Abstract
Distinctive chemical signatures have the potential to serve as discriminatory cues for olfactory recognition mechanisms. Cuticular hydrocarbon (CHC) profiles are among the most prominent chemical signatures in insects that can be highly diverse even among closely related species and between populations with similar ecology. Particularly within the major insect order Hymenoptera, CHC profiles are characterized by high complexity and variation with the potential to evolve rapidly. In this study, we found two very distinct CHC chemotypes distinguishing sympatric colonies of the African carpenter ant Camponotus maculatus (Hymenoptera: Formicinae). These chemotypic differences were mainly detected on the surface profiles of eggs produced by either queens or isolated worker groups. In one chemotype, queen- and worker-laid eggs are very similar. This is largely contrasted by the other chemotype, where queen-laid eggs clearly differ from worker-laid eggs with several prominent queen-exclusive compounds. However, workers display a stable behavior of discriminating against and selectively disposing of worker-laid eggs i.e., worker policing, independent of egg chemotype. Furthermore, genetic barcoding of workers revealed a clear separation between colonies characterized by producing these two distinct egg chemotypes, which may indicate that these colonies belong to a cryptic species complex. Interestingly, worker policing behaviour appears to be evolutionarily conserved, despite the strikingly different egg surface profiles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eusocial insect societies like ants are characterized by only one or few reproducing individuals, while the majority of individuals form a distinct worker caste with no or very reduced fertility (Wilson 1971; Hölldobler and Wilson 1990). In most ant species and other eusocial Hymenoptera, reproductives or queens usually signal their reproductive status, inducing workers to remain sterile (Hoover et al. 2003; Le Conte and Hefetz 2008; Holman et al. 2010). Fertility signaling in social insects is largely mediated by cuticular hydrocarbons (CHCs) on the cuticle of both eggs and their producers (Endler et al. 2004; Smith et al. 2012; Van Oystaeyen et al. 2014). Additionally, numerous studies have shown a link between particular CHC compounds or compositions and reproductive status (Peeters and Liebig 2009; Smith et al. 2009; Yagound et al. 2014; Abril et al. 2018; Honorio et al. 2019). In several social insect species, differences in CHC surface profiles between queens and workers can be perceived and distinguished by nestmates, potentially signaling presence or absence of a fertile reproductive caste (Monnin et al. 1997; Dietemann et al. 2003, 2005).
In the absence of a fertile queen, workers of many ant species are capable of activating their ovaries to lay unfertilized eggs that can develop into males (Bourke 1988; Bourke and Franks 1995; Wenseleers and Ratnieks 2006). However, frequent observations have shown that individual workers may attempt to reproduce even in the presence of a fertile queen (Frank 1995; Wenseleers et al. 2004; Wenseleers and Ratnieks 2006). Worker reproduction is usually not in the interest of other workers, due to potential relatedness asymmetries arising in the colony, as well as bearing the costs of a generally reduced colony efficiency (Ratnieks and Wenseleers 2005; Wenseleers and Ratnieks 2006). This can lead to workers preventing each other from reproducing, which has been termed ‘worker policing’ (Foster and Ratnieks 2001; Whitfield 2002; Endler et al. 2007).
Policing behaviors either involve direct aggression towards reproductive workers (Liebig et al. 1999; Wenseleers et al. 2004, 2005) or the selective removal of worker-laid eggs (Foster and Ratnieks 2001; d'Ettorre et al. 2004; Endler et al. 2004). Because destroying queen-laid eggs would be counter-productive in the process, egg policing requires the ability to reliably discriminate between queen- and worker-laid eggs. Such discriminating mechanisms are frequently mediated by CHC profiles on the surface of eggs (Endler et al. 2004; Helanterä and d'Ettorre 2015; Saitoh et al. 2020). These generally function as an extension of the surface profiles of the respective egg layer, enabling workers to assess the egg layers’ fertility status and caste identity (Heinze et al. 2002; Endler et al. 2004; Abril et al. 2018). For example, the carpenter ant Camponotus floridanus (Hymenoptera: Formicinae) is characterized by marked differences in egg surface CHC profiles between queen- and worker-laid eggs (Endler et al. 2004). These differences are used by workers to selectively destroy worker-laid eggs, i.e., inducing worker policing. However, egg surface profile variations and related policing behaviors have not yet been investigated in other species of this highly diverse genus of carpenter ants. Additionally, it remains yet to be investigated whether fertility signals encoded by CHCs might be evolutionarily conserved across species and how universally applicable these findings are.
Here, we attempt to close these knowledge gaps by analyzing CHC profiles of both queen- (QLE) and worker-laid eggs (WLE) and their respective producers (i.e., queens and workers) in African carpenter ant colonies of Camponotus maculatus (Hymenoptera: Formicinae). Camponotus colonies are generally characterized by a single monandrous queen and workers usually refrain from reproduction in her presence (Wilson 1971; Hölldobler and Wilson 1990; Gadau et al. 1996, 1998). However, workers generally start laying eggs in the absence of a fertile queen or if the productivity of a queen decreases (Endler et al. 2004, 2007). We show that these sympatric colonies contain very distinct egg surface profiles that can be clustered into two different chemotypes. One of these chemotypes contains very few differences between queen- and worker-laid eggs which is largely contrasted by the other chemotype, where queen-laid eggs clearly differ from worker-laid eggs with several prominent queen-exclusive compounds. We further show a highly selective brood discrimination ability of C. maculatus workers for the first time, independent of the respective egg chemotype. Finally, we use a genetic barcoding analysis correlating genetic and chemical divergence, which may indicate a cryptic species complex. Our results show highly conserved worker policing behavior contrasted by a low conservation of distinct chemical cues involved in the discrimination of queen- and worker-laid eggs across colonies in this potentially cryptic species complex, challenging the assumption of universally detectable chemical signatures of reproductive status in ants.
Materials and methods
We characterized the chemotypes (CHC profiles) of seven laboratory colonies founded by queens collected during their nuptial flight. From these colonies, we created 25 queenless nests that produced worker laid eggs, and 61 queenless nests that were used in worker policing assays discriminating between queen-laid eggs (QLE) and worker-laid eggs (WLE).
Ant colonies
Founding queens of C. maculatus were collected using a blacklight at the Comoé National Park in the northeastern part of the Ivory Coast (8°46′11′′ N, 3° 47′21′′W) after nuptial flights in 2017 (about 10 founding queens), 2018 (about 20 founding queens) and 2019 (about 50 founding queens) during nighttime. The genus Camponotus may reach high colony densities of up to 0.045/m2 (Lange et al. 2019). Assuming similarly high colony densities for our sample location and coordinated mating flights, the queens are most likely from several unrelated colonies. Mated queens were transferred to our laboratory at the University of Münster, Germany, housed in plastic boxes (25 × 25 × 10 cm3) containing a plastered floor, and fed twice a week with honey water and cockroaches (Blaptica dubia). The colonies were maintained at a day/night cycle of 30 °C/25 °C for 12 h each at 60% relative humidity for at least a year prior to carrying out the worker policing experiment and extracting chemical data from colonies that had grown to at least 1000 workers. All required collection and export permits were provided by the Directeur Général of the Office Ivoirien des Parcs et Réserves (OIPR), Côte d’Ivoire (N°018/MINEDD/OIPR/DZ).
Egg and ant surface extractions and chemical analysis
Pilot analysis of representative chemical profiles of queens and workers and their respective eggs from two colonies grown to more than 1000 individuals (17–01 and 18–02, Table S1) revealed two very distinct colony-specific chemotypes. Batches of 20 eggs per group (QLE and WLE) were extracted in 80 µl of MS pure hexane (UniSolv, Darmstadt, Germany) in a 2 ml GC-vial (Agilent, Santa Clara, California, U.S.A.) while being swirled on an orbital shaker (IKA KS 130 Basic, Staufen, Germany) for 10 min. For single C. maculatus workers (2 for each chemotype, respectively), the same procedure was used except of applying 250 µl of MS pure hexane to cover the workers entirely during the extractions. Extracts were then transferred to a 250 µl conical insert (Agilent, Santa Clara, California, U.S.A.) and evaporated under a constant flow of CO2. The dried extracts were resuspended with 20 µl of an MS pure hexane solution containing 7.5 ng/μl of n-dodecane (EMD Millipore Corp., Billerica, Massachusetts, U.S.A.) as an internal standard. Five μl of the extract were injected into a GC-QQQ Triple Quad (GC: 7890B, Triple Quad: 7010B, Agilent, Waldbronn, Germany) with a PAL Autosampler system operating in electron impact ionization mode with 70 eV. The split/splitless injector was operated at 300 °C in Pulsed splitless mode at 20 psi until 0.75 min with the Purge Flow to Split Vent set at 50 ml/min at 0.9 min. For the queens of the two colonies, we used solid-phase microextraction (SPME), a non-lethal method to extract cuticular chemicals. The individual queens were immobilized with ice for 10 min and their abdomens inserted through a small slit cut into filter paper to hold them in place. A 7 µm thick Polydimethylsiloxane (PDMS) SPME fiber (Restek GmbH, Bad Homburg, Germany), pre-conditioned on a temperature program starting from 60 °C, held for 2 min, increasing by 60 °C per min to 200 °C, followed by an increase of 4 °C per min to 325 °C, was gently rubbed over the abdomen of the queen for 5 min. Then the SPME fiber was inserted into the GC-Inlet using the same method as the liquid injections. Chromatographic results obtained with a 7 µm PDMS fiber have been shown to be the closest to the corresponding liquid injections, albeit without the possibility to conduct direct quantitative comparisons (Tentschert et al. 2002; Geiselhardt et al. 2009; Gerhardt et al. 2015).
Separation of compounds was performed on a 30 m × 0.25 mm ID × 0.25 μm DB-5MS ultra inert fused silica column or a 30 m × 0.25 mm ID × 0.25 μm HP-1 Dimethylpolysiloxane column (Agilent J&W GC columns, Santa Clara, California, U.S.A), which both have very similar, comparable separation properties (Agilent, Waldbronn, Germany, pers. comm.). The temperature program started from 60 °C, held for 2 min, and increasing by 60 °C per min to 200 °C, followed by an increase of 4 °C per min to 325 °C, where it was held for 5 min. Helium served as carrier gas with a constant flow of 1.2 ml per min and a pressure of 10.42 psi. CHC peak detection and identification were performed using the Qualitative Analysis Navigator of the MassHunter Workstation Software (Version B.08.00/Build 8.0.8208.0, Agilent Technologies, Santa Clara, California, U.S.A). Peaks were identified according to their diagnostic ions and retention indices calculated with a C21-C40 alkane standard solution (Merck, Darmstadt, Germany). For CHC quantification, Quantitative Analysis MassHunter Workstation Software (Version B.09.00/Build 9.0.647.0, Agilent Technologies, Santa Clara, California, USA) was used. Peaks were quantified using the most abundant diagnostic ion in their mass spectra as quantifiers (m/z = 57.0) and several characteristic diagnostic ions as qualifiers to allow for unambiguous detection by the quantification software. The pre-defined integrator Agile 2 was used for the peak integration algorithm to allow for maximum flexibility. All peaks were then additionally checked for correct integration and quantification, and, where necessary, re-integrated manually. To standardize the peak areas, they were divided by total peak area sum per chromatogram, resulting in relative ratios. The chemotypes of QLE and WLE of the other five colonies with over 1000 individuals (18–05, 19–10, 19–13, 19–16, 19–17, Table S1) were verified and quantified under a different set-up where the gas chromatograph (GC: 7890B) was simultaneously coupled to a flame ionization detector (FID: G3440B) and the above tandem mass spectrometer (MS/MS: 7010B, all provided by Agilent Technologies, Waldbronn, Germany). The system was equipped with a DB-5MS fused silica column as indicated above at a temperature of 300 °C with helium used as a carrier gas under a constant flow of 1.8 ml/min. The FID had a temperature of 300 °C and used nitrogen with a 20 mL/min flow rate as make-up gas and hydrogen with a 30 mL/min flow rate as fuel gas. The column was split at an auxiliary electronic pressure control (Aux EPC) module into an additional deactivated fused silica column piece (0.9 m × 150 μm) with a flow rate of 0.8 ml/min leading into the FID detector, and another deactivated fused silica column piece (1.33 m × 150 μm) at a flow rate of 1.33 ml/min into the mass spectrometer. The column temperature program was set as described above. This set-up allowed for the combination of the best-suited method for hydrocarbon quantification (Agilent Technologies, Waldbronn, Germany, pers. comm.) with reliable compound identifications. However, due to the differences to the initial set-up (see above), the resulting chromatograms could not be directly compared quantitatively to the ones received in the pilot analysis of the first two colonies, which were therefore excluded from the subsequent linear discriminant analysis (LDA). We performed an LDA to visualize the chemical differences between QLE and WLE of the five representative colonies (Chemotype 1:18–05 and 19–03; Chemotype 2:19–10, 19–16, 19–17) with the R package “MASS” and calculated an approximated Wilk's λ to measure the quality of the LDA.
Worker policing experiment
From the seven chemotyped laboratory colonies, we established in total 61 queen-less nests of orphaned minor workers with group sizes of ten individual workers each (Table S1). For each of the three colonies of chemotype 1 (17–01, 18–05, 19–13), we established five worker groups receiving QLE and five worker groups receiving WLE, except for colony 17–01 where we only established three queenless groups per QLE and WLE treatment, respectively. For each of the four colonies of chemotype 2 (18–02, 19–10, 19–16, 19–17) we also established five replicates per egg type, except for colony 18–02, where two replicates received QLE, and three replicates received WLE. One hour after isolation from their natal nest, each isolated group was provided with a batch of ten eggs, derived either from the queen of their own colony (QLE) or from their sisters in one of the 25 queenless nests used for producing worker-laid eggs (WLE), depending on the respective treatment of each discriminator colony. As we never observed egg-laying in freshly isolated worker groups before approximately 30 days had passed, we could exclude the occurrence of additional WLE from within the respective discrimination groups in this time frame. Egg batches were transferred with fine forceps to a piece of plastic (3 × 3 cm2) and checked under a stereo microscope to confirm that no eggs were damaged before starting the experiment. The egg batches were then transferred into the isolated nests 1 cm away from their entrance on the glass plate that covered the indention of the plaster floor. Twenty-four hours after introduction, any remaining eggs were removed and counted. Selective egg removal has frequently been demonstrated to occur by oophagy in ants (Monnin and Peeters 1997; Foster and Ratnieks 2001; d'Ettorre et al. 2004), thus we regarded non-recovered eggs as consumed (or destroyed) by the workers. Mean differences in the number of remaining eggs recovered after 24 h in all 61 isolated worker groups were compared according to the origins of the eggs (QLE vs. WLE) with Mann–Whitney U tests, performed with R (Version 4.1.2) in R Studio (Version 2022.07.1) (R Development Core Team 2018).
DNA barcoding/sequencing of cytochrome c oxidase I (COI)
We used COI as a standardized single molecular marker gene, which is present in all animals, not considered to be under selection and has a mutation rate fast enough to distinguish between closely related species but is conserved among conspecifics (Hebert et al. 2003). The Chelex procedure as described by Gadau (2009) was used for our DNA extraction of one worker per colony. PCRs were subsequently performed based on Folmer et al. (1994) with 1 µl of the DNA extracts in 2 µl Colorless GoTaq Reaction Buffer (pH 8.5, 7.5 mM MgCl2; Promega, Walldorf, Germany), 1 µl 25 mM MgCl2, 0.6 µl 1.25 mM dNTPs, 0.2 µl of each primer (20 µM, HCO: TAA ACT TCA GGG TGA CCA AAA AAT CA; LCO: GGT CAA CAA ATCATA AAG ATA TTG G), 0.06 µl GoTaq G2 DNA Polymerase Buffer (5u/µl; Promega, Walldorf, Germany) and 4.94 µl demineralized H2O. Samples were heated to 94 °C for 3 min and passed through 38 cycles of 94 °C for 1 min, 45 °C for 1 min, and 72 °C for 1.5 min. Final extension occurred at 72 °C for 5 min. Five µl of the PCR products were purified using 0.25 µl Exonuclease 1 (5u; Thermofisher, Dreieich, Germany) and 0.5 µl Shrimp alkaline phosphatase (5u; Thermofisher, Dreieich, Germany) for 15 min at 37 °C and another 15 min at 85 °C. Five µl of purified DNA solution were added to 5 µl of one primer (5 µM; HCO or LCO) and sequenced at the European Custom Sequencing Centre, Cologne, Germany. Neighbor Joining calculations based on the mtDNA sequences were conducted using the program MEGA 7.0 (Kumar et al. 2016). Sequences were aligned by ClustalW (Thompson et al. 1994) and a Neighbor-Joining (Juke-Cantor) tree was created including 100 bootstraps. The gene tree was rooted with the published sequences from Camponotus floridanus (NCBI Reference number: NW_020229323.1).
Results
Distinct egg surface profiles separate into two chemotypes while worker policing behavior remains conserved
We discovered very distinct egg surface CHC patterns clearly distinguishing the seven experimental colonies into two distinct chemotypes, namely Cmt 1 and Cmt 2 (Figs. 1, 2, Table 1). In the first chemotype (Cmt 1) shared by three colonies (17–01, 18–05, 19–13), both queen- (QLE) and worker-laid egg (WLE) surface profiles contained the same set of compounds up to carbon chain length C31, and QLE surface profiles additionally had compounds with longer chain lengths of up to C35 (Fig. 1, Table 1). Furthermore, the profiles of both QLE and WLE in Cmt 1 were mostly composed of methyl-branched alkanes (see also Fig. 4a). Conversely, the second chemotype (Cmt 2), detected in four colonies (18–02, 19–10, 19–16, 19–17), was characterized by mostly shared compounds between WLE and QLE (Fig. 2, Table 1). Moreover, the egg surface profiles of Cmt 2 appeared to be dominated by unsaturated CHC compounds (see also Fig. 4b), largely contrasting them with Cmt 1. Irrespective of these two very distinct chemotypes, numbers of remaining eggs in isolated worker groups from our seven experimental colonies representing both Cmt 1 (three colonies, n = 26, W = 13, P < 0.001, Mann–Whitney U test) and Cmt 2 (four colonies, n = 35, W = 33, P < 0.001, Mann–Whitney U test) were significantly lower for WLE than for QLE 24 h after egg introduction (Fig. 3, Table S1).
Comparison of queen- and worker laid eggs for chemotype 1 reveal queen-laid egg exclusive longer chained compounds. Chemical profile comparison of queen-laid eggs (QLE, red, top) and worker-laid eggs (WLE, blue, bottom) for chemotype (Cmt) 1. CHC compound classes are indicated by different symbols. Compounds exclusively occurring in one of the two egg types are marked with an asterisk
Comparison of queen- and worker laid eggs for chemotype 2 show mostly shared compounds across a wide chain-length range. Chemical profile comparison of queen-laid eggs (QLE, red, top) and worker-laid eggs (WLE, blue, bottom) for chemotype (Cmt) 2. CHC compound classes are indicated by different symbols. QLE-exclusive compounds were only detected in trace amounts and are marked with an asterisk
Isolated C. maculatus workers discriminate strongly against worker laid eggs. Mean relative egg survival rates (± SD) of queen-laid eggs (QLE, red) and worker-laid eggs (WLE, blue) from both distinct chemotypes (Cmt 1 and Cmt 2) after 24 h in isolated worker groups. The average absolute differences between initially presented and remaining eggs compared between QLE and WLE were assessed with Mann-Whitney U tests and were highly significant for both Cmt 1 (n = 26, W = 13, P < 0.001) and Cmt 2 (n = 35, W = 33, P < 0.001)
Comprehensive chemical comparison of surface profiles
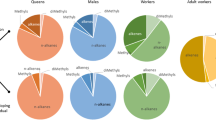
The chemical profiles of workers and queens that we examined from the two initial exemplary colonies were generally similar to the profiles of the eggs they produced for both chemotypes, albeit with no complete quantitative and qualitative congruence (Figs. 4 and 5). The distribution of CHC compound classes was similar, particularly the dominance of methyl-branched alkanes for Cmt 1 and the exclusive occurrence of unsaturated compounds for Cmt 2 (Fig. 4). However, closer inspection revealed that worker and queen surface profiles differ from the surface profiles of their eggs. In Cmt 1, for instance, the proportions of both n-alkanes and mono-methyl-branched alkanes are almost twice as large on WLE (around 80% in total) when directly compared to the profiles of the workers (around 50%), which, in turn, displayed far greater proportions of di- and tri-methyl-branched alkanes (Fig. 4a). Conversely, for Cmt 2, WLE and worker profiles displayed very similar compound class distributions, whereas QLE and the queen profile showed more differences (Fig. 4b). No tri-methyl-branched alkanes and only a small fraction of di-methyl-branched alkanes could be detected on QLE despite being clearly present on the queen’s surface in Cmt 2. As apparent from the chromatogram comparison (see Fig. 2), the chain length ranges of WLE and QLE were vastly different in Cmt 1, which was generally mirrored by both queen and worker profiles (Fig. 5a). Queen and QLE profiles of Cmt 1 mostly span between chain lengths of C23 and C35, whereas both worker and WLE profiles only ranged between C23 and C31. Conversely, Cmt 2 showed more congruence of egg surface profiles with their respective producers, reflecting the general similarity of QLE and WLE which largely contrasted them with Cmt 1 (see also Figs. 1 and 2). Focusing solely on the egg surface profiles, a linear discriminant analysis (LDA) clearly showed a strong separation of WLE and QLE for Cmt 1, whereas the two egg types clustered much more closely together for Cmt 2 (Fig. 6, approximated Wilk’s λ < 0.0001, χ2 = -38.032).
Comparison of CHC compound class ratios between egg surface profiles and their respective producers. Relative distributions (%) of the seven detected CHC compound classes (n-alkanes, n-alkenes, alkadienes, mono-, di-, tri- and tetra-methyl branched alkanes) for a) chemotype (Cmt) 1 and b) chemotype (Cmt) 2, indicated by different colors, across the surface profiles of queens, QLE, WLE, and workers (top to bottom). Note that for queen surface profiles, a different, non-lethal extraction method (SPME) had to be applied
Comparison of relative CHC lengths distributions between egg surface profiles and their respective producers. Relative distributions (%) of CHC compound chain lengths (C21-C39) for a) chemotype (Cmt) 1 and b) chemotype (Cmt) 2, indicated by a color gradient, across the surface profiles of Queens, QLE, WLE, and Workers (Top to bottom) Note that for queen surface profiles, a different, non-lethal extraction method (SPME) had to be applied
Linear discriminant analysis shows clear separation of egg surface profiles according to chemotypes and caste. Linear discriminant analysis (LDA) based on 49 cuticular hydrocarbon (CHC) profiles from queen- (QLE) and worker-laid eggs (WLE) of five laboratory colonies initially identified as Camponotus maculatus, separated according to the two detected chemotypes (Cmt 1 and Cmt 2) and the respective castes of their producers. The respective contributions of the two discriminant functions to the total chemical variation are indicated in percentages (approximated Wilk’s λ < 0.0001, χ2 = −38.032)
Genetic analysis of the investigated colonies
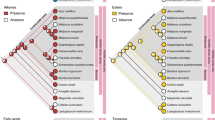
A genetic barcoding analysis based on partial mitochondrial cytochrome c oxidase I (COI) sequences showed two distinct clusters clearly separating all tested colonies in line with the two egg chemotypes which the investigated C. maculatus colonies produce (Fig. 7). Well-supported by bootstrap values based on 100 iterations, the seven colonies clearly clustered according to the distinct egg surface profiles.
C. maculatus egg chemotypes correlate with distinct clusters after genetic barcoding. Neighbor-Joining tree (Juke-Cantor) based on partial mitochondrial COI sequences for Camponotus maculatus, with bootstrap values based on 100 iterations at each node. The tree was routed with Camponotus floridanus, a carpenter ant native to North America, as an outgroup. The respective egg chemotypes characterizing the two distinct clusters are indicated as well
Discussion
Despite two very distinct egg surface chemotypes detected in different African carpenter ant colonies initially identified as Camponotus maculatus, worker policing behavior appears to be remarkably similar and conserved (Fig. 3). In all tested colonies from both chemotypes, WLE are significantly less accepted, unlike QLE which always show a higher survival rate in isolated worker groups. This clearly demonstrates the capability of isolated Camponotus workers to discriminate against and selectively dispose of WLE while the numbers of QLE remain significantly higher. Interestingly, only in one chemotype (Cmt 1), numerous qualitative CHC differences between QLE and WLE (Fig. 1 and 4a, Table 1) could be detected. In contrast, Cmt 2 shows very few prominent qualitative differences between QLE and WLE (Fig. 2 and Table 1), which generally appear to be very similar. Additionally, Cmt 1 and Cmt 2 are also very distinct from each other overall with very few shared CHC compounds (Table 1).
Our comparisons of egg surface profiles with their respective producers generally corroborate the highly distinct chemotypes for both compound class and chain length distributions (Figs. 4, 5). However, the egg surface profiles appear in no instance entirely reflective of the profiles of their producers, independent of stemming from either worker or queen in both chemotypes. This contrasts with findings in the related ant species Camponotus floridanus, where the chemical profiles of egg surfaces generally function as an extension of the profiles of their producers and match them very closely (Endler et al. 2004). Our results suggest a more selective deposition of CHC compounds on the egg surfaces by their respective producers in C. maculatus, potentially explaining the deviations in both compound class ratios and chain length distributions (Figs. 4, 5). Intriguingly, QLE surface profiles have been found to be more dominated by short-chained CHCs in C. floridanus, a pattern reversed in our findings for Cmt 1 with more long-chained QLE-exclusive compounds (Figs. 2 and 5a, Table 1), but also for the QLE-exclusive trace components in Cmt 2 (Fig. 3 and Table 1).
This challenges the hypothesis that shorter-chain compounds generally function as conserved queen fertility cues in the evolution of the ant genus Camponotus. Concordantly, recent findings have put the universality of queen fertility signals across eusocial insect taxa into question, even within the same genus (Smith and Liebig 2017; Smith et al. 2018; Steitz and Ayasse 2020). Notably, CHC profiles of most investigated hymenopteran species appear to be either dominated by methyl-branched or unsaturated CHCs, and closely related species have frequently been found to opposingly display either one of these two fundamentally different chemotypes (van Wilgenburg et al. 2011; Kather and Martin 2015; Sprenger and Menzel 2020). This matches the two distinct chemotypes revealed in our study, with Cmt 1 being proportionally dominated by methyl-branched alkanes, and Cmt 2 by n-alkenes and dienes (Figs. 1, 2, 4, and Table 1). Moreover, our genetic barcoding analysis clearly shows that the genetic divergence between the investigated carpenter ant colonies is in accordance with the two distinct chemotypes (Fig. 6). It has been shown that CHC profiles have the potential to evolve rapidly (Menzel et al. 2017; Hartke et al. 2019). For instance, differences in CHC profiles can occur due to local adaptation to microclimatic conditions, e.g., higher humidity or radiation, within a few generations (Menzel et al. 2018). CHC differences might also occur due to assortative mating and are correlated with speciation events in other insects, where species boundaries are maintained by chemical differences (Schwander et al. 2013; Chung and Carroll 2015). Since C. maculatus has long been known as a notoriously difficult ant species to characterize taxonomically (Baroni-Urbani 1972; Bolton 1994, 2019), our results support the hypothesis that C. maculatus constitutes a cryptic species complex that can be differentiated using chemical and molecular, but not morphological characteristics. Moreover, since we detected these two distinct chemotypes consistently and independently of the respective collection years of the founding queens, it appears to be a stable discriminatory trait within the sampled range. As an evolutionary consequence, the chemical profiles of these cryptic species may be selected as one of the first cues to differentiate and evolve rapidly within the sympatric speciation process of C. maculatus subspecies, thus comprising a potential source for brood discrimination and to detect colony membership. A rapid change in egg surface profiles might also be an adaptive strategy to counteract the risk of brood parasitism at certain locations. Quickly changing surface profiles might impede social parasites to stably imitate them, and potentially prevent the exploitation through brood parasitism (Guillem et al. 2014; Casacci et al. 2019).
Our study further demonstrates that worker policing behavior can be remarkably conserved even between populations with no concretely discernable queen fertility signal on the egg surfaces. A few studies have already hinted at evolutionarily stable worker policing and brood discrimination abilities from a behavioral perspective (Endler et al. 2004; Helanterä and d'Ettorre 2015; Saitoh et al. 2020). However, a common, unambiguous fertility signal could not be ultimately verified so far (Smith and Liebig 2017; Smith et al. 2018). For instance, in the black garden ant Lasius niger (Hymenoptera: Formicinae), 3-MeC31 has been shown to function as queen-specific fertility signal, that reduces both worker ovarian activation and aggression (Holman et al. 2010). This hints at the general potential of mono-methyl-branched alkanes as fertility cues. In contrast, in the trap-jaw ant Odontomachus brunneus Patton (Hymenoptera: Ponerinae), a C29 alkene (9-nonacosene) has been identified as queen fertility signal across multiple geographically varied populations (Smith et al. 2013, 2015). In comparison, we found three C29 alkene isomers, one in Cmt 1 and two in Cmt 2, yet all three of them are clearly detectable in both WLE and QLE, with no recognizable queen-specific pattern (Table 1).
Thus, universal features of signals encoding and conveying the same fundamental biological information, in this case fertility and reproductive status, appear to be hard to ultimately verify (Smith and Liebig 2017; Smith et al. 2018). It has been postulated that mainly saturated straight-chain and methyl-branched alkanes constitute conserved queen pheromonal compounds across the order Hymenoptera (Van Oystaeyen et al. 2014). Interestingly, the only shared compounds between Cmt 1 and 2 are, in fact, a subset of straight-chain and methyl-branched alkanes (Table 1). Only two compounds, 7-MeC35 and 5-MeC33, were found exclusively in small traces in QLE. The latter were shared between both chemotypes, potentially hinting at the rarely investigated capability of this compound class to encode and convey information (Holze et al. 2021), despite their comparatively low quantities.
Our study design cannot completely discriminate whether QLE display distinguishable fertility cues preventing them from being policed, or WLE display differential cues rendering them more prone to policing. The physiological, perceptual and cognitive processes for egg- and nestmate recognition are still largely unknown. It is suggested that queen odors are the dominant recognition cues (Carlin and Hölldobler 1987; Van Oystaeyen et al. 2014; Smith and Liebig 2017), but discrimination patterns may largely vary between species and the relative influences of genetic, environmental, and chemical factors may be different – even between populations of the same or closely related species. Additionally, in an environment with a high number of sympatric species such as in the present study’s sampling location (Comoé National Park, Ivory Coast), individuals may experience reinforced selective pressure to constantly fine-tune their template for recognition (Leonhardt et al. 2007; Smith and Liebig 2017).
To obtain more detailed insights into the exact mechanisms of egg discrimination hinted at by our study, other recognition cues distinguishing QLE from WLE should be investigated, e.g., subtle visual or morphometric differences (Ratnieks and Visscher 1989; Monnin and Peeters 1997; Kikuta and Tsuji 1999), though we have been unable so far to confirm any distinctive traits apart from their surface chemistry. In honey bees, egg discrimination occurs even in complete darkness, and cues of QLE can be transferred to WLE (Martin et al. 2004), delivering more empirical evidence that the main communication modalities used by social insects are chemical cues rather than optical or morphological cues. Therefore, possibly undetected chemical signaling compounds should be considered in future studies as well. For instance, several studies have revealed the presence of longer chained CHCs generally eluding detection with standard analytical methods (Akino 2006; Cvacka et al. 2006; Bien et al. 2019; Golian et al. 2022). Thus, egg surface profile differences might have gone undetected in the present study due to hitherto undetected CHCs with longer chain lengths. Additionally, we cannot exclude the reliance on non-CHC compounds for egg recognition and fertility signaling (e.g. Hanus et al. 2010; Smith et al. 2016; Steitz et al. 2019). For instance, it has been shown in termites of the genus Reticulitermes that specific enzymes can also be used for egg recognition (Matsuura et al. 2009). Future studies should also include large scale analyses between both sympatric and allopatric carpenter ant populations as well as their degree of interfertility and reproductive isolation. Rigorous comparisons of the capability of isolated worker groups from these colonies to discriminate QLE from WLE and corresponding chemical analyses should reveal whether our study unveiled a more wide-spread and consistent egg discrimination capability with no discernable recognition pattern.
In conclusion, we detected two very distinct chemotypes in African carpenter ant colonies initially identified as Camponotus maculatus. These chemotypes are mainly recognizable on the cuticular surface profiles of queen- and worker-laid eggs, and also, in part, on the cuticle of the respective egg producers. The highly divergent egg surface profiles greatly differed in both dominant compound classes as well as chain length distributions. Nevertheless, workers were clearly able to discriminate between queen- and worker-laid eggs and displayed selective policing behavior against the latter. The largely distinct chemotypes are also corroborated by the segregation of genetic clusters based on mitochondrial DNA sequences of the investigated carpenter ant populations, hinting at a cryptic species complex most strongly characterized by chemical divergence with conserved worker policing behavior.
Data and material availability
All data underlying the presented study is provided as Supplementary Material.
Code availability
The code underlying the Linear Discriminant Analysis is available at the GitHub repository (https://github.com/janina-rinke/Chemical-Policing-Code).
References
Abril S, Diaz M, Lenoir A, Ivon Paris C, Boulay R, Gómez C (2018) Cuticular hydrocarbons correlate with queen reproductive status in native and invasive Argentine ants (Linepithema humile, Mayr). PLoS ONE 13:e0193115
Akino T (2006) Cuticular hydrocarbons of Formica truncorum (Hymenoptera: Formicidae): description of new very long chained hydrocarbon components. Appl Entomol Zool 41:667–677
Baroni-Urbani C (1972) Studi sui Camponotus (Hymenoptera, Formicidae). Verh Naturforsch Ges Basel 82:122–135
Bien T, Gadau J, Schnapp A, Yew JY, Sievert C, Dreisewerd K (2019) Detection of very long-chain hydrocarbons by laser mass spectrometry reveals novel species-, sex-, and age-dependent differences in the cuticular profiles of three Nasonia species. Anal Bioanal Chem 411:2981–2993
Bolton B (1994) Identification guide to the ant genera of the world. Harvard University Press, Cambridge, Mass
Bolton B (2019) AntCat. An online catalog of the ants of the world. http://antcat.org/
Bourke AFG (1988) Worker reproduction in the higher eusocial Hymenoptera. Q Rev Biol 63:291–311
Bourke AFG, Franks NR (1995) Social evolution in ants. Princeton University Press, Princeton, N.J.
Carlin NF, Hölldobler B (1987) The kin recognition system of carpenter ants (Camponotus spp.): II Larger Colonies. Behav Ecol Sociobiol 20:209–217
Casacci LP, Schönrogge K, Thomas JA, Balletto E, Bonelli S, Barbero F (2019) Host specificity pattern and chemical deception in a social parasite of ants. Sci Rep 9:1619
Chung H, Carroll SB (2015) Wax, sex and the origin of species: dual roles of insect cuticular hydrocarbons in adaptation and mating. BioEssays 37:822–830
Cvacka J, Jiros P, Sobotnik J, Hanus R, Svatos A (2006) Analysis of insect cuticular hydrocarbons using matrix-assisted laser desorption/ionization mass spectrometry. J Chem Ecol 32:409–434
d’Ettorre P, Heinze J, Ratnieks FLW (2004) Worker policing by egg eating in the ponerine ant Pachycondyla inversa. Proc R Soc B 271:1427–1434
Dietemann V, Liebig J, Hölldobler B, Peeters C (2005) Changes in the cuticular hydrocarbons of incipient reproductives correlate with triggering of worker policing in the bulldog ant Myrmecia gulosa. Behav Ecol Sociobiol 58:486–496
Dietemann V, Peeters C, Liebig J, Thivet V, Hölldobler B (2003) Cuticular hydrocarbons mediate discrimination of reproductives and nonreproductives in the ant Myrmecia gulosa. Proc Natl Acad Sci USA 100:10341–10346
Endler A, Hölldobler B, Liebig J (2007) Lack of physical policing and fertility cues in egg-laying workers of the ant Camponotus floridanus. Anim Behav 74:1171–1180
Endler A, Liebig J, Schmitt T, Parker JE, Jones GR, Schreier P, Hölldobler B (2004) Surface hydrocarbons of queen eggs regulate worker reproduction in a social insect. Proc Natl Acad Sci USA 101:2945–2950
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Foster K, Ratnieks F (2001) Convergent evolution of worker policing by egg eating in the honeybee and common wasp. Proc R Soc B 268:169–174
Frank SA (1995) Mutual policing and repression of competition in the evolution of cooperative groups. Nature 377:520–522
Gadau J (2009) DNA isolation from ants. Cold Spring Harbor Protoc 7:pdb.prot5245
Gadau J, Gertsch PJ, Heinze J, Pamilo P, Hölldobler B (1998) Oligogyny by Unrelated Queens in the Carpenter Ant, Camponotus ligniperdus. Behav Ecol Sociobiol 44:23–33
Gadau J, Heinze J, Hölldobler B, Schmid M (1996) Population and colony structure of the carpenter ant Camponotus floridanus. Mol Ecol 5:785–792
Geiselhardt SF, Geiselhardt S, Peschke K (2009) Comparison of tarsal and cuticular chemistry in the leaf beetle Gastrophysa viridula (Coleoptera: Chrysomelidae) and an evaluation of solid-phase microextraction and solvent extraction techniques. Chemoecology 19:185
Gerhardt H, Schmitt C, Betz O, Albert K, Lämmerhofer M (2015) Contact solid-phase microextraction with uncoated glass and polydimethylsiloxane-coated fibers versus solvent sampling for the determination of hydrocarbons in adhesion secretions of Madagascar hissing cockroaches Gromphadorrhina portentosa (Blattodea) by gas chromatography-mass spectrometry. J Chromatogr A 1388:24–35
Golian M, Bien T, Schmelzle S, Esparza-Mora MA, McMahon DP, Dreisewerd K, Buellesbach J (2022) Neglected very long-chain hydrocarbons and the incorporation of body surface area metrics reveal novel perspectives for cuticular profile analysis in insects. InSects 13:83
Guillem RM, Drijfhout F, Martin SJ (2014) Chemical deception among ant social parasites. Curr Zool 60:62–75
Hanus R, Vrkoslav V, Hrdy I, Cvacka J, Sobotnik J (2010) Beyond cuticular hydrocarbons: evidence of proteinaceous secretion specific to termite kings and queens. Proc R Soc B Biol Sci 277:995–1002
Hartke J, Sprenger PP, Sahm J, Winterberg H, Orivel J, Baur H, Beuerle T, Schmitt T, Feldmeyer B, Menzel F (2019) Cuticular hydrocarbons as potential mediators of cryptic species divergence in a mutualistic ant association. Ecol Evol 9:9160–9176
Hebert PDN, Cywinska A, Ball SL, deWaard JR (2003) Biological identifications through DNA barcodes. Proc R Soc B 270(1512):313–321. https://doi.org/10.1098/rspb.2002.2218.
Heinze J, Stengl B, Sledge MF (2002) Worker rank, reproductive status and cuticular hydrocarbon signature in the ant. Pachycondyla Cf Inversa Behav Ecol Sociobiol 52:59–65
Helanterä H, d’Ettorre P (2015) A comparative study of egg recognition signature mixtures in Formica ants. Evolution 69:520–529
Hölldobler B, Wilson EO (1990) The ants. Belknap Press of Harvard University Press, Cambridge, Mass
Holman L, Jørgensen CG, Nielsen J, d’Ettorre P (2010) Identification of an ant queen pheromone regulating worker sterility. Proc R Soc B Biol Sci 277:3793–3800
Holze H, Schrader L, Buellesbach J (2021) Advances in deciphering the genetic basis of insect cuticular hydrocarbon biosynthesis and variation. Heredity 126:219–234
Honorio R, Châline N, Chameron S (2019) Pre-existing differences in putative fertility signals give workers the upper hand in ant reproductive hierarchies. Anim Behav 157:129–140
Hoover SER, Keeling CI, Winston ML, Slessor KN (2003) The effect of queen pheromones on worker honey bee ovary development. Sci Nat 90:477–480
Kather R, Martin SJ (2015) Evolution of cuticular hydrocarbons in the Hymenoptera: a meta-analysis. J Chem Ecol 41:871–883
Kikuta N, Tsuji K (1999) Queen and worker policing in the monogynous and monandrous ant, Diacamma sp. Behav Ecol Sociobiol 46:180–189
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Lange D, Calixto ES, Rosa BB, Sales TA, Del-Claro K (2019) Natural history and ecology of foraging of the Camponotus crassus Mayr, 1862 (Hymenoptera: Formicidae). J Nat Hist 53:1737–1749
Le Conte Y, Hefetz A (2008) Primer pheromones in social hymenoptera. Annu Rev Entomol 53:523–542
Leonhardt SD, Brandstaetter AS, Kleineidam CJ (2007) Reformation process of the neuronal template for nestmate-recognition cues in the carpenter ant Camponotus floridanus. J Comp Physiol A 193:993–1000
Liebig J, Peeters C, Hölldobler B (1999) Worker policing limits the number of reproductives in a ponerine ant. Proc R Soc B 266:1865–1870
Martin SJ, Châline N, Oldroyd BP, Jones GR, Ratnieks FLW (2004) Egg marking pheromones of anarchistic worker honeybees (Apis mellifera). Behav Ecol 15(5):839–844. https://doi.org/10.1093/beheco/arh089
Matsuura K, Yashiro T, Shimizu K, Tatsumi S, Tamura T (2009) Cuckoo fungus mimics termite eggs by producing the cellulose-digesting enzyme β glucosidase. Curr Biol 19:30–36
Menzel F, Blaimer BB, Schmitt T (2017) How do cuticular hydrocarbons evolve? Physiological constraints and climatic and biotic selection pressures act on a complex functional trait. Proc R Soc B Biol Sci 284(1850):20161727
Menzel F, Zumbusch M, Feldmeyer B (2018) How ants acclimate: impact of climatic conditions on the cuticular hydrocarbon profile. Funct Ecol 32:657–666
Monnin T, Malosse C, Peeters C (1997) Solid-phase microextraction and cuticular hydrocarbon differences related to reproductive activity in queenless ant. J Chem Ecol 24:473–490
Monnin T, Peeters C (1997) Cannibalism of subordinates’ eggs in the monogynous queenless ant Dinoponera quadriceps. Sci Nat 84:499–502
Peeters C, Liebig J (2009) Fertility signaling as a general mechanism of regulating reproductive division of labor in ants. Pp. 220–242. Organization of insect societies: From genome to socio-complexity. University Press, Harvard
R Development Core Team (2018) R: A language and environment for statistical computing. R foundation for statistical computing, http://www.R-project.org, Vienna, Austria
Ratnieks FLW, Visscher PK (1989) Worker policing in the honeybee. Nature 342:796–797
Ratnieks FLW, Wenseleers T (2005) Policing insect societies. Science 307:54–56
Saitoh F, Janssen A, Choh Y (2020) The use of volatile cues in recognition of kin eggs by predatory mites. Ecol Entomol. https://doi.org/10.1111/een.12872
Schwander T, Arbuthnott D, Gries R, Gries G, Nosil P, Crespi BJ (2013) Hydrocarbon divergence and reproductive isolation in Timema stick insects. BMC Evol Biol 13:151
Smith AA, Hölldober B, Liebig J (2009) Cuticular hydrocarbons reliably identify cheaters and allow enforcement of altruism in a social insect. Curr Biol 19:78–81
Smith AA, Liebig J (2017) The evolution of cuticular fertility signals in eusocial insects. Curr Opin Insect Sci 22:79–84
Smith AA, Millar JG, Hanks LM, Suarez AV (2012) Experimental evidence that workers recognize reproductives through cuticular hydrocarbons in the ant Odontomachus brunneus. Behav Ecol Sociobiol 66:1267–1276
Smith AA, Millar JG, Hanks LM, Suarez AV (2013) A conserved fertility signal despite population variation in the cuticular chemical profile of the trap-jaw ant Odontomachus brunneus. J Exp Biol 216:3917–3924
Smith AA, Millar JG, Suarez AV (2015) A social insect fertility signal is dependent on chemical context. Biol Lett 11:20140947
Smith AA, Millar JG, Suarez AV (2016) Comparative analysis of fertility signals and sex-specific cuticular chemical profiles of Odontomachus trap-jaw ants. J Exp Biol 219:419–430
Smith AA, Suarez AV, Liebig J (2018) Queen pheromones out of context: a comment on Holman. Behav Ecol 29:1212–1212
Sprenger PP, Menzel F (2020) Cuticular hydrocarbons in ants (Hymenoptera: Formicidae) and other insects: how and why they differ among individuals, colonies, and species. Myrmecol News 30:1–26
Steitz I, Ayasse M (2020) Macrocyclic lactones act as a queen pheromone in a primitively eusocial sweat bee. Curr Biol 30:1136-1141.e1133
Steitz I, Brandt K, Biefel F, Minat Ä, Ayasse M (2019) Queen recognition signals in two primitively eusocial halictid bees: evolutionary conservation and caste-specific perception. InSects 10:416
Tentschert J, Bestmann HJ, Heinze J (2002) Cuticular compounds of workers and queens in two Leptothorax ant species—a comparison of results obtained by solvent extraction, solid sampling, and SPME. Chemoecology 12:15–21
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl Acids Res 22:4673–4680
Van Oystaeyen A, Oliveira RC, Holman L, van Zweden JS, Romero C, Oi CA, d’Ettorre P, Khalesi M, Billen J, Wäckers F et al (2014) Conserved class of queen pheromones stops social insect workers from reproducing. Science 343:287–290
van Wilgenburg E, Symonds MRE, Elgar MA (2011) Evolution of cuticular hydrocarbon diversity in ants. J Evol Biol 24:1188–1198
Wenseleers T, Helanterä H, Hart A, Ratnieks FLW (2004) Worker reproduction and policing in insect societies: An ESS analysis. J Evol Biol 17:1035–1047
Wenseleers T, Ratnieks FLW (2006) Comparative analysis of worker reproduction and policing in eusocial hymenoptera supports relatedness theory. Am Nat 168:E163–E179
Wenseleers T, Tofilski A, Ratnieks FLW (2005) Queen and worker policing in the tree wasp Dolichovespula sylvestris. Behav Ecol Sociobiol 58:80–86
Whitfield J (2002) Social insects: the police state. Nature 416:782–784
Wilson EO (1971) The insect societies. Belknap Press of Harvard University Press, Cambridge, Mass
Yagound B, Blacher P, Fresneau D, Poteaux C, Châline N (2014) Status discrimination through fertility signalling allows ants to regulate reproductive conflicts. Anim Behav 93:25–35
Acknowledgements
We thank the Comoé National Park Research Station and its staff for the use of their facilities for field and laboratory research and the park management of Office Ivoirien des Parcs et Réserves for enabling field research in the park. Special thanks go to Bonnie Blaimer for generously providing her taxonomic expertise in morphologically examining workers from our studied colonies.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was partly funded by the German Research Foundation (DFG) as part of the SFB TRR 212 (NC3) – Project numbers 316099922 and 396780988.
Author information
Authors and Affiliations
Contributions
Conceptualization, JG and URE; methodology, JB, JR, URE, VH and MP; validation, JB, JR and URE; formal analysis, JB, URE and JR; investigation, JB, JR, VH, LR, JMT and MP; resources, JG; data curation, JB and JR; writing—original draft preparation, JB and JR; writing—review and editing, JB, JR, JG and URE; visualization, JB and JR; supervision, JG and URE; project administration, JG and URE; funding acquisition, JG.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
There is no ethics committee overseeing experimental research on carpenter ants. However, all efforts have been made to treat the animals as humanely as possible. The research was carried out under research permit number N°018/MINEDD/OIPR/DZ from the Office Ivoirien des Parcs et Réserves and adhered to the requirements of the relevant research guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Buellesbach, J., Rinke, J., Reuter, L. et al. Conserved worker policing in African carpenter ants with drastically different egg chemotypes. Evol Ecol 37, 815–834 (2023). https://doi.org/10.1007/s10682-023-10245-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-023-10245-5