Abstract
Pears (Pyrus) represent an important part of consumer diets, and have the fourth highest production of non-citrus fruits, measured by fresh weight, in the U.S. They are maintained clonally and grown as composite plants, consisting of a scion (fruit bearing) cultivar grafted onto a rootstock cultivar. Up to 98% of existing production relies on only a few scion and rootstock cultivars, leaving the standing crop vulnerable to threats. Pears are faced with a wide range of biotic and abiotic threats and production vulnerabilities, some of which can be limited by integrating resistance and horticultural traits from wild and cultivated materials from around the world. The National Clonal Germplasm Repository (NCGR Corvallis), part of the USDA-ARS National Plant Germplasm System, maintains a large Pyrus collection from across the globe, consisting of 2793 Pyrus accessions from 37 species. The collection represents an important resource for preservation, research, and breeding efforts for pears. The crop vulnerability status of pears in the U.S. is currently moderate to high, with increasing threats and challenges. Breeding and preservation efforts, along with genetic, crop protection and production research are, however, actively targeting these needs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction to the crop
Pears (Pyrus) are clonally propagated Rosaceous tree crops, consumed as fresh and processed fruit and juice. Commercial cultivars can be grouped into two types: European and Asian pears, characterized by their soft, melting flesh or crisp, juicy flesh, respectively. In the U.S., commercial pear fruit production (including both European and Asian cultivars) was valued at $353 million in 2022, and is declining (USDA NASS 2004, 2014, 2023). The U.S. pear industry attributes lower production to inconsistent consumption, increasing production costs, and competition from imported fruit (Elkins et al. 2012; Mitcham and Elkins 2007). Further, the industry has relatively few options for high-density production, due in part to the lack of dwarfing rootstocks. Genetic uniformity is high among commercially grown cultivars, and wild populations are facing threats to genetic erosion (Montanari et al. 2020; USA Pears 2023). Nationally, there are only a few pear scion and rootstock breeding programs, and they aim to develop elite lines and new cultivars that address industry and consumer needs. The National Plant Germplasm System maintains a large Pyrus collection, focused on preserving both cultivar and species diversity.
Production
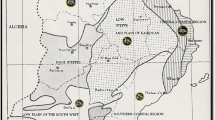
Between 2012 and 2022, U.S. pear acreage dropped from 50,100 to 40,600 and production from 851,130 to 642,910 metric tons (utilized production, (USDA NASS 2020); USDA NASS (2023)). Per acre production has remained steady at around 16.0 tons per acre. Over 99% of the total was produced by Washington (17,500 acres, 285,000 tons), Oregon (13,700 acres, 198,500 tons), and California (9,400 acres, 160,500 tons) (USDA NASS 2023). Washington and Oregon are heavily weighted toward the fresh market while California sells about evenly to fresh and processed markets (USDA NASS 2023). Other states with minor commercial production include New York, Michigan, and Pennsylvania. Major commercial cultivars (in order of volume) are ‘Beurré d’Anjou’, ‘Bartlett’, ‘Beurré Bosc’, ‘Red Anjou’, ‘Starkrimson’, and ‘Doyenné du Comice’, according to the pear crop estimate five-year average (Fig. 1, and communicated from USA Pears 2023).
Pears are produced commercially in mid-latitude temperate regions internationally. Top pear producers in 2021/2022 (by weight) were China, the European Union, Argentina, the United States, Türkiye, South Africa, India, Russia, South Korea, and Chile (Table 1, USDA FAS (2023)).
Industry representation
There are several national and regional industry and professional grower organizations that represent the interests of U.S. pear production and marketing. The Pear Bureau Northwest is a marketing organization representing growers in Washington and Oregon and the California Pear Advisory Board (CPAB) represents California growers. Various entities are established to review and determine funding for research proposals aimed at improving pear production and quality. These include the Fresh and Processed Pear Committees in the Washington and Oregon region, and the CPAB and Pear Pest Management Research Fund (co-funded by growers and processors) in California. The Pear Bureau Northwest and the CPAB also provides marketing and consumer-related information through their respective websites (USA Pears 2023).
Primary products
Pear trees are cultivated for four primary products: food for human consumption (fresh fruit or processed), beverage (juice or fermented cider), ornamental landscape planting, and occasionally pear wood used for products such as firewood and furniture.
Most of the fruit crop is sold fresh or processed, which is mainly in the form of canned pears, and to a lesser extent, baby food and dried. Dried pears are now primarily sourced from South America but are processed in the U.S. as a niche product. Pear cultivars commercially grown for fruit are valued for traits related to fruit quality and ease of production. Targets for fruit breeding include traits to improve fruit quality, uniform ripening requirements, size, and productivity, as well as disease and insect resistance.
Fermented pear cider, or ‘perry’, traditionally produced in the U.K. and France, has increased in popularity in the U.S. Many perry pear cultivars were introduced into the U.S. from Europe in recent years to meet this increased demand. Desired traits for perry pears are different from those for fresh fruit consumption. Cultivars of both groups should have high fruit production but the fruit of a perry pear typically contains high tannins content and/or acidity, combined with good flavor that is retained throughout the fermentation process (Luckwill and Pollard 1963). Traditional perry pear cultivars have a higher sorbitol content than other sugars (He et al. 2022). Presence of hard stone cells in fruits of many pear species limit their use in breeding fruit for eating but have no impact on fermented juice products.
Pyrus germplasm is also used to develop ornamental trees. For pear trees planted as landscape specimens, small, obscure, and unpalatable fruit are usually valued. Many cultivars and selections of the Callery pear (P. calleryana Decne.) have been produced in the nursery trade for use as flowering street trees; however, in recent years profuse reseeding of these cultivars has caused them to be considered invasive in some locations (Culley 2017). Selections of the willow-leaf pear (P. salicifolia Pall.) are appreciated as landscape trees for their fine texture, gray, pubescent foliage, and some for their weeping growth habit (Dirr 1997). Other pear species, including P. betulifolia Bunge, P. dimorphophylla Makino, P. elaeagrifolia Pall., P. regelii Rehder, and P. syriaca Boiss., have striking foliage, unusual flowers, or unique environmental adaptations and should be evaluated for landscape use.
Wood of various Pyrus species is used for making furniture, musical instruments, and kitchen implements, but there have been no deliberate efforts to select cultivars for genetic traits desirable for these purposes.
Dietary
Pears are known for being high in fiber (3.1 g per 100 g of raw ‘Bartlett’) (USDA ARS 2022). In comparison with other fruits and some vegetables, ascorbic acid content is low (4.3 mg vitamin C per 100 g). They are a relatively low source of energy (59 kcal per 100 g), due to the low content of sugars (9.73 g per 100 g, or nearly 10% by weight). Total lipids are only 0.15 g per 100 g. Comparative research has shown that nutritional contents vary substantially depending on cultivar (Galvis Sanchez et al. 2003; Li et al. 2016). A relatively high percentage of the sugars in pears are in the forms of fructose and sorbitol (Reiland and Slavin 2015). Combined with the high fiber content, pear fruit can aid gut health, but high levels of consumption can have a laxative effect (Nazir et al. 2020; Reiland and Slavin 2015). Because pears should be eaten as part of a balanced diet, there are no deficiencies that constitute a dietary threat to the population. In fact, increased consumption of whole, fresh fruits, including pears, is part of the USDA Dietary Guideline for Americans (USDA and DHHS 2020).
Pears also contain phytonutrients, particularly phenolics and antioxidants (Macheix 1990; Nazir et al. 2020), which have been found to have disease-fighting properties (Ames et al. 1993). These compounds are particularly enriched in the peel (Galvis Sanchez et al. 2003). Reviews on pear nutrition demonstrate a wide range of total phenolic content across cultivars (Li et al. 2016). The phenolic content, antioxidant activity, and enzyme inhibitory activity in pears can help in dietary management of early stages of hyperglycemia linked to type 2 diabetes and diabetes-associated hypertension (Sarkar et al. 2015). These authors also found that fermented pears inhibit the bacteria associated with stomach ulcers and promote probiotic organisms.
Biology and distribution
Pyrus and the related rootstock genera are members of the Rosaceae, subfamily Amygdaloideae, tribe Maleae, subtribe Malineae. The basic chromosome number of the Maloideae (x = 17) is high compared to other Rosaceae subfamilies (x = 7–9), indicating a polyploid origin (Bell and Itai 2011). All species are functionally diploid (2n = 34), but some triploid and tetraploid cultivars exist. Aneuploidy is characteristic of the naturally-occurring interspecific hybrid species P. × nivalis Jacq. Speciation in Pyrus has proceeded without a change in chromosome number (Zielinski and Thompson 1967). Reticulation caused by interspecific hybridization is a major evolutionary process for Pyrus (Zheng et al. 2014). The Germplasm Resources Information Network (GRIN) taxonomy database (USDA ARS 2023a) recognizes 37 primary species, plus 15 interspecific hybrids, three of which may be artificial or “arboretum hybrids” (Table S1). Morphology differs substantially between cultivated germplasm and wild crop relatives (Fig. 2).
Representatives of the diversity of Pyrus fruit morphology. A sampling of fruit from a range of European pear cultivars, B Asian pear cultivars, and C crop wild relatives found in the NCGR Corvallis Pyrus collection. A ‘Bartlett’ and ‘Buerré Bosc’ fruit were included in the crop wild relatives photograph for reference, denoted by a * and + symbol, respectively
Due to a high level of genetic heterozygosity, pears do not breed true from seed, and cultivars are propagated by grafting vegetative buds onto a rootstock. In some cases, when the scions and rootstocks are incompatible, a mutually compatible interstock, such as ‘Old Home’, ‘Beurré Hardy’, or ‘Comice’, is used. Within Pyrus, most species are graft-compatible (Westwood and Bjornstad 1971). Quince (Cydonia oblonga Mill.) is the only non-Pyrus species which has been used commercially as a rootstock, though it is incompatible with dominant cultivars such as ‘Bartlett’ and requires an interstock. Additional genera which are potential rootstocks include Amelanchier, Crataegus, Sorbus, × Sorbopyrus and Mespilus.
The Pyrus genus originated in the mountainous area of western and southwestern China during the Tertiary periods (65–55 million years ago) (Rubstov 1944). The genus has two sections, Pyrus for the western species and Pashia for the Asian species. Vavilov (1951) identified three centers of diversity for pears: the Chinese center, where forms of P. pyrifolia (Burm.) Nak. and P. ussuriensis Maxim. are grown, the central Asiatic center, consisting of Tadjikistan, Uzbekistan, India, Afghanistan, and western Tian-Shan mountains, where P. korshinsyki Litv. and P. boissieriana Buhse are found, and the Near Eastern center comprising the Caucasus Mountains and Asia Minor, where wild and domesticated forms of P. communis L. occur (Table S1).
Genetic uniformity of the standing crop and current pear breeding efforts
Genetic uniformity, varietal life spans, and characteristics driving plantings
Scion genetic uniformity
The degree of genetic uniformity of the U.S. pear crop is extremely high, and thus, its production is particularly vulnerable. Approximately 46% of national commercial production in 2022 was accounted for by a single cultivar, ‘Bartlett’ (syn. ‘William’s Bon Chretien’) (USDA NASS 2023). Three additional cultivars, ‘Beurré d’Anjou’, ‘Beurré Bosc’, and ‘Red Anjou’ account for up to 98% of the remaining production. Thus, a vast majority of reported commercial U.S. production consists of only four cultivars (Fig. 1). ‘Doyenné du Comice’ is also grown substantially in southern Oregon. All major cultivars belong to a single species, Pyrus communis L., and most were selected from open-pollinated seedling populations in Western Europe between the late 17th and early nineteenth centuries. Molecular research of genetic diversity and relationships using single nucleotide polymorphism (SNP) markers among 1897 of mostly P. communis accessions from the National Plant Germplasm System (NPGS) collection indicated a relatively high degree of genetic similarity, similar to previous research using microsatellite markers (Bassil and Postman 2010; Montanari et al. 2020). Limited quantities of Asian cultivars (e.g. P. pyrifolia ‘Hosui’, ‘Shinseiki’, ‘Ya Li’) are grown mainly in the Central Valley of California (Beutel 1990). P. pyrifolia cultivars were likewise found to be relatively close (Kimura et al. 2002). Together, this work confirmed that only a few pear cultivars served as the main progenitors for commonly grown European pear cultivars today. Similarly, European pear production is dominated by eight Pyrus communis cultivars: ‘Conference’, ‘Bartlett’, ‘Abate Fetel’, ‘Spadona’, ‘Doyenne du Comice’, ‘BeurreBosc’, ‘Dr. Jules Guyot’, and ‘Coscia’ (Dondini and Sansavini 2012).
Scion varietal life spans
Varietal life spans of U.S. scion cultivars are long. All of the major scion cultivars grown in the U.S., including ‘Bartlett’, ‘Beurré d’Anjou’, ‘Beurré Bosc’, ‘Doyenné du Comice’, ‘Seckel’, and ‘Forelle’, were first selected or reported between the late 1600’s and early 1800’s (Hedrick 1921; Lombard et al. 1980). The red-skinned mutant cultivars of ‘Bartlett’, ‘Beurré d’Anjou’, and ‘Clapp’s Favorite’ all originated in the mid-twentieth century (USA Pears 2023). The only cultivar from a recent breeding program to be planted to a significant amount of acreage in this country was ‘Concorde’, which was introduced to the U.S. from the East Malling Research Station, UK, in the early 1990’s, but quickly declined in favor due to extreme susceptibility to fire blight (Good Fruit Grower 2008; Mielke et al. 2005). The situation is similar in all countries where European pear cultivars are grown. Of the Japanese Asian pear cultivars, ‘Chojuro’ was introduced in 1859, ‘Nijisseiki’ in 1898, ‘Niitaka’ in 1927, ‘Shinseiki’ in 1945, ‘Kousui’ in 1959, and ‘Housui’ in 1972 (Kanato et al. 1982; Saito 2016). Of the major Korean cultivars, ‘A Ri Rang’ (syn. ‘Dan Bae’ or ‘Olympic’) was released in 1969, and four other cultivars were released from 1984 through 1994 (Shin et al. 2002). ‘A Ri Rang’ is grown mostly by amateur growers in the U.S. Pear trees can produce commercial crops for 50–75 years. The high costs of new plantings and the 4–5 year delay in fruit bearing and economic return inhibits renewal of orchards, resulting in long orchard life spans (Gallardo et al. 2022).
Rootstock genetic uniformity
The genetic base of pear rootstocks is even more narrow than that of scions. Nearly all rootstocks currently used for commercial production in the U.S. are derived from the cultivar ‘Old Home’. This includes the ‘Old Home’ × ‘Farmingdale’ (OH × F) series developed in Oregon, and ‘Pyrodwarf®’ and ‘Pyro™ 2–33’, two rootstocks from Germany (Elkins et al. 2012; Jacob 2002). ‘Horner 4’ was developed in Oregon through open pollination of OH × F clones (Mielke and Smith 2002). Analyses based on microsatellite fingerprinting (Postman et al. 2013) and more recent SNP array genotyping (Montanari et al. 2020) have shown that the pollen parent of the most common OH × F clones is actually ‘Bartlett’.
Seedling rootstocks were used almost exclusively in the U.S. until about 30 years ago. Because seedling rootstocks are derived from parent cultivars, which are highly heterozygous and self-incompatible, they are not genetically identical, although they are usually sufficiently uniform in important traits. Seedlings of 'Bartlett' predominate in older plantings, with seedlings of 'Winter Nelis’ being the next most widely used seedling rootstock, both P. communis cultivars (Elkins et al. 2012). Seedlings of P. betulifolia are used when high vigor is needed in clay or poorly drained soils. Certain selections of P. betulifolia (i.e., “Reimer” selections) are also the predominant choice for Asian pear cultivars, along with ‘OH × F 97’ more recently. Seedlings of P. calleryana are also used as rootstocks in warmer regions (Reil et al. 2007).
In Southern Oregon, quince (Cydonia) is used commercially as a rootstock for ‘Doyenné du Comice’. BA29C is the predominant clone, and limited plantings include other quince cultivars (‘Adams’, ‘EMC’, ‘EMH’, ‘Sydo’, ‘Quince A’, and ‘Quince C’) (Elkins et al. 2012; Sugar and Basile 2011). These cultivars were derived from different populations in France, or bred and released by East Malling Research Station (Tukey 1964; Webster 2003). Use of microsatellite markers has shown a high degree of similarity between quince cultivars (Bassil et al. 2011; Yamamoto et al. 2004).
Characteristics driving rootstock plantings
Clonally propagated Pyrus communis rootstocks from the ‘Old Home’ × ‘Farmingdale’ (OH × F) series are used for most of the commercial production in the U.S., with ‘OH × F 87’ and ‘OH × F 97’ used most widely. These clonal rootstocks offer semi-dwarfing size control of scions. While ‘OH × F 333’, perhaps the most easily propagated clone, was once the most widely planted, it produces high yields of small fruit and has been surpassed. ‘OH × F 69’ has also been planted but is more difficult to propagate. ‘Pyrodwarf®’ and ‘Pyro™ 2–33’ have exhibited similar scion growth control as that of ‘OH × F 87’, though ‘Pyro™ 2–33’ has exhibited more vigor in some trials (Elkins et al. 2021; Jacob 2002). They have seen limited adoption however, particularly ‘Pyrodwarf®’, which suckers profusely. Selections of the open-pollinated ‘OH × F Horner’ series have been tested on the West Coast, with ‘Horner 4’ showing promise as a vigorous but precocious replacement for P. betulifolia and other standard rootstocks (Einhorn et al. 2014; Elkins 2019). Seedlings of P. calleryana exhibit variable growth and susceptibility to pear psylla feeding and pear decline (Reil et al. 2007). ‘Bartlett’ and ‘Winter Nelis’ are both highly susceptible to fire blight, while P. betulifolia has been used to generate seedlings resistant to fire blight (Elkins et al. 2012; Lombard and Westwood 1975).
Pear breeding efforts
Breeding of new cultivars is currently accomplished by crossing distinct accessions with desirable traits and selecting among segregating seedling populations. Major objectives of global pear breeding programs have been reviewed (Bellini and Nin 2002; Brewer and Palmer 2011). There are many shared objectives, and well as interesting specific objectives. In the U.S., there are two active pear breeding programs: a scion breeding program at the USDA Agricultural Research Service (ARS) Appalachian Fruit Research Station (AFRS), and a rootstock breeding program at Washington State University (WSU).
The USDA ARS AFRS scion breeding program has historically focused on the development of cultivars with resistance to fire blight, Fabraea leaf spot, pear scab, and pear psylla, and improved postharvest quality and storage life, utilizing P. ussuriensis x P. communis hybrids and P. communis germplasm from Eastern and Central Europe (Bell 1991; Bell and Stuart 1990; Bell and van der Zwet 1998). Current efforts are focused on evaluation of elite and prospective lines, as well as developing new populations for trait mapping and cultivars for the fresh and processing pear industries. Breeding targets for scions include fruit quality, appropriate flowering and fruiting seasons, disease and arthropod pest resistance, ripening and storage quality, and ideal architectures for production and harvest. The program released three cultivars in 1960, and six from 1992 to 2022.
One of the greatest needs of the U.S. pear industry is availability of stress-resistant rootstocks that will promote dwarfing, precocity, and productivity of fruiting cultivars (Elkins et al. 2012). The wide range of adaptation to various soil types, temperature, moisture, pH, and nutrients as well as to soil-borne insects, nematodes, and diseases of Pyrus species suggests that there are many opportunities to identify improved pear rootstocks (Lombard and Westwood 1987). Washington State University at Wenatchee, WA, initiated a new U.S. pear rootstock breeding program in 2015, with initial focus on breeding Pyrus rootstocks conferring dwarfing and precocity to scions. Supporting this effort is a new USDA ARS program, also in Wenatchee, focused on understanding mechanisms of key pear rootstock traits. The multi-state NC-140 program has evaluated multiple rootstock selections since 1987, including OH × F clones, OH × F Horner clones, and several European selections (Einhorn et al. 2013; Elkins et al. 2011; NC-140, 2023). Available rootstocks for high density pear plantings have been recently reviewed (Einhorn 2021).
In addition to public breeding programs in the U.S., there are several commercial programs, as well as both public and commercial programs internationally. The commercial breeding programs in the U.S. are Subarashii Kundamono and Virginia Gold Orchard, which breed Asian (P. pyrifolia) fruit cultivars for their own production and marketing. There are many international pear breeding programs, focused on scions, rootstocks, or both (Table S2). They are largely focused on developing cultivars and rootstocks that produce new, high-quality fruit and resistance to local biotic and abiotic threats and conditions.
Global threats of genetic erosion and conservation efforts of in situ reserves
Genetic erosion
Globally, wild populations of Pyrus are faced with threats from logging and firewood cutting, overgrazing by livestock, excessive fruit harvest, and other agricultural and urban development. In addition, indigenous landrace cultivars are being replaced by more modern cultivars. Further, hybridization between wild species and commercial cultivars threaten local wild populations. According to the International Union for the Conservation of Nature and Natural Resources (IUCN), Pyrus taxa of particular concern are five critically endangered species (Pyrus browiczii, P. gergerana, P. korshinskyi, P. tadshikistanica, and P. voronovii), seven endangered species (Pyrus cajon, P. daralagezi, P. hajastana, P. nutans, P. tamamschianae, P. sosnovskyi, and P. theodorovii), and two vulnerable species (P. complexa and P. serikensis) (Table S3 and IUCN (2022)). Pyrus taxonomy is complex, however, with many species having limited definitions and likely many synonyms. At times, initial descriptions may have come from small populations or species hybrids, the latter of which can be easily reconstituted from the parent species that is not rare or endangered. This makes it challenging to compare lists of taxa on the IUCN Redlist (IUCN 2022) to those in genebank collections, and difficult to determine true threatened status. The NPGS has only five accessions of P. korshinskyi and two accessions of P. complexa, the only species on the above lists that are listed in GRIN Taxonomy, representing a gap in the collection. Based on molecular data, P. korshinskyi in the collection may actually be P. communis subsp. caucasica or a complex hybrid, and P. complexa is a complex hybrid as the name suggests (Montanari et al. 2020). Güner and Zielinski (1996) state that P. serikensis was “known until recently as P. boissieriana subsp. crenulata”, which GRIN recognizes as a synonym of P. cordata; the NPGS has 22 accessions of P. cordata. Several other species not recognized in the IUCN have been assessed as endangered or threatened, including P. elaeagrifolia subsp. bulgarica and P. yaltirikii in Turkey (Güner and Zielinski 1996), wild populations of P. communis ssp. pyraster in Germany and the Czech Republic (Endtmann 1999; Sĭndelář, 2002), P. calleryana in Japan (Ohba 1996), and P. kawakamii in Taiwan (Lear and Hunt 1996). The International Dendrological Society also lists P. magyarica as either endangered, vulnerable, or rare; however, it is only described in Hungary and likely a synonym of P. communis subsp. pyraster, (Barina and Kiraly 2014). Together, these mixed assessments highlight the importance improving access to accessions of these potential species, so that geneticists can better address their taxonomic positions and threatened status.
In situ reserves conservation efforts
In situ conservation, which refers to the preservation of wild populations of taxa in their natural range, is rare. In Germany, surveys have been made to identify endangered natural populations of P. communis subsp. pyraster (Fellenberg et al. 2000; Wolf et al. 2000). Six populations have been conserved in Germany and additional in situ populations have been conserved in the Czech Republic (Kleinschmidt et al. 1998; Paprštein et al. 2010, 2002; Wagner 1999). Other such efforts at in situ conservation have been planned for the Middle East (Amri et al. 2002). Surveys for natural populations of Pyrus and Cydonia have also been conducted in Albania (Kullaj et al. 2012), Azerbaijan, and Georgia (Akparov and Musayev 2012; Maghradze et al. 2012). Efforts to work with local people to conserve native forests are ongoing in Central Asia, particularly Kyrgyzstan (Eastwood et al. 2009). In a report issued by the Food and Agriculture Organization, Maxted and Kell (2009) listed in situ reserves including pear in Azerbaijan (15 sites, 6500 ha), Georgia (1 site, 6822 ha), Germany (1 site, 374,432 ha), Moldova (5 sites, 19,300 ha), and Turkey (1 site, 21,300 ha). More recent assessments additionally include protected areas in Kyrgyzstan (5 sites, 173,688 ha), Tajikistan (14 sites, 2.77 million ha), Turkmenistan (2 sites, 189,910 ha), and Uzbekistan (1 site, 122,730 ha) (Lapeña et al. 2014). In situ conservation has been extended to include on-farm conservation of landrace cultivars, either as new orchards or as preservation of existing trees and naturalized seedlings (Paprštein et al. 2010, 2011). Orchards of 91 pear landraces have been established in the eleven localities of the Czech Republic.
Current and emerging biotic, abiotic, production, and market vulnerabilities
Biotic vulnerabilities
The U.S. pear crop can be affected by many pathogens and arthropod pests (Sutton et al. 2016). Pathogens and especially arthropod pests can mutate to overcome pesticides and other control measures, and thus, constitute a continuing threat to the industry. A cost analysis determined the total cost per acre to control diseases, arthropod pests, and weeds for ‘Bartlett’ pear in southern Washington to be $1422, beginning in the 4th through 6th years after planting (Gallardo et al. 2022). The major endemic diseases and pests are discussed below and summarized in Table S4 (Elkins et al. 2023; Murray et al. 2020; Sutton et al. 2016; WSU 2023).
Bacteria, Fungus, and Virus-caused Diseases
Bacterial blossom blast or Pseudomonas blight
Incited by the bacterium Pseudomonas syringae pv. syringae Van Hall, this disease can significantly decrease the crop in all production regions but is more important where cold and wet conditions prevail in late winter and spring, such as northern Oregon. The pathogen infects blossoms and young leaves, and can spread into woody spurs and, infrequently, to branches. Most European and Asian pears are susceptible, although ‘Forelle’ and ‘El Dorado’ have been rated as moderately resistant. Red-skinned mutants of ‘Beurré d’Anjou’ and ‘Bartlett’ appear to be less susceptible than the green-skinned cultivar to canker (i.e., trunk) infections associated with cold temperature injury.
Fire blight
The most serious disease of pears is caused by the bacterium Erwinia amylovora (Burrill) Winslow et al., and can infect blossoms, shoots, leaves, scaffolds, trunks, and rootstocks, potentially resulting in tree death of susceptible cultivars. Most major cultivars in production in the U.S. and elsewhere are highly susceptible (van der Zwet and Keil 1979). The primary infection court is the blossoms, so control methods target that organ. Although rare, infection through susceptible rootstock suckers can also occur. It is difficult to prevent and control, requiring prophylactic applications of copper compounds, antibiotics, or biological controls. The biology, disease cycle, and management of the disease has been reviewed by Kharadi et al. (2021) and van der Zwet et al. (2012). It has been a major factor in the restriction of large-scale commercial production to the warmer and drier interior valleys of the Pacific coast states. Development of strains of the bacterium which are resistant to streptomycin and terramycin, antibiotics used to prevent blossom and, to some extent, shoot infections, highlight the vulnerability of the industry. Of the major European (Pyrus communis) pear cultivars, ‘Seckel’ is moderately resistant to this disease, while ‘d’Anjou’ (including ‘Gebhard Red’ and ‘Striped’), ‘Le Conte’, ‘Olia’, ‘Winter Cole’, and ‘Winter Nelis’ have been reported as both resistant and susceptible. Of the major cultivars of Asian pears, the P. x bretschneideri cultivars, ‘Ya Li’ and ‘Tzu Li’, are at least moderately resistant. The P. pyrifolia cultivars, including the widely planted ‘Housui’, ‘Nijisseiki’, and ‘Shinseiki’, are almost uniformly as susceptible as P. communis cultivars, but ‘A Ri Rang’ (syn. ‘Dan Bae’, ‘Korean Giant’, ‘Olympic’), ‘Shinko’, ‘Meigetsu’, ‘Seuri’, and ‘Immamura Aki’ appear to be less severely infected than most Asian cultivars (Bell 1991).
Collar and root rot
The disease is caused by infection of fungal Phytophtora species, principally, P. cactorum (Lebert & Cohn) Schröter. The disease is not as important in pear orchards as in apple orchards, where it can also infect rootstock crowns. Trees typically exhibit poor growth and leaf chlorosis. Necrotic and orange to red inner phloem tissue, revealed after bark removal, is symptomatic of the disease. Slow decline is typical, but sudden tree collapse can occur.
European pear scab
The fungal pathogen Venturia pirina Aderh. can cause severe infection of leaves and fruit in seven of the 11 major cultivars for which reasonably reliable or repeatable observations have been made. ‘Bartlett’, ‘Conference’, and ‘Dr. Jules Guyot’ have been reported to be resistant, although artificial inoculations in a greenhouse produced symptoms on ‘Bartlett’ as well as ‘Crimson Gem Comice’ and ‘Sensation Red Bartlett’ (Postman et al. 2005). ‘Forelle’ was the least susceptible of the major U. S. cultivars. Asian accessions were much less susceptible to both leaf and fruit infection. Westwood (1982) reports that P. pyrifolia is variable for resistance, and P. ussuriensis is resistant. Presumably, P. × bretschneideri will be heterogeneous. Control requires repeated applications of fungicide, especially in the more humid production areas.
Oak root rot
Also known as Armillaria root rot, the disease is caused by Armillaria mellea (Vahl) P. Kumm., a soil-borne fungal pathogen. The disease has been diagnosed in California and southern Oregon where orchards have replaced oak trees. It is associated with increased levels of irrigation and can infect rootstocks previously thought to be resistant. Symptoms include slow decline in vigor, and sometimes rapid wilting. Dense white mycelial plaques form around the crown under the bark, and infected wood becomes spongy. It is frequently present in cool, moist soil. When associated with irrigation, fruit rots can occur; in these cases, it is also known as sprinkler rot. Control is difficult and there are no pesticides approved for use on existing orchards. Pre-plant fumigation, traditionally with methyl bromide, but more recently using other fumigants, is the best preventative control, with shallow crown planting as an added precaution. Reducing irrigation frequency and keeping soil removed from the crown and upper root systems are cultural practices (Elkins et al. 1998). The University of California, Davis, has also developed an in vitro screening procedure for oak root rot (Tweedy 2021).
Powdery mildew
Powdery mildew, caused by the fungal species Podosphaera leucotricha (Ell. & Ev.) Salm., can cause damage to leaves, russet on fruit, and is particularly a problem in the Pacific Northwest. ‘Bartlett’ and ‘Beurré d’Anjou’ are moderately susceptible and susceptible, respectively, while ‘Winter Nelis’ is reportedly moderately resistant (Fisher 1922). In a study of the Pyrus core collection, the most susceptible cultivars were ‘Bartlett’, ‘Crimson Gem Comice’, ‘Gebhard Red d’Anjou’, and ‘Untoase de Geoagiu’, while Asian accessions were in general more susceptible, but heterogenous in their response (Kanato et al. 1982; Serdani et al. 2006; Westwood 1982).
Cankers
European canker is caused by the fungus Neonectria ditissima (Tul. & C. Tul.) Samuels and Rossman, and is primarily found along the Pacific coast, where rainfall is highest. Pear is less susceptible than apple; however, the fungus can cause eye rot and bull’s-eye rot, similar to other cankers (Murray et al. 2020). Valsa canker, caused by Valsa ceratosperma (Tode ex Fr.) Maire, is common in Japan, Korea, and China, and has also been observed in North America. It affects the bark, resulting in branch girdling and death. No curative fungicides are available, but some fungicides may provide preventative control. It is widespread on P. ussuriensis and P. × bretschneideri cultivars but is less severe on P. pyrifolia cultivars. For the purposes of this report and assessing impact, P. communis will be assumed to be susceptible. The disease was reported to cause serious damage to pear in Italy in 2001 (Montuschi and Collina 2003). Other cankers have been identified that are mainly minor threats, for example pear branch canker, caused by Diplodia seriata De Not. (Choudhury et al. 2014).
Pacific Coast pear rust
Caused by the fungus Gymnosporangium libocedri (Henn.) F. Kern, this rust alternates between conifer hosts and hosts in the Rosaceae family. Typically, incense cedar, Calocedrus decurrens (Torr.) Florin, is the conifer host. Symptoms include fruit that becomes malformed and drops early and yellow-to-orange spots and pustules on the fruit surface (Murray et al. 2020).
Leaf, branch, and fruit disease
This fungal pathogen, Guignardia piricola (Nose) Yamamoto (syn. Botryosphaeria berengeriana f. sp. piricola (Nose) Koganezawa & Sakuma or Physalospora piricola (Nose)), exists in Japan, and is related to apple ring rot. It is listed by APHIS as an exotic pathogen, but it has been reported to be identical to Botryosphaeria dothidae (Moug.: Fr.) Ces. & De Not., which is present in the U.S. (Farr et al. 1989; Slippers et al. 2004). The Japanese authors, however, consider it to be distinct. It is presumed that European pears are susceptible. It may be subject to control by the same fungicides used to control white rot (i.e., “bot rot”), caused by Botryosphaeria dothidea, a common disease in the U.S.
Other fungal leaf and fruit spot diseases
Fabraea leaf spot, Fabraea maculata Atk., also known as Diplocarpon mespili (Sor.) Sutton (anamorph Entomosporium maculatum Lev.) can cause severe defoliation and fruit spots on most major European pears, although ‘Bartlett’ is moderately resistant. Asian cultivars are generally more resistant, but not immune. A second pathogen, Mycosphaerella sentina (Fckl.) Schroet., causes a minor leaf spot which is primarily a problem in Europe. Both of these diseases can be controlled by frequent fungicide application, and they are not of concern in the dry Pacific coast production regions. Black spot is caused by the fungal pathogen Alternaria alternata (Fr.) Keissler and is primarily a postharvest disease.
Postharvest fruit rot diseases
Several fungal pathogens which infect the fruit, either pre-harvest or postharvest, can account for as much as 30% loss of fruit in storage. The major diseases of pears are blue mold (Penicillium expansum Link), gray mold (Botrytis cinerea Pers.), Coprinus psychromorbidus Redhead & Traquair (especially in the Hood River, OR, and Wenatchee, WA, districts), Mucor rot (Mucor piriformis E. Fischer), side rot (Phialophora malorum (Kidd & Beaumont) McColloch and Cladosporium herbarum (Pers.) Link), and bull's-eye rot (Neofabraea malicorticis [syn. Pezicula malicorticis (H. Jacks.) Nannf,]). Other minor diseases that also cause fruit decay in the orchard include Alternaria rot (Alternaria alternata (Fr.) Keissler), bot rot (Botryosphaeria obtusa), black rot (Sphaeropsis malorum), white rot (Botryosphaeria dothidea (Mong.) Ces. & De Not.), bitter rot (Colletotrichum gloeosporiodes (Penz.) Penz. & Sacc.; teleomorph Glomerella cingulata (Stonem.) Spauld. & Schrenk), brown rot (Monilinia fructicola (Wint.) Honey), and sprinkler rot (Phytophthora cactorum (Lebert & Cohn) Schröter). All major cultivars are susceptible to these diseases. ‘Beurré Bosc’, in particular, is highly susceptible to side rot. Further, there are three species of brown rot pathogens that cause fruit rots of pear. Monilinia fructicola is present in the U.S., whereas M. fructigena Honey is the most common species in Europe and is the one of quarantine significance. A third species, M. laxa (Aderh. & Ruhl.) Honey rarely causes fruit rot on pear. All European pear cultivars are presumed to be susceptible to these pathogens; however, no information on Asian cultivars could be found.
Three new postharvest pathogens have been reported on ‘Beurre d’Anjou’ in Washington State. One is caused by Phacidiopycnis piri (Fucke) Weindlymayr, the anamorph of Potebniamyces pyri (Berkeley & Broome) Dennis, which is associated with bark necrosis and twig cankers of pear in the Pacific Northwest (Xiao and Boal 2004, 2005; Xiao et al. 2005). The second, caused by Sphaeropsis pyriputrescens sp. nov., occurs at a low and sporadic level in some Washington orchards (Xiao and Rogers 2004). Stem and calyx end rots develop in storage. The pathogen overwinters in cankers and twigs. The third, a recently reported and quarantined disease, Yellow-Lambertella rot, is caused by Lambertella corni-maris von Höhnel (Amiri et al. 2017). Infection occurs through skin cuticle wounds, so avoiding stem punctures is important for control. Infected fruit have spongy lesions with white mycelium, which changes to compact yellow mycelium.
In the case of pathogens which produce incipient or quiescent symptoms in the orchard or harvest bins (gray mold, bull’s eye rot, white rot, black rot, bitter rot, brown rot, sprinkler rot, and probably Sphaeropsis rot), orchard sanitation, including pruning diseased branches, and prophylactic fungicide sprays will significantly reduce the amount of fruit rot developing later during storage. Other pathogens (blue mold, Mucor rot) primarily infect wounds caused by stem punctures and bruises during harvest or postharvest handling and packing. Fruit loss to these diseases can be reduced by fungicide dips and the practice of wrapping individual fruit in copper sulfate impregnated papers. Both types of control measures may be effective against Phacidiopycnis rot.
Pear decline
This disease occurs throughout North America and was responsible for the extensive death of commercial pear orchards in the 1950’s to early 1970’s until the causal pathogen, vector, and biology were elucidated and control methods investigated (Gubler et al. 2007). It is caused by a phytoplasma, ‘Candidatus Phytoplasma pyri’ (Seemüller and Schneider 2004) transmitted primarily by the pear psylla, Cacopsylla spp. (Hibino and Schneider 1970). It causes sieve-tube necrosis below the graft union and is particularly severe when scion cultivars of the generally tolerant species, P. communis, are grafted onto rootstocks of the sensitive species P. pyrifolia or P. ussuriensis. Use of these latter rootstocks has been rare since the problem was recognized and tolerant rootstock substituted, along with adequate suppression of the pear psylla vector (Mitcham and Elkins 2007). All of the major P. communis cultivars are apparently moderately tolerant to varying degrees, with the exception of ‘Clapp Favorite’ and ‘Conference’, which are susceptible (Graf 1977).
Viruses and viroids
Viruses and viroids are typically only graft-transmissible and are not known to be insect or seed-transmissible. Therefore, the use of certified pathogen-free budwood for propagation is the primary defense against these diseases. They vary in the degree of deleterious effects, and symptoms of virus-caused diseases for which agents have been identified are summarized in Table S4. Molecular tests and identification (i.e., immunoassay, PCR, and qPCR) are available to help determine whether viruses or viroids are present in a symptomatic sample. Efforts to move from immunoassays and PCR methods to more high-throughput qPCR and next-generation sequencing (NGS) have been made. Currently, imported pome fruit are screened for over 19 + viruses and viroids before release. Diseases for which no known agents have been determined with solid evidence have been avoided here. Diseases with known agents that are of quarantine significance are listed in Table S5.
Arthropod pests
Integrated pest management programs and collaborations for U.S. pear growing areas have developed detailed and regularly updated histories and descriptions of common arthropod pests of the standing crop (Elkins et al. 2023; Murray et al. 2020; Sutton et al. 2016; WSU 2023). Descriptions of pests, damage symptoms, and treatment and mitigation strategies are summarized below and in Table S4.
Pear psylla
Cacopsylla pyricola Förster is the single most expensive pest to control in many pear production districts in North America, where it is not under natural biological control. Psylla, both nymphs and adults, primarily feed on the phloem and xylem of leaves and young shoots, and secrete a sticky honeydew, which coats the fruit. The honeydew itself can russet fruit, as well as act as a growth medium for sooty mold. Severe infestation can cause psylla shock, stunted trees, defoliation, and fruit drop. Pear psylla transmit the pear decline phytoplasma. Progress in biological control and integrated pest management (IPM) has been made; however, the level of control necessary still requires the use of substantial amounts of insecticide where biological control is inadequate.
Codling moth
Cydia pomonella L. is a serious pest, damage from which results in unmarketable fruit. It is considered the primary pest of pear in most IPM programs. Eggs are laid on the fruit surface, and the larvae feed directly on the fruit and bore into the flesh, frequently as far as the core where they feed on the seeds, leaving holes with frass protruding. As many as three broods per season can be produced. Insecticide resistance has become an issue, but advances in pheromone mating disruption and integrated pest management have improved control.
Webspinning spider mites
Two spotted mite (Tetranychus urticae Koch) leaf feeding causes necrotic areas on the leaves and defoliation if uncontrolled. Fall defoliation may lead to fall blooming and reduced crop the following year, as well as reduced winter hardiness. European red mite, Panonychus ulmi (Koch), can be an occasional pest, causing mottled leaves, leaf bronzing, and rarely, defoliation. Biological controls (i.e., predatory mites and other insects) can effectively control mites, especially with the use of mating disruption for codling moth and “soft” pesticides.
Eriophyid mites
The pear rust mite (Epitrimeris pyri (Nalepa)) causes fruit russeting and damage to leaves (e.g. bronzing). It is a cyclical pest and can be a particular problem in organic orchards. Pear leaf blister mite (Eriophyes pyri (Pagenstecher)) leaf feeding causes blisters which lead to late season leaf necrosis and premature leaf drop. The loss of leaves can weaken the tree, retard fruit maturation, and reduce fruit bud development. There can also be some damage to fruit caused by pre-bloom feeding on buds. Leaf blister mite is also primarily a problem in organic orchards.
Grape mealy bug
Pseudococcus maritimus (Ehrhorn) honeydew secretions cause a rough fruit russeting. Infestation of the calyx can cause fruit rots in storage. Various biological control insects may help to control this pest, and there are several insecticides that provide good control, while sucker removal reduces overwintering sites.
Leafrollers
There are four species of leafrollers, which are the larvae of torticid moths. They are sporadic pests in California and Pacific Northwest orchards. The obliquebanded (Choristoneura rosaceana (Harris)), fruit tree (Archips argyrospila (Walker)), European (Archips rosana (Linnaeus)), and pandemis (Pandemis pyrusana Kearfott) leafrollers are the principal pear pests. The larvae roll the leaves to provide shelter, and feed on leaves, buds, and the fruit skin. Early season fruit feeding results in fruit abortion or deformed fruit with corky scars. Late season damage often occurs between fruit in clusters. Leafrollers can develop resistance to insecticides very quickly.
Sawflies (pear slug)
Three species of sawfly are found as minor pests for pears (Caliroa cerasi, Pristophora abbreviate, and Ametastegia glabrata), as their larvae eat round holes in the leaves. When feeding is heavy, only the midribs of leaves may remain. Regular spray programs, however, keep sawflies under control in most cases. Occasionally, high populations may occur in organic pear orchards.
San Jose scale
Quadraspidiotus perniciosus (Comstock) feeds on bark, leaves and fruit, and large populations can kill shoots. Fruit damage includes a red halo on the fruit skin around the scale’s body, which usually results in fruit culling. Thorough dormant sprays usually control this pest.
Stink bugs and Lygus bugs
The consperse stink bug (Euschistus consperus Uhler) is a common stink bug. Damage is expressed as shallow dimples on the fruit, with a brown, pithy area in the flesh. The tarnished plant bug, Lygus lineolaris (Palisot de Beauvois), the brown lygus bug (Lygus hespersus), and the green lygus bug (Lygus elisus) are the common lygus bugs. Feeding damage to developing buds is usually not serious, but early-season feeding on the fruit results in raised pustules and deformed fruit, and late season feeding leaves depressions in the fruit similar to stink bug damage. A recent and potentially serious pest (Leskey et al. 2012) is the brown marmorated stink bug (Halyomorpha halys Stål). Feeding results in fruit sunken areas of the fruit and brown corky tissue under the skin and in the flesh (Leskey et al. 2009). It is an invasive pest which was introduced in the mid-1990s in Pennsylvania and has spread throughout the Mid-Atlantic into New York, westward into Michigan, and throughout the pear growing areas of eastern Washington, the Willamette Valley in Oregon, and central California.
Abiotic and physiological vulnerabilities
Climate-related vulnerabilities
Nationally, Pyrus accessions are grown across a range of diverse geographical areas and climates, which can affect key developmental processes such as entrance into dormancy, cold-hardiness of wood and buds, accumulation of chilling hours, and bud break. Climate change has introduced more unpredictability and extreme temperature events that can have major impacts on these processes. Late fall and early spring warming can shorten the number of chilling hours accumulated, particularly in warmer climates, which leads to issues with bud break (Vyse et al. 2019). In cooler climates, where conditions for chilling hour accumulation are more reliably met, spring warming in combination with late spring frosts can cause extensive damage to buds that have broken dormancy. Further, successful pollination or parthenocarpic fruit set earlier in the season can shift the entire developmental timeline, such that harvest occurs earlier and at a warmer part of the summer, with higher risks for heat-related injury to fruit. In fact, a study on pome fruit in Romania demonstrated significant shifts in the timing of phenological stages of pears and apples from 1969 to 2018 (Chitu and Paltineanu 2020). While extreme summer heat events are also becoming more frequent (Coumou and Rahmstorf 2012), the effect of heat stress on pear fruit is not well known. As orchards adopt more high-density systems, fruit exposure to sunlight may become more of an issue. A warming climate is also predicted to lead to decreased and/or unpredictable rainfall, or decreased irrigation water supplies in areas that depend on snow melt-off (IPCC 2023). Therefore, drought resistance will become more critical for both scion and rootstock cultivars. Climate predictions for Corvallis, Oregon, the location of the Pyrus germplasm collection, include increasing minimum and maximum temperatures, and decreased precipitation in the drier parts of the year (Fig. 3). Climate extremes impact both scion and rootstock health. The effects of winter warming on fruit tree phenology will be complex and difficult to predict or make generalized statements across growing regions (Darbyshire et al. 2014). A few investigations have begun to identify genes and genetic regions associated with breaking dormancy in Pyrus species (Anh Tuan et al. 2016; Gabay et al. 2019; Zhang et al. 2015). Further study of climate impact on pears, and application of research from other pomes, such as apple, will be necessary to better prepare for future abiotic stresses. Important abiotic factors for pears also include soil characteristics. Iron chlorosis due to high pH soils continues to be a problem, particularly in California; therefore, evaluation of germplasm leading to the selection or development of rootstocks with tolerance to this soil factor will be needed. Likewise, adaptation to a range of soil types and moisture content is needed. The interaction of abiotic and biotic threats should also be noted, as climate change alters factors like pest life cycles and soil microbiome composition (IPCC 2023; Singh et al. 2023).
Climate predictions for Corvallis, OR, location of the NCGR Corvallis Pyrus collection. A Maximum temperature for the warmest month, under historical temperatures (1970–2000) and four Shared Socioeconomic Pathways (SSP) prediction scenarios (cite IPCC report). SSPs have been used in the IPCC Sixth Assessment Report on climate change and are determined by Integrated Assessment Models, which consider socioeconomic and climate pathways. SSP1-2.6 represents a model in which greenhouse gas (GHG) emissions decline to a net zero by 2070, SSP2-4.5 represents GHG emissions remaining around current levels, and SSP3-7 and SSP5-8.5 represent models where GHG emissions double by 2100 and 2050, respectively (IPCC report). B Predicted minimum temperature for the coolest month. C Precipitation for the driest months predicted for Corvallis, OR. Graphs were generated by the NPGS Climate Futures application (https://geocentroid.shinyapps.io/npgsclimatefutures). (Color figure online)
Physiological disorders
Pear fruit are subject to several physiological postharvest disorders. Core breakdown, characterized by a softening and browning of the flesh, is associated with overmature fruit, but cultivars differ in their susceptibility. Superficial scald is characterized by the development of brown skin following removal from cold storage. ‘Beurre d’Anjou’ is particularly susceptible. Predisposing factors are immaturity at harvest, low calcium, high fruit nitrogen, and other handling factors. Senescent scald affects the entire fruit, which fails to ripen. Cork spot of ‘Anjou’ pears results in an uneven fruit surface, with corky lesions under the skin. It is associated with low fruit calcium and high nitrogen content of the fruit. Pink calyx of ‘Bartlett’ is a premature ripening disorder associated with cool growing conditions starting about a month before harvest (Mellenthin and Wang 1977). Sunburn and heat damage is associated with water stress and poor canopy vigor exposing fruit mainly on south and west exposures in the Northern hemisphere.
Production and market vulnerabilities
Production related vulnerabilities
The lack of adapted rootstocks that induce precocious and high sustained yields while controlling tree size, to a level which is easier to manage and harvest, is perhaps the single most important vulnerability of pear production in the U.S. (Elkins et al. 2012). To maintain an economically viable industry, rootstocks are needed that produce trees 50% the size of those on Pyrus communis seedling (e.g. ‘Bartlett’ and ‘Winter Nelis’ seedling) rootstock, induce cropping in the third year after planting, have resistance to fire blight and pear decline, and are cold-hardy in temperate regions of the country.
Market vulnerabilities and consumer preference
Pear consumption in the U.S. has generally deceased over the last 20 years. Per capita consumption of fresh pears was highest in the year 2000 at 3.39 pounds and decreased to a low of 2.67 pounds in 2015 (ERS and USDA 2022). Since 2015, per capita consumption has risen back to 3.12 pounds in 2021 (ERS and USDA 2022). This overall decrease in fresh pear consumption and lack of full return to peak levels could be attributed to a number of reasons, including the difficulty in postharvest storage, ripening, supply chain processes associated with the pear market, and consumer preference.
The storage and ripening (cold conditioning) requirements of European pears have been long-term vulnerabilities to the crop. The vast majority of European pear cultivars and cultivars require a subjective assessment of harvest timing and complicated cold storage ripening requirements (Sugar 2007). For harvest timing, most pear cultivars are picked when they reach a target firmness, sugar level, and to a lesser extent, exhibit desirable coloration. Complications from fruit position within the tree, tree size, rootstock, and seasonal environmental conditions, however, can hinder fruit maturation processes. Moreover, the cold condition requirement is highly genotype-dependent and is not always uniform between growing sites within a season (Kupferman et al. 2010; Villalobos-Acuna and Mitcham 2008). Once in storage, markets demand variable removal times. The use of 1-Methylcyclopropene (MCP) is abundant in extending storage times of apple and pear, but the effect in pear is complicated by the delicate balance of delaying ripening versus complete inhibition of ripening (Zhang et al. 2020). Considering that, 1-MCP has been used to delay ripening for ‘Bartlett’, ‘Beurré Bosc’, and ‘Beurré d’Anjou’ and preserve eating qualities, but has been found to negatively affect eating quality of ‘Doyenné du Comice’ (DeEll and Ehsani-Moghaddam 2011; Guo et al. 2020; KC et al., 2022).
Many points throughout the supply chain highlight the vulnerabilities of pears. Firstly, pears are highly susceptible to mechanical and frictional damage. This damage typically occurs during the shipping and handling process, such as scuffing due to rough surfaces and coarse movements on conveyor belts, peel-to-peel or peel-to-crate contact, and bruising during the shipping process due to improper packaging and, again, coarse or high-frequency movements of the transport vehicles (Berardinelli et al. 2005; Meheriuk et al. 1994). Due to the high phenolic content of the peels and activity of polyphenol oxidase (PPO), browning of the damage area occurs rapidly when membrane integrity is damaged (Franck et al. 2007; He and Luo 2007; Meheriuk et al. 1994). Following enzymatic browning, the fruit becomes unmarketable due to its appearance and increased susceptibility to postharvest rots (Franck et al. 2007; He and Luo 2007). There have been attempts to address these challenges, however, through application of antioxidant coats to protect the fruit from browning with some success (Feng et al. 2004).
Consumer preferences also shape the vulnerability of Pyrus. The United States has been traditionally a European pear market. Turner et al. (2005) and Elkins et al. (2008) published research using 6–10 cultivars for consumer preference tests with European pears. The most important factors to determine consumer preference were related to texture, tartness and sourness, and juiciness. The top performing cultivars, alternative to ‘Bartlett’, were ‘Concorde’, ‘Blake’s Pride’, ‘Sunrise’, and US71665-014 (‘Gem’). A more recent evaluation of six early and late season pear cultivars found consumers remain interested in juiciness, melting texture, and sweetness, in that order (Colonna et al. 2023). More interestingly, consumers highly valued pears that change color during ripening. This result suggests consumers lack knowledge on determining optimal eating time for store bought fruit. Additionally, this study found ‘Paragon’ and ‘Bartlett’ as the most desirable cultivars (Colonna et al. 2023). The changing preferences in cultivars but consistent desire for melting flesh, sweet, and juicy European pears present an opportunity to market new cultivars. Another consideration is the increasing population of migrants from Asian countries resulting in an increase in production and sale of Asian pear types. Here, consumers have communicated their willingness to purchase cultivars such as ‘Yoinashi’, ‘Olympic’, ‘Shinko’, and ‘Atago’ (Walsh et al. 2016).
NPGS pear germplasm collection
Germplasm collection
The National Clonal Germplasm Repository (NCGR Corvallis) in Corvallis, Oregon houses the germplasm collections for Pyrus and related rootstock genera (USDA NPGS NCGR Corvallis 2023). It is a facility of the National Plant Germplasm System (NPGS), a program of the U.S. Department of Agriculture’s Agricultural Research Service. The facility was established in 1980, with the purpose of conserving temperate fruit and nut crops as living, ex situ collections. The NCGR Corvallis Pyrus collection spans the 37 species listed in GRIN. Pyrus and all related rootstock genera, with the exception of Amelanchier species and Sorbus americana Marshall, are not native to the United States, thus the collection is important for developing diverse gene pools for direct use and crop improvement. Germplasm is evaluated for desirable traits, as well as tested for viruses, viroids, bacterial, and fungal diseases. The climate in Corvallis is ideal for maintaining a globally diverse set of Pyrus germplasm, given its relatively mild weather and disease pressure.
Holdings, maintenance, security backups, and passport information
The NCGR Corvallis collection currently holds 2793 Pyrus germplasm accessions (Tables 2 and S6). These represent holdings from 59 countries, including 101 accessions from uncertain geographical origin. Additionally, the NCGR Corvallis collection holds 146 Cydonia oblonga L. (Quince) accessions from 19 countries of origin. The Pyrus collection is maintained as 392 seedlots stored at – 18 °C and 2111 clonal or seedling trees growing in 5.5 hectares of field plantings. Seedlots generally represent wild collected samples of primary Pyrus species. Approximately 48% of the field trees represent European pear cultivars, 9% are Asian cultivars, 5% are hybrid (Asian × European) cultivars, and 38% represent wild Pyrus species. Backup trees for about 500 less cold hardy or fire blight susceptible clones are maintained in small tube pots in a glasshouse. The Cydonia collection is maintained as 28 seedlots stored at – 18 °C, and 176 clonal or seedling trees growing in a 0.5 hectare orchard (USDA ARS 2023a).
Clonally propagated collections maintained in the field or greenhouse are particularly vulnerable to abiotic and biotic stresses. NPGS collections can be securely backed-up at the National Laboratory for Genetic Resources Preservation (NLGRP) in Fort Collins, Colorado. NLGRP offers both − 18 °C seed storage capacity as well as liquid nitrogen storage for cryopreserved materials. Pyrus seeds are classified as orthodox and can be stored at either − 18 °C or in liquid nitrogen (SER et al. 2023). At this time, seeds from 16 Pyrus accessions (representing original seeds from collection trips) are present at NLGRP.
Vegetatively propagated materials valued for their specific allelic combinations (i.e., cultivars) can be cryopreserved as either 1 mm shoot tips or as dormant bud segments. A slow-cooling method was developed and implemented to cryopreserve shoot tips from in vitro-grown pear plants (Chang and Reed 2000, 2001; Reed et al. 2013). The same slow-cooling method was also used at NLGRP to continue to cryopreserve accessions in the NCGR Corvallis Pyrus collection. More recently, a Pyrus dormant bud cryopreservation method has been implemented at NLGRP (Tanner et al. 2021). In total, 228 unique Pyrus accessions are preserved in liquid nitrogen at NLGRP as either shoot tips or dormant buds.
Passport data are recorded in GRIN-Global and are publicly available. Passport data include: collection site, general description of the site and the accessions, latitude, longitude, GPS coordinates, elevation, and habitat information. Other information recorded in GRIN-Global include accession number (PI, which refers to the unique Plant Introduction Number and/ or CPYR, for Corvallis Pyrus, a prefix for each pear inventory conserved at the NCGR Corvallis), collector (if from an exploration), date the accession was received, backup status, accession name, availability, narrative (about the accession), source history (development or collection information), pedigree, observation (phenotypic and genotypic data), images (mostly leaves or fruit), and vouchers (herbarium specimen), if available.
Distribution and outreach
Pear genetic resources are distributed as seed, dormant budwood (scion and budwood), leaves, fruit, pollen, and DNA. Scionwood is distributed during the dormant season in mid-winter in the United States and by far exceeds all other forms of pear material distributed. Lyophilized leaf material and/or DNA are increasingly shipped particularly overseas for genetic research. Since 2006, the NCGR Corvallis has distributed a minimum of 950 accessions per year to researchers worldwide (Fig. 4). While pear has been distributed to 24 foreign countries, distribution in the U.S. exceeds that sent to all other countries by tenfold. In 2020, the NPGS reduced distribution to home-gardeners, resulting in a significant drop in NCGR Corvallis Pyrus distributions. Most requested accessions over the past 40 years are summarized in Table S8.
Distributions of Pyrus germplasm from the NCGR Corvallis between 2000 and 2022. CPYR stands for Corvallis Pyrus, a prefix for each pear inventory conserved at the NCGR Corvallis. Numbers of accessions each year that are distributed nationally and internationally as in vitro propagules (CPYR_IV, blue), scion (CPYR_SC, orange), or seed (CPYR_SD, grey). (Color figure online)
U.S. and international visitors include students, researchers, breeders, growers, and other stakeholder groups. Occasionally open houses are held in the fall season during fruiting to highlight the diversity of the pear collection.
Acquisitions and explorations
Since 2007, 436 accessions have been acquired by the NCGR Corvallis. These include accessions from two species that were not previously available, P. × neoserrulata, and P. × sinkiangensis. We have also increased our holdings of the following species: P. communis and its subspecies communis, caucasica, and pyraster; P. korshinskyi; P. pyrifolia; P. salicifolia; P. spinosa (= P. amygdaliformis); P. syriaca; P. ussuriensis; P. × bretschneideri; as well as Pyrus species hybrid accessions. Acquisitions and most requested clones are reported in Tables S7 and S8.
Exploration excursions funded by the USDA over the past 100 + years have served as an important mechanism for acquiring germplasm and address gaps in the collection (Table S9) (Arnold Arboretum 2023; USDA ARS NCGR Corvallis 2022; van der Zwet et al. 1989, 1987). Trips taken in the early 1900’s contributed accessions that built the foundation of the collection as it exists today (Fig. 5). Pyrus centers of origin and diversity are widespread, and policies specific to each country affect access to their genetic resources. All legal frameworks must be followed for any exchange or exploration activities, including receiving appropriate permissions from landowners and governments, as well as abiding by the International Treaty on Plant Genetic Resources for Food and Agriculture (FAO 2009). Phytosanitary restrictions (Kinard 2020) and detailed guidelines on planning and conducting NPGS explorations must be followed (Williams 2020a, b). Seed and budwood is imported into the U.S. according to USDA-APHIS regulations and policies. A total of 50 vegetatively propagated accessions (budwood) of pome fruit species (apples, pears, quince, etc.) per year are accepted by the APHIS quarantine program. These clonal importations undergo a multiyear process of indexing and clean-up prior to provisional release. Seeds are also imported through the USDA quarantine program but are usually released much more quickly, because there are no known seed transmitted pathogens of pear. Importation of seed would be appropriate for wild populations.
Photographs from Frank C. Reimer’s expedition to China, Japan, and Korea in 1919. A Reimer traveling by donkey while investigating pears in northern China. B P. calleryana, medium sized, near Yihsien, Shantung, China. C trees in a P. betulifolia grove near Ping Ku, China. D fruit identified as P. uyematsuana, photographed at Yokkaichi, Japan. E P. betulifolia branch, leaves, and fruit at Nantai, China. F fruit of the ‘Ya Kuang Li’ cultivar, obtained at the market in Peking, China
Genetic coverage and gaps
World Pyrus taxa are very well represented in USDA genebank holdings. Several endangered or critically endangered species noted above are not present at the NCGR Corvallis, but the validity of these taxa are in question and living specimens are not available to study. Pyrus taxa recognized by USDA ARS that are not well represented include: P. cossonii (5 accessions), P. gharbiana (7 accessions), P. korshinskyi (18 accessions), P. mamorensis (12 accessions), and P. pseudopashia (2 accessions) (Table 2). Some of these numbers over-represent genetic coverage in cases where individual trees were assigned unique accessions but originated from a single or very few seed samples. For example, a number of single tree accessions of P. gharbiana and P. mamorensis were received from a field collection at Oregon State University, but these were discovered to have originated from a single seedlot for each species. Likewise, individual P. korshinskyi clonal accessions originated as seedlings from a very small number of seed samples. Several hybrid species such as P. × canescens, P. × complexa, P. × neoserrulata, P. × phaeocarpa and P. × uyematsuana are also poorly represented at the NCGR Corvallis but are derived from other species that are well represented (Table 2).
The natural ranges of some represented Pyrus taxa span geographic areas that are not well represented in the collection. For example, P. communis cultivars and wild relatives are present throughout Europe, but the NCGR Corvallis has very few accessions from Scandinavia or the Baltic countries and not a single accession from Finland, Latvia, Lithuania, or Norway. Many traditional pear cultivars are grown in Greece and several wild relative species are native there, but Greece is poorly represented at the NCGR Corvallis. Native pears from North Africa are also poorly represented, except for several accessions from Tunisia. The drought resistant species P. syriaca is native throughout the Middle East, but the NCGR Corvallis only has a few accessions that came from Syria and Israel. Populations from surrounding countries (Lebanon, Jordan, Iraq, Iran, Saudi Arabia, etc.) are not represented. There is a long history of pear cultivation in Afghanistan, Iran, and Iraq, but neither cultivars nor wild relatives from this region are well represented at the NCGR Corvallis, if at all (Table S6).
Genotypic and phenotypic characterization of the collection
Simple sequence repeat (SSR) and single nucleotide polymorphism (SNP) markers have been used to genotype accessions at the NCGR Corvallis. Volk et al. (2006) used 13 microsatellite markers to differentiate 145 P. communis subsp. pyraster and subsp. caucasica in the NPGS. In 2009, 10 Expressed Sequence Tag (EST)-SSRs were developed from Genbank sequences and used to identify 81 P. communis, 13 P. pyrifolia, and 20 P. ussuriensis or P. x bretshneideri accessions (Bassil and Postman 2010). An easy to use and cost-effective, multiplexed DNA fingerprinting set was developed in 2020, named the “U.S. Pyrus Genetic Resources” (USPGR) set, comprised of 10 SSR primer pairs and included eight primer pairs with long core repeats and two dinucleotide-containing SSRs (Zurn et al. 2020). It was used to genotype 237 accessions from the NCGR Corvallis collection, and has been routinely used to genotype further accessions (Zurn et al. 2020). Genotypes are currently available for 568 accessions. Furthermore, 1,650 pear accessions from the NCGR Corvallis were genotyped with an Axiom™ array of 70,000 single nucleotide polymorphism (SNP) markers (Montanari et al. 2019, 2020). A total of 1,331 pear trees had unique genotypes, and a pedigree was reconstructed for 637 duos/trios (sets of two or three cultivars that have parent–offspring relationships). Pedigrees were confirmed for 139 cultivars and elucidated for 498 cultivars that did not have previous parentage information. Montanari et al. (2020) identified 218 groups of duplicates representing 534 trees, of which 54 were previously unknown. Comparison of fruit phenotypes of 56 Asian x European hybrid cultivars that are widely grown in warm climates, such as India, Pakistan, the Middle East, and the South Eastern U.S., due to their low chilling requirements and tolerance to fire blight confirmed that: 11 each are duplicates of ‘Kieffer’ or ‘Le Conte’; nine each are duplicates of ‘Garber’, ‘La Providence’, or ‘Naspate’; and seven are duplicates of ‘Baldwin’ in the NCGR Corvallis collection (Bassil et al. 2023). The remaining duplicates are being confirmed through phenotypic assessments. Chloroplast sequences have also been used to characterize diversity and relationship among Pyrus accessions in the collection, as well as place them within the larger collection of Maleae taxa within the NPGS (Volk et al. 2019).
The pear NPGS collection has been phenotypically evaluated for incidence of diseases and insects, as well as architectural, production, fruit, and phenology traits of interest to breeders, researchers and growers (Table S10). Ploidy was estimated for by flow cytometry and resulted in identification of one tetraploid, 87 triploid, two diploid/tetraploid chimera, one aneuploid, and 1170 diploid accessions. Disease evaluations included fruit scab, leaf scab, Fabraea leaf spot, mildew, rust, and bacterial blossom blast. Rating for damage caused by blister mites was also estimated. Tree and architecture traits recorded include: tree symmetry and shape; branch angle, density, and stiffness; central leader; and bark characteristics and texture. Some of the fruit traits that were phenotyped consist of: flavor; quality; amount and location of russeting; flesh color and texture; calyx, core, and cavity traits; number of carpels; size and number of lenticels; and percent of fruit surface with overcolor. Phenological descriptors consisted of bloom (first, full, and last) as well as full ripening dates. Production traits assessed include chilling requirement, precocity, and yield.
Crop Germplasm Committee and additional germplasm characterization
The Pyrus Crop Germplasm Committee provides recommendations and information to the National Plant Germplasm System and the National Clonal Germplasm Repository on matters related to the germplasm collection, including composition, evaluation, exploration and acquisition, and maintenance. It also reviews proposals for the NPGS germplasm evaluation grant program, ranks the proposals, and recommends funding decisions (Table S11). Information about the Pyrus CGC activities is available online (Pyrus CGC 2023).
Genetic and genomic databases
NCGR Corvallis Pyrus collection data are maintained in GRIN-Global, the information system for the National Plant Germplasm System. Public information relating to inventory, passport data, and phenotypic and genotypic data are available online (USDA ARS 2023a). The NPGS Pyrus taxonomic information is available through GRIN-Taxonomy (USDA ARS 2023b). Pyrus genotypic and genomic data are also available in the Genome Database for Rosaceae (GDR) (GDR 2023; Jung et al. 2019). Genomic data is also often deposited in the National Center for Biotechnology Information (NCBI 2023). There are several national research organizations that deal with genomic resources and breeding, such as the U.S. Rosaceae Genomics, Genetics, and Breeding Executive Committee (RosEXEC) and the Rosaceae International Genomics Initiative (RosIGI).
Other genetic resource capacities
Germplasm collections outside of NPGS
Large pear germplasm collections are maintained throughout the world. Collections in Europe, the U.K., and Asia are summarized in Table S12 (Maggioni et al. 2004; Morgan 2015; NIHHS 2016). The European Cooperative Programme for Plant Genetic Resources (ECPGR) coordinates long-term conservation and utilization of plant genetic resources, including Pyrus, in Europe (ECPGR 2023). Many non-government organizations throughout the world also maintain significant pear germplasm collections, for example, the heirloom cultivar collection at Filoli Gardens in California, and collections held by individual members of amateur grower organizations, such as the North American Fruit Explorers (NAFEX) and the California Rare Fruit Growers (CRFG). Additionally, pear breeding programs throughout the world generally maintain small collections related to their breeding goals. Long- and short-term university collections and trials, mainly housed on experiment station land but also on private land, have diminished due to the high maintenance costs and competition for scarce land and labor resources.
Genomic resources
With decreasing costs of sequencing, genomic resources for Pyrus are becoming more abundant. The first genome sequence made available was the Asian pear P. × bretschneideri ‘Dangshansuli’, which was assembled using a hybrid approach of bacterial artificial chromosome (BAC)-by-BAC method using next-generation sequencing (NGS) (Wu et al. 2013), and a later revision provided some quality improvements using genetic maps to re-scaffold the genome (Xue et al. 2018). Shortly thereafter, a draft assembly of the European pear P. communis ‘Bartlett’ was released (Chagne et al. 2014). This first wave of genomes led the way for genomic pear research, but exhibited a high degree of fragmentation due to reliance on early NGS sequencing technology. Over the next five years sequencing technologies advanced through broadening access to long-reads, more advanced scaffolding methods such as high-throughput chromatin conformation capture (Hi-C) sequencing, and optical mapping, along with improvements in genome assembly and annotation software. As a result, in 2019 the first chromosome-scale Pyrus assembly was released for a hybrid P. ussuriensis × P. communis ‘Zhongai 1’ (Ou et al. 2019). Within the same year an assembly of doubled haploid ‘Bartlett’ was released (Linsmith et al. 2019) followed by a P. betulifolia, another Asian pear cultivar (Dong et al. 2020). These three genome assemblies represent vast improvements over the previous genomes, with high contiguity. We summarize quality parameters of currently available genomes in Table S13. Since 2021, three more genomes have been released, two P. pyrifolia assemblies for ‘Nijisseiki’ and ‘Cuifuan’, and P. communis ‘Beurré d’Anjou’ (Gao et al. 2021; Shirasawa et al. 2021; Zhang et al. 2022). Further, the P. communis cv. ‘Bartlett’ doubled haploid genome was polished to improve gene models (Zhang et al. 2022).
Although genome resources are increasing in availability for Pyrus, a need to broaden the diversity in sequenced species is needed. Currently, three species genomes are available (P. betulifolia, P. communis, and P. pyrifolia) along with two hybrids. These genomes represent less than 15% of the known species and hybrids available. Indications from the currently available genomes suggest that Pyrus genome size is considerably variable, ranging from 427 to 600 Mb when comparing chromosome-scale assemblies. Predicted gene content is highly variable with a minimum of 37,445 and a maximum of 59,552 predicted genes reported (Table S13). Furthermore, haplotypic variation has not been explored as most of the available genomes underwent limited phasing or represent a mix of haplotypes. Taken together, these results indicate a tremendous potential for novel sequences and genes within the genus and more efforts are needed to expand the available genomic resources for Pyrus.
Beyond nuclear genome sequences, chloroplast genomes include P. pyrifolia ‘Housui’, P. pyrifolia ‘Wonwhang’, P. ussuriensis, P. phaeocarpa, P. hopeiensis, and the invasive P. calleryana species (Cho et al. 2019; Chung et al. 2017; Gil et al. 2019; Li et al. 2018; Nowicki et al. 2022; Terakami et al. 2012; Xiang et al. 2019). Chloroplast sequences have been used to explore genetic diversity of threatened Pyrus, such as P. hopeiensis (Li et al. 2018).
DNA markers including microsatellite (simple sequence repeats or SSRs) and SNP (single nucleotide polymorphisms) markers were developed in pear and have been used for cultivar identification, diversity assessment, linkage mapping, QTL analyses, and genome wide association, among others (Li et al. 2022). There are over 1000 SSRs in pear (Yamamoto 2021) and three SNP arrays: IRSC 1 K (Montanari et al. 2013), Axiom 70 K (Montanari et al. 2019), and the 200 K Axiom PyrSNP array (Li et al. 2019). Data from these SNP arrays, as well as some SSR data, are currently integrated into the Genome Database for Rosaceae (GDR), so that genotypes for different accessions are more easily searchable (Jung et al. 2019). At this time, GRIN-Global has genotype data from 23 SSRs in up to 292 pear accessions from the NCGR Corvallis.
Prospects and future developments
The major commercial U.S. pear industry is vulnerable due to the small number of cultivars, all of which are susceptible to various diseases and arthropod pests. Fruit physiological postharvest disorders present production and marketing problems and consequent loss of income, resulting in increased production costs. Further, the adoption of new pear cultivars and rootstocks for commercial use is hindered by multiple factors, including limited demand for new options by retailers and processors, long delay to attain return on orchard investment, and complex ripening protocols that hinder consumer acceptance relative to competing fruits. While there are many cultivars that have performed well in consumer evaluations and have been successful in small-scale settings (e.g. farm stands, farmers markets and CSAs), they have failed to become more broadly retailed to the wider public. Positive consumer response demonstrated in the above-mentioned trials suggests real opportunity to improve growing, postharvest, and handling characteristics to expand offerings and demand for pears.
The diversity of genetic material provided by pear germplasm collections is crucial for successful research and breeding programs. Introduction of new traits for pear production and fruit quality depend on genetic diversity and integration of resistance and horticultural traits. In particular, rootstock traits may benefit from a wide diversity, given that germplasm selection does not rely on fruit quality. Similarly to other countries, the U.S. pear scion cultivar breeding effort is limited to only the USDA ARS AFRS and two small private programs for Asian pear cultivars. Rootstock breeding is limited to the program at Washington State University. Beyond breeding, the only systematic field evaluation program is NC-140, within which pear is an increasingly minor component.
The U.S. Pyrus germplasm collection can benefit from interactions and germplasm exchange with international germplasm repositories, and exploration targeted at underrepresented or absent crop wild relatives, particularly if they are potential sources of traits of interest. Germplasm with the potential for drought and heat resistance, based on environmental conditions in their native habitats, would be especially valuable given the threats posed by climate change. Taxa classified as critically endangered, endangered, vulnerable, or even near endangered, and confirmed as unique species, should be sought through germplasm acquisition or exchange, or exploration. The NCGR Corvallis, USDA ARS, and other pear scion and rootstock breeding programs may be able to import or exchange improved cultivars, breeding selections and germplasm from foreign breeding programs with appropriate intellectual property agreements to enhance U.S. breeding programs. New research of industry needs and priorities will help guide germplasm exploration and acquisition, and new research of consumer preferences would also guide acquisitions by the NCGR Corvallis and breeding program goals and acquisitions.
Genomic resources to characterize pear germplasm are becoming increasingly accessible, thanks to the integration of -omics and horticultural research, and major decreases in sequencing costs. This will be important for identification and confirmation of genetic diversity, as well as expanding molecular knowledge of trait function. As we generate more genomic data, however, we face challenges in assuring the usability of that data. For example, integrating genomic resources and making them accessible will require that databases are linked to one another, and that more scientists are trained to use and analyze data. Development of tools allowing visualization or searchability of data and linking genomic to phenomic data will also be important.
Phenotyping, on the other hand, continues to be a bottleneck across crops and disciplines. Much phenotyping still depends on manual labor, and major efforts to phenotype the U.S. germplasm will require collaborative labor and funding resources. Standardization of data collection is difficult yet required for comparisons of germplasm performance across locations. Further, the work of managing phenotypic data and curation for public accessibility must be considered. Phenotyping of fruit quality, disease traits, abiotic responses, and rootstock traits represent gaps to be filled. Systematic field evaluation programs, such as the NC-140 regional rootstock project, as well as CGC-funded germplasm evaluations represent limited but concerted efforts that address phenotyping gaps. Identification and utilization of biomarkers (e.g., gene activity) or other novel approaches to estimate ripening could help overcome the challenges of determining harvest times and cold requirements for fruit quality (Honaas et al. 2021). Additionally, further use of sensory panels to evaluate fruit quality could help ensure that breeding parents, selections, and prospective cultivars meet consumer preferences (Colonna et al. 2023; Lozano et al. 2023).
In conclusion, pear germplasm collections provide a diverse source of genetic material important for research and breeding programs. Access to ex situ collections of plants and seeds is necessary for developing improved scion and rootstock cultivars. Access to correctly identified and diverse living collections of Pyrus germplasm will assure that advances in breeding and genetic research will continue to be made long into the future, and vulnerability of the crop will continue to be addressed.
References
Akparov ZI, Musayev MK (2012) Diversity of fruit genetic resources in Azerbaijan. Acta Hortic 948:217–222
Ames BN, Shigenaga MK, Hagen TM (1993) Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA 90:7915–7922
Amiri A, Hawkins AW, Mulvaney KA (2017) Study of fitness, virulence, and fungicidal sensitivity of Lambertella corni-maris causing yellow rot on apple. Plant Dis 101:738–743
Amri A, Valkoun J, Shehadeh A (2002) Promoting in situ conservation of agrobiodiversity in West Asia. ICARDA Caravan 17:31–33
KC AN, Rasmussen AL, DeShields JB (2022) 1-methylcyclopropene preharvest application and its effect on storability of ‘'Comice’ and ‘Bosc’ pears. HortScience 57:24–31
Anh Tuan P, Bai S, Saito T, Imai T, Ito A, Moriguchi T (2016) Involvement of EARLY BUD-BREAK, an AP2/ERF transcription factor gene, in bud break in Japanese pear (Pyrus pyrifolia Nakai) lateral flower buds: expression, histone modifications and possible target genes. Plant Cell Physiol 57:1038–1047
Arnold Arboretum (2023) Campaign in China and Central Asia. https://arboretum.harvard.edu/expeditions/campaign-in-china-and-central-asia/. Accessed 20 Sept 2023
USDA ARS (2022) USDA food and nutrient database for dietary studies 2019–2020. In: USDA (ed) Food Surveys Research Group home page, http://www.ars.usda.gov/nea/bhnrc/fsrg: U.S. Department of Agriculture, Agricultural Research Service
USDA ARS (2023a) Germplasm Resources Information Network (GRIN). USDA Agricultural Research Service, https://npgsweb.ars-grin.gov/gringlobal/search. Accessed 21 Sept 2023
USDA ARS (2023b) GRIN taxonomy. USDA Agricultural Research Service, https://npgsweb.ars-grin.gov/gringlobal/taxon/taxonomysearch. Accessed 18 Sept 2023
Barina Z, Kiraly G (2014) Taxonomic re-evaluation of the enigmatic Pyrus magyarica (Rosaceae). Phytotaxa 167:133–136
Bassil N, Postman JD (2010) Identification of European and Asian pears using EST-SSRs from Pyrus. Genet Resour Crop Evol 57:357–370
Bassil NV, Postman JD, Hummer KE (2011) Quince (Cydonia oblonga) genetic relationships determined using microsatellite markers. Acta Hortic 909:75–83
Bassil N, King R, Nyberg A, Zurn J, Clare S, Reinhold L, Postman J, Gilmore G, Flores G, Volk G, Jenderek M, Montanari S, Chagne D, Bus V, Brewer L, Dardick C, Gottschalk C, Durel CE, Denance C (2023) Progress on the molecular characterization of the USDA National pear collection. In: 31st international horticultural congress, August 14–20, 2022. Acta Horticulturae, Angers, France
Bell RL, Itai A (2011) Pyrus. In: Kole C (ed) Wild crop relatives: genomic and breeding resources, temperate fruits. Springer, Berlin, pp 147–177
Bell RL, Stuart LC (1990) Resistance in Eastern-European Pyrus germplasm to pear psylla nymphal feeding. HortScience 25:789–791
Bell RL, van der Zwet T (1998) Breeding for resistance to pear psylla: evaluation of parental germplasm. Acta Hort 484:471–475
Bell RL (1991) Pears (Pyrus). In: Moore JN, Ballington Jr. (eds) Genetic Resources of Temperate Fruit and Nut Crops, vol 290. ISHS Acta Horticulturae, p. 657–700
Bellini E, Nin S (2002) Breeding for new traits in pear. Acta Hortic 596:217–224
Berardinelli A, Donati V, Giunchi A, Guarnieri A, Ragni L (2005) Damage to pears caused by simulated transport. J Food Eng 66:219–226
Beutel J (1990) Asian pears. In: Janick J, Simon JE (eds) First National Symposium on New Crops, held October 23–26, 1988. Indianapolis. Timber Press, Portand, OR, p. 304–309
Brewer LR, Palmer JW (2011) Global pear breeding programmes: goals, trends and progress for new cultivars and new rootstocks. Acta Hortic 909:105–119
Chagne D, Crowhurst RN, Pindo M, Thrimawithana A, Deng C, Ireland H, Fiers M, Dzierzon H, Cestaro A, Fontana P, Bianco L, Lu A, Storey R, Knabel M, Saeed M, Montanari S, Kim YK, Nicolini D, Larger S, Stefani E, Allan AC, Bowen J, Harvey I, Johnston J, Malnoy M, Troggio M, Perchepied L, Sawyer G, Wiedow C, Won K, Viola R, Hellens RP, Brewer L, Bus VGM, Schaffer RJ, Gardiner SE, Velasco R (2014) The draft genome sequence of European pear (Pyrus communis L. ‘Bartlett’). PLoS ONE 9:e92644
Chang YJ, Reed BM (2000) Extended alternating-temperature cold acclimation and culture duration improve pear shoot cryopreservation. Cryobiology 40:311–322
Chang YJ, Reed BM (2001) Preculture conditions influence cold hardiness and regrowth of Pyrus cordata shoot tips after cryopreservation. HortScience 36:1329–1333
Chimeric plants perspective.pdf
Chitu E, Paltineanu C (2020) Timing of phenological stages for apple and pear trees under climate change in a temperate-continental climate. Int J Biometeorol 64:1263–1271
Cho MS, Kim Y, Kim SC, Park J (2019) The complete chloroplast genome of Korean Pyrus ussuriensis Maxim. (Rosaceae): providing genetic background of two types of P. ussuriensis. Mitochondrial DNA Part B Resour 4:2424–2425
Choudhury R, Modi P, Hanstad J, Elkins R, Gubler WD (2014) First report of Diplodia seriata causing pear branch canker dieback in California. Plant Dis 98:688–689
Chung HY, Lee TH, Kim YK, Kim JS (2017) Complete chloroplast genome sequences of Wonwhang (Pyrus pyrifolia) and its phylogenetic analysis. Mitochondrial DNA Part B Resour 2:325–326
Colonna AE, Gutierrez J, Montero ML, Gallardo RK, Ross CF (2023) An investigation of consumer pear knowledge, consumer preferences, and desirable sensory traits to increase pear consumption. Acta Horticulturae (In Press)
Coumou D, Rahmstorf S (2012) A decade of weather extremes. Nat Clim Change 2:491–496
Culley T (2017) The rise and fall of the ornamental Callery pear tree. Arnoldia 74:2–11
Darbyshire R, Webb L, Goodwin I, Barlow EW (2014) Challenges in predicting climate change impacts on pome fruit phenology. Int J Biometeorol 58:1119–1133
DeEll JR, Ehsani-Moghaddam B (2011) Timing of postharvest 1-methylcyclopropene treatment affects Bartlett pear quality after storage. Can J Plant Sci 91:853–858
Dirr M (1997) Dirr’s Hardy trees and shrubs: an illustrated encyclopedia. Timber Press
Dondini L, Sansavini S (2012) European pear. In: Badenes M, Byrne D (eds) Fruit breeding. Handbook of plant breeding, vol 8. Springer, Boston, pp 369–413
Dong XG, Wang Z, Tian LM, Zhang Y, Qi D, Huo HL, Xu JY, Li Z, Liao R, Shi M, Wahocho SA, Liu C, Zhang SM, Tian ZX, Cao YF (2020) De novo assembly of a wild pear (Pyrus betuleafolia) genome. Plant Biotechnol J 18:581–595
Eastwood A, Lazkov G, Newton AC (2009) The red list of trees of central Asia. Flora and Fauna International, Cambridge
ECPGR (2023) ECPGR Malus/Pyrus working group. https://www.ecpge.cgiar.org/working-groups/maluspyrus. Accessed 19 Sept 2023
Einhorn T (2021) A review of recent Pyrus, Cydonia, and Amelanchier rootstock selections for high-density pear plantings. Acta Hortic 1303:185–196
Einhorn TC, Castagnoli S, Smith TJ, Turner J, Meilke E (2013) Summary of the 2002 Pacific Northwest of USA pear rootstock Trials: performance of ‘d’Anjou’ and ‘Golden Russet Bosc’ pear on eight Pyrus rootstocks. J Am Pomol Soc 67:80–88
Einhorn T, Auvil T, Castagnoli S (2014) Horner rootstock grower evaluation trials. In: Washington tree fruit research commission research report
Elkins RB, Rizzo DM, Whiting EC (1998) Biology and management of Armillaria root disease in pear in California. Acta Hortic 475:453–458
Elkins RB, Turner J, Seavert CF, Castagnoli S, Mitcham EJ, Biasi WV, Colonna A, Bell R (2008) Evaluation of potential alternative European pear cultivars for U.S West Coast Growers. Acta Hortic 800:483–490
Elkins RB, Castagnoli S, Embree C, Parra-Quezada R, Robinson TL, Smith TL, Ingels CA (2011) Evaluation of potential rootstocks to improve pear tree precocity and productivity. Acta Hortic 909:183–194
Elkins R, Bell R, Einhorn T (2012) Needs assessment for future US pear rootstock research directions based on the current state of pear production and rootstock research. J Am Pomol Soc 66:153–163
Elkins RB, Varela LG, Roncoroni JA, Adaskaveg JE, Gubler WD, Hanson B, Ingles CA, Van Steenwyk RA (2023) UC pest management guidelines: pear. UC ANR Publication, Davis, CA, p 3455
Elkins R, Shaffer C, Lampinen B (2021) High density orchard systems for European pear: the 2013 NC-140 regional rootstock project (2020 Report). In: California pear research report. California Pear Advisory Board Sacramento, CA
Elkins R (2019) Comparison of Horner 4, OHxF 87 and OHxF 97 rootstocks under varying growing conditions and cultural practices in Lake County, California. In: California pear advisory board research report
Endtmann KJ (1999) Taxonomy and nature conservation of wild pear (Pyrus pyraster) and its congeneric taxa. Beitrage Fur Forstwirtschaft Und Landschaftsokologie 33:123–131
ERS, USDA (2022) Economic research service. Per capita consumption of fresh pears in the United States from 2000 to 2021 (in pounds) [Graph]. https://www.statista.com/statistics/257226/per-capita-consumption-of-fresh-pears-in-the-us/. Accessed 19 Sept 2023
FAO (2009) International treaty on plant genetic resources for food and agriculture. In: Nations FaAOotU, ed. Food and Agriculture Organization of the United Nations, Rome, Italy
Farr DF, Bills GF, Chamuris GP, Rossman AY (1989) Fungi on plants and plant products in the United States. APS Press, St. Paul, MN
USDA FAS (2023) USDA Foreign Agricultural Service—global market analysis—June 2023. https://apps.fas.usda.gov/psdonline/circulars/fruit.pdf. Accessed 15 Sept 2023
Fellenberg U, Kadolsky M, Rumpf H, Soppa B (2000) Wild fruit trees and shrubs as an integrated part of conservation and utilization of forest genetic resources in north west Germany. Acta Hortic 538:63–66
Feng XQ, Biasi B, Mitcham EJ (2004) Effects of various coatings and antioxidants on peel browning of ‘Bartlett’ pears. J Sci Food Agric 84:595–600
Fisher DF (1922) An outbreak of powdery mildew (Podosphaera leucotricha) on pears. Phytopathology 12:103
Franck C, Lammertyn J, Ho QT, Verboven P, Verlinden B, Nicolai BA (2007) Browning disorders in pear fruit. Postharvest Biol Technol 43:1–13
Gabay G, Faigenboim A, Dahan Y, Izhaki Y, Itkin M, Malitsky S, Elkind Y, Flaishman MA (2019) Transcriptome analysis and metabolic profiling reveal the key role of alpha-linolenic acid in dormancy regulation of European pear. J Exp Bot 70:1017–1031
Gallardo RK, Pedroso-Galinato S, Nottingham L (2022) 2022 cost estimates of producing and packing fresh-market Bartlett pears in South Washington. Washington State University Extension, pp 1–8
Galvis Sanchez AC, Gil-Izquierdo A, Gil MI (2003) Comparative study of six pear cultivars in terms of their phenolic and vitamin C contents and antioxidant capacity. J Sci Food Agric 83:995–1003
Gao YH, Yang QS, Yan XH, Wu XY, Yang F, Li JZ, Wei J, Ni JB, Ahmad M, Bai SL, Teng YW (2021) High-quality genome assembly of ‘Cuiguan’ pear (Pyrus pyrifolia) as a reference genome for identifying regulatory genes and epigenetic modifications responsible for bud dormancy. Hortic Res 8:197
GDR (2023). Genome Database for Rosaceae. https://www.rosaceae.org/, MainLab Bioinformatics, Washington State University, Accessed 20 Sept 2023
Gil HY, Kim Y, Kim SH, Jeon JH, Kwon Y, Kim SC, Park J (2019) The complete chloroplast genome of Pyrus Ussuriensis Maxim. (Rosaceae). Mitochondrial DNA Part B Resour 4:1000–1001
Good Fruit Grower (2008). Concorde’s fatal flaw. https://www.goodfruit.com/concordes-fatal-flaw/. Accessed 22 Sept 2023
Graf H (1977) Birnbaumsterben im obstbaugebiet an der Niederelbe. Erwerbsobstbau 19:110–114
Gubler WD, Zoller B, Duncan R, Lindow S (2007) Diseases. In: Mitcham EJ, Elkins RB (eds) Pear production and handling manual. University of California, Agriculture and Natural Resources Publication, Davis, CA, p 3483
Güner A, Zielinski J (1996) The conservation status of Turkish woody flora. In: Hunt DR (ed) International dendrological society symposium on the conservation status of temperate trees. University of Bonn, Germany, p 12
Guo JM, Wei XY, Lu EL, Wang Y, Deng ZL (2020) Ripening behavior and quality of 1-MCP treated d’Anjou pears during controlled atmosphere storage. Food Control 117:107364
He Q, Luo Y (2007) Enzymatic browning and its control in fresh-cut produce. Stewart Postharvest Rev 6:3
He WJ, Laaksonen O, Tian Y, Haikonen T, Yang BR (2022) Chemical composition of juices made from cultivars and breeding selections of European pear (Pyrus communis L.). J Agric Food Chem 70:5137–5150
Hedrick UP (1921) The Pears of New York. State of New York, Department of Agriculture, Albany, NY
Hibino H, Schneider H (1970) Mycoplasmalike bodies in sieve tubes of pear trees affected with pear decline. Phytopathology 60:499–501
Honaas L, Hargarten H, Hadish J, Ficklin SP, Serra S, Musacchi S, Wafula E, Mattheis J, dePamphilis CW, Rudell D (2021) Transcriptomics of differential ripening in ‘d’Anjou’ pear (Pyrus communis L.). Front Plant Sci 12:609684
IPCC (2023) Climate change 2023: synthesis report. In: Core writing team HLaJR (ed) Contribution of Working groups I, II and III to the sixth assessment report of the intergovernmental panel on climate change. IPCC, Geneva, Switzerland, p. 35–115
IUCN (2022) The IUCN red list of threatened species. Version 2022-2. https://www.iucnredlist.org. Accessed 15 Sept 2023
Jacob H (2002) New pear rootstocks from Geisenheim, Germany. Acta Hortic 596:337–344
Jung S, Lee T, Cheng CH, Buble K, Zheng P, Yu J, Humann J, Ficklin SP, Gasic K, Scott K, Frank M, Ru SS, Hough H, Evans K, Peace C, Olmstead M, DeVetter LW, McFerson J, Coe M, Wegrzyn JL, Staton ME, Abbott AG, Main D (2019) 15 years of GDR: new data and functionality in the genome database for Rosaceae. Nucleic Acids Res 47:D1137–D1145
Kanato K, Kajiura I, McKenzie DW (1982) The ideal Japanese pear. In: van der Zwet TNFC (ed) The pear: Cultivars to marketing. Horticultural Publications, Gainesville, FL, pp 138–155
Kharadi RR, Schachterle JK, Yuan X, Castiblanco LF, Peng J, Slack SM, Zeng Q, Sundin GW (2021) Genetic dissection of the Erwinia amylovora disease cycle. Annu Rev Phytopathol 59:191–212
Kimura T, Shi YZ, Shoda M, Kotobuki K, Matsuta N, Hayashi T, Ban Y, Yamamoto T (2002) Identification of Asian pear varieties by SSR analysis. Breed Sci 52:115–121
Kinard G (2020) Phytosanitary and regulatory issues in the movement of plant genetic resources: A U.S. perspective. In: Volk GM (ed) Fundamentals of plant genebanking. Colorado State University, Fort Collins, CO
Kleinschmidt J, Soppa B, Wagner I, Fellenberg U, Schmidt J, Brotje H, Schute G, Meier-Dinkel A (1998) Die wildbirne—Baum des jahres. Forst Und Holz 53:35–39
Kullaj D, Shahini S, Çakalli A (2012) Inventory of fruit crop wild relatives of Albania. Acta Hortic 948:289–294
Kupferman E, Sater C, Walter M, Buchanan N (2010) Conditioning Anjou pears: summary of research conducted by the WSU Tree Fruit Postharvest Laboratory. Washington State University, Wenatchee, WA, pp 1–36
Lapeña I, Turdieva M, López Noriega I, Ayad WG (2014) Conservation of fruit tree diversity in Central Asia: Policy options and challenges. Bioversity International, Rome, Italy
Lear M, Hunt D (1996) Updating the threatened temperate tree list. In: Hunt DR (ed) Internation dendrological society symposium on the conservation status of temperate trees. University of Bonn, Germany, pp 161–171
Leskey TC, Short BD, Wright SE, Brown MW (2009) Diagnosis and variation in appearance of brown stink bug (Hemiptera: Pentatomidae) injury on apple. J Entomol Sci 44:314–322
Leskey TC, Short BD, Butler BR, Wright SE (2012) Impact of the invasive brown marmorated stink bug, Halymorpha halys (Stal), in Mid-Atlantic tree fruit orchards in the United States: case studies of commercial management. Psyche 2012:1–4
Li YT, Zhang J, Li LF, Gao LJ, Xu JT, Yang MS (2018) Structural and Comparative analysis of the complete chloroplast genome of Pyrus hopeiensis”Wild Plants with a Tiny Population” and three other Pyrus Species. Int Mol Sci 19:3262
Li XL, Singh J, Qi MF, Li SW, Zhang X, Zhang MY, Khan A, Zhang SL, Wu J (2019) Development of an integrated 200 K SNP genotyping array and application for genetic mapping, genome assembly improvement and genome wide association studies in pear (Pyrus). Plant Biotechnol J 17:1582–1594
Li JM, Zhang MY, Li XL, Khan A, Kumar S, Allan AC, Kui LW, Espley RV, Wang CH, Wang RZ, Xue C, Yao GF, Qin MF, Sun MY, Tegtmeier R, Liu HN, Wei WL, Ming ML, Zhang SL, Zhao KJ, Song BB, Ni JP, An JP, Korban SS, Wu J (2022) Pear genetics: recent advances, new prospects, and a roadmap for the future. Hortic Res 9:uhab040
Li X, Li X, Wang T, Gao W (2016) Nutritional composition of pear cultivars (Pyrus spp.). In: Simmonds MSJ and Preedy VR (eds) Nutritional Composition of Fruit Cultivars. Academic Press, London, UK, pp 573–608
Linsmith G, Rombauts S, Montanari S, Deng CH, Celton JM, Guerif P, Liu C, Lohaus R, Zurn JD, Cestaro A, Bassil NV, Bakker LV, Schijlen E, Gardiner SE, Lespinasse Y, Durel CE, Velasco R, Neale DB, Chagne D, Van de Peer Y, Troggio M, Bianco L (2019) Pseudo-chromosome-length genome assembly of a double haploid “Bartlett” pear (Pyrus communis L.). Gigascience 8:giz138
Lombard PB, Westwood MN (1975) Pear rootstock research in Oregon. Am Pomol Soc 29:17–19
Lombard PB, Westwood MN (1987) Pear rootstocks. In: Rom RC, Carlson RF (eds) Rootstocks for fruit crops. John Wiley & Sons, New York, pp 145–183
Lombard P, Hull J, Westwood MN (1980) Pear cultivars of North-America. Fruit Var J 34:74–83
Lozano L, Iglesias I, Puy J, Echeverria G (2023) Performance of an expert sensory panel and instrumental measures for assessing eating fruit quality attributes in a pear breeding programme. Foods 12:1426
Luckwill LC, Pollard A (1963) Perry pears. University of Bristol, Bristol, U.K.
Macheix JJ (1990) Fruit phenolics. CRC Press, Boca Raton
Maggioni L, Fischer M, Lateur M, Lamont EJ, Lipman E, compilers (2004) Report of a working group on Malus/Pyrus. In: Second meeting, May 2002, Dresden-Pillnitz, Germany. IPGRI, Rome, Italy
Maghradze D, Bobokashvili Z, Akparov ZI, Musayev MK, Mammadov A (2012) Importance, usage and prospective of CWR for fruits, grapevine and nuts in Georgia and Azerbaijan. Acta Hortic 948:33–40
Maxted N, Kell SP (2009) Establishment of a global network for the in situ conservation of crop wild relatives: status and needs. FAO Commission on Genetic Resources for Food and Agriculture, Rome, Italy
Meheriuk M, Prange RK, Lidster PD, Porritt SW (1994) Postharvest disorders of apples and pears. Agriculture and Agri-Food Canada, Pub. 1737/E, Canada
Mellenthin WM, Wang CY (1977) The relationship of premature ripening of Bartlett pears to preharvest temperatures. Acta Hortic 69:281–286
Mielke EA, Smith L (2002) Evaluation of the horner rootstocks. Acta Hortic 596:325–330
Mielke EA, Drake SR, Elfving DC (2005) ‘Concorde’ pear flavor, texture, and storage quality improved by manipulating harvest maturity. Acta Hortic 671:361–367
Mitcham EJ, Elkins RB (2007) Pear production and handling manual. University of California Division of Agriculture and Natural Resources, Oakland, CA
Montanari S, Saeed M, Knabel M, Kim Y, Troggio M, Malnoy M, Velasco R, Fontana P, Won K, Durel CE, Perchepied L, Schaffer R, Wiedow C, Bus V, Brewer L, Gardiner SE, Crowhurst RN, Chagne D (2013) Identification of Pyrus single nucleotide polymorphisms (SNPs) and evaluation for genetic mapping in European pear and interspecific Pyrus hybrids. PLoS ONE 8:e77022
Montanari S, Bianco L, Allen BJ, Martinez-Garcia PJ, Bassil NV, Postman J, Knabel M, Kitson B, Deng CH, Chagne D, Crepeau MW, Langley CH, Evans K, Dhingra A, Troggio M, Neale DB (2019) Development of a highly efficient Axiom 70 K SNP array for Pyrus and evaluation for high-density mapping and germplasm characterization. BMC Genom 20:331
Montanari S, Postman J, Bassil NV, Neale DB (2020) Reconstruction of the largest pedigree network for pear cultivars and evaluation of the genetic diversity of the USDA-ARS National Pyrus Collection. G3 (bethesda) 10:3285–3297
Montuschi C, Collina M (2003) First record of Valsa ceratosperma on pear in Italy. Inf Agrar 59:55–57
Morgan J (2015) The book of pears: the definitive history and guide to over 500 varieties. Chelsea Green Publishing
Murray K, Jepson P, Hedstrom C (2020) Integrated pest management strategic plan for Oregon and Washington pears. Oregon State University Extension Service
Nass USDA (2004) Noncitrus fruits and nuts final estimates 1997–2002. USDA National Agricultural Statistics Service
Nass USDA (2014) Noncitrus fruits and nuts final estimates 2007–2012. USDA National Agricultural Statistics Service
Nass USDA (2023) Noncitrus fruits and nuts 2022 summary. USDA National Agricultural Statistics Service
USDA NASS (2020) Statistics by subject. https://www.nass.usda.gov/Statistics_by_Subject/index.php, USDA National Agricultural Statistics Service. Accessed 12 Feb 2024
Nazir N, Nisar S, Mubarak S, Khalil A, Javeed K, Banjaree S, Kour J, Nayik GA (2020) Antioxidants in fruits: Properties and Health Benefits. In: Nayik GA and Gull A (eds) Antioxidants in fruits: properties and health benefits. Springer, Singapore, p 435–447
NC-140 (2023). NC-140 regional rootstock research project. http://www.nc140.org/. Accessed 20 Sept 2023
NCBI (2023). National Center for Biotechnology Information. https://www.ncbi.nlm.nih.gov/, National Library of Medicine (US), NCBI. Accessed 21 Sept 2023
NIHHS (2016). National Institute of Horticultural and Herbal Science Locations. https://www.nihhs.go.kr/eng/about/nihhsLocation.do. Accessed 15 Sept 2023
Nowicki M, Huff ML, Staton ME, Trigiano RN (2022) Chloroplast genome of the invasive Pyrus calleryana underscores the high molecular diversity of the species. J Appl Genet 63:463–467
Ohba H (1996) A brief overview of the woody vegetation of Japan and its conservation status. In: Hunt DR (ed) International dendrological society symposium on the conservation status of temperate trees. University of Bonn, Germany, pp 89–107
Ou C, Wang F, Wang J, Li S, Zhang Y, Fang M, Ma L, Zhao Y, Jiang S (2019) A de novo genome assembly of the dwarfing pear rootstock Zhongai 1. Sci Data 6:281
Paprštein F, Kloutvor J, Holubec V (2002) Mapping of the regional cultivars of fruit woody species in the Czech Republic. In: Swiecicki W, Naganowska B, Wolko B (eds) XVIth EUCARPIA genetic resources section workshop. Poznan, Poland, pp 71–76
Paprštein E, Sedlák J, Holubec V (2010) In situ conservation of fruit landraces. Czech J Genet Plant Breed 46:S57-59
Paprštein F, Kloutvor J, Sedlák J, Holubec V (2011) In situ and on-farm conservation of Central European fruit genetic resources. Acta Hortic 918:85–90
USA Pears (2023) USA pears. Pear Bureau Northwest, https://trade.usapears.com/crop-information/. Accessed 21 Sept 2023
Postman JD, Spotts RA, Calabro J (2005) Scab resistance in Pyrus germplasm. Acta Hortic 671:601–608
Postman J, Kim D, Bassil N (2013) OH x F paternity perplexes pear producers. J Am Pomol Soc 67:157–167
Pyrus CGC (2023) Crop Germplasm Committees. https://www.ars-grin.gov/CGC. Accessed 19 Sept 2023
Reed BM, Denoma J, Wada S, Postman J (2013) Micropropagation of pear (Pyrus sp.). Methods Mol Biol 11013:3–18
Reil W, Ireland J, Elkins RB (2007) Propagation and rootstock selection. In: Mitcham EJ, Elkins RB (eds) Pear production and handling manual. University of California Agriculture and Natural Resources Publication, Davis, CA, p 3483
Reiland H, Slavin J (2015) Systematic review of pears and health. Nutr Today 50:301–305
Rubstov GA (1944) Geographical distribution of the genus Pyrus and trends and factors in its evolution. Am Nat 78:358–366
Saito T (2016) Advances in Japanese pear breeding in Japan. Breed Sci 66:46–59
Sarkar D, Ankolekar C, Pinto M, Shetty K (2015) Dietary functional benefits of Bartlett and Starkrimson pears for potential management of hyperglycemia, hypertension and ulcer bacteria Helicobacter pylori while supporting beneficial probiotic bacterial response. Food Res Int 69:80–90
Seemüller E, Schneider B (2004) ‘Candidatus Phytoplasma mali’, ‘Candidatus Phytoplasma pyri’ and ‘Candidatus phytoplasma prunorum’, the causal agents of apple proliferation, pear decline and European stone fruit yellows, respectively. Int J Syst Evol Microbiol 54:1217–1226
SER, INSR, RBGK (2023). Seed information database. Society for ecological restoration and international network for seed based restoration and Royal Botanic Gardens Kew, https://ser-sid.org/species/a773d8bc-635f-4374-8357-859e167f1f15. Accessed 21 Sept 2023
Serdani M, Spotts RA, Calabro JM, Postman JD, Qu AP (2006) Evaluation of the USDA national clonal Pyrus germplasm collection for resistance to Podosphaera leucotricha. HortScience 41:717–720
Shin IS, Kim WC, Hwang HS, Shin YU (2002) Achievements of pear breeding in Korea. Acta Hortic 596:247–250
Shirasawa K, Itai A, Isobe S (2021) Chromosome-scale genome assembly of Japanese pear (Pyrus pyrifolia) variety ‘Nijisseiki.’ DNA Res 28:dsab001
Sĭndelář J (2002) Toward a threatened forest tree species preservation on the example of crab apple (Malus sylvestris L.) and wild pear (Pyrus pyraster L. [Burgsdorf]). Zpravy Lesnickeho Vyzkumu 47:199–203
Singh BK, Delgado-Baquerizo M, Egidi E, Guirado E, Leach JE, Liu HW, Trivedi P (2023) Climate change impacts on plant pathogens, food security and paths forward. Nat Rev Microbiol 21:640–656
Slippers B, Crous PW, Denman S, Coutinho TA, Wingfield BD, Wingfield MJ (2004) Combined multiple gene genealogies and phenotypic characters differentiate several species previously identified as Botryosphaeria dothidea. Mycologia 96:83–101
Sugar D (2007) Postharvest handling of winter pears. In: Mitcham EJE, Elkins RB (eds) Pear production and handling manual. University of California, Agriculture and Natural Resources Publication, Davis, CA, p 3483
Sugar D, Basile SR (2011) Performance of ‘Comice’ pear on quince rootstocks in Oregon, USA. Acta Hortic 909:215–218
Sutton TB, Aldwinckle HS, Agnello AM, Walgenbach JF (2016) PART III: arthropod pests. Compendium of apple and pear diseases and pests, 2nd edn. APS Publications, pp 133–204
Tanner JD, Chen KY, Jenderek MM, Wallner SJ, Minas IS (2021) Determining the effect of pretreatments on freeze resistance and survival of cryopreserved temperate fruit tree dormant buds. Cryobiology 101:87–94
Terakami S, Matsumura Y, Kurita K, Kanamori H, Katayose Y, Yamamoto T, Katayama H (2012) Complete sequence of the chloroplast genome from pear (Pyrus pyrifolia): genome structure and comparative analysis. Tree Genet Genomes 8:841–854
Tukey HB (1964) Dwarfed fruit trees: for orchard garden and home. Macmillan
Turner J, Bai J, Marin A, Colonna A (2005) Consumer sensory evaluation of pear cultivars in the Pacific Northwest, USA. Acta Hortic 671:355–360
Tweedy CA (2021) Assessment of pear rootstocks for Armillaria Resistance, University of California Davis. ProQuest Dissertations and Theses, p. 92
USDA ARS NCGR Corvallis (2022) NCGR Corvallis plant collection expeditions. https://www.ars.usda.gov/pacific-west-area/corvallis-or/national-clonal-germplasm-repository/docs/plant-collecting-expeditions/expedition-reports/. Accessed 20 Sept 2023
USDA NPGS NCGR Corvallis (2023) National Clonal Germplasm Repository: Corvallis, OR. https://www.ars.usda.gov/pacific-west-area/corvallis-or/national-clonal-germplasm-repository/. Accessed 20 Sept 2023
USDA, DHHS (2020) Dietary guidelines for Americans, 2020–2025. ServicesDietaryGuidelines.gov, US Department of Agriculture and US Department of Health and Human
van der Zwet T, Keil HL (1979) Fire blight: a bacterial disease of rosaceous plants. USDA, Washington, D.C.
van der Zwet T, Stankovic D, Ristevski B (1987) Collecting Pyrus Germplasm in Yugoslavia. HortScience 22:15–21
van der Zwet T, Cociu V, Czarnecki B, Nyeki J, Blazek J (1989) Collecting Pyrus Germplasm in Romania, Poland, Hungary, and Czechoslovakia. HortScience 24:420–424
van der Zwet T, Orolaza-Halbrendt N, Zeller W (2012) Fire blight: History, biology, and management. American Phytopathology Society, St. Paul, MN
Vavilov NI (1951) The origin, variation, immunity and breeding of cultivated plants. Chron Bot 13:1–366
Villalobos-Acuna M, Mitcham EJ (2008) Ripening of European pears: the chilling dilemma. Postharvest Biol Technol 49:187–200
Volk GM, Richards CM, Henk AD, Reilley AA, Bassil NV, Postman JD (2006) Diversity of wild Pyrus communis based on microsatellite analyses. J Am Soc Hortic Sci 131:408–417
Volk GM, Henk AD, Richards CM, Bassil N, Postman J (2019) Chloroplast sequence data differentiate Maleae, and specifically Pyrus, species in the USDA-ARS National Plant Germplasm System. Genet Resour Crop Evol 66:5–15
Vyse K, Pagter M, Zuther E, Hincha DK (2019) Deacclimation after cold acclimation-a crucial, but widely neglected part of plant winter survival. J Exp Bot 70:4595–4604
Wagner I (1999) Schutz und nutzen von wildobst – Probleme bei der direkten nutzung von Wukdibstrekukten. [Conservation and yield of wild fruit trees – problems regarding direct uses of relics of wild fruit trees]. Forstarchiv 70:23–27
Walsh CS, Harshman JM, Wallis AE, Williams AB, Newell MJ, Welsh GR (2016) Asian pear: a potential alternative fruit crop for growers in the Mid-Atlantic Region. HortScience 51:1325–1328
Webster AD (2003) Breeding and selection of apple and pear rootstocks. Acta Hortic 622:499–512
Westwood MN (1982) Pear germplasm of the new national clonal repository: it’s evaluation and uses. Acta Hortic 124:54–65
Westwood MN, Bjornstad HO (1971) Some fruit characteristics of interspecific hybrids and extent of self-sterility in Pyrus. Bull Torrey Bot Club 98:22–24
Williams K (2020a) Conduction and exploration for plant genetic resources to be incorporated into the USDA National Plant Germplasm System. In: Volk GM (ed) Fundamentals of plant genebanking. Colorado State University, Fort Collins CO
Williams K (2020b) Planning an exploration for plant genetic resources to be incorporated into the USDA National Plant Germplasm System. In: Volk GM (ed) Fundamentals of plant genebanking. Colorado State University, Fort Collins, CO
Wolf H, Arenhovel W, Behm A, Franke A, Kleinschmit J, Rogge M, Schneck D, Schneck V, Schykzke R, Tabel U (2000) Conservation and breeding of wild fruit tree species in forestry. Acta Hortic 538:57–62
WSU (2023) Crop protection guides for tree fruits in Washington. Pear programs. Washington State University Extension, https://cpg.treefruit.wsu.edu/pear-programs. Accessed 18 Sept 2023
Wu J, Wang ZW, Shi ZB, Zhang S, Ming R, Zhu SL, Khan MA, Tao ST, Korban SS, Wang H, Chen NJ, Nishio T, Xu X, Cong L, Qi KJ, Huang XS, Wang YT, Zhao X, Wu JY, Deng C, Gou CY, Zhou WL, Yin H, Qin GH, Sha YH, Tao Y, Chen H, Yang YA, Song Y, Zhan DL, Wang J, Li LT, Dai MS, Gu C, Wang YZ, Shi DH, Wang XW, Zhang HP, Zeng L, Zheng DM, Wang CL, Chen MS, Wang GB, Xie L, Sovero V, Sha SF, Huang WJ, Zhang SJ, Zhang MY, Sun JM, Xu LL, Li Y, Liu X, Li QS, Shen JH, Wang JY, Paull RE, Bennetzen JL, Wang J, Zhang SL (2013) The genome of the pear (Pyrus bretschneideri Rehd.). Genome Res 23:396–408
Xiang QH, Zhang DX, Wang Q, Wang XR, Guan WB (2019) The complete chloroplast genome sequence of Pyrus phaeocarpa Rehd. Mitochondrial DNA Part B Resour 4:1370–1371
Xiao CL, Boal RJ (2004) Prevalence and incidence of Phacidiopycnis rot in d’Anjou pears in Washington State. Plant Dis 88:413–418
Xiao CL, Boal RJ (2005) Distribution of Potebniamyces pyri in the Pacific Northwest and its association with bark necrosis and canker on twigs of pear trees. Plant Dis 89:920–925
Xiao CL, Rogers JD (2004) A postharvest fruit rot in d’Anjou pears caused by Sphaeropsis pyriputrescens sp. Plant Dis 88:114–118
Xiao CL, Rogers JD, Kim YK, Liu Q (2005) Phacidiopycnis washingtonensis—a new species associated with pome fruits from Washington State. Mycologia 97:464–473
Xue HB, Wang S, Yao JL, Deng CH, Wang L, Su YL, Zhang HR, Zhou HK, Sun MS, Li XG, Yang J (2018) Chromosome level high-density integrated genetic maps improve the Pyrus bretschneideri ‘DangshanSuli’ v1.0 genome. BMC Genom 19:833
Yamamoto T (2021) DNA markers and molecular breeding in pear and other Rosaceae fruit trees. Hortic J 90:1–13
Yamamoto T, Kimura T, Soejima J, Sanada T, Ban Y, Hayashi T (2004) Identification of quince varieties using SSR markers developed from pear and apple. Breed Sci 54:239–244
Zhang Q-j, Tao S-t, Li M, Qi X-x, Wu J, Yin H, Deng J-l, Zhang S-l (2015) Identification of differentially expressed genes using digital gene expression profiles in Pyrus pyrifolia Nakai cv. Hosui bud release following early defoliation. Tree Genet Genomes 11:34
Zhang J, Ma YC, Dong C, Terry LA, Watkins CB, Yu ZF, Cheng ZM (2020) Meta-analysis of the effects of 1-methylcyclopropene (1-MCP) treatment on climacteric fruit ripening. Hortic Res 7:208
Zhang HT, Wafula EK, Eilers J, Harkess AE, Ralph PE, Timilsena PR, dePamphilis CW, Waite JM, Honaas LA (2022) Building a foundation for gene family analysis in Rosaceae genomes with a novel workflow: a case study in Pyrus architecture genes. Front Plant Sci 13:975942
Zheng XY, Cai DY, Potter D, Postman J, Liu J, Teng YW (2014) Phylogeny and evolutionary histories of Pyrus L. revealed by phylogenetic trees and networks based on data from multiple DNA sequences. Mol Phylogenet Evol 80:54–65
Zielinski QB, Thompson MM (1967) Speciation in Pyrus: chromosome number and meiotic behavior. Bot Gaz 128:109–112
Zurn JD, Norelli JL, Montanari S, Bell R, Bassil NV (2020) Dissecting genetic resistance to fire blight in three pear populations. Phytopathology 110:1305–1311
Acknowledgements
We would like to thank Kate Evans, Soon Li Teh Chris Adams, and Oscar Hurtado-Gonzalez for helpful feedback on the manuscript.
Funding
Agricultural Research Service.
Author information
Authors and Affiliations
Contributions
All authors wrote the main text, reviewed the manuscript, and prepared tables. JMW, CG, NB and LR prepared figures.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rachel B. Elkins, Joseph D. Postman, and Richard L. Bell are currently retired.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Waite, J.M., Gottschalk, C., Reinhold, L.A. et al. Vulnerability of pear (Pyrus) genetic resources in the U.S.. Genet Resour Crop Evol (2024). https://doi.org/10.1007/s10722-024-01990-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10722-024-01990-9