Abstract
Natural hybridization provides valuable insights into evolutionary processes, such as speciation and the forces driving or hindering it. Sulawesi tarsiers Tarsius dentatus and T. lariang hybridize within a limited area, suggesting selection against hybrids. Their species- and sex-specific duet songs might serve as a premating barrier in sympatry, especially if differences are strengthened by character displacement. Individuals of mixed origin might face disadvantages if they inherit intermediate song traits. To shed light on the processes shaping this hybrid zone, we analysed 55 duet songs from within and outside the zone. For females and males, we identified temporal and frequency-related parameters that differ between species. We inspected hybrid songs for intermediate characteristics and analysed purebred songs for character displacement in sympatry. Female hybrid songs (N = 2) were intermediate in four to five of six parameters; interpretation of male hybrid songs (N = 2) was inconclusive, because only two parameters were reliably quantifiable. There was no character displacement in female songs in sympatry (N = 11) compared with monospecific areas (N = 17). In male songs, interspecific differences in note rate were significantly larger within the hybrid zone (N = 8) compared with outside (N = 13). Intermediate song traits indicate inheritance and may disadvantage hybrids during mate choice. Character displacement in male songs is consistent with female mate choice, because females should opt for unmistakable signals to avoid costly hybridization. Our findings thus suggest that duet songs of T. lariang and T. dentatus play an important role in limiting this hybrid zone.
Abstrak
Persilangan alami memberikan pemahaman berharga ke dalam proses-proses evolusi seperti spesiasi dan kekuatan pendorong atau penghambatnya. Tarsius Sulawesi Tarsius dentatus dan T. lariang bersilangan dalam area terbatas, menggambarkan adanya seleksi terhadap para silangan. Nyanyian duetnya yang spesifik-jenis kelamin dan -spesies diduga bertindak sebagai penghalang pra-perkawinan dalam simpatri, terutama jika perbedaan diperkuat oleh perpindahan karakter. Individu campuran diduga akan menghadapi kerugian jika mereka mewarisi sifat nyanyian perantara. Untuk menjelaskan proses pembentukan zona persilangan ini, kami menganalisa 55 nyanyian duet dari dalam dan luar zona. Untuk betina dan jantan, kami mengidentifikasi parameter terkait temporal dan frekuensi yang berbeda antar spesies. Kami memeriksa nyanyian silangan untuk karakteristik perantara dan menganalisis nyanyian ras murni untuk perpindahan karakter dalam simpatri. Nyanyian betina silangan (N = 2) merupakan perantara dalam empat hingga lima dari enam parameter; interpretasi nyanyian jantan silangan (N = 2) tidak dapat disimpulkan karena hanya dua parameter yang dapat diukur secara terpercaya. Tidak ada perpindahan karakter pada nyanyian betina dalam simpatri (N = 11) dibandingkan dengan daerah monospesifik (N = 17). Pada nyanyian jantan, perbedaan interspesifik dalam tingkat nada secara signifikan lebih besar di dalam zona silangan (N = 8) dibandingkan dengan di luar (N = 13). Sifat nyanyian perantara menunjukkan pewarisan dan diduga dapat merugikan silangan selama pemilihan pasangan. Perpindahan karakter dalam nyanyian jantan konsisten dengan pilihan pasangan betina, karena betina harus memilih sinyal yang jelas untuk menghindari persilangan yang merugikan. Temuan kami menyarankan bahwa nyanyian duet T. lariang dan T. dentatus memainkan peran penting dalam membatasi zona persilangan ini. *The translated abstract was not copy-edited by Springer Nature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Speciation is based on the evolution of reproductive barriers between populations. When populations come into secondary contact before reproductive isolation is complete, hybridization—interbreeding—is likely to occur (Abbott et al., 2013; Brandler et al., 2021). Hybridization may thus provide insights into the evolution of species, making hybrid zones “natural laboratories for evolutionary studies” (Hewitt, 1988, p. 158). Therefore, hybridization has been in the focus of evolutionary research ever since Charles Darwin realized the significance of “crossing species” in the evolutionary context (Zinner et al., 2011). The consequences of hybridization depend on various factors, such as habitat properties, hybrid fitness, frequency of assortative mating, and backcrossings (Arnold, 1997; Cortés-Ortiz et al., 2019; den Hartog et al., 2007; Stebbins, 1959; Zinner et al., 2011). Hybridization can push, delay, or even prevent speciation (Abbott et al., 2013). If hybrids exhibit adaptive traits leading to fitness higher than or similar to the parent species (e.g., in the periphery of the latter's ecological range) and if backcrossings into the parental populations are common (den Hartog et al., 2007), the hybrid zone is likely to expand. As a result, both parental species will eventually be superseded or will fuse back into a single species (“speciation reversal”; Seehausen, 2006). If hybrids exhibit fitness similar to or even better than the original species but mainly breed assortatively without crossing back, they may establish as a third species (“hybrid speciation”) and coexist with both parent taxa (Abbott et al., 2013; Lamichhaney et al., 2018; Mallet, 2007; Zinner et al., 2011). Most frequently, however, hybridization in animals results in no offspring or offspring with lower fitness than the parent species and thus is selected against (Barton & Hewitt, 1985; Hoskin & Higgie, 2013). The latter case results in a stable, narrow hybrid zone, a so-called “tension zone” (Barton, 2001; Barton & Hewitt, 1985; Buggs, 2007; den Hartog et al., 2007; Merker et al., 2009) where backcrosses with the parental populations cause introgression of foreign genes from one species’ gene pool into the other.
Selection against disadvantageous heterospecific matings reinforces reproductive barriers in the parental species (Hoskin & Higgie, 2013; Howard et al., 1993). These barriers will preferentially take effect before mating, promoting the recognition of conspecific mates (Abbott et al., 2013; Coyne & Orr, 2004; den Hartog et al., 2007; Ortiz-Barrientos et al., 2009; Servedio & Noor, 2003). An outcome of reinforced premating barriers might be displaced reproductive characters—the phenomenon of diverging phenotypes in sympatric populations of closely related species that experience reduced fitness due to reproductive interaction (Brown & Wilson, 1956; Pfennig & Pfennig, 2009; Wilkins et al., 2013). If premating barriers are not invincible, postmating barriers might contribute to drive reproductive isolation. Complications for individuals of mixed ancestry can be intermediate, possibly unattractive signals (El-Shehaby et al., 2011; Rundle & Nosil, 2005; Segura et al., 2011; Xue et al., 2018). As a result, hybrid ⨯ hybrid matings may be more common than backcrosses (Lamichhaney et al., 2018). However, intermediate signals can as well be more attractive to one or both parental species (Coyne et al., 1994; Vander Meer et al., 1985), which would increase the number of backcrossings.
In many vocalizing species, such as birds and nocturnal mammals, acoustic signals are prevailing traits for mate recognition and mate choice and thus can act as premating reproductive barriers. For this reason, acoustic mate choice signals are prone to reproductive character displacement (Braune et al., 2008; den Hartog et al., 2007; Kenyon et al., 2011): If vocal signals are used for species recognition and if hybridization is disadvantageous in terms of fitness, the calls of closely related species might diverge where populations co-occur. If an individual’s vocal repertoire is inherited rather than being learned from its consexual parent or its social neighbourhood, such “strange tunes” of hybrids might be unfavourable in the course of mate choice. While reproductive character displacement in vocal traits has been observed in several animal taxa (insects: Marshall & Cooley, 2000; anurans: Gordon et al., 2017; Micancin & Wiley, 2014; Pfennig & Rice, 2014; birds: Demko et al., 2019; Kirschel et al., 2009; Seddon, 2005; mammals: Campbell et al., 2019), it has not yet been clearly demonstrated in primates. Evidence for ecological or reproductive character displacement occurs in visual signals in guenons (Allen et al., 2014) and ecological character displacement occurs in acoustic signals in tamarins (Sobroza et al., 2021).
One of the most complex and elaborate—and still little understood—forms of vocal communication in animals is duetting, as the necessary coordination is cognitively highly demanding (Nieder & Mooney, 2019). Duets are defined as overlapping, more or less precisely coordinated and stereotype song displays of two, usually mated, individuals—a leader/initiator and a follower/responder (Dahlin & Benedict, 2014; Farabaugh, 1982; Hall, 2004; Logue & Krupp, 2016). Duet songs often are loud and emitted mainly at dawn, thus optimizing long distance transmission (Adret et al., 2018; Seibt & Wickler, 1977); they are mostly sex-specific (Todt & Naguib, 2000). Beside the emitters’ locations, details transferred in a duet can comprise crucial information for mate choice, such as species and individual identity, sex, reproductive status, mate quality, or pair-bond-status (Hall, 2004). Duetting might have more than one function, such as joint resource (e.g., territory) defence and pair bond strengthening or mate guarding, even within a single species (Hall, 2004).
The most common mammal duetters are pair-bonded, territorial primates living in dense vegetation, i.e., tropical forests (Adret et al., 2018; Geissmann, 2002; Müller & Anzenberger, 2002). Duetting is known from various primate taxa, namely gibbons (Geissmann, 2002; Marshall & Sugardjito, 1986), langurs (Tilson & Tenaza, 1976), lemurs (Méndez-Cárdenas & Zimmermann, 2009; Pollock, 1986), titi monkeys (Adret et al., 2018; Caselli et al., 2014; Müller & Anzenberger, 2002) and tarsiers (Burton & Nietsch, 2010; Shekelle et al., 2008, 2017). The latter are a family of small, nocturnal primates inhabiting Southeast Asian archipelagos. Their hotspot of diversification lies on the Indonesian island of Sulawesi. Apart from the special case of Tarsius pumilus, an enigmatic, small-bodied species living on Central (and possibly South) Sulawesi’s mountain tops (Grow & Gursky-Doyen, 2010; Hagemann et al., 2022), ranges of currently known Sulawesi tarsier species do not overlap. Tarsiers communicate intensively via olfactory and vocal signals (Driller et al., 2015; Gursky-Doyen, 2010; MacKinnon & MacKinnon, 1980; Merker & Groves, 2006; Niemitz et al., 1991; Nietsch, 1999; Shekelle & Salim, 2009). Whereas olfactory information is limited in range, vocalizations have the advantage of reaching over larger distances, which might give them more importance for mate attraction. While they are very similar in morphology, ecology, and behaviour, many tarsier species exhibit a distinct and complex vocal repertoire. In particular, the Eastern tarsiers, i.e., those from Sulawesi, are known for their far-reaching and sex- as well as species-specific duet songs (Burton & Nietsch, 2010; Clink et al., 2020; Grow, 2019; Haimoff, 1986; Merker & Groves, 2006; Merker et al., 2010; Niemitz et al., 1991; Nietsch, 1999; Shekelle et al., 2008, 2019). These are uttered by adult and occasionally also subadult family members when they disperse from their sleeping site at dusk and especially before they regather at dawn (Driller et al., 2009; Merker et al., 2004; Nietsch, 1999; Nietsch & Kopp, 1998; Shekelle, 2008). Little is known about the functions of Eastern tarsiers’ duets. Possible purposes include territory declaration and defence, information about individual or pair identity, and pair reunion after dispersed foraging at night (Clink et al., 2020; Méndez-Cárdenas & Zimmermann, 2009). A function that is considered certain is species recognition, which means that duets also provide important information in mate choice where multiple species come into contact (Burton & Nietsch, 2010; Merker et al., 2009; Nietsch, 1999; Nietsch & Kopp, 1998; Shekelle et al., 1997).
While, despite the recognition of its evolutionary significance, our knowledge about primate hybridization is in general fragmentary (Cortés-Ortiz et al., 2019), this particularly applies to tarsiers (Zinner et al., 2011). The first evidence of natural hybridization in tarsiers concerns the two Eastern tarsier species Tarsius lariang and T. dentatus (Merker et al., 2009). Both species are endemic to Sulawesi, where they are parapatrically distributed. They are similar in morphology, ecology, and behaviour; they sleep in small groups (mostly one adult pair plus their offspring), and their mating system can be described as predominantly monogamous with occasional extra-pair matings (Driller et al., 2009; Merker et al., 2004; Tremble et al., 1993; Bohr, unpublished data). T. lariang and T. dentatus diverged ca. 1 Mio. years ago (Driller et al., 2015) and came into secondary contact before their reproductive isolation was completed. Genetic evidence points to a narrow hybrid tension zone (Merker et al., 2009) and thus to selection against hybrids. Duet songs might play an important role in shaping the hybrid zone by acting as a reproductive barrier—before mating (possibly strengthened by character displacement) or after mating in terms of “strange tunes”, i.e., unattractive signals, if songs show intermediate characteristics (Rundle & Nosil, 2005).
We focussed on a narrow contact zone of ca. 1 km width between Tarsius lariang and T. dentatus (Merker et al., 2009) to further elucidate the role of duet songs in tarsier speciation processes. Subsequent to an assessment of the discriminative ability of parameters measured for male and female tarsier songs, we addressed the following questions:
-
1)
Do individuals of mixed ancestry show purebred or intermediate song traits? As vocal learning in nonhuman primates is highly limited, we hypothesize that also tarsier songs are to a large extent inherited. We thus predict intermediate traits for the songs of hybrids compared with those of purebreds. If tarsiers learn their vocal repertoire from their consexual parent, then songs of hybrid individuals should clearly resemble those of purebreds.
-
2)
Is there evidence for vocal character displacement in male or female songs in areas of sympatry compared with allopatric occurrences, i.e., monospecific areas? Because females have higher reproduction costs, they should opt for clearly discernible signals during mate choice. Therefore, we predict character displacement in male but not or less in female songs.
Methods
Study Sites and Sampling
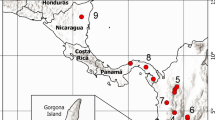
Between 2005 and 2012, we recorded duet songs of Tarsius lariang and T. dentatus at seven different locations in Central Sulawesi, Indonesia (Fig. 1; Tables S1, S2), at several monospecific sites and within a hybrid zone described by Merker et al. (2009). To account for a possible movement of the hybrid zone over time, we extensively monitored its soundscape in 2005/2006 and 2012. Yvonne Bohr consistently spent a full year in the hybrid zone, and Stefan Merker spent 7 months there in total; fieldwork that included song monitoring took place almost every day. We were thus able to observe in each period where tarsier groups were living in audible range of calls of the other species (considered as hybrid zone) or not (considered as monospecific area). Because we monitored each group several times, we were highly familiar with the local soundscape. We only included vocalizations in the study whose assignment to the hybrid zone or to a monospecific area was unambiguous. We recorded all but one duet between 0500–0630 h when tarsiers rejoined at their sleeping sites. One recording took place at 1735–1745 h when the group dispersed. Recording distance ranged between ca. 5 and 30 m.
Tentative distribution of T. lariang (light grey) and T. dentatus (dark grey) on Sulawesi, Indonesia (adapted from Merker et al., 2010) and song recording sites (2005–2012; 1 = Winatu, 2 = Marena, 3 = Make, 4 = Peana, 5 = Pombewe, 6 = Kebun Kopi, 7 = Luwuk; TL = monospecific area T. lariang, TD = monospecific area T. dentatus, * for the sites Winatu and Marena, we differentiated calls as stemming from the hybrid zone or a monospecific area on the basis of smaller-scale criteria, see text). For site-specific sample sizes, see Table S2.
Recording Equipment and Acoustic Analysis
Stefan Merker recorded tarsier songs at locations 1–6 (Fig. 1). Two recordings from Luwuk (location 7) were provided by Christine Driller. Both used a Sony MZ-NH900 Hi-MD Walkman (Sony Corporation, Tokyo, Japan) with a frequency range of 20 Hz – 20 kHz, connected to a hand-held Røde NT3 condenser microphone (RØDE Microphones, Silverwater, NSW, Australia), covering a range of 20 Hz – 20 kHz. After recording, they converted linear PCM files into Waveform Audio File Format (.wav) using Sony’s SonicStage 4 software. Yvonne Bohr recorded tarsier songs at Winatu (location 1; Fig. 1). She employed an Olympus LS-3 Linear PCM recorder (Olympus Corporation, Tokyo, Japan), with a frequency range of 40 Hz – 21 kHz, connected to the handheld, highly directional gun microphone Sennheiser ME 67 (Sennheiser electronic GmbH & Co. KG, Wedemark, Germany), which covers a frequency range of 40 Hz – 20 kHz. Conversion of files was not needed here. We sampled all signals at a rate of 44.1 kHz and digitized them with 16-bit resolution, resulting in a dynamic range of 96 dB. As the dominant frequencies of T. lariang and T. dentatus duets in general range between 3 and 20 kHz (Merker & Groves, 2006; Niemitz et al., 1991; Grow, 2019; see Gursky, 2015 for nonduet ultrasonic vocalizations in Sulawesi tarsiers), our equipment adequately covered the frequency range of the vocalizations examined in this study. We visualized and analyzed the final stereo .wav files in Raven Pro 1.4 and 1.5 (The Cornell Lab of Ornithology, 2011). We only analyzed spectrograms of completely recorded duet songs where all relevant song parameters were clearly discernible, which lowered our sample size considerably. We generated spectrograms in Raven Pro with a fast Fourier transformation (FFT) applying a Hann window shape (sine-squared) with a size of 256 samples, 3 dB bandwidth of 248 Hz, an overlap of 50% and a frequency grid spacing of 172 Hz. The term “duet” refers to the conjoined vocalization of female and male tarsiers. We refer to one individual’s part in a duet as “song” or “duet song”. A “note” is the basic unit of a song and consists of a single, continuous, up- or downwardly modulated trace in a spectrogram. We analyzed 32 songs from female and 23 songs from male tarsiers. All songs stem from different individuals to ensure independence of the samples. Due to pronounced divocalism (Fig. 2; MacKinnon & MacKinnon, 1980; Merker & Groves, 2006; Niemitz et al., 1991; Nietsch, 1999), it was easy to distinguish female and male songs, both audibly and visually.
Spectrograms of T. lariang, T. dentatus, and hybrid songs (edited for publication to improve visual traceability and to eliminate noise), recorded 2005–2012 in Central Sulawesi, Indonesia. Additional spectrograms are shown in Fig. S1. Detailed descriptions of the species’ spectrograms can be found in Niemitz et al. (1991), Nietsch (1999), and Nietsch and Kopp (1998) for T. dentatus and for T. lariang in Merker and Groves (2006).
For female songs, we manually drew selection frames around the emphasized frequency. This energy-richest or dominant frequency of a harmonic series is in general identical to the fundamental or first harmonic, referring to the lowest frequency in a harmonic series (Whitehead, 1995). We then extracted the following measurements: begin time, end time, delta time, lowest frequency, highest frequency, delta frequency. From these measurements, we calculated six parameters applying to the whole duet song or to single notes (Table I). Because we found notes of male songs difficult to visually identify and to frame consistently over a whole song, we restricted framing to the 10 central notes of each male song (the number of notes in male songs ranged between ca. 35 and 240 notes). This limited the number of parameters compared with female songs. We extracted begin time, end time, lowest frequency, and highest frequency of each note, and we then calculated the number of notes per second and the mean frequency height of notes (Table I). In tarsier duet songs, notes of males and females partly overlap. We therefore refrained from an automatic placement of selection frames but framed manually instead. To minimize bias from placing selection frames in Raven Pro by eye (and thereby to reduce bias in the extracted parameters), a single researcher (Yvonne Bohr) checked and, if necessary, adjusted the final placement of frames.
Genetic Assignment Methods
We performed genetic analyses after song recordings were completed. We used eight microsatellite markers (Tl2301, Tl2407, Tl2350, Tl2491, Tl2487, Tl2328, Tl2325, Tl2457; see Tables 1 and 2 in Merker et al., 2012) to characterize a total sample of 60 tarsiers from the Winatu region as purebreds or hybrids (Bohr et al., in prep.). We performed DNA extraction, Whole Genome Amplification (WGA) and Multiplex-PCR as described in Merker et al. (2012). We determined microsatellite allele lengths using a Beckman Coulter capillary sequencer CEQ 2000. With these data, we conducted a population assignment test in Structure v2.3 (100,000 burn-in period, 1,000,000 repetitions, population information not used, ancestry model “admixture”, allele frequency model “correlated”). We considered tarsiers with Structure admixture coefficients (q) as purebred Tarsius lariang if 0 ≤ q ≤ 0.1, as purebred T. dentatus if 0.9 ≤ q ≤ 1.0, and as hybrids if q > 0.1 and < 0.9. Assignments using NEWHYBRIDS 1.1 beta (Anderson & Thompson, 2002) confirmed this threshold. The four hybrids whose songs we examined in this study had the following assignment scores: Hybrid 1, 0.543; Hybrid 2, 0.320; Hybrid 3, 0.774; Hybrid 4, 0.565.
Assignment of Songs
Before acoustic and statistical analyses, we assigned songs to sex, species (or hybrid origin), and area. We could easily distinguish male and female duet songs based on spectrograms. To assign female and male songs to either Tarsius lariang, T. dentatus, or hybrid origin, we used geographic information (calls close to or far from the common species border, i.e., hybridization possible or unlikely) and—in the hybrid zone—genetic data (microsatellite-based assignment scores) and acoustic monitoring findings. We only labelled songs to be of hybrid origin if the emitting animal was unambiguously identified and genotyped as a hybrid. Both female and both male hybrids were adult at the time of recording. We labelled songs that we could not assign to T. lariang, T. dentatus, or hybrid origin as “unassigned”. If not stated otherwise, samples used in statistical analyses comprise assigned (not unassigned) songs. Concerning the area, we assigned recordings as stemming from the hybrid zone if T. lariang and T. dentatus both occurred in that area, i.e., when we had heard duets of both species. We denoted recordings as stemming from a monospecific area if we had heard vocalizations of only one of both species. For more details of the assignment procedure, see Supplement.
Statistical Analyses
We performed all statistical analyses using IBM SPSS Statistics, Version 26.0. If not stated otherwise, tests were two-tailed with α set at 0.05. We report means with standard errors (\(\overline{\mathrm x}\) ± SE). We tested data for normal distribution using Shapiro-Wilk test and by visually examining Q-Q plots. To test homogeneity of variances, we used Levene’s test based on medians and visually inspected scatterplots of standardized predicted values versus standardized residuals (zpred vs. zresid).
We used independent t-tests to test for interspecific differences in song parameters in both sexes. As appropriate for small sample sizes, we report effect size for t-tests as Hedge’s g* (Hedges & Olkin, 1985). For a qualitative interpretation of g*, we use Cohen’s (1992) and Rosenthal’s (1996) classification (0.2 being a small effect, 0.5 being a medium effect, and 0.8 being a large effect).
To identify the most significant song parameters discriminating between Tarsius lariang and T. dentatus females, to classify yet unassigned female songs, and to evaluate female hybrid songs, we applied a linear discriminant function analysis (DFA), embedding the six variables (Table I) in a stepwise manner. We opted for the stepwise method in order to exclude variables that do not enhance the discriminant function’s power. Variables entered the stepwise DFA according to their impact on Wilks’ lambda with “F to enter” = 3.84 and “F to remove” = 2.71. Classification results are based on equal prior probabilities. To examine the classification’s robustness, we applied a leave-one-out cross-validation. For female songs, we used the described assignment as “actual group membership”. We interpreted squared canonical correlation coefficients (Rc2) as effect sizes for DFA. Because we used only two parameters for male tarsiers (Table I), we evaluated male hybrid songs by examination of scatterplots and raw data of male song characteristics.
To assess potential reproductive character displacement in the hybrid zone, we tested whether the difference between purebred T. dentatus and T. lariang songs was larger for individuals in the hybrid zone (HZ) compared with monospecific areas (MSA). We therefore created a new variable (“displacement”), calculated as the absolute difference between an individual’s parameter value and the other species’ mean for this parameter in the respective area. We compared “displacement” between the monospecific areas and the hybrid zone by means of a one-tailed, independent samples t-test where H0: displacement HZ ≤ displacement MSA, and Ha: displacement HZ > displacement MSA.
In addition, to examine the details of intraspecific character displacement, we used Mann-Whitney U tests to compare MSA with HZ for each song parameter. We report r as effect size estimate with 0.1 being a small effect, 0.3 a medium effect, and 0.5 a large effect (Fritz et al., 2012). For correct interpretation of effect sizes, we provide formulas for g* and r in the Supplement.
Ethical Note
This study complied to the legal requirements for foreign researchers in Indonesia and has been approved by the Indonesian Institute of Sciences (LIPI; research permit 4538/SU/KS/2005), the Indonesian State Ministry of Research and Technology (RISTEK; research permits no. 190/FRP/SM/II/2008 and 050/SIP/FRP/SM/II/2012) and by the Ministry of Forestry, Directorate General of Forest Protection and Nature Conservation (PHKA; capture permits no. S.1147/IV-Sek/HO/2005, SI.292/IV.K.26/1/2008 and S.340/IV.K-26/1/2012). In addition, our research followed the IPS Code of Best Practices for Field Primatology; all methods for data acquisition were non-invasive. The authors affirm that they have no conflict of interest with any entities described herein.
Results
Female Songs
In total (monospecific areas and hybrid zone combined), we assigned seven female songs to Tarsius dentatus, 14 songs to T. lariang, and two songs to females of mixed origin (Hybrid 1 and 2). Songs of nine individuals remained “unassigned” (Table S2).
Songs of female T. dentatus (N = 7) and T. lariang (N = 14) differed significantly in all six song parameters examined, with very strong effect sizes (Table II; Fig. S2a-f). T. dentatus females uttered shorter notes at a higher rate; their songs started at a higher frequency, they reached higher frequencies over the entire song, and both their songs and single notes had a larger frequency range (Table II) than in T. lariang females. Because sample size of female hybrids (N = 2) was small for statistical analyses, we provide values only (Table II; Fig. S2a–f). Hybrid 1 lay intermediate (between CIs) for all parameters, except Mean frq. span of notes, where it barely grouped with T. lariang. Hybrid 2 was intermediate for all parameters, except Notes/s and Max. frq. of call, where it grouped with T. dentatus.
DFA (Fig. 3) retained three predictor variables that discriminate best between populations: Max. frq. of 1st note, Mean note length, and Mean frq. span of notes. Goodness of fit statistics show that predictor variables significantly discriminate between groups (Table III). Discriminant function (DF) 1 can be regarded as the “frequency function”, DF2 as the “temporal function”. Effect sizes (Rc2) point towards strong effects, especially for the “frequency function” DF1.
Discriminant scores resulting from stepwise discriminant function analysis, including the parameters Max. frq. of 1st note, Mean note length, and Mean frq. span of notes of female T. dentatus (TD, N = 7), T. lariang (TL, N = 14), and hybrid (H, N = 2) songs (recorded 2005–2012 in Central Sulawesi, Indonesia). Group centroids are group means of DF1 and DF2 for TD, TL, and H. Unassigned cases (N = 9) are not considered in the calculation of group centroids.
Based on original cases, DFA classified all individuals correctly. The nine unassigned individuals were classified as follows: two as Tarsius dentatus; five as T. lariang; and two as hybrids (Table IV; Fig. S3a–f). Cross-validation classified 96% of all cases correctly. It assigned all T. dentatus and all hybrids to the right group, but classified one T. lariang as a hybrid (Table IV).
Character Displacement in Female Songs
We recorded 17 female songs in monospecific areas. Of these, we assigned five to Tarsius dentatus and 12 to T. lariang. In the hybrid zone, we originally assigned only two female songs to T. dentatus and two songs to T. lariang. In order to increase sample size for the hybrid zone, we included the initially unassigned recordings according to their DFA classification. This resulted in 11 songs for the hybrid zone, including four T. dentatus and seven T. lariang songs. The one-tailed t-test to compare “displacement” between the monospecific areas and the hybrid zone did not reveal reproductive character displacement in any of the six parameters (i.e., “displacement” was not larger in the hybrid zone; Table V; Fig. S5a–f).
Male Songs
We assigned (monospecific areas and the hybrid zone combined) 13 male songs to Tarsius dentatus, eight songs to T. lariang, and two songs to males of mixed origin (Hybrid 3 and 4); see also Table S2.
Male duet songs differed significantly between species: Songs of T. lariang males were faster and lower in frequency than songs of T. dentatus males (Fig. 4; Table VI; Fig. S6a-b). The effect of species identity was very strong for both parameters, but it was larger for frequency height than for note rate. As sample size for male hybrids was too small (N = 2) for statistical analyses, we give only descriptive values. While Hybrid 4 was intermediate in frequency (lying between CIs), Hybrid 3 grouped with T. lariang. For note rate, Hybrid 4 grouped with T. lariang, while Hybrid 3 was intermediate (Fig. 4; Table VI; Fig. S6a-b).
Character Displacement in Male Songs
Testing for differences in the newly created variable “displacement” between male songs from the hybrid zone (N = 8; thereof NTD = 4, NTL = 4) and monospecific areas (N = 13; thereof NTD = 9, NTL = 4) using one-tailed t-tests revealed character displacement in the parameter Notes/s (Fig. 5a): “displacement” was larger in the hybrid zone (\(\overline{\mathrm x}\) = 1.70 ± 0.17), than in the monospecific area (\(\overline{\mathrm x}\) = 0.90 ± 0.13). This difference was significant (t = 3.78, df = 19, P < 0.001, one-tailed) and represented a very large effect (g* = 1.63). There was no significant difference between the hybrid zone (\(\overline{\mathrm x}\) = 5.17 ± 0.28 kHz) and the monospecific area (\(\overline{\mathrm x}\) = 4.68 ± 0.31 kHz) in Mean frq. height of notes. (t = − 1.10, df = 19, P = 0.14 one-tailed; Fig. 5b). Effect size was g* = 0.14, indicating a small effect.
a-b. The variable “displacement” (the absolute difference between an individual’s parameter value and the other species’ mean for this parameter in the area) in a) note rate (Notes/s), and b) Mean frq. height of notes of male T. lariang and T. dentatus songs recorded 2005–2012 in a hybrid zone (N = 8) and in monospecific areas (N = 13) in Central Sulawesi, Indonesia.
Most of the shift in Notes/s can be attributed to Tarsius lariang (Fig. 6). In songs of male T. dentatus, a Mann-Whitney U test showed no significant difference in Notes/s between monospecific areas (N = 9, Mrank = 7.33) and the hybrid zone (N = 4, Mrank = 6.25), U = 15.00, Z = − 0.46, P = 0.71 (using the exact sampling distribution of U, Dinneen & Blakesley, 1973) and a small effect of r = 0.13. For T. lariang, although there was no statistically significant difference between monospecific areas (N = 4, Mrank = 3.00) and the hybrid zone (N = 4, Mrank = 6.00), U = 2.00, Z = − 1.73, P = 0.114, there was a large effect of r = 0.61.
Discussion
In this study, we determined specific vocal traits for males and females that distinguish the duet songs of Tarsius lariang and T. dentatus. We also provide an initial assessment of how songs of tarsier hybrids relate to songs of parental species. Songs of female hybrids were intermediate in most parameters. A clear interpretation of male hybrid songs was not possible. While Hybrid 4 was intermediate in frequency (lying between confidence intervals), Hybrid 3 grouped with T. lariang. For note rate, Hybrid 4 grouped with T. lariang, while Hybrid 3 was intermediate. Furthermore, we provide evidence for asymmetric reproductive character displacement in a hybrid zone of nonhuman primates: T. lariang males seem to sing faster in the mixed zone compared to outside, whereas the song characteristics of females and T. dentatus males are not reinforced in the hybrid zone.
Our analyses of female and male duet songs illustrate the discriminative power of selected temporal and frequency related song traits. They further corroborate the significance of duet songs in discriminating tarsier species. The notion that duet songs of Sulawesi tarsiers are species-specific has long been held (MacKinnon & MacKinnon, 1980; Niemitz, 1984). Commonly, identification of new tarsier species begins with the detection of unfamiliar vocalizations in the field and their comparison with spectrograms of known species. Morphological and anatomical studies follow (Merker & Groves, 2006; Shekelle et al., 2008, 2017, 2019), eventually combined with or followed by genetic analyses (Driller et al., 2015; Merker et al., 2010; Shekelle et al., 2010). As was already known from the descriptions of Tarsius dentatus (formerly T. dianae, Niemitz et al., 1991) and T. lariang (Merker & Groves, 2006) and as visual evaluation of spectrograms suggest, our analyses of single parameters prove songs of female T. dentatus and T. lariang to be highly distinct. There are striking differences in temporal as well as frequency-related traits. Discrimination of male songs, both in the field and visually, is less obvious. Our study provides two easily measurable song parameters for male T. dentatus and T. lariang—one temporal and one frequency-related trait—to discern male songs clearly.
T. dentatus females and males sing at higher frequencies compared with T. lariang. The use of different frequency levels might relate to different habitat preferences of species, as vocalizations at lower frequencies reach farther in dense vegetation than higher frequencies (Brown & Waser, 2017; Brown et al., 1995; Masali et al., 1992; Peters & Peters, 2010). So far, however, there is no evidence of striking differences in natural habitats used by T. lariang and T. dentatus (Driller et al., 2009; Yvonne Bohr and Stefan Merker, personal observation). Vocalization frequency might be also related to body size or body mass of the different taxa; in mammals, body size often is negatively correlated with frequency (Bowling et al., 2017; Martin et al., 2017; Peters & Peters, 2010). Correspondingly, species with a smaller head size are better at locating signals of higher frequencies (Heffner, 2004; Masterton et al., 1969; Ramsier & Rauschecker, 2017). Although T. lariang apparently has a slightly larger mean skull length compared with T. dentatus (Merker & Groves, 2006), there were no significant differences in head length, nor in head-body-size in our sampling of both species in Winatu (Yvonne Bohr, unpublished data). While T. dentatus males had a higher body mass than T. lariang males, females did not differ. Therefore, the correlation effect of body mass and frequency does not seem to be applicable here. The effect of body size on the fundamental frequency is based on the correlation between body size and larynx size: a larger body holds a longer larynx, which produces sounds of lower fundamental frequency (Garcia et al., 2017). However, the size of the larynx can be decoupled from body size (Garcia et al., 2017). Therefore, a shorter larynx of T. dentatus compared with that of T. lariang might explain the higher frequencies in former species’ songs. The true rationale behind the use of different frequencies in T. dentatus and T. lariang remains unknown for now. The clear interspecific differences in the vocal output of females and males (Fig. 2; Burton & Nietsch, 2010), however, might facilitate the tarsiers’ mate choice or avoid interspecific conflicts in areas of contact, such as in the studied hybrid zone. Playback experiments could further unveil the role of tarsier songs in these and other contexts.
Genetic analyses done after the song recordings showed that we had good quality records from only two female and two male hybrids. This sample size is too low for sound statistical analyses. Therefore, the performed statistical analysis (DFA) and our interpretation of hybrid songs have to be considered preliminary. While we tentatively labelled one of the genetically identified female hybrids (Hybrid 1) as T. lariang during monitoring in the field, the other female hybrid (Hybrid 2) had already attracted attention during monitoring, because her song appeared to start like T. dentatus and to end like T. lariang. Likewise, we already noted both previously “unassigned” songs of non-genotyped females that DFA later characterized as hybrid songs to be peculiar in the field. Acoustic and visual inspection of spectrograms as well as statistical analyses point towards a song structure of female hybrid calls that differs from their parental species. A comparison of their parameter means with confidence intervals of T. lariang and T. dentatus and the DFA point towards an intermediate vocal pattern. While songs of male hybrids partly showed intermediate values with respect to the parent species, the two parameters analyzed are not sufficient to characterize male hybrids’ songs clearly. Especially because male tarsier notes are in general more similar between species than are female notes.
There are several examples of intermediate song traits in avian hybrids (de Kort et al., 2002; Moore & Coulson, 2020; Shipilina et al., 2017). For mammals, intermediate vocal traits have been shown for mice (Hahn et al., 1998), fur seals (Page et al., 2001), deer (Long et al., 1998), and primates. In nonhuman primates, intermediate song traits are so far known only from gibbons (Brockelman & Schilling, 1984; Geissmann, 1984; Maples & Haraway, 1982; Marler & Tenaza, 1977; Tenaza, 1985) and howler monkeys (Kitchen et al., 2019). As in ours, the sample sizes in those studies were low, each including one or two hybrid individuals. A female Hylobates muelleri (father) ⨯ H. agilis (mother) hybrid’s song resembled the females’ song of her father’s species, which she had never heard before (Maples & Haraway, 1982). While some song traits of a male H. pileatus (father) ⨯ H. lar (mother) hybrid were very similar to purebred songs of either parental species, others were intermediate or even showed new structures. In the female hybrid’s song, some traits equaled those of her father’s species, but others were intermediate (Geissmann, 1984). The song of a female H. lar (father) ⨯ H. muelleri (mother) hybrid differed from both parental songs, whereas the male hybrid’s song was lar-like except for one trait that resembled H. muelleri (Tenaza, 1985). A genetically intermediate male hybrid of Alouatta palliata and A. pigra vocalized like A. palliata in temporal traits, while frequency-related song characteristics were intermediate (Kitchen et al., 2019). For tarsiers, our results show a similar “mixed” pattern: the two female hybrids were, compared with confidence intervals of purebred T. dentatus and T. lariang, intermediate for the majority of the parameters. For the non-intermediate song characters, there was individual variation in the similarity to purebreds. One male hybrid was intermediate in the temporal parameter and grouped with T. lariang concerning the frequency trait. The other male hybrid was intermediate in the frequency parameter and grouped with T. lariang concerning the temporal trait.
Intermediate song traits indicate genetic inheritance, rather than learned vocal patterns (Page et al., 2001). Across the animal kingdom, there is far less evidence for learned vocalizations, i.e., by imitation of other individuals, compared with genetically determined sound, even in birds (Boves et al., 2010; Janik & Slater, 1997; Nieder & Mooney, 2019; Päckert, 2018; ten Cate, 2021; Woolley & Sakata, 2019). In mammals, the clearest evidence of vocal production learning, i.e., “the ability to modify the structure of vocalizations as a result of hearing those of others” (Janik & Knörnschild, 2021), which includes modifications of frequency properties (Boughman & Moss, 2003; Janik & Slater, 1997), derives from cetaceans (Janik, 2014), pinnipeds (Reichmuth & Casey, 2014), bats (Knörnschild, 2014; Vernes & Wilkinson, 2020), elephants (Stoeger & Manger, 2014), and the human genus (Janik & Slater, 1997; ten Cate, 2021; Tyack, 2020). In nonhuman primates, this ability is debated and seems to be, at best, very limited (Fischer & Hammerschmidt, 2019; Janik & Knörnschild, 2021; Lameira et al., 2013). Several researchers concluded that vocalizations of nonhuman primates are to a large extent innate and have a highly conserved structure within species (Cheney & Seyfarth, 2018; Fischer & Hammerschmidt, 2019; Fischer & Price, 2017; Janik & Knörnschild, 2021; Jürgens, 2002; Owren et al., 1992). Neurobiological studies suggest that nonhuman primates are not able to produce new sounds (in terms of voiced calls) as they lack a direct connection between the primary motor cortex and the laryngeal motoneurons (Cheney & Seyfarth, 2018; Jürgens, 2009). Our analysis gives a first glance at vocalization patterns in hybrid tarsiers and points towards intermediate song traits. This finding suggests that tarsier duet songs are largely innate and that tarsier hybrids may not be capable of learning purebred structured vocalizations from their consexual parent. This is in accordance with current knowledge on vocal learning in nonhuman primates and on the acquisition of their vocal repertoire.
In the hybrid zone of T. lariang and T. dentatus, purebreds may perceive intermediate songs, or more precisely songs with intermediate characteristics, as “strange tunes”. Not being perceived as attractive mates will result in fitness disadvantages for hybrids (“extrinsic behavioral hybrid dysfunction”; Irwin, 2020; Servedio & Noor, 2003). In various taxa, purebred females respond less to hybrid vocalizations compared with those of conspecific males (e.g., anurans: Doherty & Gerhardt, 1984; Höbel & Gerhardt, 2003; birds: Derégnaucourt & Guyomarc'h, 2003). Nevertheless, responses to mixed individuals’ signals can still be stronger than to heterospecific individuals due to higher similarity or even single “sexy traits” in the intermediate vocalization (Wyman et al., 2016; e.g., anurans: Gerhardt, 1974; Höbel & Gerhardt, 2003; birds: Collins & Goldsmith, 1998; Derégnaucourt & Guyomarc'h, 2003). The resulting reduced discrimination against hybrids enables backcrossings and can lead to intensive introgression (Randler, 2002; Wyman et al., 2016). In the case studied here, the limited width of the hybrid zone between T. lariang and T. dentatus (Merker et al., 2009) suggests selection and thus discrimination against hybrids. It remains unclear whether the intermediate/mixed character of hybrid songs goes along with an increased attractiveness to parental species as compared to heterospecific songs, possibly promoting backcrossing.
A larger number of good quality recordings from mixed tarsier individuals, at best in combination with detailed genetic information, would provide more insights into how and to what extent hybrid vocalizations differ from purebreds. As species have, over time, acquired characteristic vocal repertoires and mate recognition systems, purebred song traits are well-predictable over time and space. In contrast, the multitude of ways hybridization can take provides for a great potential of variability in hybrid song traits. An increased sample size of hybrid songs is therefore important. We also encourage future studies to investigate how hybrid tarsiers perform with respect to other characteristics of their duetting performance, such as timing or coordination of song length (Clink et al., 2020). Playback experiments to explore perception of hybrid, con- and heterospecific signals of the same or the opposite sex by purebred and mixed individuals could shed more light on premating reproductive isolation between T. lariang and T. dentatus in the common hybrid zone.
The accentuated interspecific contrast in male song characteristics in the hybrid zone, compared with monospecific sites, is consistent with a key role of tarsier song in mate recognition. Post-mating barriers to gene flow, e.g., reduced fitness of hybrids, can lead to selection of premating barriers, like the reinforcement of mate choice relevant signals. Our finding of character displacement in males but not in females might be interpreted as a result of female mate choice; due to their higher investment in reproduction (Gursky, 2002), female Sulawesi tarsiers should opt for high quality males, i.e., for good genes. If mating with hybrids is disadvantageous, purebred females should choose males with unmistakable signals. Their preference for vocalizations that can be clearly assigned to a conspecific male would then drive character displacement. For males, in contrast, the large interspecific differences in evolved female songs may simply make further differentiation unnecessary. In addition, males generally encounter fewer disadvantages from unfavorable matings. In view of observed extra-pair offspring (Driller et al., 2009; Yvonne Bohr, unpublished data), this might hold true for T. lariang and T. dentatus. In addition, contrary to the predictions of reproductive character displacement, female songs seem to be more similar within the hybrid zone than in monospecific areas. This may be due to a lack of need for reinforcement, on the one hand, and introgression in the course of hybridization, on the other (Merker et al., 2009; Moran & Fuller, 2018).
Interestingly, T. lariang males seem to be responsible for the notable shift in Notes/s (Fig. 6), which is underlined by the effect size estimate of r = 0.61 compared with 0.13 in T. dentatus. T. lariang males in general “sing faster” than T. dentatus males, i.e., they show higher values for Notes/s. In the hybrid zone, they seem to sing even faster compared to monospecific areas, whereas T. dentatus males show no difference. This might indicate that T. lariang females have a greater interest in avoiding hybridization than T. dentatus females and choose conspecific males that differ most clearly from T. dentatus. The rationale behind this asymmetric pattern might include differences in selection pressure against hybridization between the two species, possibly because of “unequal hybridization costs, biases in likelihood of hybridization, asymmetrical effects of reproductive interference, evolutionary constraints, or historical accidents” (Cooley, 2007). As Smadja and Ganem (2005) reason for house mice, the potential competitive asymmetry in favour of the probably more opportunistic T. dentatus compared with T. lariang (Merker et al., 2009) might promote migration of T. dentatus into T. lariang range, which would favour stronger selectivity in female T. lariang. In view of previous observations (sampling period 2001–2006) that T. dentatus males successfully mate (or have mated) with T. lariang females but no indication that T. lariang males reproduce with T. dentatus females (Merker et al., 2009), our findings of stronger (but probably not yet sufficient) discrimination of T. lariang females against T. dentatus males may thus be interpreted as a reaction to the invasion of the T. lariang range first and foremost by T. dentatus males (and only subsequently by T. dentatus females). This tentative movement of the hybrid zone further into T. lariang range might explain the asymmetric pattern of mitochondrial introgression between the two species (Merker et al., 2009). Further genetic analyses and playback experiments may shed more light on this matter.
The observed displacement in male song traits in the hybrid zone concerned the temporal but not the frequency parameter. This matches the prediction that temporal traits can be easier modified than frequency-related traits (Boughman & Moss, 2003). The former can be modified by behavioral changes and are thus under control of the singing individual, whereas the latter require control of the vocal apparatus, which is restricted in nonhuman primates (Janik & Slater, 1997, 2000; ten Cate, 2021).
The results of this study are in accordance with previous observations that tarsier vocalizations play an important role in mate recognition (Burton & Nietsch, 2010; Merker et al., 2009; Nietsch, 1999; Shekelle et al., 1997). In summary, we identified temporal and frequency-related parameters to discriminate between duet songs of Tarsius lariang and T. dentatus and likely between other Sulawesi tarsier species as well. We report intermediate patterns of hybrid tarsier vocalizations in terms of quantitative acoustic characters and provide evidence of asymmetric reproductive character displacement in male songs. These findings suggest that duets songs play a key role in directing hybridization between T. lariang and T. dentatus.
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Abbott, R., Albach, D., Ansell, S., Arntzen, J. W., Baird, S. J., Bierne, N., Boughman, J., Brelsford, A., Buerkle, C. A., Buggs, R., Butlin, R. K., Dieckmann, U., Eroukhmanoff, F., Grill, A., Cahan, S. H., Hermansen, J. S., Hewitt, G., Hudson, A. G., Jiggins, C., … Zinner, D. (2013). Hybridization and speciation. Journal of Evolutionary Biology, 26(2), 229–246. https://doi.org/10.1111/j.1420-9101.2012.02599.x
Adret, P., Dingess, K. A., Caselli, C. B., Vermeer, J., Martínez, J. M., Luna Amancio, J. C., van Kuijk, S. M., Hernani Lineros, L. M., Wallace, R. B., Fernandez-Duque, E., & Di Fiore, A. (2018). Duetting patterns of titi monkeys (Primates, Pitheciidae: Callicebinae) and relationships with phylogeny. Animals, 8(10). https://doi.org/10.3390/ani8100178.
Allen, W. L., Stevens, M., & Higham, J. P. (2014). Character displacement of Cercopithecini primate visual signals. Nature Communications, 5, 4266. https://doi.org/10.1038/ncomms5266
Anderson, E. C., & Thompson, E. A. (2002). A model-based method for identifying species hybrids using multilocus genetic data. Genetics, 160, 1217–1229. https://doi.org/10.1093/genetics/160.3.1217
Arnold, M. L. (1997). Natural Hybridization and Evolution. Oxford University Press.
Barton, N. H. (2001). The role of hybridization in evolution. Molecular Ecology, 10(3), 551–568. https://doi.org/10.1046/j.1365-294x.2001.01216.x
Barton, N. H., & Hewitt, G. M. (1985). Analysis of hybrid zones. Annual Review of Ecology and Systematics, 16, 113–148. https://doi.org/10.1146/annurev.es.16.110185.000553
Boughman, J. W., & Moss, C. F. (2003). Social sounds: Vocal learning and development of mammal and bird calls. In A. M. Simmons, A. N. Popper, & R. R. Fay (Eds.), Acoustic communication (pp. 138–224). Springer. https://doi.org/10.1007/0-387-22762-8_4.
Boves, T. J., Buehler, D. A., & Massey, P. C. (2010). Interspecific song imitation by a Cerulean Warbler. Wilson Journal of Ornithology, 122(3), 583–587. https://doi.org/10.1676/09-157.1
Bowling, D. L., Garcia, M., Dunn, J. C., Ruprecht, R., Stewart, A., Frommolt, K.-H., & Fitch, W. T. (2017). Body size and vocalization in primates and carnivores. Scientific Reports, 7, 41070. https://doi.org/10.1038/srep41070
Brandler, O. V., Kapustina, S. Y., Nikol’skii, A. A., Kolesnikov, V. V., Badmaev, B. B., & Adiya, Y. (2021). A study of hybridization between Marmota baibacina and M. Sibirica in their secondary contact zone in Mongolian Altai. Frontiers in Ecology and Evolution, 9, 1–16, 555341. https://doi.org/10.3389/fevo.2021.555341.
Braune, P., Schmidt, S., & Zimmermann, E. (2008). Acoustic divergence in the communication of cryptic species of nocturnal primates (Microcebus ssp.). BMC Biology, 6(19), 1–10. https://doi.org/10.1186/1741-7007-6-19.
Brockelman, W. Y., & Schilling, D. (1984). Inheritance of stereotyped gibbon calls. Nature, 312(5995), 634–636. https://doi.org/10.1038/312634a0
Brown, C. H., Gomez, R., & Waser, P. M. (1995). Old-world monkey vocalizations: Adaptation to the local habitat? Animal Behaviour, 50(4), 945–961. https://doi.org/10.1016/0003-3472(95)80096-4
Brown, C. H., & Waser, P. M. (2017). Primate habitat acoustics. In R. Quam, M. Ramsier, R. R. Fay, & A. N. Popper (Eds.), Primate hearing and communication (pp. 231). Springer. https://doi.org/10.1007/978-3-319-59478-1.
Brown, W. L., & Wilson, E. O. (1956). Character Displacement. Systematic Zoology, 5(2), 49–64. https://doi.org/10.2307/2411924
Buggs, R. J. A. (2007). Empirical study of hybrid zone movement. Heredity, 99(3), 301–312. https://doi.org/10.1038/sj.hdy.6800997
Burton, J. A., & Nietsch, A. (2010). Geographical variation in duet songs of sulawesi tarsiers: Evidence for new cryptic species in South and Southeast Sulawesi. International Journal of Primatology, 31(6), 1123–1146. https://doi.org/10.1007/s10764-010-9449-8
Campbell, P., Arévalo, L., Martin, H., Chen, C., Sun, S., Rowe, A. H., Webster, M. S., Searle, J. B., & Pasch, B. (2019). Vocal divergence is concordant with genomic evidence for strong reproductive isolation in grasshopper mice (Onychomys). Ecology and Evolution, 9(22), 12886–12896. https://doi.org/10.1002/ece3.5770
Caselli, C. B., Mennill, D. J., Bicca-Marques, J. C., & Setz, E. Z. F. (2014). Vocal behavior of black-fronted titi monkeys (Callicebus nigrifrons): Acoustic properties and behavioral contexts of loud calls. American Journal of Primatology, 76(8), 788–800. https://doi.org/10.1002/ajp.22270
Cheney, D. L., & Seyfarth, R. M. (2018). Flexible usage and social function in primate vocalizations. Proceedings of the National Academy of Sciences of the United States of America, 115(9), 1974–1979. https://doi.org/10.1073/pnas.1717572115
Clink, D. J., Tasirin, J. S., & Klinck, H. (2020). Vocal individuality and rhythm in male and female duet contributions of a nonhuman primate. Current Zoology, 66(2), 173–186. https://doi.org/10.1093/cz/zoz035
Cohen, J. (1992). A power primer. Psychological Bulletin, 112(1), 155–159. https://doi.org/10.1037/0033-2909.112.1.155
Collins, S. A., & Goldsmith, A. R. (1998). Individual and species differences in quail calls (Coturnix c. japonica, c. c. coturnix and a hybrid). Ethology, 104(12), 977–990. https://doi.org/10.1111/j.1439-0310.1998.tb00047.x.
Cooley, J. R. (2007). Decoding asymmetries in reproductive character displacement. Proceedings of the Academy of Natural Sciences of Philadelphia, 156(1), 89–96, 88. https://doi.org/10.1635/0097-3157(2007)156[89:DAIRCD]2.0.CO;2.
Cortés-Ortiz, L., Roos, C., & Zinner, D. (2019). Introduction to Special Issue on Primate Hybridization and Hybrid Zones. International Journal of Primatology, 40(1), 1–8. https://doi.org/10.1007/s10764-019-00076-z.
Coyne, J. A., Crittenden, A. P., & Mah, K. (1994). Genetics of a pheromonal difference contributing to reproductive isolation in Drosophila. Science, 265(5177), 1461–1464. https://doi.org/10.1126/science.8073292
Coyne, J. A., & Orr, H. A. (2004). Speciation. Sinauer.
Dahlin, C. R., & Benedict, L. (2014). Angry birds need not apply: A perspective on the flexible form and multifunctionality of avian vocal duets. Ethology, 120(1), 1–10. https://doi.org/10.1111/eth.12182
de Kort, S., den Hartog, P., & ten Cate, C. (2002). Vocal signals, isolation and hybridization in the vinaceous dove (Streptopelia vinacea) and the ring-necked dove (S. capicola). Behavioral Ecology and Sociobiology, 51(4), 378–385. https://doi.org/10.1007/s00265-001-0449-8.
Demko, A. D., Sosa-López, J. R., & Mennill, D. J. (2019). Subspecies discrimination on the basis of acoustic signals: A playback experiment in a Neotropical songbird. Animal Behaviour, 157, 77–85. https://doi.org/10.1016/j.anbehav.2019.08.021
den Hartog, P. M., de Kort, S. R., & ten Cate, C. (2007). Hybrid vocalizations are effective within, but not outside, an avian hybrid zone. Behavioral Ecology, 18(3), 608–614. https://doi.org/10.1093/beheco/arm018
Derégnaucourt, S., & Guyomarc'h, J. C. (2003). Mating call discrimination in female European (Coturnix c. coturnix) and Japanese quail (Coturnix c. japonica). Ethology, 109(2), 107–119. https://doi.org/10.1046/j.1439-0310.2003.00854.x.
Dinneen, L. C., & Blakesley, B. C. (1973). Algorithm AS 62: A Generator for the Sampling Distribution of the Mann-Whitney U Statistic. Journal of the Royal Statistical Society. Series C (Applied Statistics), 22(2), 269–273. https://doi.org/10.2307/2346934.
Doherty, J. A., & Gerhardt, H. C. (1984). Acoustic communication in hybrid treefrogs: Sound production by males and selective phonotaxis by females. Journal of Comparative Physiology A, 154(3), 319–330. https://doi.org/10.1007/bf00605231
Driller, C., Merker, S., Perwitasari-Farajallah, D., Sinaga, W., Anggraeni, N., & Zischler, H. (2015). Stop and go – Waves of tarsier dispersal mirror the genesis of Sulawesi island. PLoS One, 10(11), e0141212. https://doi.org/10.1371/journal.pone.0141212.
Driller, C., Perwitasari-Farajallah, D., Zischler, H., & Merker, S. (2009). The social system of Lariang tarsiers (Tarsius lariang) as revealed by genetic analyses. International Journal of Primatology, 30(2), 267–281. https://doi.org/10.1007/s10764-009-9341-6
El-Shehaby, M., Salama, M. S., Brunner, E., & Heinze, J. (2011). Cuticular hydrocarbons in two parapatric species of ants and their hybrid. Integrative Zoology, 6(3), 259–265. https://doi.org/10.1111/j.1749-4877.2011.00255.x
Farabaugh, S. M. (1982). The ecological and social significance of duetting. In D. E. Kroodsma & E. H. Miller (Eds.), Acoustic communication in birds (Vol. 2, pp. 85–124). Academic Press.
Fischer, J., & Hammerschmidt, K. (2019). Towards a new taxonomy of primate vocal production learning. Philosophical Transactions of the Royal Society B, 375(1789), 20190045. https://doi.org/10.1098/rstb.2019.0045
Fischer, J., & Price, T. (2017). Meaning, intention, and inference in primate vocal communication. Neuroscience and Biobehavioral Reviews, 82, 22–31. https://doi.org/10.1016/j.neubiorev.2016.10.014
Fritz, C. O., Morris, P. E., & Richler, J. J. (2012). Effect size estimates: Current use, calculations, and interpretation. Journal of Experimental Psychology: General, 140(1), 2–18. https://doi.org/10.1037/a0024338
Garcia, M., Herbst, C. T., Bowling, D. L., Dunn, J. C., & Fitch, W. T. (2017). Acoustic allometry revisited: Morphological determinants of fundamental frequency in primate vocal production. Scientific Reports, 7(10450), 10450. https://doi.org/10.1038/s41598-017-11000-x
Geissmann, T. (1984). Inheritance of song parameters in the gibbon song, analyzed in 2 hybrid gibbons (Hylobates pileatus x Hylobates lar). Folia Primatologica, 42(3–4), 216–235. https://doi.org/10.1159/000156165
Geissmann, T. (2002). Duet-splitting and the evolution of gibbon songs. Biological Reviews, 77(1), 57–76. https://doi.org/10.1017/s1464793101005826
Gerhardt, H. C. (1974). Vocalizations of some hybrid treefrogs: Acoustic and behavioral analyses. Behaviour, 49(1–2), 130–151. https://doi.org/10.1163/156853974X00435
Gordon, N. M., Ralph, M. Z., & Stratman, K. D. (2017). Rapid character displacement of different call parameters in closely related treefrogs (Hyla cinerea and H. gratiosa). Behavioral Ecology and Sociobiology, 71(112), 1–8. https://doi.org/10.1007/s00265-017-2341-1.
Grow, N., & Gursky-Doyen, S. (2010). Preliminary data on the behavior, ecology, and morphology of pygmy tarsiers (Tarsius pumilus). International Journal of Primatology, 31(6), 1174–1191. https://doi.org/10.1007/s10764-010-9456-9
Grow, N. B. (2019). Cryptic communication in a montane nocturnal haplorhine, Tarsius pumilus. Folia Primatologica, 90(5), 404–421. https://doi.org/10.1159/000497427
Gursky-Doyen, S. (2010). Intraspecific variation in the mating system of spectral tarsiers. International Journal of Primatology, 31(6), 1161–1173. https://doi.org/10.1007/s10764-010-9450-2
Gursky, S. (2002). The behavioral ecology of the spectral tarsier, Tarsius spectrum. Evolutionary Anthropology: Issues, News, and Reviews, 11(6), 226–234. https://doi.org/10.1002/evan.10035
Gursky, S. (2015). Ultrasonic vocalizations by the spectral tarsier, Tarsius spectrum. Folia Primatologica, 86(3), 153–163. https://doi.org/10.1159/000371885
Hagemann, L., Grow, N., Bohr, Y.E.-M.B., Perwitasari-Farajallah, D., Duma, Y., Gursky, S. L., & Merker, S. (2022). Small, odd and old: The mysterious Tarsius pumilus is the most basal Sulawesi tarsier. Biology Letters, 18(3), 20210642. https://doi.org/10.1098/rsbl.2021.0642
Hahn, M. E., Karkowski, L., Weinreb, L., Henry, A., Schanz, N., & Hahn, E. M. (1998). Genetic and developmental influences on infant mouse ultrasonic calling. II. Developmental patterns in the calls of mice 2–12 days of age. Behavior Genetics, 28(4), 315–325. https://doi.org/10.1023/a:1021679615792.
Haimoff, E. H. (1986). Convergence in the duetting of monogamous old-world primates. Journal of Human Evolution, 15(1), 51–59. https://doi.org/10.1016/s0047-2484(86)80065-3
Hall, M. L. (2004). A review of hypotheses for the functions of avian duetting. Behavioral Ecology and Sociobiology, 55(5), 415–430. https://doi.org/10.1007/s00265-003-0741-x
Hedges, L. V., & Olkin, I. (1985). Statistical methods for meta-analysis. Academic Press.
Heffner, R. S. (2004). Primate hearing from a mammalian perspective. Anatomical Record Part A, 281A(1), 1111–1122. https://doi.org/10.1002/ar.a.20117
Hewitt, G. M. (1988). Hybrid zones – Natural laboratories for evolutionary studies. Trends in Ecology & Evolution, 3(7), 158–167. https://doi.org/10.1016/0169-5347(88)90033-x
Höbel, G., & Gerhardt, H. C. (2003). Reproductive character displacement in the acoustic communication system of green tree frogs (Hyla cinerea). Evolution, 57(4), 894–904. https://doi.org/10.1111/j.0014-3820.2003.tb00300.x
Hoskin, C. J., & Higgie, M. (2013). Hybridization: Its varied forms and consequences. Journal of Evolutionary Biology, 26(2), 276–278. https://doi.org/10.1111/jeb.12035
Howard, D. J., Waring, G. L., Tibbets, C. A., & Gregory, P. G. (1993). Survival in a mosaic hybrid zone. Evolution, 47(3), 789–800. https://doi.org/10.2307/2410184
Irwin, D. E. (2020). Assortative mating in hybrid zones is remarkably ineffective in promoting speciation. American Naturalist, 195(6), E150–E167. https://doi.org/10.5061/dryad.k98sf7m30
Janik, V. M. (2014). Cetacean vocal learning and communication. Current Opinion in Neurobiology, 28, 60–65. https://doi.org/10.1016/j.conb.2014.06.010
Janik, V. M., & Knörnschild, M. (2021). Vocal production learning in mammals revisited. Philosophical Transactions of the Royal Society b: Biological Sciences, 376(1836), 20200244. https://doi.org/10.1098/rstb.2020.0244
Janik, V. M., & Slater, P. J. B. (1997). Vocal learning in mammals. In P. J. B. Slater, J. S. Rosenblatt, C. T. Snowdon, & M. Milinski (Eds.), Advances in the Study of Behavior (Vol. 26, pp. 59–99). Academic Press. https://doi.org/10.1016/S0065-3454(08)60377-0.
Janik, V. M., & Slater, P. J. B. (2000). The different roles of social learning in vocal communication. Animal Behaviour, 60(1), 1–11. https://doi.org/10.1006/anbe.2000.1410
Jürgens, U. (2002). Neural pathways underlying vocal control. Neuroscience & Biobehavioral Reviews, 26(2), 235–258. https://doi.org/10.1016/S0149-7634(01)00068-9
Jürgens, U. (2009). The neural control of vocalization in mammals: A review. Journal of Voice, 23(1), 1–10. https://doi.org/10.1016/j.jvoice.2007.07.005
Kenyon, H. L., Toews, D. P. L., & Irwin, D. E. (2011). Can song discriminate between MacGillivray’s and Mourning Warblers in a narrow hybrid zone? The Condor, 113(3), 655–663. https://doi.org/10.1525/cond.2011.100182
Kirschel, A. N. G., Blumstein, D. T., & Smith, T. B. (2009). Character displacement of song and morphology in African tinkerbirds. Proceedings of the National Academy of Sciences of the United States of America, 106(20), 8256–8261. https://doi.org/10.1073/pnas.0810124106
Kitchen, D. M., Bergman, T. J., Dias, P. A. D., Ho, L., Canales-Espinosa, D., & Cortés-Ortiz, L. (2019). Temporal but not acoustic plasticity in hybrid howler monkey (Alouatta palliata x A. pigra) loud calls. International Journal of Primatology, 40(1), 132–152. https://doi.org/10.1007/s10764-017-0004-8.
Knörnschild, M. (2014). Vocal production learning in bats. Current Opinion in Neurobiology, 28, 80–85. https://doi.org/10.1016/j.conb.2014.06.014
Lameira, A. R., Hardus, M. E., Kowalsky, B., Vries, H. d., Spruijt, B. M., Sterck, E. H. M., Shumaker, R. W., & Wich, S. A. (2013). Orangutan (Pongo spp.) whistling and implications for the emergence of an open-ended call repertoire: A replication and extension. The Journal of the Acoustical Society of America, 134(3), 2326–2335. https://doi.org/10.1121/1.4817929.
Lamichhaney, S., Han, F., Webster, M. T., Andersson, L., Grant, B. R., & Grant, P. R. (2018). Rapid hybrid speciation in Darwin’s finches. Science, 359(6372), 224–227. https://doi.org/10.1126/science.aao4593
Logue, D. M., & Krupp, D. B. (2016). Duetting as a collective behavior. Frontiers in Ecology and Evolution, 4(7), 1–12. https://doi.org/10.3389/fevo.2016.00007
Long, A. M., Moore, N. P., & Hayden, T. J. (1998). Vocalizations in red deer (Cervus elaphus), sika deer (Cervus nippon), and red x sika hybrids. Journal of Zoology, 244, 123–134. https://doi.org/10.1111/j.1469-7998.1998.tb00014.x
MacKinnon, J., & MacKinnon, K. (1980). The behavior of wild spectral tarsiers. International Journal of Primatology, 1(4), 361–379. https://doi.org/10.1007/BF02692280
Mallet, J. (2007). Hybrid speciation. Nature, 446(7133), 279–283. https://doi.org/10.1038/nature05706
Maples, E. G., & Haraway, M. M. (1982). Taped vocalization as a reinforcer of vocal behavior in a female agile gibbon (Hylobates agilis). Psychological Reports, 51(1), 95–98. https://doi.org/10.2466/pr0.1982.51.1.95
Marler, P., & Tenaza, R. R. (1977). Signaling behavior of apes with special reference to vocalization. In T. A. Sebeok (Ed.), How Animals Communicate (pp. 965–1033). Indiana University Pres.
Marshall, D. C., & Cooley, J. R. (2000). Reproductive character displacement and speciation in periodical cicadas, with description of a new species, 13-year Magicicada neotredecim. Evolution, 54(4), 1313–1325. https://doi.org/10.1111/j.0014-3820.2000.tb00564.x
Marshall, J. T., & Sugardjito, J. (1986). Gibbon systematics. In D. Swindler & J. Erwin (Eds.), Comparative primate biology (Vol. 1, pp. 137–185). Alan R. Liss.
Martin, K., Tucker, M. A., & Rogers, T. L. (2017). Does size matter? Examining the drivers of mammalian vocalizations. Evolution, 71(2), 249–260. https://doi.org/10.1111/evo.13128
Masali, M., Borgognini Tarli, S., & Maffei, M. (1992). Auditory ossicles and the evolution of the primate ear: A biomechanical approach. In J. Wind, B. Chiarelli, B. Bichakjian, A. Nocentini, & A. Jonker (Eds.), Language origin: A multidisciplinary approach (Vol. 61, pp. 67–86). Springer. https://doi.org/10.1007/978-94-017-2039-7.
Masterton, B., Heffner, H., & Ravizza, R. (1969). The evolution of human hearing. Journal of the Acoustical Society of America, 45(4), 966–985. https://doi.org/10.1121/1.1911574
Méndez-Cárdenas, M. G., & Zimmermann, E. (2009). Duetting – A mechanism to strengthen pair bonds in a dispersed pair-living primate (Lepilemur edwardsi)? American Journal of Physical Anthropology, 139(4), 523–532. https://doi.org/10.1002/ajpa.21017
Merker, S., Boucsein, D., Feldmeyer, B., Perwitasari-Farajallah, D., & Streit, B. (2012). Novel tetra- and pentanucleotide microsatellite markers allow for multiplexed genotyping of Sulawesi tarsiers (Tarsius spp.). Conservation Genetics Resources, 4(2), 343–345. https://doi.org/10.1007/s12686-011-9543-z.
Merker, S., Driller, C., Dahruddin, H., Wirdateti, Sinaga, W., Perwitasari-Farajallah, D., & Shekelle, M. (2010). Tarsius wallacei: A new tarsier species from Central Sulawesi occupies a discontinuous range. International Journal of Primatology, 31(6), 1107–1122.https://doi.org/10.1007/s10764-010-9452-0.
Merker, S., Driller, C., Perwitasari-Farajallah, D., Pamungkas, J., & Zischler, H. (2009). Elucidating geological and biological processes underlying the diversification of Sulawesi tarsiers. Proceedings of the National Academy of Sciences of the United States of America, 106(21), 8459–8464. https://doi.org/10.1073/pnas.0900319106
Merker, S., & Groves, C. P. (2006). Tarsius lariang: A new primate species from western Central Sulawesi. International Journal of Primatology, 27(2), 465–485. https://doi.org/10.1007/s10764-006-9038-z
Merker, S., Yustian, I., & Mühlenberg, M. (2004). Loosing ground but still doing well – Tarsius dianae in human-altered rainforests of Central Sulawesi. In G. Gerold, M. Fremerey, & E. Guhardja (Eds.), Land use, nature conservation and the stability of rainforest margins in Southeast Asia (pp. 299–311). Springer. https://doi.org/10.1007/978-3-662-08237-9.
Micancin, J. P., & Wiley, R. H. (2014). Allometric convergence, acoustic character displacement, and species recognition in the syntopic cricket frogs Acris crepitans and A. gryllus. Evolutionary Biology, 41(3), 425–438. https://doi.org/10.1007/s11692-014-9274-7.
Moore, S., & Coulson, J. O. (2020). Intergeneric hybridization of a vagrant Common Black Hawk and a Red-shouldered Hawk. Journal of Raptor Research, 54(1), 74–80. https://doi.org/10.3356/0892-1016-54.1.74
Moran, R. L., & Fuller, R. C. (2018). Agonistic character displacement of genetically based male colour patterns across darters. Proceedings of the Royal Society B: Biological Sciences, 285(1884), 20181248. https://doi.org/10.1098/rspb.2018.1248
Müller, A. E., & Anzenberger, G. (2002). Duetting in the titi monkey Callicebus cupreus: Structure, pair specificity and development of duets. Folia Primatologica, 73(2–3), 104–115. https://doi.org/10.1159/000064788
Nieder, A., & Mooney, R. (2019). The neurobiology of innate, volitional and learned vocalizations in mammals and birds. Philosophical Transactions of the Royal Society B, 375(1789), 1–21. https://doi.org/10.1098/rstb.2019.0054
Niemitz, C. (1984). Vocal communication of two tarsier species (Tarsius bancanus and Tarsius spectrum). In C. Niemitz (Ed.), Biology of tarsiers (pp. 129–141). Fischer.
Niemitz, C., Nietsch, A., Warter, S., & Rumpler, Y. (1991). Tarsius dianae: A new primate species from Central Sulawesi (Indonesia). Folia Primatologica, 56(2), 105–116. https://doi.org/10.1159/000156534
Nietsch, A. (1999). Duet vocalizations among different populations of Sulawesi tarsiers. International Journal of Primatology, 20(4), 567–583. https://doi.org/10.1023/a:1020342807709
Nietsch, A., & Kopp, M.-L. (1998). Role of vocalization in species differentiation of Sulawesi tarsiers. Folia Primatologica, 69, 371–378. https://doi.org/10.1159/000052725
Ortiz-Barrientos, D., Grealy, A., & Nosil, P. (2009). The genetics and ecology of reinforcement: Implications for the evolution of prezygotic isolation in sympatry and beyond. Annals of the New York Academy of Sciences, 1168(1), 156–182. https://doi.org/10.1111/j.1749-6632.2009.04919.x
Owren, M. J., Dieter, J. A., Seyfarth, R. M., & Cheney, D. L. (1992). Food calls produced by adult female rhesus (Macaca mulatta) and Japanese (M. fuscata) macaques, their normally-raised offspring, and offspring cross-fostered between species. Behaviour, 120(3–4), 218–231. https://doi.org/10.1163/156853992x00615.
Päckert, M. (2018). Song: The learned language of three major bird clades. In D. T. Tietze (Ed.), Bird species: How they arise, modify and vanish (pp. 75–94). Springer International Publishing. https://doi.org/10.1007/978-3-319-91689-7_5.
Page, B., Goldsworthy, S. D., & Hindell, M. A. (2001). Vocal traits of hybrid fur seals: Intermediate to their parental species. Animal Behaviour, 61(5), 959–967. https://doi.org/10.1006/anbe.2000.1663
Peters, G., & Peters, M. K. (2010). Long-distance call evolution in the Felidae: Effects of body weight, habitat, and phylogeny. Biological Journal of the Linnean Society, 101(2), 487–500. https://doi.org/10.1111/j.1095-8312.2010.01520.x
Pfennig, K. S., & Pfennig, D. W. (2009). Character displacement: Ecological and reproductive responses to a common evolutionary problem. Quarterly Review of Biology, 84(3), 253–276. https://doi.org/10.1086/605079
Pfennig, K. S., & Rice, A. M. (2014). Reinforcement generates reproductive isolation between neighbouring conspecific populations of spadefoot toads. Proceedings of the Royal Society B, 281(1789), 20140949. https://doi.org/10.1098/rspb.2014.0949
Pollock, J. I. (1986). The song of the Indris (Indri indri; Primates: Lemuroidea): Natural history, form, and function. International Journal of Primatology, 7(3), 225–264. https://doi.org/10.1007/bf02736391
Ramsier, M. A., & Rauschecker, J. P. (2017). Primate audition: Reception, perception, and ecology. In R. Quam, M. Ramsier, R. R. Fay, & A. N. Popper (Eds.), Primate hearing and communication (pp. 231). Springer. https://doi.org/10.1007/978-3-319-59478-1.
Randler, C. (2002). Avian hybridization, mixed pairing and female choice. Animal Behaviour, 63(1), 103–119. https://doi.org/10.1006/anbe.2001.1884
Reichmuth, C., & Casey, C. (2014). Vocal learning in seals, sea lions, and walruses. Current Opinion in Neurobiology, 28, 66–71. https://doi.org/10.1016/j.conb.2014.06.011
Rosenthal, J. A. (1996). Qualitative descriptors of strength of association and effect size. Journal of Social Service Research, 21(4), 37–59. https://doi.org/10.1300/J079v21n04_02
Rundle, H. D., & Nosil, P. (2005). Ecological speciation. Ecology Letters, 8(3), 336–352. https://doi.org/10.1111/j.1461-0248.2004.00715.x
Seddon, N. (2005). Ecological adaptation and species recognition drives vocal evolution in neotropical suboscine birds. Evolution, 59(1), 200–215. https://doi.org/10.1111/j.0014-3820.2005.tb00906.x
Seehausen, O. (2006). Conservation: Losing biodiversity by reverse speciation. Current Biology, 16(9), R334–R337. https://doi.org/10.1016/j.cub.2006.03.080
Segura, D. F., Vera, M. T., Rull, J., Wornoayporn, V., Islam, A., & Robinson, A. S. (2011). Assortative mating among Anastrepha fraterculus (Diptera: Tephritidae) hybrids as a possible route to radiation of the fraterculus cryptic species complex. Biological Journal of the Linnean Society, 102(2), 346–354. https://doi.org/10.1111/j.1095-8312.2010.01590.x
Seibt, U., & Wickler, W. (1977). Duettieren als Revier-Anzeige bei Vögeln. Zeitschrift Für Tierpsychologie, 43(2), 180–187. https://doi.org/10.1111/j.1439-0310.1977.tb00067.x
Servedio, M. R., & Noor, M. A. F. (2003). The Role of reinforcement in speciation: Theory and data. Annual Review of Ecology, Evolution, and Systematics, 34(1), 339–364. https://doi.org/10.1146/annurev.ecolsys.34.011802.132412
Shekelle, M. (2008). Distribution of tarsier acoustic forms, North and Central Sulawesi: With notes on the primary taxonomy of Sulawesi’s tarsiers. In M. Shekelle, C. P. Groves, I. Maryanto, H. Schulze, & H. Fitch-Snyder (Eds.), Primates of The Oriental Night (pp. 35–50). LIPI Press.
Shekelle, M., Groves, C., Merker, S., & Supriatna, J. (2008). Tarsius tumpara: A new tarsier species from Siau Island, North Sulawesi. Primate Conservation, 23(1), 55–64. https://doi.org/10.1896/052.023.0106
Shekelle, M., Groves, C. P., Maryanto, I., & Mittermeier, R. A. (2017). Two new tarsier species (Tarsiidae, Primates) and the biogeography of Sulawesi, Indonesia. Primate Conservation, 31, 61–69.
Shekelle, M., Groves, C. P., Maryanto, I., Mittermeier, R. A., Salim, A., & Springer, M. S. (2019). A new tarsier species from the Togean Islands of Central Sulawesi, Indonesia, with references to Wallacea and conservation on Sulawesi. Primate Conservation, 33, 65–73.
Shekelle, M., Leksono, S. M., Ichwan, L. L. S., & Masala, Y. (1997). Natural history of the tarsiers of North and Central Sulawesi. Sulawesi Primate Newsletter, 4(2), 3–11.
Shekelle, M., Meier, R., Wahyu, I., Wirdateti, & Ting, N. (2010). Molecular phylogenetics and chronometrics of Tarsiidae based on 12S mtDNA haplotypes: Evidence for miocene origins of crown tarsiers and numerous species within the Sulawesian clade. International Journal of Primatology, 31(6), 1083–1106.https://doi.org/10.1007/s10764-010-9457-8.
Shekelle, M., & Salim, A. (2009). An acute conservation threat to two tarsier species in the Sangihe Island chain, North Sulawesi, Indonesia. Oryx, 43(03). https://doi.org/10.1017/s0030605309000337.
Shipilina, D., Serbyn, M., Ivanitskii, V., Marova, I., & Backstrom, N. (2017). Patterns of genetic, phenotypic, and acoustic variation across a chiffchaff (Phylloscopus collybita abietinus/tristis) hybrid zone. Ecology and Evolution, 7, 2169–2180. https://doi.org/10.1002/ece3.2782
Smadja, C., & Ganem, G. (2005). Asymmetrical reproductive character displacement in the house mouse. Journal of Evolutionary Biology, 18(6), 1485–1493. https://doi.org/10.1111/j.1420-9101.2005.00944.x
Sobroza, T. V., Gordo, M., Pequeno, P. A. C. L., Dunn, J. C., Spironello, W. R., Rabelo, R. M., & Barnett, A. P. A. (2021). Convergent character displacement in sympatric tamarin calls (Saguinus spp.). Behavioral Ecology and Sociobiology, 75(5), 88. https://doi.org/10.1007/s00265-021-03028-x.
Stebbins, G. L. (1959). The role of hybridization in evolution. Proceedings of the American Philosophical Society, 103, 231–251.
Stoeger, A. S., & Manger, P. (2014). Vocal learning in elephants: Neural bases and adaptive context. Current Opinion in Neurobiology, 28, 101–107. https://doi.org/10.1016/j.conb.2014.07.001
ten Cate, C. (2021). Re-evaluating vocal production learning in non-oscine birds. Philosophical Transactions of the Royal Society b: Biological Sciences, 376(1836), 20200249. https://doi.org/10.1098/rstb.2020.0249
Tenaza, R. (1985). Songs of hybrid gibbons (Hylobates lar x H. muelleri). American Journal of Primatology, 8(3), 249–253. https://doi.org/10.1002/ajp.1350080307.
Tilson, R. L., & Tenaza, R. R. (1976). Monogamy and duetting in an Old World monkey. Nature, 263(5575), 320–321. https://doi.org/10.1038/263320a0
Todt, D., & Naguib, M. (2000). Vocal interactions in birds: The use of song as a model in communication. In P. J. B. Slater, J. S. Rosenblatt, C. T. Snowdon, & T. J. Roper (Eds.), Advances in the Study of Behavior (Vol. 29, pp. 247–296). Academic Press. https://doi.org/10.1016/S0065-3454(08)60107-2.
Tremble, M., Muskita, Y., & Supriatna, J. (1993). Field observations of Tarsius dianae at Lore Lindu Nation Park, Central Sulawesi, Indonesia. Tropical Biodiversity, 1(2), 67–76.
Tyack, P. L. (2020). A taxonomy for vocal learning. Philosophical Transactions of the Royal Society b: Biological Sciences, 375(1789), 20180406. https://doi.org/10.1098/rstb.2018.0406
Vander Meer, R. K., Lofgren, C. S., & Alvarez, F. M. (1985). Biochemical evidence for hybridization in fire ants. Florida Entomologist, 68(3), 501–506. https://doi.org/10.2307/3495147
Vernes, S. C., & Wilkinson, G. S. (2020). Behaviour, biology and evolution of vocal learning in bats. Philosophical Transactions of the Royal Society b: Biological Sciences, 375(1789), 20190061. https://doi.org/10.1098/rstb.2019.0061
Whitehead, J. M. (1995). Vox alouattinae: A preliminary survey of the acoustic characteristics of long-distance calls of howling monkeys. International Journal of Primatology, 16(1), 121–144. https://doi.org/10.1007/bf02700156
Wilkins, M. R., Seddon, N., & Safran, R. J. (2013). Evolutionary divergence in acoustic signals: Causes and consequences. Trends in Ecology & Evolution, 28(3), 156–166. https://doi.org/10.1016/j.tree.2012.10.002
Woolley, S. C., & Sakata, J. T. (2019). Mechanisms of species diversity in birdsong learning. Plos Biology, 17(12), e3000555. https://doi.org/10.1371/journal.pbio.3000555.
Wyman, M. T., Locatelli, Y., Charlton, B. D., & Reby, D. (2016). Female sexual preferences toward conspecific and hybrid male mating calls in two species of polygynous deer, Cervus elaphus and C. nippon. Evolutionary Biology, 43, 227–241. https://doi.org/10.1007/s11692-015-9357-0
Xue, H.-J., Segraves, K. A., Wei, J., Zhang, B., Nie, R.-E., Li, W.-Z., & Yang, X.-K. (2018). Chemically mediated sexual signals restrict hybrid speciation in a flea beetle. Behavioral Ecology, 29(6), 1462–1471. https://doi.org/10.1093/beheco/ary105
Zinner, D., Arnold, M. L., & Roos, C. (2011). The strange blood: Natural hybridization in primates. Evolutionary Anthropology, 20(3), 96–103. https://doi.org/10.1002/evan.20301
Acknowledgements
The authors thank the Indonesian authorities at the Indonesian Institute of Sciences (LIPI), the Ministry of Research and Technology (RISTEK), the Directorate General of Forest Protection and Nature Conservation (PHKA), and the Central Sulawesi Natural Resources Conservation Agency (BKSDA Central Sulawesi), as well as local administrations of several villages for granting research, capture, and export permits. They thank Dr. Joko Pamungkas, then-director of the Primate Research Center (PSSP) at Bogor Agricultural University (IPB), Dr. Ir Entang Iskandar, and the late Wahyu Sudrajat from PSSP for their administrative support. Baso helped with bureaucratic affairs in Palu. The authors also are very grateful to their field assistants for their indispensable help in situ: the late Pak Frans, Amar, Amos, Baso, Ferdi, I. B. Ari Surya, Ifan, Leo, Om Harun, Raimon, Sapri, and Thony. They thank Dr. Christine Driller for providing recordings from Luwuk. Kai Schmale and Alexander Daniel contributed valuable preparative work for final acoustic analysis. Last but not least, the authors thank two anonymous reviewers and the editor-in-chief Prof Joanna “Jo” M Setchell for their valuable comments and suggestions during the review process.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by Deutsche Forschungsgemeinschaft (ME 2730/2-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest whatsoever.
Declaration of Authorship
Author contributions (following the taxonomy of CRediT): YB is responsible for investigation, formal analysis, visualization, writing of the original draft, and project administration; AP is responsible for investigation and abstract translation; DP is responsible for project administration, and provision of resources; JG is responsible for supervision, validation, as well as review and editing of the original draft; SM is responsible for conceptualization, methodology, validation, funding acquisition, provision of resources, project administration, supervision, as well as review and editing of the original draft.
Inclusion and Diversity Statement
The author list includes contributors from the location where the research was conducted, who participated in investigation and administration.
Additional information
Handling Editor: Joanna (Jo) M. Setchell
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bohr, Y.EM.B., Purbatrapsila, A., Perwitasari-Farajallah, D. et al. Strange Tunes—Acoustic Variation and Character Displacement in a Tarsier Hybrid Zone. Int J Primatol 44, 581–612 (2023). https://doi.org/10.1007/s10764-023-00351-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-023-00351-0