Abstract
Novel therapies are urgently needed to address the rising incidence and prevalence of acute kidney injury (AKI) and chronic kidney disease (CKD). Mesenchymal stem/stromal cells (MSCs) have shown promising results in experimental AKI and CKD, and have been used in the clinic for more than a decade with an excellent safety profile. The regenerative effects of MSCs do not rely on their differentiation and ability to replace damaged tissues, but are primarily mediated by the paracrine release of factors, including extracellular vesicles (EVs), composed of microvesicles and exosomes. MSC-derived EVs contain genetic and protein material that upon transferring to recipient cells can activate several repair mechanisms to ameliorate renal injury. Recent studies have shown that MSC-derived EV therapy improved renal outcomes in several animal models of AKI and CKD, including ischemia-reperfusion injury, drug/toxin-induced nephropathy, renovascular disease, ureteral obstruction, and subtotal nephrectomy. However, data about the renoprotective effects of EV therapy in patients with renal failure are scarce. This review summarizes current knowledge of MSC-derived EV therapy in experimental AKI and CKD, and discusses the challenges that need to be addressed in order to consider MSC-derived EVs as a realistic clinical tool to treat patients with these conditions.
Similar content being viewed by others
Background
Kidney disease is a prominent challenge for health care systems. Incidence and mortality rates of both acute kidney injury (AKI) and chronic kidney disease (CKD) have increased in recent decades [1]. It is estimated that during a hospital admission one in five adults and one in three children experience AKI, a sudden episode of kidney failure or kidney damage [2]. CKD, a condition characterized by a gradual loss of kidney function, is estimated to be quite prevalent. In the US alone, its predicted prevalence rate is 13.6%, with more than 670,000 patients in end-stage renal disease (ESRD) [3, 4], the final stage of CKD when irreversible loss of renal function mandates dialysis or kidney transplantation. Both AKI and CKD consume considerable healthcare resources and are associated with significant economic costs. AKI is responsible for more than 5% of overall hospital expenses [5], and more than $80 billion of the Medicare budget is spent to care for CKD and ESRD patients, accounting for over 18% of its total expenditure [4, 6]. AKI can cause ESRD directly, and increase the risk of developing CKD and worsening of underlying CKD [7]. Importantly, AKI and CKD are risk factors for developing cardiovascular disease and mortality [8]. Therefore, the rising incidence and prevalence of AKI and CKD and their deleterious complications underscore the need to identify more effective therapeutic strategies to attenuate renal injury and prevent its progression to ESRD.
Mesenchymal stem/stromal cells (MSCs) are multipotent cells with robust self-renewal, regenerative, proliferative, and multi-lineage differentiation potential [9]. By definition, MSCs are characterized by the expression of MSC markers and the ability to differentiate into adipocytes, chondrocytes, and osteocytes [10]. Emerging evidence supports the existence of kidney-resident MSCs, which originate from renal pericytes that form an extensive network around the microvasculature [11]. Although the entire spectrum of their function still remains to be elucidated, they play key roles in regulation of renal blood flow, capillary permeability, endothelial survival, and immunologic surveillance [12]. In addition, MSCs with potent proangiogenic and immunomodulatory properties can also be isolated from various extrarenal sources, including adipose tissue, making them ideal candidates for renal regenerative therapy [13, 14].
According to ClinicalTrials.gov there are currently 46 ongoing or completed clinical trials using MSC therapy for AKI and CKD, including diabetic nephropathy, focal segmental glomerulosclerosis, systemic lupus erythematous, and kidney transplantation [15,16,17] (Table 1). In an ongoing phase I clinical trial, patients with cisplatin-induced AKI and solid organ cancer are followed for 1 month after a single systemic infusion of allogeneic bone marrow-derived MSCs (NCT01275612). Primary and secondary end points include the rate of decline in renal function and urinary injury markers, respectively. Cardiac surgery patients at high risk of postoperative AKI were treated safely with allogeneic MSCs [18, 19]. Systemic administration of autologous bone marrow-derived MSCs in patients with autosomal dominant polycystic kidney disease did not cause any serious adverse events and decreased serum creatinine levels after 12 months of follow-up [20]. Preliminary results of a randomized clinical trial in patients with diabetic nephropathy also showed stabilized or improved glomerular filtration rate (GFR) after 3 months of treatment with allogenic MSCs [21]. Likewise, intra-arterial infusion of autologous MSCs in patients with renovascular disease (RVD) increased cortical perfusion and renal blood flow (RBF), and reduced renal tissue hypoxia in the post-stenotic kidney [22]. Clinical trials are also testing the immunomodulatory and renoprotective properties of MSCs after renal transplantation (NCT02409940). Autologous MSCs were found to be superior to conventional immunosuppressive therapy in preventing acute rejection, decreasing opportunistic infections, and preserving renal function in patients undergoing renal transplant [23]. Taken together, these studies indicate that MSC therapy is safe, feasible, well tolerated, and effectively ameliorates renal pathology in a wide range of diseases.
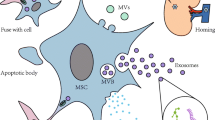
Mounting evidence supports the notion that MSCs exert their reparative effects by releasing extracellular vesicles (EVs), including exosomes with a diameter of 30–120 nm, and micro-vesicles ranging from 100 nm to 1 μm in size [24]. Exosomes arise form endocytic compartments, known as microvesicular bodies, and are released into extracellular space through fusion with plasma membrane [25]. In contrast, microvesicles originate from outward buddings of cell membrane and their release is controlled by calcium influx and cytoskeletal reorganization, among several other factors [25]. We have previously shown that porcine MSCs release EVs (Fig. 1) that are selectively packed with proteins, mRNAs, and microRNAs [26,27,28]. Furthermore, we recently proposed that genes, proteins, and microRNAs enriched in EVs have the potential to modulate selective cellular pathways in recipient cells [29]. Therefore, MSC-derived EVs may exert trophic and reparative effects, representing an attractive non-cellular approach for treating renal disease. Indeed, recent studies have shown that delivery of MSC-derived EVs is safe and can improve kidney function in several models of AKI and CKD. The purpose of this review is to summarize the current knowledge of MSC-derived EV therapy in experimental AKI and CKD, and discusses the challenges that need to be addressed in order to consider MSC-derived EVs as a realistic clinical tool to treat patients with these conditions.
MSC-derived EVs in experimental AKI
Ischemia-reperfusion injury
Renal ischemia-reperfusion injury (IRI), a condition caused by initial sudden cessation of blood flow to the kidney followed by restoration of blood flow and re-oxygenation, is one of the primary causes of AKI associated with significant morbidity and mortality [30]. Although the pathophysiology of renal IRI remains obscure, both hypoxia at ischemic phase and subsequent generation of reactive oxygen species at reperfusion initiate a cascade of deleterious responses characterized by inflammation and cell death that subsequently leads to AKI [31]. A number of studies have recently tested the efficacy of MSC-derived EVs to blunt experimental IRI-induced AKI (Table 2). Lindoso et al. [32] tested the biological effect of EVs in an in vitro model of renal IRI induced by ATP depletion of tubular cells, which were subsequently co-incubated with MSC-derived EVs. EVs progressively incorporated into damaged tubular cells, suggesting higher uptake under stressful conditions. EVs decreased cell death and restored proliferation of ATP-depleted tubular cells. This was paralleled with downregulated expression of a specific set of microRNAs involved in apoptosis, hypoxia, and cytoskeletal reorganization, suggesting that EVs can protect tubular cells against metabolic stress by mechanisms involving post-transcriptional regulation.
The renoprotective effects of MSC-derived EVs have also been investigated in several in vivo models of renal IRI. In rats subjected to unilateral nephrectomy and renal artery occlusion for 45 min, intravenous MSC-derived EVs immediately after ischemia significantly reduced epithelial tubular cell damage and apoptosis and enhanced their proliferation, improving renal function [33]. Interestingly, the beneficial effect of EVs was mediated in part by the transfer of RNA-based information to recipient cells. Similarly, in rats with renal IRI systemic administration of autologous bone marrow MSC-derived EVs decreased renal injury and improved function, extending the benefits of EVs to ameliorate IRI-induced renal damage and contribute to cellular repair in vivo [34].
EVs harvested from human umbilical cord MSCs have also shown renoprotective benefits in rats with IRI. Intravenous delivery of EVs immediately after the ischemic phase of IRI mitigated renal oxidative damage by decreasing the expression of the pro-oxidant NADPH oxidase-2 [35]. MSC-derived EV-induced attenuation of renal oxidative stress was associated with enhanced renal cell proliferation, decreased apoptosis, and normalized serum creatinine levels 2 weeks after the ischemic insult. Consistent with these findings, intravenous injection of EVs isolated from the conditioned medium of human umbilical cord MSCs after unilateral renal ischemia preserved kidney function and decreased serum levels of the AKI marker neutrophil gelatinase-associated lipocalin [36]. EVs also decreased renal expression of nuclear factor E2-related factor-2, a transcription factor that modulates cellular oxidative stress, which in turn resulted in decreased tubular damage.
Studies in experimental renal IRI have also shown that MSC-derived EVs exert renoprotection by modulating renal angiogenesis. Systemic administration of MSC-derived EVs in rats with renal IRI increased renal capillary density and reduced fibrosis by direct transfer of the proangiogenic factor vascular endothelial growth factor (VEGF) and mRNAs involved in this process [37]. In a similar study, delivery of EVs in rats with IRI increased gene and protein expression of the proangiogenic hepatocyte growth factor, associated with decreased tubular fibrosis [38]. Interestingly, the renoprotective effects of EVs were abolished when EVs were pretreated with RNase, implying that mRNA transfer of proangiogenic factors mediated EV-induced renal repair. The proangiogenic effects of EVs were not limited to those isolated from umbilical cord MSCs. EVs isolated from kidney resident MSCs have been shown to contain several proangiogenic genes, including VEGF, basic fibroblast growth factor, and insulin-like growth factor (IGF)-1 [39]. Systemic administration of allogeneic kidney-resident MSC-derived EVs into mice with renal IRI was followed by engraftment in ischemic kidneys and improvement in renal function, suggesting that delivery of proangiogenic transcripts may contribute to EV-induced renal repair.
Furthermore, administration of MSC-derived EVs has been proved to ameliorate the inflammation that follows IRI. Intravenous delivery of EVs following unilateral renal ischemia in rats decreased the number of kidney macrophages and the expression of the macrophage chemo-attractant factor chemokine C-X-C motif ligand-1 (CXCL1), possibly by transferring into recipient cells microRNAs capable of modulating CXCL1 expression [40]. This treatment boosted tubular proliferation, attenuated fibrosis, and preserved kidney function. Likewise, in rats with IRI induced by bilateral renal artery occlusion and reperfusion, treatment with intravenous MSCs or their EV progeny decreased expression of inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-1-β [41]. Combined MSC and MSC-derived EV therapy resulted in an additive effect on amelioration of tubular injury, extending their value to preserve the kidney when delivered in conjunction with MSCs.
MSC-derived EVs may also confer protection against IRI that occurs in kidney donation after circulatory death, preserving renal function prior to kidney transplantation. In a recent study, incubation of donated kidneys with EVs in buffering solution after harvest and prior to transplant decreased ischemic damage by altering the expression of genes encoding enzymes known to improve cell energy metabolism and ion transport [42]. However, it remains to be determined whether the renoprotective effect of MSC-derived EVs is confined to a specific cell type or may prolong graft survival after kidney transplantation. Therefore, these studies suggest that the beneficial effect of MSC-derived EVs in renal IRI is attributable to their antioxidant, immunomodulatory, and proangiogenic properties, and their ability to modulate cell metabolism and several cellular pathways.
Drug-induced nephropathy
Drug-induced nephropathy (DIN) is a common etiology of AKI that accounts for as high as 60% of both community- and hospital-acquired episodes [43]. Non-steroidal anti-inflammatory drugs, antibiotics, angiotensin converting enzyme inhibitors, and contrast agents have been associated with renal cell toxicity, and may compromise renal function by promoting tubulo-interstitial nephritis and altering intra-glomerular hemodynamics [44]. Recently, the efficacy of MSC-derived EVs has been tested in models of DIN (Table 3). Co-incubation of cisplatin-damaged tubular cells with MSC-derived EVs increased cell proliferation, partly by transferring IGF-1 and IGF receptor-1 [45]. These observations were supported by in vivo studies in animal models of DIN, in which delivery of MSC-derived EVs prevented tubular cell death and enhanced proliferation. For example, administration of MSC-derived EVs into the renal capsule of rats with cisplatin-induced AKI attenuated renal injury and dysfunction partly by reducing formation of pro-oxidants and suppressing activation of pro-apoptotic pathways [46]. Likewise, in mice after cisplatin-induced [47] and glycerol-induced AKI [48, 49] single and multiple intravenous administration of MSC-derived EVs ameliorated tubular injury and improved kidney function. Modulation of apoptosis was implicated in EV-induced renoprotection, which was abolished after degradation of EV mRNA content, suggesting that anti-apoptotic genes shuttled by EVs are the final effectors of their biologic actions.
Modulation of renal inflammation is an important mechanism by which MSC-derived EVs protect the kidney from toxic drug injury. In rats with gentamycin-induced AKI, EV delivery preserved renal function by preventing the rise in several pro-inflammatory cytokines, including IL-6 and TNF-α, whereas levels of the anti-inflammatory cytokine IL-10 were restored in EV-treated animals [50]. In line with this observation, in mice with glycerol-induced AKI, EV delivery was associated with downregulation of pro-inflammatory genes [51]. However, these studies did not explore whether renal parenchymal or infiltrating inflammatory cells were direct targets of the immunomodulatory effects of EVs. Interestingly, both studies reported that renoprotective effects of MSC-derived EVs were blunted in mice treated with RNA depleted EVs, suggesting an important role for mRNA and/or microRNA shuttling in mediating EV-induced renal recovery after AKI. In line with this notion, a recent study suggested that the anti-apoptotic and immunomodulatory effects of MSC-derived EVs in DIN-AKI are partly mediated by their ability to transfer genes that activate autophagy [52]. Authors found that administration of MSC-derived EVs in the renal capsule of rats with cisplatin-induced AKI increased renal expression of several autophagy-related genes and improved renal function. Taken together, these results indicate that EVs are capable of modulating several pathways involved in the pathogenesis of DIN, and may serve as a novel therapeutic approach in these patients.
MSC-derived EVs in experimental CKD
Renovascular disease
Renovascular disease (RVD) is an important cause of secondary hypertension and ESRD in the elderly population [53]. RVD frequently coexists with metabolic syndrome (MetS), a constellation of cardiovascular risk factors that accentuates renal injury and is associated with poor renal outcomes [54]. Recently, our group took advantage of a novel porcine model of coexisting MetS and RVD (MetS + RVD) to test whether intrarenal delivery of autologous MSC-derived EVs would ameliorate structural and functional decline in MetS + RVD kidney [55]. MetS was induced by feeding pigs a high fat/high fructose diet for 16 weeks, whereas RVD was achieved by placing an irritant coil in the main renal artery. We found that a single intrarenal administration of MSC-derived EVs in these pigs attenuated renal inflammation, disclosed by decreased renal vein levels of several pro-inflammatory cytokines, including TNF-α, IL-6, and IL-1-β. Contrarily, renal vein levels of IL-10 increased in EV-treated pigs, associated with a shift from pro-inflammatory to reparative macrophages populating the stenotic kidney, underscoring the immunomodulatory potential of EVs. EVs also improved medullary oxygenation and fibrosis, and restored RBF and GFR, yet animals treated with IL-10 knock-down EVs showed limited renal recovery, implying that this cytokine mediates at least part of their protective effects (Table 4).
Unilateral ureteral obstruction
Although complete ureteral obstruction is not a common cause of human renal disease, the unilateral ureteral obstruction (UUO) model, which promotes renal parenchymal inflammation, apoptosis, and fibrosis, offers a unique opportunity to study mechanisms responsible for kidney injury [56]. Lately, studies in mouse models of UUO achieved by unilateral ureteral ligation have tested the efficacy of MSC-derived EVs in preventing renal injury (Table 5). Intravenous administration of MSC-derived EVs mitigated tubular injury and fibrosis and improved renal function 2 weeks after UUO [57]. EVs transferred microRNAs capable of modulating fibrosis and epithelial to mesenchymal transition (EMT). In agreement, in vitro experiments in tubular cells treated with the pro-fibrotic transforming growth factor (TGF)-β1 showed that co-incubation with kidney-resident MSC-derived EVs reversed EMT and TGF-β1-induced morphological changes. This mechanism was also confirmed by another study on TGF-β1-treated endothelial cells, in which MSC-derived EVs ameliorated endothelial to mesenchymal transformation and improved cell proliferation 7 days after UUO [58]. Therefore, these studies underscore important anti-fibrotic and renoprotective properties of MSC-derived EVs in experimental UUO.
Subtotal nephrectomy
The renoprotective effects of MSC-derived EVs were also studied in a mouse model of subtotal nephrectomy (STN; Table 6), one of the most widely used experimental models of CKD which is characterized by progressive loss of renal mass and deteriorating renal function [59]. STN was induced by removing one kidney and resecting 5/6 of upper and lower poles of the remaining kidney. Delivery of EVs into the mouse caudal vein 2 days after STN mitigated lymphocyte infiltration and prevented tubular atrophy and fibrosis within 1 week after treatment [60]. Decreased proteinuria, serum creatinine, blood urea nitrogen (BUN), and uric acid levels underscored the potential of MSC-derived EV delivery in preserving the remaining renal function.
Challenges of MSC-derived EV delivery in human CKD
As discussed above, several studies in animal models of AKI and CKD suggest that MSC-derived EVs can effectively preserve renal structure and function. So far, however, only one clinical trial has tested the renoprotective effects of MSC-derived EVs on the progression of CKD [61]. In this phase II/III pilot study, 40 patients with estimated GFR (eGFR) between 15 and 60 ml/min were randomized to receive either placebo or EVs derived from allogenic cord blood MSCs. Patients were treated with two doses of EVs and followed for 12 months. EV therapy improved eGFR, serum creatinine, and BUN levels, as well as urinary albumin/creatinine ratio. Plasma levels of TNF-α decreased, whereas levels of IL-10 increased in EV-treated patients. Renal biopsy findings 3 months after intervention revealed that EV-treated kidneys showed upregulated expression of cell regeneration and differentiation markers. Importantly, participants did not experience any significant adverse events during or after EV therapy throughout the study period. Therefore, this study suggests that MSC-derived EV therapy is safe and can ameliorate renal inflammation and improve function in patients with CKD. Nevertheless, future long-term follow-up clinical studies need to confirm the persistence of the beneficial effects of this approach in patients with CKD.
Furthermore, significant translational challenges need to be faced before adopting MSC-derived EVs as a useful therapy for AKI and CKD (Table 7). Theoretically, cell-free therapies such as EVs might offer superior advantages over delivery of their parent MSCs in terms of safety. EVs are small particles with no proliferative capacity. Being acellular, EVs should be exempted from adverse effects. Unlike MSCs, EVs can be stored for a long time, allowing their use as “off the shelf” products. Nevertheless, long-term follow-up studies for closely monitoring EVs are needed to determine their safety.
According to recent methodological guidelines [62], several methods could be used to isolate EVs which may impact on EV purity, concentration, morphology, size range, and functional activity [63]. EV handling and storage may also affect their concentration, composition, and function [64]. Therefore, additional studies are needed to test whether renal outcomes vary as a function of EV collection, storage, and isolation methods, and optimize standard protocols for clinical studies.
Few studies have tracked the fate of EVs after systemic in vivo administration, but data from IRI [39] and UUO [58] animal models showed that 24 h after infusion EVs primarily engrafted into the damaged kidney and to a lesser extent in the non-affected kidney [40]. The majority of EVs were taken up by renal tubular epithelial cells (RTECs) and peritubular capillaries [39, 58], but some were identified in glomeruli [33]. In our MetS + RVD model, EV retention was higher in post-stenotic kidney than contralateral kidneys, and EVs engrafted tubular cells and macrophages 4 weeks after administration [55]. This suggests enhanced tissue uptake of EVs under stressful conditions, which may be mediated by infiltrated immune cells or altered expression of surface markers on parenchymal cells. EVs were also observed in the heart, and in large quantities in the lungs, liver, and spleen. Development of kidney-targeted EVs can facilitate their systemic delivery and enhance their regenerative benefits.
The duration and long-term term effects of MSC-derived EVs are important to consider before moving towards their clinical application. In most experimental studies, follow-up ranged from 1 day to 2 weeks post-injection, and only one study in rats with renal IRI found a lower incidence of CKD 6 months after EV therapy [33]. It is clear that EVs can alter transcription profiles in recipient cells, and modulate tissue metabolism and several cellular pathways. Thus, the long-term implications of these post-transcriptional modifications, especially with continuous or repetitive administration of EVs, need to be elucidated. In this respect, their lack of cellular machinery and inability to proliferate in the recipient tissue might limit the duration of their effects and necessitate repeated administration.
There is also uncertainty regarding the optimal dose regimen of MSC-derived EVs, which might influence their capacity to home and engraft damaged cells, and thereby their efficacy for renal repair. Macrophages may promptly target and remove exogenously administered EVs [65], so multiple doses may be needed to achieve and sustain EV-induced renoprotection. A single study found that a multiple dose regimen was superior in decreasing mortality and improving renal function [47]. Administration of larger doses of MSCs was not necessarily associated with better outcomes, and even an inverse dose–response relationship may occur following a high MSC dose [66, 67]. Administration of both low (1 × 105 cells/kg) and high (2.5 × 105 cells/kg) dose of autologous MSCs improved renal blood flow and kidney perfusion to the same magnitude in patients with RVD [22]. However, no study has reported the in vivo efficacy of escalating doses of EVs or determined a threshold dose in experimental renal disease. Therefore, a standard regimen of EV delivery needs to be established in order to test their efficacy in randomized clinical trials. Furthermore, the adequate number of EV injections and the interval between them need to be determined in future studies.
Cardiovascular risk factors may impair the functionality of MSCs and diminish the regenerative benefits of autologous MSC implantation [68]. However, whether EVs isolated from MSCs are also susceptible remains unknown. We have recently found that MetS interferes with the packaging of cargo of porcine adipose tissue MSC-derived EVs, altering the expression of microRNAs that control genes implicated in the development of MetS and its complications [28]. These observations suggest that cardiovascular risk factors may limit the therapeutic efficacy of autologous MSCs and EVs in subjects with coexisting MetS and renal disease. Further preclinical studies and thoughtfully designed and sufficiently powered clinical trials are urgently needed to clarify these uncertainties and overcome the challenges associated with EV therapy in patients with AKI and CKD.
Lastly, emerging evidence suggests that renal cell-derived EVs might also exert tissue protective properties in experimental renal disease. RTECs that line the renal tubules play a crucial role in renal function. Similar to MSCs, RTECs release EVs that serve as intercellular communication messengers and may accelerate renal recovery by eliciting tissue regenerative responses. RTEC-derived EVs similarly contain a rich cargo of mRNAs, microRNAs, and proteins that transmit regenerative signals. TGF-β1-treated RTECs release multiple EVs containing microRNA-21 that enhance PTEN-Akt signaling, which modulates several important biological processes [69]. However, EVs released by injured RTECs also contain TGF-β1 mRNA and microRNAs that activate fibroblasts, and their co-incubation with them promoted collagen production [70]. Speculatively, this function might be related to the injury resolution phase. Unfortunately, none of these studies tested the in vivo protective effects of RTEC-derived EVs.
More recently, intravenous administration of EVs derived from RTECs in rats with renal IRI improved the renal microvasculature and decreased tubular damage and fibrosis [71]. EVs from hypoxia preconditioned RTECs were more effective compared to those obtained from normoxic cells, possibly due to their inhibitory effects on apoptosis following ATP depletion [72]. Fibroblast-derived EVs failed to ameliorate kidney damage in glycerol-induced AKI [48], suggesting that EV-induced renoprotection depends on their cellular source. Therefore, in vitro modifications of RTECs may enhance the protective properties of their daughter EVs. Future studies are needed to confirm these findings and compare the renoprotective potential of MSC- with non-MSC-derived EVs.
Conclusions
AKI and CKD remain global public health challenges, associated with an increased risk for progression to ESRD and cardiovascular complications. Several characteristics of MSCs tested pre-clinically make them attractive to preserve the kidney suffering from AKI and CKD. There are currently several ongoing or completed clinical trials using MSCs for a wide range of renal diseases and preliminary results suggest that MSCs are safe, well tolerated, and effectively ameliorate renal pathology. MSCs exert their reparative effects by releasing EVs, and recent studies in experimental models of AKI and CKD have shown that MSC-derived EVs offer an effective modern treatment option for these patients. MSC-derived EVs contain genetic and protein material that upon transferring to recipient cells can activate several repair mechanisms to ameliorate renal injury (Fig. 2). Furthermore, these particles offer some exciting advantages over MSCs. However, clinical data are limited and several challenges need to be addressed as we move towards clinical translation. To date, the primary uncertainties for MSC-derived EV therapy for renal disease include insufficient scientific data to support their safety, and the need to identify the most appropriate EV cellular source, isolation method, and dose regimen, and to assess the impact of co-morbidities on their cargo and renoprotective effect. Alternatively, RTEC-derived EVs may also contribute to cellular repair in AKI and CKD, but the beneficial effects of this approach in patients with CKD remain unknown. Therefore, further basic and translational studies need to continue exploring the potential therapeutic applications of MSC-derived and renal cell-derived EVs for AKI and CKD.
Mesenchymal stem cell (MSC)-derived extracellular vesicles (EVs) are taken up by renal proximal and distal tubular cells, macrophages, and endothelial cells. MSC-derived EVs transfer their protein, mRNA, and microRNA content into recipient cells. This in turn modulates several pathways involved in the pathophysiology of renal disease, including vascular rarefaction, inflammation, oxidative stress, fibrosis, extracellular matrix remodeling, apoptosis, and cell proliferation. This figure is original for this article
Abbreviations
- AKI:
-
Acute kidney injury
- BUN:
-
Blood urea nitrogen
- CKD:
-
Chronic kidney disease
- CXCL1:
-
C-X-C motif ligand 1
- DIN:
-
Drug-induced nephropathy
- eGFR:
-
estimated glomerular filtration rate
- EMT:
-
Epithelial to mesenchymal transition
- ESRD:
-
End stage renal disease
- EV:
-
Extracellular vesicle
- GFR:
-
Glomerular filtration arte
- IGF:
-
Insulin-like growth factor
- IL:
-
Interleukin
- IRI:
-
Ischemia reperfusion injury
- MetS:
-
Metabolic syndrome
- MSC:
-
Mesenchymal stem cell
- RBF:
-
Renal blood flow
- ROS:
-
Reactive oxygen species
- RTEC:
-
Renal tubular epithelial cell
- RVD:
-
Renovascular disease
- STN:
-
Subtotal nephrectomy
- TGF:
-
Transforming growth factor
- TNF-α:
-
Tumor necrosis factor-alpha
- UUO:
-
unilateral ureteral obstruction
- VEGF:
-
Vascular endothelial growth factor.
References
Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope III CA, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128.
Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, Jaber BL. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8(9):1482–93.
Hsu RK, Powe NR. Recent trends in the prevalence of chronic kidney disease: not the same old song. Curr Opin Nephrol Hypertens. 2017;26(3):187–96.
Saran R, Li Y, Robinson B, Abbott KC, Agodoa LY, Ayanian J, Bragg-Gresham J, Balkrishnan R, Chen JL, Cope E, Eggers PW, Gillen D, Gipson D, Hailpern SM, Hall YN, He K, Herman W, Heung M, Hirth RA, Hutton D, Jacobsen SJ, Kalantar-Zadeh K, Kovesdy CP, Lu Y, Molnar MZ, Morgenstern H, Nallamothu B, Nguyen DV, O'Hare AM, Plattner B, Pisoni R, Port FK, Rao P, Rhee CM, Sakhuja A, Schaubel DE, Selewski DT, Shahinian V, Sim JJ, Song P, Streja E, Kurella Tamura M, Tentori F, White S, Woodside K. US Renal Data System 2015 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2016;67(3 Suppl 1):Svii. S1–305.
Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–70.
Honeycutt AA, Segel JE, Zhuo X, Hoerger TJ, Imai K, Williams D. Medical costs of CKD in the Medicare population. J Am Soc Nephrol. 2013;24(9):1478–83.
Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82(5):516–24.
Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. American Heart Association Councils on Kidney in Cardiovascular Disease HBPRCC, Epidemiology, Prevention. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42(5):1050–65.
Charbord P. Bone marrow mesenchymal stem cells: historical overview and concepts. Hum Gene Ther. 2010;21(9):1045–56.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7.
Bruno S, Chiabotto G, Camussi G. Concise review: different mesenchymal stromal/stem cell populations reside in the adult kidney. Stem Cells Transl Med. 2014;3(12):1451–5.
Kramann R, Humphreys BD. Kidney pericytes: roles in regeneration and fibrosis. Semin Nephrol. 2014;34(4):374–83.
Griffin MD, Ryan AE, Alagesan S, Lohan P, Treacy O, Ritter T. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: what have we learned so far? Immunol Cell Biol. 2013;91(1):40–51.
Hass R, Kasper C, Bohm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12.
Hickson LJ, Eirin A, Lerman LO. Challenges and opportunities for stem cell therapy in patients with chronic kidney disease. Kidney Int. 2016;89(4):767–78.
Peired AJ, Sisti A, Romagnani P. Mesenchymal stem cell-based therapy for kidney disease: a review of clinical evidence. Stem Cells Int. 2016;2016:4798639.
Westenfelder C, Togel FE. Protective actions of administered mesenchymal stem cells in acute kidney injury: relevance to clinical trials. Kidney Int Suppl. 2011;1(3):103–6.
Togel FE, Westenfelder C. Kidney protection and regeneration following acute injury: progress through stem cell therapy. Am J Kidney Dis. 2012;60(6):1012–22.
Kaushal GP, Shah SV. Challenges and advances in the treatment of AKI. J Am Soc Nephrol. 2014;25(5):877–83.
Makhlough A, Shekarchian S, Moghadasali R, Einollahi B, Hosseini SE, Jaroughi N, Bolurieh T, Baharvand H, Aghdami N. Safety and tolerability of autologous bone marrow mesenchymal stromal cells in ADPKD patients. Stem Cell Res Ther. 2017;8(1):116.
Packham DK, Fraser IR, Kerr PG, Segal KR. Allogeneic mesenchymal precursor cells (MPC) in diabetic nephropathy: a randomized, placebo-controlled, dose escalation study. EBioMedicine. 2016;12:263–9.
Saad A, Dietz AB, Herrmann SMS, Hickson LJ, Glockner JF, McKusick MA, Misra S, Bjarnason H, Armstrong AS, Gastineau DA, Lerman LO, Textor SC. Autologous mesenchymal stem cells increase cortical perfusion in renovascular disease. J Am Soc Nephrol. 2017;28(9):2777–85.
Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, Sun X, Chen J, Yang S, Cai J, Gao X, Pileggi A, Ricordi C. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA. 2012;307(11):1169–77.
Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med. 2011;6(4):481–92.
Koniusz S, Andrzejewska A, Muraca M, Srivastava AK, Janowski M, Lukomska B. Extracellular vesicles in physiology, pathology, and therapy of the immune and central nervous system, with focus on extracellular vesicles derived from mesenchymal stem cells as therapeutic tools. Front Cell Neurosci. 2016;10:109.
Eirin A, Riester SM, Zhu XY, Tang H, Evans JM, O'Brien D, van Wijnen AJ, Lerman LO. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene. 2014;551(1):55–64.
Eirin A, Zhu XY, Puranik AS, Woollard JR, Tang H, Dasari S, Lerman A, van Wijnen AJ, Lerman LO. Comparative proteomic analysis of extracellular vesicles isolated from porcine adipose tissue-derived mesenchymal stem/stromal cells. Sci Rep. 2016;6:36120.
Meng Y, Eirin A, Zhu XY, Tang H, Chanana P, Lerman A, Van Wijnen AJ, Lerman LO. The metabolic syndrome alters the miRNA signature of porcine adipose tissue-derived mesenchymal stem cells. Cytometry A. 2017. doi:10.1002/cyto.a.23165.
Nargesi AA, Lerman LO, Eirin A. Mesenchymal stem cell-derived extracellular vesicles for renal repair. Curr Gene Ther. 2017;17(1):29–42.
Srisawat N, Kellum JA. Acute kidney injury: definition, epidemiology, and outcome. Curr Opin Crit Care. 2011;17(6):548–55.
Malek M, Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J Renal Inj Prev. 2015;4(2):20–7.
Lindoso RS, Collino F, Bruno S, Araujo DS, Sant'Anna JF, Tetta C, Provero P, Quesenberry PJ, Vieyra A, Einicker-Lamas M, Camussi G. Extracellular vesicles released from mesenchymal stromal cells modulate miRNA in renal tubular cells and inhibit ATP depletion injury. Stem Cells Dev. 2014;23(15):1809–19.
Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C, Camussi G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant. 2011;26(5):1474–83.
Wang R, Lin M, Li L, Qi G, Rong R, Xu M, Zhu T. Bone marrow mesenchymal stem cell-derived exosome protects kidney against ischemia reperfusion injury in rats. Zhonghua Yi Xue Za Zhi. 2014;94(42):3298–303.
Zhang G, Zou X, Miao S, Chen J, Du T, Zhong L, Ju G, Liu G, Zhu Y. The anti-oxidative role of micro-vesicles derived from human Wharton-Jelly mesenchymal stromal cells through NOX2/gp91(phox) suppression in alleviating renal ischemia-reperfusion injury in rats. PLoS One. 2014;9(3):e92129.
Zhang G, Zou X, Huang Y, Wang F, Miao S, Liu G, Chen M, Zhu Y. Mesenchymal stromal cell-derived extracellular vesicles protect against acute kidney injury through anti-oxidation by enhancing Nrf2/ARE activation in rats. Kidney Blood Press Res. 2016;41(2):119–28.
Zou X, Gu D, Xing X, Cheng Z, Gong D, Zhang G, Zhu Y. Human mesenchymal stromal cell-derived extracellular vesicles alleviate renal ischemic reperfusion injury and enhance angiogenesis in rats. Am J Transl Res. 2016;8(10):4289–99.
Ju GQ, Cheng J, Zhong L, Wu S, Zou XY, Zhang GY, Gu D, Miao S, Zhu YJ, Sun J, Du T. Microvesicles derived from human umbilical cord mesenchymal stem cells facilitate tubular epithelial cell dedifferentiation and growth via hepatocyte growth factor induction. PLoS One. 2015;10(3):e0121534.
Choi HY, Moon SJ, Ratliff BB, Ahn SH, Jung A, Lee M, Lee S, Lim BJ, Kim BS, Plotkin MD, Ha SK, Park HC. Microparticles from kidney-derived mesenchymal stem cells act as carriers of proangiogenic signals and contribute to recovery from acute kidney injury. PLoS One. 2014;9(2):e87853.
Zou X, Zhang G, Cheng Z, Yin D, Du T, Ju G, Miao S, Liu G, Lu M, Zhu Y. Microvesicles derived from human Wharton's Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res Ther. 2014;5(2):40.
Lin KC, Yip HK, Shao PL, Wu SC, Chen KH, Chen YT, Yang CC, Sun CK, Kao GS, Chen SY, Chai HT, Chang CL, Chen CH, Lee MS. Combination of adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes for protecting kidney from acute ischemia-reperfusion injury. Int J Cardiol. 2016;216:173–85.
Gregorini M, Corradetti V, Pattonieri EF, Rocca C, Milanesi S, Peloso A, Canevari S, De Cecco L, Dugo M, Avanzini MA, Mantelli M, Maestri M, Esposito P, Bruno S, Libetta C, Dal Canton A, Rampino T. Perfusion of isolated rat kidney with Mesenchymal Stromal Cells/Extracellular Vesicles prevents ischaemic injury. J Cell Mol Med. 2017. doi:10.1111/jcmm.13249.
Ghane Shahrbaf F, Assadi F. Drug-induced renal disorders. J Renal Inj Prev. 2015;4(3):57–60.
Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930–6.
Tomasoni S, Longaretti L, Rota C, Morigi M, Conti S, Gotti E, Capelli C, Introna M, Remuzzi G, Benigni A. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2013;22(5):772–80.
Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, Zhang B, Wang M, Mao F, Yan Y, Gao S, Gu H, Zhu W, Qian H. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 2013;4(2):34.
Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C, Camussi G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7(3):e33115.
Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Tetta C, Camussi G. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20(5):1053–67.
Bruno S, Tapparo M, Collino F, Chiabotto G, Deregibus MC, Soares Lindoso R, Neri F, Kholia S, Giunti S, Wen S, Quesenberry P, Camussi G. Renal regenerative potential of different extracellular vesicle populations derived from bone marrow mesenchymal stromal cells. Tissue Eng Part A. 2017;23(21-22):1262–73.
Reis LA, Borges FT, Simoes MJ, Borges AA, Sinigaglia-Coimbra R, Schor N. Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PLoS One. 2012;7(9):e44092.
Collino F, Bruno S, Incarnato D, Dettori D, Neri F, Provero P, Pomatto M, Oliviero S, Tetta C, Quesenberry PJ, Camussi G. AKI Recovery induced by mesenchymal stromal cell-derived extracellular vesicles carrying microRNAs. J Am Soc Nephrol. 2015;26(10):2349–60.
Wang B, Jia H, Zhang B, Wang J, Ji C, Zhu X, Yan Y, Yin L, Yu J, Qian H, Xu W. Pre-incubation with hucMSC-exosomes prevents cisplatin-induced nephrotoxicity by activating autophagy. Stem Cell Res Ther. 2017;8(1):75.
Hansen KJ, Edwards MS, Craven TE, Cherr GS, Jackson SA, Appel RG, Burke GL, Dean RH. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg. 2002;36(3):443–51.
Zhang X, Li ZL, Woollard JR, Eirin A, Ebrahimi B, Crane JA, Zhu XY, Pawar AS, Krier JD, Jordan KL, Tang H, Textor SC, Lerman A, Lerman LO. Obesity-metabolic derangement preserves hemodynamics but promotes intrarenal adiposity and macrophage infiltration in swine renovascular disease. Am J Physiol Renal Physiol. 2013;305(3):F265–76.
Eirin A, Zhu XY, Puranik AS, Tang H, McGurren KA, van Wijnen AJ, Lerman A, Lerman LO. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017;92(1):114–24.
Ucero AC, Benito-Martin A, Izquierdo MC, Sanchez-Nino MD, Sanz AB, Ramos AM, Berzal S, Ruiz-Ortega M, Egido J, Ortiz A. Unilateral ureteral obstruction: beyond obstruction. Int Urol Nephrol. 2014;46(4):765–76.
He J, Wang Y, Lu X, Zhu B, Pei X, Wu J, Zhao W. Micro-vesicles derived from bone marrow stem cells protect the kidney both in vivo and in vitro by microRNA-dependent repairing. Nephrology (Carlton). 2015;20(9):591–600.
Choi HY, Lee HG, Kim BS, Ahn SH, Jung A, Lee M, Lee JE, Kim HJ, Ha SK, Park HC. Mesenchymal stem cell-derived microparticles ameliorate peritubular capillary rarefaction via inhibition of endothelial-mesenchymal transition and decrease tubulointerstitial fibrosis in unilateral ureteral obstruction. Stem Cell Res Ther. 2015;6:18.
Santos LS, Chin EW, Ioshii SO, Tambara FR. Surgical reduction of the renal mass in rats: morphologic and functional analysis on the remnant kidney. Acta Cir Bras. 2006;21(4):252–7.
He J, Wang Y, Sun S, Yu M, Wang C, Pei X, Zhu B, Wu J, Zhao W. Bone marrow stem cells-derived microvesicles protect against renal injury in the mouse remnant kidney model. Nephrology (Carlton). 2012;17(5):493–500.
Nassar W, El-Ansary M, Sabry D, Mostafa MA, Fayad T, Kotb E, Temraz M, Saad AN, Essa W, Adel H. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater Res. 2016;20:21.
Coumans FAW, Brisson AR, Buzas EI, Dignat-George F, Drees EEE, El-Andaloussi S, Emanueli C, Gasecka A, Hendrix A, Hill AF, Lacroix R, Lee Y, van Leeuwen TG, Mackman N, Mager I, Nolan JP, van der Pol E, Pegtel DM, Sahoo S, Siljander PRM, Sturk G, de Wever O, Nieuwland R. Methodological guidelines to study extracellular vesicles. Circ Res. 2017;120(10):1632–48.
Yuana Y, Boing AN, Grootemaat AE, van der Pol E, Hau CM, Cizmar P, Buhr E, Sturk A, Nieuwland R. Handling and storage of human body fluids for analysis of extracellular vesicles. J Extracell Vesicles. 2015;4:29260.
Eldh M, Lotvall J, Malmhall C, Ekstrom K. Importance of RNA isolation methods for analysis of exosomal RNA: evaluation of different methods. Mol Immunol. 2012;50(4):278–86.
Imai T, Takahashi Y, Nishikawa M, Kato K, Morishita M, Yamashita T, Matsumoto A, Charoenviriyakul C, Takakura Y. Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. J Extracell Vesicles. 2015;4:26238.
Lee JW, Rocco PR, Pelosi P. Mesenchymal stem cell therapy for acute respiratory distress syndrome: a light at the end of the tunnel? Anesthesiology. 2015;122(2):238–40.
Golpanian S, Schulman IH, Ebert RF, Heldman AW, DiFede DL, Yang PC, Wu JC, Bolli R, Perin EC, Moye L, Simari RD, Wolf A, Hare JM. Review and perspective of cell dosage and routes of administration from preclinical and clinical studies of stem cell therapy for heart disease. Stem Cells Transl Med. 2016;5(2):186–91.
Badimon L, Onate B, Vilahur G. Adipose-derived mesenchymal stem cells and their reparative potential in ischemic heart disease. Rev Esp Cardiol (Engl Ed). 2015;68(7):599–611.
Zhou Y, Xiong M, Fang L, Jiang L, Wen P, Dai C, Zhang CY, Yang J. miR-21-containing microvesicles from injured tubular epithelial cells promote tubular phenotype transition by targeting PTEN protein. Am J Pathol. 2013;183(4):1183–96.
Borges FT, Melo SA, Ozdemir BC, Kato N, Revuelta I, Miller CA. Gattone 2nd VH, LeBleu VS, Kalluri R. TGF-beta1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol. 2013;24(3):385–92.
Dominguez JH, Liu Y, Gao H, Dominguez JM, 2nd, Xie D, Kelly KJ. Renal tubular cell-derived extracellular vesicles accelerate the recovery of established renal ischemia reperfusion injury. J Am Soc Nephrol. 2017. doi:10.1681/ASN.2016121278.
Zhang W, Zhou X, Yao Q, Liu Y, Zhang H, Dong Z. HIF-1-mediated production of exosomes during hypoxia is protective in renal tubular cells. Am J Physiol Renal Physiol. 2017;313(4):F906–13.
Acknowledgements
Not applicable.
Funding
This study was partly supported by NIH grant numbers DK100081, DK104273, HL123160, DK102325, and DK106427, and the Mayo Clinic Foundation: Mary Kathryn and Michael B. Panitch Career Development Award.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
AAN researched data for the article, wrote the article, and provided substantial contributions to discussions of its content. LOL reviewed and/or edited of the manuscript before submission and provided substantial contributions to discussions of its content. AE reviewed and/or edited of the manuscript before submission and provided substantial contributions to discussions of its content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Aghajani Nargesi, A., Lerman, L.O. & Eirin, A. Mesenchymal stem cell-derived extracellular vesicles for kidney repair: current status and looming challenges. Stem Cell Res Ther 8, 273 (2017). https://doi.org/10.1186/s13287-017-0727-7
Published:
DOI: https://doi.org/10.1186/s13287-017-0727-7