEDICINES9111507
Lötvall J, Hill AF, Hochberg F et al (2014) Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the international society for extracellular vesicles. J Extracell Vesicles 3:26913. https://doi.org/10.3402/JEV.V3.26913
Lou G, Yang Y, Liu F et al (2017) MiR-122 modification enhances the therapeutic efficacy of adipose tissue-derived mesenchymal stem cells against liver fibrosis. J Cell Mol Med 21:2963–2973. https://doi.org/10.1111/JCMM.13208
Lozano-Ramos I, Bancu I, Oliveira-Tercero A et al (2015) Size-exclusion chromatography-based enrichment of extracellular vesicles from urine samples. J Extracell Vesicles 4:1–11. https://doi.org/10.3402/JEV.V4.27369
Ma J, Li Y, Chen M et al (2023) hMSCs-derived exosome circCDK13 inhibits liver fibrosis by regulating the expression of MFGE8 through miR-17-5p/KAT2B. Cell Biol Toxicol 39:1–22. https://doi.org/10.1007/S10565-022-09714-4
Madrigal M, Rao KS, Riordan NH (2014) A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J Transl Med 12:260. https://doi.org/10.1186/S12967-014-0260-8
Mahmoudi A, Butler AE, Jamialahmadi T, Sahebkar A (2022) The role of exosomal miRNA in nonalcoholic fatty liver disease. J Cell Physiol 237:2078–2094. https://doi.org/10.1002/JCP.30699
Maji S, Yan IK, Parasramka M et al (2017) In vitro toxicology studies of extracellular vesicles. J Appl Toxicol 37:310–318. https://doi.org/10.1002/JAT.3362
Mao F, Wu Y, Tang X et al (2017) Exosomes derived from human umbilical cord mesenchymal stem cells relieve inflammatory bowel disease in mice. Biomed Res Int 2017:5356760. https://doi.org/10.1155/2017/5356760
Moayedfard Z, Sani F, Alizadeh A et al (2022) The role of the immune system in the pathogenesis of NAFLD and potential therapeutic impacts of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther 131(13):1–16. https://doi.org/10.1186/S13287-022-02929-6
Moeinabadi-Bidgoli K, Babajani A, Yazdanpanah G et al (2021) Translational insights into stem cell preconditioning: from molecular mechanisms to preclinical applications. Biomed Pharmacother 142:112026. https://doi.org/10.1016/J.BIOPHA.2021.112026
Momen-Heravi F, Balaj L, Alian S et al (2012) Impact of biofluid viscosity on size and sedimentation efficiency of the isolated microvesicles. Front Physiol 3:162. https://doi.org/10.3389/FPHYS.2012.00162
Monsel A, Zhu YG, Gudapati V et al (2016) Mesenchymal stem cell derived secretome and extracellular vesicles for acute lung injury and other inflammatory lung diseases. Expert Opin Biol Ther 16:859–871. https://doi.org/10.1517/14712598.2016.1170804
Mortezaee K, Khanlarkhani N, Sabbaghziarani F et al (2017) Preconditioning with melatonin improves therapeutic outcomes of bone marrow-derived mesenchymal stem cells in targeting liver fibrosis induced by CCl4. Cell Tissue Res 369:303–312. https://doi.org/10.1007/S00441-017-2604-1
Motterlini R, Foresti R, Bassi R, Green CJ (2000) Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med 28:1303–1312. https://doi.org/10.1016/S0891-5849(00)00294-X
Musiał-Wysocka A, Kot M, Majka M (2019) The pros and cons of mesenchymal stem cell-based therapies. Cell Transplant 28:801. https://doi.org/10.1177/0963689719837897
Nakai W, Yoshida T, Diez D et al (2016) A novel affinity-based method for the isolation of highly purified extracellular vesicles. Sci Reports 61(6):1–11. https://doi.org/10.1038/srep33935
Natasha G, Gundogan B, Tan A et al (2014) Exosomes as immunotheranostic nanoparticles. Clin Ther 36:820–829. https://doi.org/10.1016/J.CLINTHERA.2014.04.019
Niu Q, Wang T, Wang Z et al (2022) Adipose-derived mesenchymal stem cell-secreted extracellular vesicles alleviate non-alcoholic fatty liver disease via delivering miR-223-3p. Adipocyte 11:572. https://doi.org/10.1080/21623945.2022.2098583
Noronha Nc NDC, Mizukami A, Caliári-Oliveira C et al (2019) Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res Ther 10:131. https://doi.org/10.1186/S13287-019-1224-Y
Ohara M, Ohnishi S, Hosono H et al (2018) Extracellular vesicles from amnion-derived mesenchymal stem cells ameliorate hepatic inflammation and fibrosis in rats. Stem Cells Int 2018:3212643. https://doi.org/10.1155/2018/3212643
Olén O, Erichsen R, Sachs MC et al (2020) Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet (london, England) 395:123–131. https://doi.org/10.1016/S0140-6736(19)32545-0
Onishi R, Ohnishi S, Higashi R et al (2015) Human amnion-derived mesenchymal stem cell transplantation ameliorates dextran sulfate sodium-induced severe colitis in rats. Cell Transplant 24:2601–2614. https://doi.org/10.3727/096368915X687570
Ono M, Ohnishi S, Honda M et al (2015) Effects of human amnion-derived mesenchymal stromal cell transplantation in rats with radiation proctitis. Cytotherapy 17:1545–1559. https://doi.org/10.1016/J.JCYT.2015.07.003
Oshima T, Miwa H (2015) Epidemiology of functional gastrointestinal disorders in Japan and in the World. J Neurogastroenterol Motil 21:320–329. https://doi.org/10.5056/JNM14165
Owusu G, Obiri DD, Ainooson GK et al (2020) Acetic acid-induced ulcerative colitis in sprague dawley rats is suppressed by hydroethanolic extract of cordia vignei leaves through reduced serum levels of TNF- α and IL-6. Int J Chronic Dis 2020:1–11. https://doi.org/10.1155/2020/8785497
Pinky GS, Krishnakumar V et al (2021) Mesenchymal stem cell derived exosomes: a nano platform for therapeutics and drug delivery in combating COVID-19. Stem Cell Rev Rep 17:33–43. https://doi.org/10.1007/S12015-020-10002-Z
Pouwels S, Sakran N, Graham Y et al (2022) Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr Disord 22:63. https://doi.org/10.1186/S12902-022-00980-1
Psaraki A, Ntari L, Karakostas C et al (2022) Extracellular vesicles derived from mesenchymal stem/stromal cells: The regenerative impact in liver diseases. Hepatology 75:1590–1603. https://doi.org/10.1002/HEP.32129
Qu Y, Zhang Q, Cai X et al (2017) Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med 21:2491–2502. https://doi.org/10.1111/JCMM.13170
Quante M, Tu SP, Tomita H et al (2011) Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 19:257–272. https://doi.org/10.1016/J.CCR.2011.01.020
Racchetti G, Meldolesi J (2021) Extracellular vesicles of mesenchymal stem cells: therapeutic properties discovered with extraordinary success. Biomedicines 9:667. https://doi.org/10.3390/BIOMEDICINES9060667
Rahimi B, Panahi M, Saraygord-Afshari N et al (2021) The secretome of mesenchymal stem cells and oxidative stress: challenges and opportunities in cell-free regenerative medicine. Mol Biol Rep 48:5607–5619. https://doi.org/10.1007/S11033-021-06360-7
Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200:373–383. https://doi.org/10.1083/JCB.201211138
Razavi M, Rezaee M, Telichko A et al (2020) The paracrine function of mesenchymal stem cells in response to pulsed focused ultrasound. Cell Transplant 29:963689720965478. https://doi.org/10.1177/0963689720965478
Ren S, Pan L, Yang L et al (2021) miR-29a-3p transferred by mesenchymal stem cells-derived extracellular vesicles protects against myocardial injury after severe acute pancreatitis. Life Sci 272:119189. https://doi.org/10.1016/J.LFS.2021.119189
Revenfeld ALS, Bæk R, Nielsen MH et al (2014) Diagnostic and prognostic potential of extracellular vesicles in peripheral blood. Clin Ther 36:830–846. https://doi.org/10.1016/J.CLINTHERA.2014.05.008
Rezaie J, Nejati V, Mahmoodi M, Ahmadi M (2022) Mesenchymal stem cells derived extracellular vesicles: a promising nanomedicine for drug delivery system. Biochem Pharmacol 203:115167. https://doi.org/10.1016/J.BCP.2022.115167
Rong X, Liu J, Yao X et al (2019) Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res Ther 10:98. https://doi.org/10.1186/S13287-019-1204-2
Rostom DM, Attia N, Khalifa HM et al (2020) The Therapeutic potential of extracellular vesicles versus mesenchymal stem cells in liver damage. Tissue Eng Regen Med 17:537. https://doi.org/10.1007/S13770-020-00267-3
Sameri MJ, Savari F, Hoseinynejad K et al (2022) The hepato-protective effect of H2S-modified and non-modified mesenchymal stem cell exosomes on liver ischemia-reperfusion injury in mice: The role of MALAT1. Biochem Biophys Res Commun 635:194–202. https://doi.org/10.1016/J.BBRC.2022.09.111
Scammahorn JJ, Nguyen ITN, Bos EM et al (2021) Fighting oxidative stress with sulfur: hydrogen sulfide in the renal and cardiovascular systems. Antioxidants 10:1–21. https://doi.org/10.3390/ANTIOX10030373
Sengupta V, Sengupta S, Lazo A et al (2020) Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev 29:747–754. https://doi.org/10.1089/SCD.2020.0080
Shah TG, Predescu D (2019) Mesenchymal stem cells-derived extracellular vesicles in acute respiratory distress syndrome: a review of current literature and potential future treatment options. Clin Transl Med 81(8):1–6. https://doi.org/10.1186/S40169-019-0242-9
Shao H, Im H, Castro CM et al (2018) New technologies for analysis of extracellular vesicles. Chem Rev 118:1917–1950. https://doi.org/10.1021/ACS.CHEMREV.7B00534
Shao M, Xu Q, Wu Z et al (2020) Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate IL-6-induced acute liver injury through miR-455-3p. Stem Cell Res Ther 11:37. https://doi.org/10.1186/S13287-020-1550-0
Shi Y, Yang X, Wang S et al (2022) Human umbilical cord mesenchymal stromal cell-derived exosomes protect against MCD-induced NASH in a mouse model. Stem Cell Res Ther 13:517. https://doi.org/10.1186/S13287-022-03201-7
Shiha G, Nabil A, Lotfy A et al (2020) Antifibrotic effect of combination of nilotinib and stem cell-conditioned media on CCl4-induced liver fibrosis. Stem Cells Int 2020:6574010. https://doi.org/10.1155/2020/6574010
Sidhom K, Obi PO (2020) A review of exosomal isolation methods: is size exclusion chromatography the best option? Int J Mol Sci 21:6466. https://doi.org/10.3390/IJMS21186466
Soubh AA, Abdallah DM, El-Abhar HS (2015) Geraniol ameliorates TNBS-induced colitis: involvement of Wnt/β-catenin, p38MAPK, NFκB, and PPARγ signaling pathways. Life Sci 136:142–150. https://doi.org/10.1016/J.LFS.2015.07.004
Štulík K, Pacáková V, Tichá M (2003) Some potentialities and drawbacks of contemporary size-exclusion chromatography. J Biochem Biophys Methods 56:1–13. https://doi.org/10.1016/S0165-022X(03)00053-8
Sun L, Xu R, Sun X et al (2016) Safety evaluation of exosomes derived from human umbilical cord mesenchymal stromal cell. Cytotherapy 18:413–422. https://doi.org/10.1016/J.JCYT.2015.11.018
Sun CK, Chen CH, Lo CC et al (2017) Melatonin treatment enhances therapeutic effects of exosomes against acute liver ischemia-reperfusion injury. Am J Transl Res 9:1543–1560
Sun H, Zhang T, Gao J (2022) Extracellular vesicles derived from mesenchymal stem cells: a potential biodrug for acute respiratory distress syndrome treatment. BioDrugs 36:701–715. https://doi.org/10.1007/S40259-022-00555-5
Szatanek R, Baran J, Siedlar M, Baj-Krzyworzeka M (2015) Isolation of extracellular vesicles: determining the correct approach (Review). Int J Mol Med 36:11–17. https://doi.org/10.3892/IJMM.2015.2194
Takeuchi S, Tsuchiya A, Iwasawa T et al (2021) Small extracellular vesicles derived from interferon-γ pre-conditioned mesenchymal stromal cells effectively treat liver fibrosis. NPJ Regen Med 61(6):1–14. https://doi.org/10.1038/s41536-021-00132-4
Talebjedi B, Tasnim N, Hoorfar M et al (2021) Exploiting microfluidics for extracellular vesicle isolation and characterization: potential use for standardized embryo quality assessment. Front Vet Sci 7:620809. https://doi.org/10.3389/FVETS.2020.620809
Tamura R, Uemoto S, Tabata Y (2016) Immunosuppressive effect of mesenchymal stem cell-derived exosomes on a concanavalin A-induced liver injury model. Inflamm Regen 36:26. https://doi.org/10.1186/S41232-016-0030-5
Tan Y, Huang Y, Mei R et al (2022) HucMSC-derived exosomes delivered BECN1 induces ferroptosis of hepatic stellate cells via regulating the xCT/GPX4 axis. Cell Death Dis 134(13):1–11. https://doi.org/10.1038/s41419-022-04764-2
Tatsumi K, Ohashi K, Matsubara Y et al (2013) Tissue factor triggers procoagulation in transplanted mesenchymal stem cells leading to thromboembolism. Biochem Biophys Res Commun 431:203–209. https://doi.org/10.1016/J.BBRC.2012.12.134
Tawfeek GAE, Kasem HA (2023) Curcumin preconditioned mesenchymal stem cells derived exosomes transplantation ameliorate and protect against non- alcoholic steatohepatitis by regulation the expression of key genes of inflammation and oxidative stress. Transpl Immunol 78:101837. https://doi.org/10.1016/J.TRIM.2023.101837
Tetta C, Deregibus MC, Camussi G (2020) Stem cells and stem cell-derived extracellular vesicles in acute and chronic kidney diseases: mechanisms of repair. Ann Transl Med 8:570–570. https://doi.org/10.21037/atm.2020.03.19
Tian J, Zhu Q, Zhang Y et al (2020) Olfactory ecto-mesenchymal stem cell-derived exosomes ameliorate experimental colitis via modulating Th1/Th17 and treg cell responses. Front Immunol 11:598322. https://doi.org/10.3389/FIMMU.2020.598322
Tian S, Zhou X, Zhang M et al (2022) Mesenchymal stem cell-derived exosomes protect against liver fibrosis via delivering miR-148a to target KLF6/STAT3 pathway in macrophages. Stem Cell Res Ther 13:330. https://doi.org/10.1186/S13287-022-03010-Y
Tilkorn DJ, Davies EM, Keramidaris E et al (2012) The in vitro preconditioning of myoblasts to enhance subsequent survival in an in vivo tissue engineering chamber model. Biomaterials 33:3868–3879. https://doi.org/10.1016/J.BIOMATERIALS.2012.02.006
Touani FK, Borie M, Azzi F et al (2021) Pharmacological preconditioning improves the viability and proangiogenic paracrine function of hydrogel-encapsulated mesenchymal stromal cells. Stem Cells Int 2021:6663467. https://doi.org/10.1155/2021/6663467
Trounson A, McDonald C (2015) Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell 17:11–22. https://doi.org/10.1016/J.STEM.2015.06.007
Van Niel G, D’Angelo G (2018) Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 194(19):213–228. https://doi.org/10.1038/nrm.2017.125
Van Deun J, Mestdagh P, Agostinis P et al (2017) EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods 14:228–232. https://doi.org/10.1038/NMETH.4185
Varderidou-Minasian S, Lorenowicz MJ (2020) Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: challenges and opportunities. Theranostics 10:5979–5997. https://doi.org/10.7150/THNO.40122
Wagner W, Wein F, Seckinger A et al (2005) Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol 33:1402–1416. https://doi.org/10.1016/J.EXPHEM.2005.07.003
Wang F, Pu C, Zhou P et al (2015) Cinnamaldehyde prevents endothelial dysfunction induced by high glucose by activating Nrf2. Cell Physiol Biochem 36:315–324
Wang G, Yuan J, Cai X et al (2020) HucMSC-exosomes carrying miR-326 inhibit neddylation to relieve inflammatory bowel disease in mice. Clin Transl Med 10:e113. https://doi.org/10.1002/ctm2.113
Wang J, Chen D, Ho EA (2021) Challenges in the development and establishment of exosome-based drug delivery systems. J Control Release 329:894–906. https://doi.org/10.1016/J.JCONREL.2020.10.020
Wang R, Li Z, Liu S, Zhang D (2023) Global, regional, and national burden of 10 digestive diseases in 204 countries and territories from 1990 to 2019. Front Public Heal 11:1061453. https://doi.org/10.3389/FPUBH.2023.1061453
Watanabe T, Tsuchiya A, Takeuchi S et al (2020) Development of a non-alcoholic steatohepatitis model with rapid accumulation of fibrosis, and its treatment using mesenchymal stem cells and their small extracellular vesicles. Regen Ther 14:252–261. https://doi.org/10.1016/J.RETH.2020.03
Wei X, Zheng W, Tian P et al (2020) Administration of glycyrrhetinic acid reinforces therapeutic effects of mesenchymal stem cell-derived exosome against acute liver ischemia-reperfusion injury. J Cell Mol Med 24:11211–11220. https://doi.org/10.1111/JCMM.15675
Wei H, Chen Q, Lin L et al (2021) Regulation of exosome production and cargo sorting. Int J Biol Sci 17:163. https://doi.org/10.7150/IJBS.53671
Wendon J et al (2017) EASL clinical practical guidelines on the management of acute (fulminant) liver failure. J Hepatol 66:1047–1081. https://doi.org/10.1016/J.JHEP.2016.12.003
Weng Z, Zhang B, Wu C et al (2021) Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. J Hematol Oncol 141(14):1–22. https://doi.org/10.1186/S13045-021-01141-Y
Wiklander OPB, Nordin JZ, O’Loughlin A et al (2015) Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles 4:1–13. https://doi.org/10.3402/JEV.V4.26316
Williams T, Salmanian G, Burns M et al (2023) Versatility of mesenchymal stem cell-derived extracellular vesicles in tissue repair and regenerative applications. Biochimie 207:33–48. https://doi.org/10.1016/J.BIOCHI.2022.11.011
Wu Y, Qiu W, Xu X et al (2018) Exosomes derived from human umbilical cord mesenchymal stem cells alleviate inflammatory bowel disease in mice through ubiquitination. Am J Transl Res 10:2026–2036
Wu H, Fan H, Shou Z et al (2019) Extracellular vesicles containing miR-146a attenuate experimental colitis by targeting TRAF6 and IRAK1. Int Immunopharmacol 68:204–212. https://doi.org/10.1016/J.INTIMP.2018.12.043
Wu HY, Zhang XC, Jia BB et al (2021) Exosomes derived from human umbilical cord mesenchymal stem cells alleviate acetaminophen-induced acute liver failure through activating ERK and IGF-1R/PI3K/AKT signaling pathway. J Pharmacol Sci 147:143–155. https://doi.org/10.1016/J.JPHS.2021.06.008
Xia C, Dai Z, Jin Y, Chen P (2021) Emerging antioxidant paradigm of mesenchymal stem cell-derived exosome therapy. Front Endocrinol (lausanne) 12:727272. https://doi.org/10.3389/FENDO.2021.727272
Xie K, Liu L, Chen J, Liu F (2019a) Exosomes derived from human umbilical cord blood mesenchymal stem cells improve hepatic ischemia reperfusion injury via delivering miR-1246. Cell Cycle 18:3491. https://doi.org/10.1080/15384101.2019.1689480
Xie K, Liu L, Chen J, Liu F (2019b) Exosomal miR-1246 derived from human umbilical cord blood mesenchymal stem cells attenuates hepatic ischemia reperfusion injury by modulating T helper 17/regulatory T balance. IUBMB Life 71:2020–2030. https://doi.org/10.1002/IUB.2147
Xu R, Simpson RJ, Greening DW (2017) A protocol for isolation and proteomic characterization of distinct extracellular vesicle subtypes by sequential centrifugal ultrafiltration. Methods Mol Biol 1545:91–116. https://doi.org/10.1007/978-1-4939-6728-5_7
Xu R, Bai Y, Min S et al (2020) In vivo monitoring and assessment of exogenous mesenchymal stem cell-derived exosomes in mice with ischemic stroke by molecular imaging. Int J Nanomedicine 15:9011–9023. https://doi.org/10.2147/IJN.S271519
Yan Y, Jiang W, Tan Y et al (2017) hucMSC exosome-derived GPX1 is required for the recovery of hepatic oxidant injury. Mol Ther 25:465–479. https://doi.org/10.1016/J.YMTHE.2016.11.019
Yang J, Liu XX, Fan H et al (2015) Extracellular vesicles derived from bone marrow mesenchymal stem cells protect against experimental colitis via attenuating colon inflammation, oxidative stress and apoptosis. PLoS ONE 10:e0140551. https://doi.org/10.1371/JOURNAL.PONE.0140551
Yang R, Huang H, Cui S et al (2020) IFN-γ promoted exosomes from mesenchymal stem cells to attenuate colitis via miR-125a and miR-125b. Cell Death Dis 11:603. https://doi.org/10.1038/S41419-020-02788-0
Yang S, Liang X, Song J et al (2021) A novel therapeutic approach for inflammatory bowel disease by exosomes derived from human umbilical cord mesenchymal stem cells to repair intestinal barrier via TSG-6. Stem Cell Res Ther 12:315. https://doi.org/10.1186/S13287-021-02404-8
Yang F, Wu Y, Chen Y et al (2023) Human umbilical cord mesenchymal stem cell-derived exosomes ameliorate liver steatosis by promoting fatty acid oxidation and reducing fatty acid synthesis. JHEP Rep. https://doi.org/10.1016/J.JHEPR.2023.100746
Yao J, Zheng J, Cai J et al (2019) Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate rat hepatic ischemia-reperfusion injury by suppressing oxidative stress and neutrophil inflammatory response. FASEB J 33:1695–1710. https://doi.org/10.1096/FJ.201800131RR
Yeo RWY, Lai RC, Zhang B et al (2013) Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev 65:336–341. https://doi.org/10.1016/J.ADDR.2012.07.001
Yin T, Liu Y, Ji W et al (2023) Engineered mesenchymal stem cell-derived extracellular vesicles: a state-of-the-art multifunctional weapon against Alzheimer’s disease. Theranostics 13:1264. https://doi.org/10.7150/THNO.81860
Yoshida T, Ishidome T, Hanayama R (2017) High purity isolation and sensitive quantification of extracellular vesicles using affinity to TIM4. Curr Protoc Cell Biol. https://doi.org/10.1002/CPCB.32
Yousefi-Ahmadipour A, Rashidian A, Mirzaei MR et al (2019) Combination therapy of mesenchymal stromal cells and sulfasalazine attenuates trinitrobenzene sulfonic acid induced colitis in the rat: The S1P pathway. J Cell Physiol 234:11078–11091. https://doi.org/10.1002/JCP.27944
Yu H, Yang X, Xiao X et al (2021) Human adipose mesenchymal stem cell-derived exosomes protect mice from DSS-induced inflammatory bowel disease by promoting intestinal-stem-cell and epithelial regeneration. Aging Dis 12:1423–1437. https://doi.org/10.14336/AD.2021.0601
Zarovni N, Corrado A, Guazzi P et al (2015) Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods 87:46–58. https://doi.org/10.1016/J.YMETH.2015.05.028
Zhang S, Jiang L, Hu H et al (2020) Pretreatment of exosomes derived from hUCMSCs with TNF-α ameliorates acute liver failure by inhibiting the activation of NLRP3 in macrophage. Life Sci 246:117401. https://doi.org/10.1016/J.LFS.2020.117401
Zhang W, Liu R, Chen Y et al (2022) Crosstalk between oxidative stress and exosomes. Oxid Med Cell Longev 2022:3553617. https://doi.org/10.1155/2022/3553617
Zhang Q, Liu S, Li T, et al (2016) Preconditioning of bone marrow mesenchymal stem cells with hydrogen sulfide improves their therapeutic potential. Oncotarget 7:58089-58104. https://doi.org/10.18632/oncotarget.11166
Zhao S, Liu Y, Pu Z (2019) Bone marrow mesenchymal stem cell-derived exosomes attenuate D-GaIN/LPS-induced hepatocyte apoptosis by activating autophagy in vitro. Drug Des Devel Ther 13:2887–2897. https://doi.org/10.2147/DDDT.S220190
Zhao Y, Zhu X, Zhang L et al (2020a) Mesenchymal stem/stromal cells and their extracellular vesicle progeny decrease injury in poststenotic swine kidney through different mechanisms. Stem Cells Dev 29:1190–1200. https://doi.org/10.1089/SCD.2020.0030
Zhao Z, Zhong L, Li P et al (2020b) Cholesterol impairs hepatocyte lysosomal function causing M1 polarization of macrophages via exosomal miR-122-5p. Exp Cell Res 387:111738. https://doi.org/10.1016/J.YEXCR.2019.111738
Zhao J, Li Y, Jia R et al (2021) Mesenchymal stem cells-derived exosomes as dexamethasone delivery vehicles for autoimmune hepatitis therapy. Front Bioeng Biotechnol 9:650376. https://doi.org/10.3389/FBIOE.2021.650376
Zhao S, Huang M, Yan L et al (2022) Exosomes derived from baicalin-pretreated mesenchymal stem cells alleviate hepatocyte ferroptosis after acute liver injury via the Keap1-NRF2 pathway. Oxid Med Cell Longev 2022:8287227. https://doi.org/10.1155/2022/8287227
Zhou CH, Li ML, Qin AL et al (2013) Reduction of fibrosis in dibutyltin dichloride-induced chronic pancreatitis using rat umbilical mesenchymal stem cells from Wharton’s jelly. Pancreas 42:1291–1302. https://doi.org/10.1097/MPA.0B013E318296924E
Zhu X, Badawi M, Pomeroy S et al (2017) Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J Extracell Vesicles 6:1324730. https://doi.org/10.1080/20013078.2017.1324730
Zhu L, Sun HT, Wang S et al (2020a) Isolation and characterization of exosomes for cancer research. J Hematol Oncol 13:152. https://doi.org/10.1186/S13045-020-00987-Y
Zhu M, Liu X, Li W, Wang L (2020b) Exosomes derived from mmu_circ_0000623-modified ADSCs prevent liver fibrosis via activating autophagy. Hum Exp Toxicol 39:1619–1627. https://doi.org/10.1177/0960327120931152
Zhu F, Wei C, Wu H et al (2022) Hypoxic mesenchymal stem cell-derived exosomes alleviate ulcerative colitis injury by limiting intestinal epithelial cells reactive oxygen species accumulation and DNA damage through HIF-1α. Int Immunopharmacol 113:109426. https://doi.org/10.1016/J.INTIMP.2022.109426
Acknowledgements
All the persons contributed to the manuscript were placed as author in the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
MAD wrote the original draft preparation and designed the tables and figures. AAS helped in writing and revising the draft and provided valuable feedback. LAA designed the original idea and developed it in detail, provided valuable feedback and helped in writing and revising the draft. All the authors participated in drafting, writing and editing the manuscript, and approving it for submission.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Didamoony, M.A., Soubh, A.A., Atwa, A.M. et al. Innovative preconditioning strategies for improving the therapeutic efficacy of extracellular vesicles derived from mesenchymal stem cells in gastrointestinal diseases. Inflammopharmacol 31, 2973–2993 (2023). https://doi.org/10.1007/s10787-023-01350-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-023-01350-6