Abstract
Orange rust caused by Puccinia kuehnii is a major emerging disease in many sugarcane-producing countries. Breeding for resistant varieties is the main strategy for controlling orange rust. The rapid spread of this disease in recently contaminated sugarcane industries offers the opportunity to use on-going breeding trials to investigate the effect of orange rust on yield traits and gauge levels of resistance required to minimize losses. Orange rust was first observed in 2018 in Reunion. This study reports the effects of the disease on cane yield (CY), recoverable sugar (RS), fiber content (FIB) and economic index (EI) in five environments of Reunion’s sugarcane breeding program located in diverse agro-climatic zones. Disease resistance assessed under natural infection had high broad-sense heritability (0.76–0.91) in multi-environment analyses. Mean infection levels differed between locations congruently with location differences for two influential climatic parameters (humidity and temperature). Maximum potential yield losses (\({YL}_{max}\)) associated with orange rust were estimated using regression analyses of yield traits versus disease susceptibility. \({YL}_{max}\) for CY and EI varied between environments and reached up to 26.0% and 24.2% respectively, in one of the most humid environments. RS was either unaffected or only slightly increased by the disease. In contrast, FIB was always reduced by the disease (\({YL}_{max}\)≤6.5%). Multi-environment analyses of yield traits of varieties common to all five environments gave insights into the impact of orange rust on the yielding ability of these varieties across all environments. All these data provide food for thoughts to efficient breeding strategies for varietal resistance to orange rust.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sugarcane orange rust, caused by Puccinia kuehnii E.J. Butler, occurs in at least 45 sugarcane-producing countries worldwide (https://www.cabi.org/isc/datasheet/45818). It is an emerging disease in many countries of Central and South America (Ovalle et al. 2008; Chavarría et al. 2009; Flores et al. 2009; Barbasso et al. 2010; Pérez-Vicente et al. 2010; Cadavid et al. 2012; Funes et al. 2016; Morante 2018). In most tropical areas, orange rust is one of the most damaging diseases of sugarcane because disease epidemics can last many months, especially during active growth of the plant. The disease was first recognized in the South West of the Indian Ocean at Reunion and Mauritius islands in 2018 (Hubert et al. 2019; Mungur et al. 2020). It is believed that orange rust arrived on these islands with spores of P. kuehnii carried by wind currents as it was hypothesized in other locations affected by orange rust outbreaks (Rott et al. 2016). The geographical origin as well as the introduction circumstances and arrival date of the pathogen are, however, unknown.
Average night temperatures of 20–22 °C associated with high relative humidity (> 90%) are the most conducive conditions for disease progress in Florida (Sanjel et al. 2019; Chaulagain et al. 2020). Such weather conditions also exist in Reunion in many sugarcane-growing areas of the island for long periods of the year, which coincide with the active growth period of the crop. Consequently, orange rust has become a new major disease in Reunion that potentially affects sugarcane production. Furthermore, the local breeding germplasm has not been evaluated and selected for resistance to this disease yet, which is indispensable to produce new orange rust resistant sugarcane varieties.
Sporulating lesions of orange rust reduce the net photosynthetic rate of sugarcane leaves. This reduced net leaf photosynthesis results from a lower assimilation of CO2 combined with a higher dark respiration rate. This phenomenon is attributed to energy demands for host plant self-defense or injury response to the disease and for urediniospore production during the sporulation phase (Zhao et al. 2011). Reductions in green leaf area and in leaf net photosynthetic rate can affect plant growth and contribute to cane yield losses. Moreover, as reported for many crops infected by Pucciniales fungi (Ayres 1978; Grimmer et al. 2012), damage of leaf epidermis caused by orange rust could affect sugarcane's ability to control water loss, thus leading to potential increases in cane yield losses. The extent of cane tonnage loss caused by orange rust will depend on the severity and persistence of the infection. Losses from 30 to 50% have been recorded in Australia, Brazil, and the USA where varieties susceptible to orange rust were grown on large scales (Magarey et al. 2001; Rott et al. 2017). In all these locations, varieties susceptible to orange rust were progressively replaced by new and resistant varieties obtained in local breeding and selection programs.
Profitability and sustainability of most sugarcane industries worldwide rely on breeding programs that aim to regularly deliver improved high-yielding varieties that are tolerant to major local diseases (Lakshmanan et al. 2022). In most sugarcane producing countries, varietal resistance is the main strategy for controlling diseases. Breeding and selecting for resistant or tolerant varieties is all the more important because, although harvested annually, a sugarcane crop is established for several years (Van Dillewjin 1952). In Reunion, sugarcane fields are usually only replanted every 10 years (Dumont et al. 2022). Therefore, the use of insufficiently resistant cultivars may result in recurrent economic losses for a long period. Sugarcane orange rust can also be efficiently controlled with fungicide treatments (Chaulagain 2019a; b). Nevertheless, this chemical control is not a desirable long-term option due to (i) economic reasons (i.e. controlling cane production costs), (ii) long-term disease management concerns (i.e. not exerting selection pressures favoring the appearance of new pathogenic races) and (iii) environmental matters (i.e. preserving natural resources).
Since its inception almost a century ago, the sugarcane variety development program (SVDP) of Reunion has always paid attention to breeding efforts for disease resistance (Dumont et al. 2022). Consequently, production of varieties resistant or tolerant to pathogens that emerged locally during the last decades resulted in successful management of the diseases they cause. For example, sugarcane varieties resistant to brown rust (Puccinia melanocephala) have been developed using the major rust resistance gene, Bru1, identified in cultivar R570 (Daugrois et al. 1996; Asnaghi et al. 2004). This major durable resistance gene, which prevents sporulation of P. melanocephala on sugarcane leaves, is present at a high frequency in the sugarcane founders that shaped the current local breeding germplasm (Costet et al. 2012). However, this source of resistance does not prevent the development of orange rust and most local genetic resources carrying the Bru1 gene are susceptible to orange rust (Hervouet and Dijoux, unpublished data).
The main objective of the SVDP in Reunion is to select varieties adapted to the different major agro-climatic regions of sugarcane production of the local industry (Dumont et al. 2019, 2022). Seven decentralized selection programs, strategically located in the major sugarcane growing areas, are run concurrently (eRcane 2009; Guilly et al. 2017). Each program undergoes a sequential process of clonal selection for 10 years in four successive stages (stages I–IV). Key selection criteria include high biomass and sugar productivity related to cane yield and recoverable sugar, fiber content and good resistance levels to major diseases present in Reunion (Dumont et al. 2022). At the end of stage IV, few elite varieties are selected from each selection site and tested across all sites in the final selection stage (stage V) based on multi-environment trials (MET) carried out for four crop cycles (plant crop to third ratoon crop). This final selection stage allows to test genotype × location (GL) interactions and to determine the yield potential and stability of sugarcane varieties across locations. Consequently, this procedure provides key information for decision-making regarding the release of new varieties (Guilly et al. 2017).
The objective of this study was to evaluate the effect of a newly occurring disease, namely orange rust, on sugarcane yield traits in five locations of Reunion representing various agro-climatic environments. To reach this goal, we used more than a hundred elite sugarcane varieties from four breeding series that recently entered the MET selection stage V of the SVDP. This elite material had been selected before 2018 at previous stage IV mainly based on cane yield and stalk sugar content but not for orange rust resistance, at a time when the disease was absent and did not allow any choice on this criterion. Visual observations and ratings of orange rust symptoms were conducted with these elite varieties naturally infected by P. kuehnii in the five sites of the MET network. The accuracy of disease ratings was assessed in replicated field trials and the correlation of the levels of resistance to orange rust among the different experimental sites was evaluated. We also determined in each site the relationship between resistance levels and four traits related to yield. Potential yield losses due to the disease were determined by assuming that the genetic determinism of yield traits and the one of resistance to orange rust were independent. A multi-local analysis, restricted to the varieties common to all five sites, was performed with the four yield traits combined with information on their resistance level. This analysis allowed us to examine disease behavior of varieties showing the highest and most stable mean performances across sites, which represented the most promising candidate varieties for commercial release. Finally, results obtained provided food for thoughts to breeding strategies for varietal resistance to orange rust in a sugarcane breeding program.
Materials and methods
Experimental sites
Field experiments were located in the following five selection sites of Reunion: Etang-Salé (ES), Le Gol (GL), La Mare (LM), Menciol (MC) and Saint-Benoit (SB). The first two sites (ES and GL) were representative of the irrigated coastal areas of the dry leeward coast of the island, and the three others were representative of the wet windward coast, either near sea level (LM, SB) or in the highlands (MC). Guilly et al. (2017) and Dumont et al. (2022) reported precise localization, climate and soil characteristics, and selection focus of each of these sites. The five sites were characterized by wet summers (November–April) and more or less dry winters (June–September). Climate graphs for rainfalls and temperatures are provided in Supplementary Information 1. Experiments conducted in the perhumid sites of SB (2968 mm of annual rainfall) and MC (3210 mm) were conducted in rainfed conditions. In addition to natural rainfall, experiments conducted in the very close (< 3 km) and dry sites of ES (676 mm) and GL (676 mm) were drip irrigated all year round. Nevertheless, the irrigation regime was adjusted at ES to compensate for rainfall deficit and to reach the daily potential evapotranspiration value of the local climate. At GL site, due to the specific selection objective to screen for sugarcane tolerance to hydric stress (Guilly et al. 2017), the irrigation regime was adjusted to reach only 50% of the daily evapotranspiration value. Experiments in the semi-dry climate of LM (1374 mm) were irrigated mainly during the hydric stress period of winter with overhead sprinklers to reach daily evapotranspiration. The mean annual temperature at the coastal experimental sites were 24.1 °C, 24.1 °C, 23.8 °C, and 23.1 °C at ES (20 m elevation), GL (20 m), LM (70 m), and SB (40 m), respectively. The mean annual temperature at MC highlands (400 m) was 21.0 °C.
Plant material and field trials
Sugarcane studied for resistance to orange rust consisted of elite varieties belonging to four successive series, namely S7–S10 tested in the final multi-environmental stage V of Reunion’s breeding program (Dumont et al. 2022). These elite varieties represented the very best performing candidates previously selected from stage IV in one of the seven experimental sites of the program based on their performance for cane yield, recoverable sugar, and economic index during three successive crop harvests (plant cane, first and second ratoon) (Dumont et al. 2022). The most recently planted variety series i.e. S10 in 2019, contains varieties selected at stage IV based on agronomic performances measured in 2015, 2016 and 2017 i.e. before the date of the first observation of symptoms of the disease in Reunion. None of the elite varieties of S7-S10 series have so far been evaluated for their behavior against orange rust.
Table 1 provides an overview of the 20 trials (4 series × 5 sites) of the study, in terms of planting year, number of tested varieties, and year of evaluation for resistance to orange rust. The four series of elite varieties (S7–S10) were successively planted in 2016–2019 in all five experimental sites. Consequently, varieties were first harvested one year later in 2017–2020 in all sites. Varieties were then harvested annually for a maximum of four years. For example, the series S8 planted in 2017 in all sites was harvested in 2018, 2019, 2020 and 2021 in plant, first, second, and third ratoon crops, respectively. Trials were established using randomized complete block (RCB) designs with four replicates. The elementary sugarcane variety plot consisted of three 10 m-rows each separated by 1.5 m (i.e. plot size of 45 m2). Successive MET series each consisted of 26–31 elite varieties and six check varieties (Supplementary Information 2). All series considered together, 112 new candidate varieties were studied. For each series, an unbalanced number of varieties was tested between sites because of constraints of land availability (Table 1). Forty-two sugarcane varieties were tested in all five screening sites. The 20 trials also included the check variety R579, which is the leading sugarcane commercial variety in Reunion and a high yielding cultivar in several foreign countries (in Africa as well as in the Caribbean and the Mascarene Islands). Check variety R587, released for commercial production in 2016, was included in 17 of the 20 trials (85%). Moreover, in each site, trials of the four series also shared one or two additional check variety(s) as commercial reference(s) for local selection of elite varieties. All experimental fields were fertilized annually with 150, 180, and 240 kg of N, P and K per ha, respectively, as recommended for sugarcane growth in commercial fields in La Reunion (Chabalier et al. 2006). All the 112 varieties and the six checks were diagnosed for the Bru1 gene using the two diagnostic PCR flanking markers 9O20-F4 and R12H16 (Costet et al. 2012). Bru1 was present in the six checks and in 74.1% of the varieties, but absent in 22.3% varieties and undetermined in the remaining 3.6% ones (Supplementary Information 2).
Field assessment of orange rust severity (ORS)
Orange rust was assessed in all field trials under natural inoculation conditions, at the end of the favorable humid and hot season. Plants were assessed in 2020 and/or in 2021 (Table 1) at the time of canopy closure when trials were at least five months old. Presence of orange rust on all varieties was established in the field by visual observations. Criteria taken into account were (i) the orange color of fresh sporulating pustules; (ii) the short size (3–4 mm) of the uredinia and (iii) the random distribution of uredinia in any parts of infected leaves (Magarey et al. 2001; Glynn et al. 2010). Based on these visual criteria, the lesions observed on the varieties carrying Bru1 were indeed found to be caused by orange rust and not a breakdown of Bru1 resistance. As for the 29 varieties lacking the Bru1 gene, visual observations in the field were supplemented by laboratory observations performed on at least 10 randomly chosen pustules per variety to confirm the presence of orange rust. Identification criteria were (i) the typical bright orange color of urediniospores observed with a binocular magnifying glass (Zeiss Stemi 508, 50–250 magnification range), (ii) the pyriform aspect of urediniospores and the typical apical thickening of their cell wall examined under the microscope (Olympus BX51, × 500, with immersion oil) (Virtudazo et al. 2001; Magarey et al. 2001; Dixon et al. 2010). Finally, the reliability of the visual diagnostic protocols of orange rust lesions in the field and the laboratory were checked using a molecular assay. Spores collected from 100 uredinia each identified as orange rust were tested by PCR with primer pair Pk1-F/Pk1-R (Glynn et al. 2010). This assay generated an amplification product (527 bp) specific to P. kuehnii, and the results of the molecular diagnosis matched 100% with the visual identification of orange rust.
Orange rust severity was assessed on 15 randomly chosen stalks in each experimental plot. Since sugarcane stalks produce new leaves continuously at their shoot apex, the lower leaves are exposed longer to successive infection events than the higher leaves. Consequently, an increasing gradient of symptoms severity toward the lower leaf stages can be observed on susceptible varieties. To take this disease severity gradient into account, the number of rust uredinia was assessed on three different leaf stages: the top visible dewlap or TVD leaf (= first fully emerged leaf at the top with a visible dewlap at the junction of the leaf blade and the leaf sheath), the fourth leaf below the TVD leaf (= TVD + 4), and the lowest non-senescent or lowest green (LG) leaf (= TVD + 5–8). Based on the mean number of uredinia recorded for each of the three leaf stages (TVD, TVD + 4 and LG) of 15 randomly chosen stalks, an orange rust severity (ORS) score was attributed to each variety plot using a 0–8 scale (Table 2).
Traits related to yield
The harvest periods for the 20 trials spanned the local harvest campaign from early July to end of November. Trials were always harvested when sugarcane was about 12 months old. The sugarcane yield traits that were measured at harvest for each crop-year trial were as follows: cane yield (CY, tons ha−1), fiber content (FIB, %), recoverable sugar (RS, %) as percentage of fresh cane weight, and an economic index (EI). All millable stalks from each variety plot were manually cut and weighted using a digital scale mounted on a hydraulic boom. CY was determined from plot weights divided by the plot area (45 m2). FIB% and RS% quality traits were determined using a cane sample of 18 randomly chosen stalks. These stalks were processed at eRcane’s laboratory using a standard protocol and a semi-automatic near-infrared spectroscopy (NIRS) device (Bruker Optik GmbH) with in-house calibrations providing highly accurate values (Roussel et al. 2015). The EI trait used to rank cultivation merit of sugarcane varieties was calculated with CY and RS data using the conventional formula EI = CY × (RS-4) of Reunion’s breeding program (Guilly et al. 2017; Dumont et al. 2022). This economic index indicates a farming profit that is justified by gains in sugar content. These gains in sugar content are economically more beneficial than the corresponding increases in cane yield as these latter ones are associated with higher production costs (harvesting, transport, and milling) (Jackson 2005).
Statistical analyses
All data analyses presented below were performed using different linear mixed models run with the MIXED procedure of SAS 9.4 (SAS Institute, Cary, NC), and variance components were evaluated using the restricted maximum likelihood (REML) procedure. Normality of residuals was evaluated and confirmed graphically as recommended by Kozak and Piepho (2018). For all these analyses, data considered for traits related to yield (CY, RS, FIB and EI) were restricted to data collected during the three years following the initial spread of orange rust throughout Reunion, i.e. data collected for the 2019, 2020 and 2021 harvest crops. Consequently, two, three, three and two crop-cycles were analyzed for yield traits for S7, S8, S9 and S10 series, respectively.
Individual trial analysis for orange rust severity (ORS)
The 20 trials, which consisted of RCB designs were analyzed individually with the following mixed linear model fitted for orange rust severity (ORS):
where \(Y_{ijk}\) represented the observation of genotype i in replication j in crop year k (if more than one); µ the grand mean, \(G_{i}\) the main random effect of genotype i, \(R_{j}\) the fixed effect of replication j, \(C_{k}\) the random effect of crop-year k (if more than one), \(GC_{ik}\) the random effect of the genotype x crop year interaction and \(e_{ijk}\) the residual error. For trials assessed in two crop-years, an unstructured (UN) matrix of variance–covariance (VCV) of random error terms (Littell et al. 2006) was chosen to allow possible error variance specific for each crop-year as well as possible non-null covariances between crop-years. Broad-sense heritability at trial level for ORS was calculated with the following generalized formula (Rodriguez-Alvarez et al. 2018):
where \(\overline{PEV}\) is the mean of the prediction error variance of best linear unbiased prediction (BLUP) of genotypes and \({\sigma }_{G}^{2}\) is the genetic variance.
Comparison of locations for orange rust infection
The five locations used for the field trials corresponded to different agronomic and/or climatic ecologies of sugarcane production. To test for possible mean differences of natural rust infections between locations, we selected ORS data of the 36 varieties that were present in all locations in 2021 (n = 16, 7, 13 in the S8, S9 and S10 series respectively). This across-location balanced set of data was then analyzed using the following model:
where \({Y}_{ijls}\) was the ORS value of a shared genotype i related to series s observed in replicate j at location l, \({S}_{s}\) the main random effect of series s, \({L}_{l}\) the main fixed effect of location l, \({R}_{j(s,l)}\) the random effect of replication j in the trial of series s in location l, \({GL}_{il(s)}\) the random effect of genotype × location interaction in series s and other terms were the same as in model 1. Independent error variances were modelled for the different trials. Multiple comparisons of location means for ORS were performed using Tukey’s test (Steel and Torrie 1980).
Multi-environment trial (MET) analysis
Considering all locations (ES, GL, LM, MC and SB) together, each series (S7, S8, S9, and S10) was analyzed separately with the following multi-environment trial (MET) model fit to ORS and to each yield trait (CY, RS, FIB and EI):
where \({Y}_{ijkl}\) was the value of genotype i for the trait of interest, at location l, in replicate j, in crop year k; \({L}_{l}\) the main effect of location l;\({R}_{j(l)}\) the effect of replication j at location l, \({C}_{k(l)}\) the effect of crop year k at location l, \({GL}_{il}\) the effect of the genotype × location interaction, \({GLC}_{ilk}\) the effect of the genotype x location x crop year interaction, while the other terms and indices were the same as in model 1. In this analysis, which aimed to estimate variance components of each trait at the MET level, all explanatory variables were considered as random effects. In each analysis, an unstructured (UN) matrix of VCV of errors was modelled for each location in order to allow VCV structures of error terms independent between trials. Broad-sense heritability at MET level was estimated for each trait using the generalized formula of Eq. 1.
Multi-series analysis within location
Taking advantage of the presence of common check varieties in all series (Supplementary Information 2), a joint analysis of the four series was performed within each location using the following model fit to each trait (ORS, CY, RS, FIB and EI):
where \({Y}_{ijks}\) was the trait value of interest of genotype i, in series s, in replicate j, in crop year k; \({R}_{j(s)}\) the main effect of replication j in series s and \({C}_{k(s)}\) the effect of crop year k in series s, while the other terms and indices are the same as in model 2. All variables in model 4 were considered as random effects, in particular the genotype effect to obtain BLUP values, which have the desirable statistical property to maximize the correlation between the ‘true’ genotypic values and the predicted genotypic values (Searle et al. 2009). To allow structures of VCV of error terms independent between the different series' trials and error variances different between crop cycles within trials, an UN matrix was modelled for VCV of errors of each trial.
This model performed across all series was used for the following three applications:
-
Estimation of Pearson’s correlation coefficients between pairwise locations for ORS based on BLUP values of genotypes common to pair-wise locations;
-
Evaluation in each location of the relationship existing between level of resistance / susceptibility to orange rust and each yield trait. For that purpose, genotypic predictions of varieties for ORS and each yield trait (CY, RS, FIB, EI) in each location were calculated by adding their BLUP values to model intercept. These predictions were used to establish linear regressions (\(y=ax+b\)) relating ORS (\(x\)-value) to each yield trait (\(y\)-value) and to calculate Pearson’s coefficient of correlations (r) with XLSTAT 2021.4 (Addinsoft, New-York, USA). The following test statistic was computed: \({t}_{n-2}= \frac{r}{1-{r}^{2}}\sqrt{n-2}\), with n being the number of observations pairs on which each correlation was based. The significance of the observed correlations was based on a comparison of this test statistic with a Student’s t distribution with n-2 degrees of freedom (Steel and Torrie 1980). For each yield trait in each location, we calculated a maximum potential yield loss (\({YL}_{max}\%\)) caused by orange rust. This calculation was based on the discrepancy between the yields of the most resistant and the most susceptible varieties predicted by the regression line (y-value when x-value is either zero or maximum) (Bhuiyan et al. 2022). Hence, this maximum potential yield loss was calculated as follows: \({YL}_{max}\left(\%\right)=\frac{\left(a{x}_{0}+b\right)-(a{x}_{max}+b)}{(a{x}_{0}+b)} \times 100=(- a{x}_{max}/b)\times 100\), where \({x}_{max}\) was the highest ORS prediction value among all varieties tested, \({x}_{0}\) was equal to zero, a was the slope the slope of the regression line and b its y-intercept. This estimation method of a maximum potential yield loss caused by orange rust using breeding trials was motivated by two favorable features: (i) the occurrence in each location of a large disease behavior segregation from very resistant to very susceptible varieties, which resulted from variety selection prior to disease arrival and (ii) the independence between the genetic determinism of orange rust resistance and the genetic determinism of yield traits. Resistance to orange rust is controlled by a few major Quantitative Trait Alleles (QTAs) (Yang et al. 2018), whereas yield traits are controlled by a very large number of QTAs each with little effect (Hoarau et al. 2002; Reffay et al. 2005; Piperidis et al. 2008; Aitken et al. 2006, 2008). Finally, the slope of the regression line was used to estimate a yield loss resistance index (YLRI5) as defined by Magarey and Bull (2003). YLRI5 is a level of resistance inferred from the regression line and for which yields are 5% lower than the yields of the most resistant varieties.
-
Visualization across all locations of the response of candidate varieties for each yield trait (CY, RS, FB, EI) through the ‘Genotype plus Genotype x Environment’ (GGE) biplot analysis methodology that allows graphical visualization of the relationships among varieties, among locations and their underlying interactions simultaneously (Yan and Tinker 2005, 2006). For that purpose and for each trait, within-location BLUP values (\({\widehat{Y}}_{il}\)) of the sole 42 varieties common to all locations and of check variety R579 were selected and organized into a two-way table of genotypic predictions x locations. Genotypic predictions were centered to the mean trait value of each location l (\({\upmu }_{l})\) and divided by its standard deviation (\({s}_{l}\)) to obtain a “standardized GGE matrix”. This standardized GGE matrix was subjected to singular value (SV) partitioning between the variety and location eigenvectors using the general model of Yan (2002):
$$\frac{{\hat{Y}_{il} - \upmu_{l} }}{{s_{l} }} = \sum\limits_{p = 1}^{n} {\lambda_{p}^{{f_{p} }} \alpha_{ip} \lambda_{p}^{{1 - f_{p} }} \gamma_{lp} + e_{il} }$$(2)
where \({\lambda }_{p}\) is the SV of the pth principal component (PC), \({\alpha }_{ip}\) and \({\gamma }_{lp}\) are the respective eigenvectors of variety i and location l and \({f}_{p}\) is a partition factor for PC p. SV decompositions and biplot figures were performed using XLSTAT 2021.4 and its principal component analysis command with the ‘distance biplot’ option to partition SV entirely into variety eigenvectors (\({f}_{p}\) = 1). With this scaling option, variety scores on PCs (\({\lambda }_{p}^{1}{\alpha }_{ip}\)) are in the original unit of the trait and environment scores (\({\lambda }_{p}^{0}{\gamma }_{lp}\)) are unit less and many times smaller than those of variety scores (Yan 2002). The distances between the varieties in biplots represent an approximation of their Euclidean distance in the space of the five environments. The position of two varieties projected on a vector of a particular location makes it possible to compare their genotypic predictions in the considered location for the studied trait. ORS values pictured in GGE biplots of yield traits represented mean genotypic predictions of varieties estimated across all five locations. In each GGE biplot, the average PC1 and PC2 scores of the five location vectors defined an average environment (AE) vector. This AE vector and its orthogonal vector defined a new reference frame in which: (i) the highest positive (or negative) abscissa should pinpoint the varieties exhibiting the highest (or lowest) mean performance across all environments, and (ii) the highest ordinates (either positive or negative) should pinpoint the varieties exhibiting the highest performance instability across environments (Yan 2002).
Results
Analyses of the ORS data
The ORS trait was analyzed (model 1) individually for each of the 20 field trials (4 series × 5 locations). Broad-sense heritability (\({H}^{2}\)) of ORS at trial level ranged from 0.80 to 0.94 and had a mean value of 0.90 (Table 3).
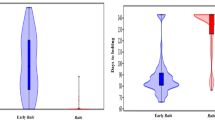
Comparison of orange rust infection levels between locations (model 2) revealed a highly significant location effect (P < 0.001). Based on mean ORS, the locations ranked as follows: SB > MC ≥ LM ≥ ES > GL (Fig. 1). The highest infection level was found at SB (4.20) and the lowest infection level at GL (2.29), and they were both different from those at the other three stations. The infection level at MC (3.60) was also statistically higher than the one at ES (2.86), while the intermediate level at LM (3.30) was comparable to the one at MC and ES.
Comparison of sugarcane orange rust severity (ORS) means between five geographical locations of Reunion (Model 2). Each data point represents the mean disease score of 36 varieties. ORS score of each variety is the mean of four replicates of 15 sugarcane stalks each (Table 2). Locations bearing the same letter are not statistically different at P = 0.05 according to Tukey’s test. Vertical bars at the top of each column represent standard error. SB = Saint-Benoît, MC = Menciol, LM = La Mare, ES = Etang-Salé, GL = Le Gol
Analyses of variance of ORS components in multi-environment trials (model 3) revealed that genotype (G) variance explained 74%, 37%, 73% and 66% of the total phenotypic variance in S7, S8, S9 and S10 series, respectively (Table 4). Whatever the series, the magnitude of variance due to location effect was small (5–18%), as well as the variance due to genotype-location interaction (4–17%). Magnitude of variance due to the two remaining genotype-crop year and genotype-location-crop year interactions was negligible (0–6%). Broad-sense heritability (\({H}^{2}\)) of ORS at the level of multi-environment trials ranged from 0.76 to 0.91, depending on the series.
Correlation coefficients of ORS between locations estimated across all series (model 4) ranged from 0.81 (GL and MC) to 0.94 (LM and ES), and they were always highly significant (P < 0.001) (Table 5).
Multi-environment analyses of yield traits
When comparing magnitudes of the different variance components of yield traits in the multi-environment analysis (model 3), some invariants were noted regardless of the variety series (Supplementary Information 3). All series taken together, the variance range due to the location effect was always much higher than the variance range due to the genotype effect: 20–55% > 6–13% for cane yield, 18–36% > 7–16% for recoverable sugar, and 17–57% > 8–12% for economic index. Exceptions were found for fiber content since variance due to genotype for this trait was greater than variance due to location in series S7 and S10. For all four traits, the variance magnitude of genotype-location interaction was always low (never higher than 10%). All traits together, variance of the crop year effect varied greatly from one series to another, from a minimum of 4% up to a maximum of 28%. Whatever the trait and the series, variances of genotype-crop year and genotype-location-crop year interactions were always negligible (not higher than 7% and 4%, respectively). Depending on the series, broad-sense heritability at level of multi-environment trials varied between 0.65 and 0.76 for cane yield, 0.54–0.76 for recoverable sugar, 0.78–0.87 for fiber content, and 0.60–0.68 for economic index.
Effect of orange rust disease on yield traits in each location
A highly significant negative correlation (P < 0.01 or 0.001) was found between disease susceptibility and cane yield in four of the five locations (GL, ES, MC and SB) (Fig. 2). Across all sites, mean correlation coefficient was − 0.32. Maximum potential yield loss (\({YL}_{max}\)) in cane tonnage associated with orange rust in the breeding trials was 10.1%, 14.2%, 21.3% and 26.0% at ES, GL, SB and MC, respectively, while it was not significant at LM (4.0%). Mean \({YL}_{max}\) was 15.1% across all sites. In the four locations where yield losses were significantly related to disease severity (ES, GL, SB and MC), YLRI5 ranged from 1.41 to 3.03. Conversely, in LM this index was greater than 8.
Effect of orange rust on cane yield (CY) in plant breeding assessment trials of the four variety series at five geographical locations of Reunion. ES = Etang-Salé, GL = Le Gol, LM = La Mare, MC = Menciol, SB = Saint-Benoît. R = coefficient of correlation. \({YL}_{max}\)= Maximum potential Yield Loss. YLRI5 = Yield Loss Resistance Index. NS = Non-Significant; significant at *P < 0.05, **P < 0.01, ***P < 0.001 according to a t-test as described in material and method (multi-series analysis within location)
An unexpected slightly positive coefficient of correlation was observed between disease susceptibility and recoverable sugar in all locations (mean value of + 0.18) which was significant (P < 0.05) in three locations (GL, ES, and LM) (Fig. 3). Consequently, \({YL}_{max}\) in recoverable sugar turned out to be a small gain between 4.0 and 5.9% at the three locations with significant relationships.
Effect of orange rust on recoverable sugar (RS) in plant breeding assessment trials of the four variety series at five geographical locations of Reunion. ES = Etang-Salé, GL = Le Gol, LM = La Mare, MC = Menciol, SB = Saint-Benoît. R = coefficient of correlation. \({YL}_{max}\) = Maximum potential Yield Loss. ‡ = the negative sign indicates a gain instead of a loss. YLRI5 = Yield Loss Resistance Index. NA = non-applicable. NS = Non-Significant; significant at *P < 0.05, **P < 0.01, ***P < 0.001 according to a t-test as described in material and method (multi-series analysis within location)
A significant (P < 0.01) negative correlation between disease susceptibility and fiber content was found for three locations (ES, LM, and SB) and a negative non-significant one for the remaining two (GL, MC) (Fig. 4). Across locations, mean correlation coefficient was − 0.26. Reduction in fiber content varied between 1.1% (GL) and 6.5% (LM) and averaged 4.3%. YLRI5 was equal or greater than 8 for three locations (GL, MC and SB) and above 5 for the two remaining ones (5.17 for LM and 5.25 for ES).
Effect of orange rust on fiber content (FIB) in plant breeding assessment trials of the four variety series at five geographical locations of Reunion. ES = Etang-Salé, GL = Le Gol, LM = La Mare, MC = Menciol, SB = Saint-Benoît. R = coefficient of correlation. \({YL}_{max}\) = Maximum potential Yield Loss. YLRI5 = Yield Loss Resistance Index. NS = Non-Significant; significant at *P < 0.05, **P < 0.01, ***P < 0.001 according to a t-test as described in material and method (multi-series analysis within location)
The correlation between disease susceptibility and the economic index was significant (P < 0.01) and negative at MC and SB but non-significant in the other three locations (GL, ES, LM) (Fig. 5). Mean coefficient of correlation was − 0.18. Given the absence of effect of the disease on recoverable sugar at MC and SB and its positive effect in the other three locations, \({YL}_{max}\) of the economic index reached 24.2%, 16.6%, 8.0% and 3.6% at MC, SB, GL, and ES, respectively, while it was a gain of 2.6% at LM.
Effect of orange rust on economic index (EI) in plant breeding assessment trials of the four variety series at five geographical locations of Reunion. ES = Etang-Salé, GL = Le Gol, LM = La Mare, MC = Menciol, SB = Saint-Benoît. R = coefficient of correlation. \({YL}_{max}\) = Maximum potential Yield Loss. ‡ = the negative sign indicates a gain instead of a loss. YLRI5 = Yield Loss Resistance Index. NA = non-applicable. NS = Non-Significant; significant at *P < 0.05, **P < 0.01, ***P < 0.001 according to a t-test as described in material and method (multi-series analysis within location)
Visualization of variety performances for their yield traits across all locations
Performances for yield traits of the 42 candidate varieties and the check R579 grown in all locations were compared using GGE biplots (Fig. 6). The first two principal components (PCs) of the biplots explained 75.4, 76.0, 86.4, and 66.6% of the total GGE variation of cane yield, recoverable sugar, fiber content, and economic index, respectively. This suggested that a biplot represented by both PC1 and PC2: (i) adequately approximated the GGE data of cane yield and recoverable sugar, (ii) represented very accurately the GGE data of fiber content, but (iii) represented less efficiently the GGE data of the economic index. For this latter trait, data variability was comparable to the other three biplots when the third PC (PC3) was also taken into account (data not shown). As a rule, location vectors in biplots of each yield trait always had high positive abscissa and the variation among them was first discriminated by PC2. The large fan-shaped distribution of location vectors from positive to negative ordinates in these three trait biplots implied changes of variety rankings between some locations, particularly between the most divergent environments (i.e. LM/MC for cane yield, SB/GL for recoverable sugar and MC/ES for economic index) as visualized by the orthogonal projection of all varieties onto their vectors.
‘Genotype plus Genotype x Environment’ (GGE) biplots of the four yield-related traits (CY = cane yield, RS = recoverable sugar, FIB = fiber content, EI = economic index) based on data of 43 varieties (including R579) grown at five locations of Reunion. PC1 and PC2 are the principal component scores on the first and second axis, respectively. The variation accounted for by the axes is given in brackets. Biplots are based on a scale representative of the distances between varieties in terms of genotypic values. Environments are indicated by arrows and varieties by numbers. The reference frame indicated by dashed arrows represents an average environment (AE) vector and its orthogonal vector. Color scale for orange rust severity (ORS) represents mean genotypic predictions of varieties for ORS estimated across all locations. Variety names corresponding to variety numbers are indicated in supplementary Information 2
Distribution pattern of varieties on GGE biplots in reference to the AE vector and its orthogonal vector (pictured by dashed arrows in Fig. 6) resulted in a clear visualization of both mean performance and stability of varieties across environments under orange rust constraint. Check variety R579 appeared moderately susceptible to orange rust with a mean genotypic prediction for ORS in the 3–4 range. The near-zero abscissa of R579 in biplots of cane yield, recoverable sugar, and economic index indicated a mean performance across all sites relatively ordinary for these three traits. However, the low ordinate of R579 in the four biplots revealed an excellent performance stability between environments for all traits.
The mean genotypic prediction for ORS over all five locations was used to rank the 42 common sugarcane varieties for their resistance to the disease. Thirteen varieties were considered resistant to orange rust (mean ORS prediction < 2), five varieties were susceptible (mean ORS prediction > 5), and the remaining 24 were classified as intermediate or moderately resistant (2 ≤ mean ORS prediction ≤ 5). In the cane yield biplot, among the five highly susceptible varieties (10, 29, 30, 41, and 42) to orange rust, four varieties had a negative abscissa while nine of the 13 resistant varieties (1, 2, 3, 11, 12, 13, 14, 25, 26, 31, 32, 33 and 34) had a positive abscissa. Conversely, in the recoverable sugar biplot, a negative abscissa was associated with nine of the 13 resistant varieties while a positive abscissa was associated with three of the five highly susceptible varieties.
Varieties with superior mean performance and good stability across all environments were visualized in each biplot by considering a threshold > 2 for the abscissa of a variety combined with a threshold < 2 for the absolute value of its ordinate. The combination of these two arbitrary thresholds allowed us to identify five superior varieties for cane yield (20, 25, 26, 31 and 34), six for recoverable sugar (9, 22, 23, 33, 35 and 36), four for fiber content (1, 31, 34 and 38), and six for the economic index (11, 14, 20, 26, 35 and 39). Two varieties were common for cane yield and fiber content (31 and 34), two for cane yield and economic index (20 and 26), and one for recoverable sugar and economic index (35). Among these 16 superior varieties for at least one yield trait, eight were resistant to orange rust (1, 11, 14, 25, 26, 31, 33 and 34) and eight showed moderate resistance (9, 20, 22, 23, 35, 36, 38 and 39). None of these varieties was susceptible to orange rust.
Finally, it is noteworthy that different varieties had a particularly high mean performance on the biplots of cane yield (variety 20), recoverable sugar (variety 22), and fiber content (variety 31), as expressed by high abscissa values (3.7, 4.6 and 5.4, respectively). These high values reflected the superiority of these three varieties for their respective traits because biplot abscissa represent a proxy of the Euclidian distance that separates each variety from the population mean (biplots’ origin), in terms of mean standard deviations of the population in the space of five environments. Among these three superior varieties, one was highly resistant to orange rust (variety 31) and the two other ones were moderately resistant (varieties 20 and 22).
Discussion
Accuracy of resistance assessment to sugarcane orange rust in field trials
Resistance to orange rust was assessed in naturally infected field trials using a disease rating scale based on symptom severity observed on different leaves. Broad-sense heritability (\({H}^{2}\)) of ORS at individual trial level was always very high since it ranged between 0.80 and 0.94. These values were obtained using a four-replication experimental design under natural plant infection. They were higher than those reported in trials using three to four replicates and artificial inoculation conditions, as well as another disease scoring scale (Yang et al. 2018, 2019; McCord et al. 2019). The very high \({H}^{2}\) values were similar to those published by Daugrois et al. (1996) and Raboin et al. (2006) (0.94–0.97) for resistance to sugarcane brown rust (caused by P. melanocephala) and assessed with a gradual disease score in naturally infected trials and three-replication designs. Consequently, resistance level to orange rust of sugarcane can be assessed very accurately in replicated field trials when natural infection is well established.
Assessment of sugarcane resistance to orange rust appeared very weakly affected by putative environmental variations within trials. This was due to (i) the rapid and massive dispersion of urediniospores by the wind currents and (ii) the relatively short infectious cycles (10–14 days) of P. kuehnii (Chapola et al. 2016; Moreira et al. 2018). The very high heritability values observed in our study suggested that selection for orange rust resistance could already be performed with some confidence in early-stages of selection programs, when thousands of varieties are screened in non-replicated large trials. Hence, screening for orange rust resistance since the very first non-replicated stage is expected to be reasonably effective as long as exposure to the disease is adequate. Such a method is already being used in breeding programs in Ecuador, Brazil and Guatemala (Castillo and Silva 2022; Cursi et al. 2022; Orozco and Queme 2022). Providing favorable epidemiological conditions, assessment of variety resistance in naturally infected field trials appears a convenient and reliable alternative to artificial inoculation techniques for screening of large populations (Sood et al. 2009; Chapola et al. 2016).
Differences in mean severity levels between locations
Natural disease pressure varied between locations of Reunion as revealed by comparison of mean ORS levels. Location ranking was consistent with the two most influential climatic parameters of orange rust epidemics identified by Sanjel et al. (2019) and Chaulagain et al. (2020) in Florida, namely a night temperature of 20–22 °C and a high relative humidity (> 90%) over a 30-day period or more. First, ranking of mean ORS of the four coastal locations (SB > LM ≥ ES > GL) was not related to differences in night temperatures (Supplementary Information 1) but to differences in relative humidity. These differences in relative humidity can be attributed to differences in mean annual rainfalls (SB = 2968 mm > LM = 1374 mm > ES = GL = 676 mm) and to differences in field irrigation regimes for the two driest locations (ES and GL). Mean ORS ranking and annual water supply ranking of the four coastal locations were identical, knowing that GL trials were half as irrigated as the ES ones (due to a specific objective of selection for drought tolerance). Second, ranking of mean ORS of the two wettest locations (SB > MC) with similar mean annual rainfalls (MC = 3210 mm and SB = 2968 mm) can be related to differences in nighttime temperatures during the wet summer season of orange rust development (January–March). This season coincides with the optimal nighttime temperatures (20–22 °C) defined in Florida in the coastal zone of SB (40 m), while the night temperatures are suboptimal (hardly exceeding 20 °C) in MC highland (400 m) (Supplementary Information 1).
Differences in ORS among the five experimental sites explained by two cardinal parameters (temperature and humidity) previously defined in Florida may reflect the existence of decisive agro-climatic differences among the studied sites. It also highlights the generic value of many weather-based models designed to predict progress of plant diseases solely with a key thermal predictor and a moisture duration requirement, regardless of the complexity of the relationship between the pathogen, the host, and the environment (Magarey et al. 2005; Bregaglio et al. 2013; Launay et al. 2014; El Jarroudhi et al. 2017). In Brazil, agro-climatic favorability zones for sugarcane orange rust at large production scales were mapped using predictive models based on long-term weather records (Sentelhas et al. 2016; Valeriano et al. 2021). These models could also be investigated to map disease risk zones for the local sugarcane industry which is characterized by extremely diverse climatic production areas (Dumont et al. 2022). Identification of agro-climatic favorability zones is critical for the decision to release new varieties for commercial production. New varieties with high yield potential but susceptible to orange rust could still be released among other commercial varieties in locations with limited impact of the disease. In contrast, locations where orange rust has a significant impact on sugarcane production, only tolerant or resistant varieties should be promoted.
Consistency of variety resistance assessment among sugarcane growing locations of Reunion
Despite differences between ORS levels, the five experimental sites provided highly congruent assessments for variety resistance as revealed by the highly significant correlation coefficients (0.81–0.94) between pairwise locations. These high coefficients reflected the major magnitude of the genotype variance in the multi-environment analyses of ORS and the resulting high to very high \({H}^{2}\) values (0.76–0.91 according to variety series). Environmental heterogeneity and interactions involving the plant genotype (genotype-location, genotype-crop year and genotype-location-crop year) were not major influential factors in the ranking of varieties based on orange rust reaction. As long as these interactions remain non influential, assessment of resistance of elite varieties commonly planted in the different locations (stage V) could be performed with confidence in only a few stations to save time. In this case, screening in the wettest stations, more prone to infection, would optimize the discrimination between varieties.
Nevertheless, variation in virulence of P. kuehnii has been reported in Australia (Magarey et al. 2001), in Brazil (Urashima et al. 2020), and in Florida (Sanjel et al. 2021). The circumstances of introduction of the pathogen into Reunion are unknown in terms of number and origin of the initial contamination event(s). Consequently, the reaction towards orange rust of a well-characterized reference set of varieties should continue to be monitored in each and between locations.
Changes in reaction to the disease of one or more varieties may reflect changes in the population structure of the pathogen and appearance or development of new races of P. kuehnii. Several varieties that were initially resistant to orange rust in Florida became susceptible to the disease after only a few years (Rott et al. 2016). Results of phenotyping sugarcane varieties for orange rust in Reunion should remain similar from one location to another as long as these locations remain affected by pathogen populations of the same race or pathogenic variant.
Effect of orange rust on yield traits in the multi-environment breeding program
The highly significant negative correlations between disease susceptibility and cane yield observed in four of the five locations confirmed the potential impact of orange rust on cane tonnage. High susceptibility to orange rust was linked to low cane yield, most likely because leaf net photosynthesis is reduced in response to orange rust infection (Zhao et al. 2011). Similar observations were made for sugarcane brown rust caused by P. melanocephala (Comstock et al. 1992; Hoy and Hollier 2009). All sites taken together, mean cane yield loss associated with orange rust in the breeding trials was 15.1%. The two most humid environments of SB and MC on the windward coast of the island had the highest rust pressures. Mean yield losses reached 21.3% and 26.0% in these two locations, respectively. Conversely, mean yield losses reached only 10.1% and 14.2% in the two driest environments of ES and GL, respectively, where the disease is less severe. These differences in cane losses between climatic zones that differ for their humidity levels are valuable information to determine selection thresholds for orange rust resistance in the different production environments and to improve local breeding efficiencies.
An unexpected positive correlation was found between orange rust susceptibility and recoverable sugar in the three locations where it rained the least (GL, ES and LM). Recoverable sugar obtained in cane processing is a trait highly correlated with the sucrose content of cane stalks (Hugot 1986). The significance and physiological mechanisms involved in this phenomenon remain to be investigated. A negative correlation between orange rust susceptibility and fiber content of stalks was found in all locations (mean of − 0.26). Nevertheless, the loss in stalk fiber content in the breeding trials of the most impacted location did not exceed 6.5%. This is the first report of the effect on P. kuehnii on fiber content and this result is in agreement with the negative effect of P. melanocephala on sugarcane dry matter (Comstock et al. 1992).
For the economic index, which was used to rank varieties for their economic merit that highly depends on cane yield [EI = CY (RS-4)], a slightly mean negative correlation (− 0.18) existed with orange rust severity across all locations. The reductions of the economic index were only significant in the two wettest locations (16.6% at SB and 24.2% at MC). The magnitude of these reductions was slightly smaller than the one of cane yield losses (21.3% at SB and 26.0% at MC). This result can be explained by a small positive but not significant effect of the disease on recoverable sugar in both locations. In contrast, the reductions of the economic index in the two driest environments (ES and GL) were markedly less important than the yield losses. This result can be attributed in these locations to the unexpected significant positive effect of the disease on recoverable sugar.
All regression analyses and impact estimates of sugarcane orange rust on yield traits reported in this study constitute valuable benchmarks for researchers and developers who face disease and decision-making, particularly when choosing varieties to promote or to discard. However, these benchmarks should only be understood as average trends. They constitute food for thoughts to breeding strategies in sugarcane breeding programs and it is important to keep in mind that disease effects were assessed using elite varieties. These varieties had already gone through four stages of selection and therefore exhibited high yield potential. The negative effects observed on cane yield, fiber content and economic index may be much higher for candidate varieties in less advanced selection stages. Appropriate higher selection thresholds for rust resistance might be required for these latter varieties during a final selection stage.
The yield loss resistance index (YLRI5) is a complementary benchmark to guide selection strategies in case of a negative impact of the disease. YLRI5 is indicative of the resistance level needed to keep yield losses below 5% of the highest yield predictable by the regression line. Low to moderate YLRI5 values for cane yield in most locations indicated that stringent selection for disease resistance is needed to minimize the risk of losses for this trait. Conversely, YLRI5 values for the fiber content that were equal or greater than 8.00 in three locations indicated that an absence of selection for rust resistance at a given location would not result in significant reduction (more than 5%) of fiber content. For the economic index, which is heavily dependent on cane yield, the lowest YLRI5 values were obtained in the two rainiest environments. Consequently, a relatively strict selection policy should be favored in these local breeding strategies for orange rust resistance to expect genetic gains based on the economic index.
Prospects for commercial selection among elite varieties
Epidemics of sugarcane orange rust developed only recently in Reunion. Consequently, the four series of elite varieties tested herein at the final selection stage (stage V) had never been screened for their resistance to the disease. Significant variation existed among these varieties regarding their reaction to orange rust, from highly resistant to highly susceptible.
In the multi-location analysis of the subset of elite varieties tested in all five environments, mean performance of each yield trait was more informative than in individual environments. This was due to some changes in variety ranking as previously reported in a similar GGE study by Guilly et al. (2017). A higher proportion of resistant varieties was observed among the varieties exhibiting superior mean performance for cane yield or economic index. None of them were susceptible or highly susceptible. This result was congruent with the negative effect of the disease assessed for cane yield and economic index in most environments of Reunion. These results indicate that in order to develop high yielding varieties for Reunion cropping system, it will be necessary to have orange rust resistance. It will be necessary to make orange rust resistance a major selection variable.
Among resistant or moderately resistant varieties exhibiting the highest mean performance for one or two yield traits across environments, several of them performed much better than the leading check cultivar (R579). Although the varieties of the four breeding series had not been initially selected for resistance to orange rust, some of the best performing varieties proved to be rather resistant to the disease. Consequently, these varieties are excellent candidates for future commercial promotion. From now on, resistance to orange rust will be taken into account starting from the first stages of selection. Only elite varieties with a good level of resistance will enter the final stages of selection. The outcome of this process should be an increase in Reunion of commercially valuable varieties that are resistant to orange rust.
Breeding prospects for orange rust resistance
Breeding for resistance will be the main and the most cost-effective strategy for controlling orange rust. As for any new trait to be improved, developing thoughtful breeding strategies for orange rust resistance would first need to acquire some basic scientific knowledge about the genetic properties of the trait in current breeding populations. In particular, it would be essential to get estimates of key genetic parameters relative to the narrow-sense heritability of the resistance trait and a comprehensive understanding of its genetic determinism in representative breeding populations. Nevertheless, acquiring this scientific knowledge requires the study of large and well-characterized segregating populations for orange resistance. Most of the time, this involves the creation of specific sugarcane populations as well as specific field and lab experiments. Nevertheless, our study demonstrated the possibility to acquire varietal resistance information from replicated field trials that produce relatively accurate and consistent data among geographical locations. This constitutes excellent and valuable news that open opportunities of direct use of varietal resistance data carefully acquired in current breeding programs to develop genetic improvement efforts for orange rust resistance in a short time.
For instance, estimates of narrow-sense heritability of the trait and breeding values of the material of interest would be helpful to define best breeding strategies and crossing choices. Getting those estimates could be straight forward attempted by directly analyzing replicated breeding trials of varieties related by pedigree with mixed models exploiting kinship information (Piepho et al. 2008; Atkin et al. 2009; Hoarau et al. 2022). Moreover, applied to the joint analysis of advanced yield trials (Oakey et al. 2007), this strategy would allow the identification of elite varieties with good breeding values for resistance to orange rust that also have good breeding and per se values for most important cane yield traits.
In addition, genetic improvement for orange rust resistance should also benefit from QTA (Quantitative Trait Allele) mapping efforts of resistance genes. This research work also relies on the availability of large and well-characterized segregating populations. Powerful SNP-based genetic analyses of traits of interest are currently performed using data obtained from next-generation sequencing technologies (such as genotyping-by-sequencing or capture sequencing) cautiously exploited for genotyping purpose in the polyploidy context of sugarcane. Using such technologies, a few QTAs controlling orange rust resistance were recently identified in modern interspecific germplasm (Yang et al. 2018; McCord et al. 2019). Identification of such QTAs represents the first step to develop valuable diagnostic markers for breeding programs to expedite the selection of superior resistant sugarcane varieties.
Conclusions
This study is the first report of effects of sugarcane orange rust on yield traits in a multi-environment breeding program. Disease resistance assessed in naturally inoculated field trials provided very accurate estimates of variety resistance levels (high broad-sense heritability values). This result was obtained thanks to the presence of two thirds of varieties in experiments exhibiting some level of susceptibility, thus ensuring adequate inoculum levels. This situation offered a unique opportunity to use multi-environment breeding trials to investigate the effect of the disease on yield and to gauge the level of resistance required in commercial germplasm to minimize losses. However, this situation should change with the increase of the resistance frequency in the sugarcane breeding population. The set-up of screening trials with susceptible checks regularly interspersed to optimize natural infection will also contribute to identify resistant plant material in a more traditional way.
Orange rust can reduce cane yields and its derived economic index, particularly in humid locations prone to disease development. Surprisingly, the disease did not reduce recoverable sugar. Our study provided valuable benchmarks of potential losses of cane yield for researchers and developers when faced with decision-making regarding variety choices depending on disease risks.
As many other rust diseases caused by Pucciniales fungi, sugarcane orange rust spreads rapidly and over an entire crop when a location is contaminated. Due to this epidemic nature, the disease can readily be investigated for yield loss estimates with on-going analyses of breeding trials. Hence, the methodologies of analyses of breeding trials reported here at the scale of a multi-environment program could serve as a guide for similar applications relative to other disease epidemics affecting sugarcane or other plant species.
Finally, the very good accuracy of the estimates of resistance levels obtained in replicated trials opens opportunities of direct valorization of data acquired from on-going breeding trials to improvement of genetic resistance to orange rust using quantitative genetics and molecular approaches.
References
Aitken K, Jackson P, McIntyre C (2006) Quantitative trait loci identified for sugar related traits in a sugarcane (Saccharum spp.) cultivar × Saccharum officinarum population. Theor Appl Genet 112:1306–1317. https://doi.org/10.1007/s00122-006-0233-2
Aitken K, Hermann S, Karno K, Bonnett G, McIntyre L, Jackson P (2008) Genetic control of yield related stalk traits in sugarcane. Theor Appl Genet 117:1191–1203. https://doi.org/10.1007/s00122-008-0856-6
Asnaghi C, Roques D, Ruffel S, Kaye C, Hoarau JY, Telismart H, Girard JC, Raboin LM, Risterucci AM, Grivet L, D’Hont A (2004) Targeted mapping of a sugarcane rust resistance gene (Bru1) using bulked segregant analysis and AFLP markers. Theor Appl Genet 108:759–764. https://doi.org/10.1007/s00122-003-1487-6
Atkin FC, Dieters MJ, Stringer JK (2009) Impact of depth of pedigree and inclusion of historical data on the estimation of additive variance and breeding values in a sugarcane breeding program. Theor Appl Genet 119:555–565. https://doi.org/10.1007/s00122-009-1065-7
Ayres PG (1978) Water relations of diseased plants. In: Kozlowski TT (ed) Water deficits and plant growth, vol 5. Academic Press, New-York, pp 1–60
Barbasso D, Jordo H, Maccheroni W, Boldini J, Bressiani J, Sanguino A (2010) First report of Puccinia kuehnii causal agent of orange rust of sugarcane, in Brazil. Plant Dis 94:1170
Bhuiyan SA, Stringer JK, Croft BJ, Olayemi ME (2022) Resistance of sugarcane varieties to smut (Sporisorium scitamineum), development over crop classes, and impact on yield. Crop Pasture Sci 73:1180–1187. https://doi.org/10.1071/CP21607
Bregaglio S, Donatelli M, Confalonieri R (2013) Fungal infections of rice, wheat, and grape in Europe in 2030–2050. Agron Sustain Dev 33:767–776. https://doi.org/10.1007/s13593-013-0149-6
Cadavid M, Ángel JC, Victoria JI (2012) First report of orange rust of sugarcane caused by Puccinia kuehnii in Colombia. Plant Dis 96:143–143. https://doi.org/10.1094/PDIS-05-11-0406
Castillo RO, Silva E (2022) Sugarcane breeding and supporting genetics research in Ecuador. Sugar Tech 24:222–231. https://doi.org/10.1007/s12355-021-01057-4
Chabalier P, Van de Kerchove V, Saint Macary H (2006) Guide de la fertilisation organique à La Réunion. CIRAD, Montpellier
Chapola RG, Hoffmann HP, Massola NS (2016) Reaction of sugarcane varieties to orange rust (Puccinia kuehnii) and methods for rapid identification of resistant genotypes. Trop Plant Pathol 41:139–146. https://doi.org/10.1007/s40858-016-0076-6
Chaulagain B, Raid RN, Dufault N, van Santen E, Rott P (2019a) Application timing of fungicides for the management of sugarcane orange rust. Crop Prot 119:141–146. https://doi.org/10.1016/j.cropro.2019.01.007
Chaulagain B, Raid RN, Rott P (2019b) Timing and frequency of fungicide applications for the management of sugarcane brown rust. Crop Prot 124:104826. https://doi.org/10.1016/j.cropro.2019.05.020
Chaulagain B, Small IM, Shine JM, Fraisse CW, Raid RN, Rott P (2020) Weather-based predictive modeling of orange rust of sugarcane in Florida. Phytopathology 110:626–632. https://doi.org/10.1094/PHYTO-06-19-0211-R
Chavarría E, Subirós F, Vega J, Ralda G, Glynn NC, Comstock JC, Castlebury LA (2009) First report of orange rust of sugarcane caused by Puccinia kuehnii in Costa Rica and Nicaragua. Plant Dis 93:425–425. https://doi.org/10.1094/PDIS-93-4-0425C
Comstock JC, Shine JM Jr, Raid RN (1992) Effect of rust on sugarcane growth and biomass. Plant Dis 76:175–177. https://doi.org/10.1094/PD-76-0175
Costet L, Le Cunff L, Royaert S, Raboin L-M, Hervouet C, Toubi L, Telismart H, Garsmeur O, Rousselle Y, Pauquet J, Nibouche S, Glaszmann JC, Hoarau JY, D’Hont A (2012) Haplotype structure around Bru1 reveals a narrow genetic basis for brown rust resistance in modern sugarcane cultivars. Theor Appl Genet 125:825–836. https://doi.org/10.1007/s00122-012-1875-x
Cursi DE, Hoffmann HP, Barbosa GVS, Bressiani JA, Gazaffi R, Chapola RG, Fernandes Junior AR, Basalobre TWA, Diniz CA, Santos JM, Carneiro MS (2022) History and current status of sugarcane breeding, germplasm development and molecular genetics in Brazil. Sugar Tech 24:112–133. https://doi.org/10.1007/s12355-021-00951-1
Daugrois JH, Grivet L, Roques D, Hoarau JY, Lombard H, Glaszmann JC, D’Hont A (1996) A putative major gene for rust resistance linked with a RFLP marker in sugarcane cultivar ‘R570.’ Theor Appl Genet 92:1059–1064. https://doi.org/10.1007/BF00224049
Dixon LJ, Castlebury LA, Aime MC, Glynn NC, Comstock JC (2010) Phylogenetic relationships of sugarcane rust fungi. Mycol Prog 9:459–468. https://doi.org/10.1007/s11557-009-0649-6
Dumont T, Thong-Chane A, Barau L, Siegmund B, Hoarau JY (2019) Genetic variabilities and genetic gains for yield components in regional sugarcane breeding programmes on Réunion Island. Sugar Tech 21:868–878. https://doi.org/10.1007/s12355-019-00718-9
Dumont T, Barau L, Thong-Chane A, Dijoux J, Mellin M, Daugrois J, Hoarau JY (2022) Sugarcane breeding in reunion: challenges, achievements and future prospects. Sugar Tech 24:181–192. https://doi.org/10.1007/s12355-021-00998-0
El Jarroudi M, Kouadio L, Bock CH, El Jarroudi M, Junk J, Pasquali M, Maraie H, Delfosse P (2017) A threshold-based weather model for predicting stripe rust infection in winter wheat. Plant Dis 101:693–703. https://doi.org/10.1094/PDIS-12-16-1766-RE
eRcane (2009) Enhancing the value of sugarcane. 2007–2009 biannual report. p 74
Flores RC, Loyo JR, Ojeda RA, Rangel OCA, Cerón FA, Márquez W, Guerra-Moreno AS, Hernandez-Ibarra HM, Gonzalez RE, Castlebury LA, Dixon LJ, Glynn NC, Comstock J, Flynn J, Amador J (2009) First report of orange rust of sugarcane caused by Puccinia kuehnii in Mexico, El Salvador, and Panama. Plant Dis 93:1347–1347. https://doi.org/10.1094/PDIS-93-12-1347B
Funes C, Pérez Gómez SG, Henriquez DD, Di Pauli V, Bertani RP, Fontana DP, Rago AM, Joya CM, Sopena RA, Gonzalez V, Babi H, Erazzu LE, Cuenya MI, Ploper LD (2016) First report of orange rust of sugarcane caused by Puccinia kuehnii in Argentina. Plant Dis 100:861. https://doi.org/10.1094/PDIS-09-15-1099-PDN
Glynn NC, Dixon LJ, Castlebury LA, Szabo LJ, Comstock JC (2010) PCR assays for the sugarcane rust pathogens Puccinia kuehnii and P. melanocephala and detection of a SNP associated with geographical distribution in P. kuehnii. Plant Pathol 59:703–711. https://doi.org/10.1111/j.1365-3059.2010.02299.x
Grimmer MK, John Foulkes M, Paveley ND (2012) Foliar pathogenesis and plant water relations: a review. J Exp Bot 63:4321–4331. https://doi.org/10.1093/jxb/ers143
Guilly S, Dumont T, Thong-Chane A, Barau L, Hoarau JY (2017) Analysis of multienvironment trials (MET) in the sugarcane breeding program of Reunion Island. Euphytica 213:213. https://doi.org/10.1007/s10681-017-1994-1
Hoarau JY, Grivet L, Offmann B, Raboin LM, Diorflar JP, Payet J, Hellmann M, D’Hont AD, Glaszmann JC (2002) Genetic dissection of a modern sugarcane cultivar (Saccharum spp.).II. Detection of QTLs for yield components. Theor Appl Genet 105:1027–1037. https://doi.org/10.1007/s00122-002-1047-5
Hoarau JY, Dumont T, Wei X, Jackson P, D’hont A (2022) Applications of quantitative genetics and statistical analyses in sugarcane breeding. Sugar Tech 24:320–340. https://doi.org/10.1007/s12355-021-01012-3
Hoy JW, Hollier CA (2009) Effect of brown rust on yield of sugarcane in Louisiana. Plant Dis 93:1171–1174. https://doi.org/10.1094/PDIS-93-11-1171
Hubert J, Jeandel C, Costet L, Hostachy B, Dupuis AS, Coddeville A, Barau L, Ioos R (2019) First report of orange rust caused by Puccinia kuehnii on sugarcane on the Island of Reunion. Plant Dis 103:2962. https://doi.org/10.1094/PDIS-04-19-0750-PDN
Hugot E (1986) Handbook of cane sugar engineering, 3rd completely, revised. Elsevier, Amsterdam
Jackson PA (2005) Breeding for improved sugar content in sugarcane. Field Crops Res 92:277–290. https://doi.org/10.1016/j.fcr.2005.01.024
Kozak M, Piepho H-P (2018) What’s normal anyway? Residual plots are more telling than significance tests when checking ANOVA assumptions. J Agron Crop Sci 204:86–98. https://doi.org/10.1111/jac.12220
Lakshmanan P, Jackson P, Hemaprabha G, Li YR (2022) Sugar tech special issue: history of sugarcane breeding, germplasm development and related molecular research. Sugar Tech 24:1–3. https://doi.org/10.1007/s12355-021-01080-5
Launay M, Caubel J, Bourgeois G, Huard F, de Cortazar-Atauri IG, Bancal MO, Brisson N (2014) Climatic indicators for crop infection risk: application to climate change impacts on five major foliar fungal diseases in Northern France. Agric Ecosys Environ 197:147–158. https://doi.org/10.1016/j.agee.2014.07.020
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006) SAS for mixed models, 2nd edn. SAS Institute Inc., Cary
Magarey RC, Bull JI (2003) Relating cultivar Pachymetra root rot resistance to sugarcane yield using breeding selection trial analyses. Aust J Exp Agric 43:617–615. https://doi.org/10.1071/EA02144
Magarey RC, Croft BJ, Willcox TG (2001) An epidemic of orange rust on sugarcane in Australia. Proc Int Soc Sugar Cane Technol 24:410–416
Magarey RD, Sutton TB, Thayer CL (2005) A simple generic infection model for foliar fungal plant pathogens. Phytopathology 95:92–100. https://doi.org/10.1094/PHYTO-95-0092
McCord P, Glynn N, Comstock J (2019) Identifying markers for resistance to sugarcane orange rust (Puccinia kuehnii) via selective genotyping and capture sequencing. Euphytica 215:1–14. https://doi.org/10.1007/s10681-019-2340-6
Morante MC (2018) Record of sugarcane orange rust in the lowland tropics of Santa Cruz. Bolivia J Plant Pathol Microbiol 9:11. https://doi.org/10.4172/2157-7471.1000459
Moreira AS, Nogueira Junior AF, Gonçalves CRNB, Souza NA, Bergamin Filho A (2018) Pathogenic and molecular comparison of Puccinia kuehnii isolates and reactions of sugarcane varieties to orange rust. Plant Pathol 67:1687–1696. https://doi.org/10.1111/ppa.12870
Mungur H, Saumtally S, Joomun N, Dookun-Saumtally A (2020) Presence of sugarcane orange rust in Mauritius. Sugar Tech 22:671–674. https://doi.org/10.1007/s12355-020-00818-x
Oakey H, Verbyla AP, Cullis BR, Wei X, Pitchford WS (2007) Joint modeling of additive and non-additive (genetic line) effects in multi-environment trials. Theor Appl Genet 114:1319–1332. https://doi.org/10.1007/s00122-007-0515-3
Orozco H, Queme J (2022) Sugarcane improvement in Central America and México with special focus on Guatemala. Sugar Tech 24:254–266. https://doi.org/10.1007/s12355-021-01072-5
Ovalle W, Comstock JC, Glynn NC, Castlebury LA (2008) First report of Puccinia kuehnii, causal agent of orange rust of sugarcane, in Guatemala. Plant Dis 92:973–973. https://doi.org/10.1094/PDIS-92-6-0973C
Pérez-Vicente L, Martín-Triana EL, Barroso F, Martínez-de la Parte E, Borrás-Hidalgo O, Hernández Estévez I (2010) Definitive identification of orange rust of sugarcane caused by Puccinia kuehnii in Cuba. Plant Pathol 59:804–804. https://doi.org/10.1111/j.1365-3059.2009.02248.x
Piepho HP, Möhring J, Melchinger AE, Büchse A (2008) BLUP for phenotypic selection in plant breeding and variety testing. Euphytica 161:209–228. https://doi.org/10.1007/s10681-007-9449-8
Piperidis N, Jackson P, D’Hont A, Besse P, Hoarau JY, Courtois B, Aitken K, McIntyre C (2008) Comparative genetics in sugarcane enables structured map enhancement and validation of marker-trait associations. Mol Breed 21:233–247. https://doi.org/10.1007/s11032-007-9124-8
Raboin LM, Oliveira KM, Lecunff L, Telismart H, Roques D, Butterfield M, Hoarau JY, D’Hont A (2006) Genetic mapping in sugarcane, a high polyploid, using bi-parental progeny: identification of a gene controlling stalk colour and a new rust resistance gene. Theor Appl Genet 112:1382–1391. https://doi.org/10.1007/s00122-006-0240-3
Reffay N, Jackson PA, Aitken KS, Hoarau JY, D’Hont A, Besse P, McIntyre CL (2005) Characterisation of genome regions incorporated from an important wild relative into Australian sugarcane. Mol Breed 15:367–381. https://doi.org/10.1007/s11032-004-7981-y
Rodriguez-Alvarez MX, Boer MP, van Eeuwijk FA, Eilers PH (2018) Correcting for spatial heterogeneity in plant breeding experiments with P-splines. Spat Stat 23:52–71. https://doi.org/10.1016/j.spasta.2017.10.003
Rott PC, Kaye C, Naranjo M, Shine JM Jr, Sood S, Comstock JC, Raid RN (2016) Controlling sugarcane diseases in Florida: a challenge in constant evolution. Proc Int Soc Sugar Cane Technol 29:595–600
Rott P, Sood S, Comstock JC, Raid RN, Glynn NC, Gilbert RA, Sandhu HS (2017) Sugarcane orange rust. Agronomy Department. UF/IFAS Extension. SS-AGR-378 https://edis.ifas.ufl.edu/sc099
Roussel C, Warembourg A, Davrieux F, Bernard J, Poser C (2015) Development of NIRS method for routine assessment of sugarcane quality in Reunion Island. Abstract In: Pushing the frontiers of sugarcane improvement. 11th Germplasm & Breeding and 8th Molecular biology ISSCT Workshop. eRcane. Saint-Gilles. Reunion. p 16
Sanjel S, Chaulagain B, Small IM, Comstock J, Hincapie M, Raid RN, Rott P (2019) Comparison of progress of brown rust and orange rust and conditions conducive for severe epidemic development during the sugarcane crop season in Florida. Plant Dis 103:825–831. https://doi.org/10.1094/PDIS-05-18-0862-RE
Sanjel S, Hincapie M, Wang Y, Todd J, Chaulagain B, Sood S, Comstock JC, Raid RN, Rott P (2021) Occurrence of two races of Puccinia kuehnii causing orange rust of sugarcane in Florida. Plant Pathol 70:1616–1625. https://doi.org/10.1111/ppa.13405
Searle SR, Casella G, McCulloch CE (2009) Variance components. John Wiley & Sons
Sentelhas PC, dos Santos DL, Monteiro LA, Soares-Colletti AR, Pallone Filho WJ, Donzelli JL, Arrigoni EB (2016) Agro-climatic favorability zones for sugarcane orange rust as a tool for cultivar choice and disease management. Crop Prot 84:88–97. https://doi.org/10.1016/j.cropro.2016.02.016
Sood SG, Comstock JC, Glynn NC (2009) Leaf whorl inoculation method for screening sugarcane rust resistance. Plant Dis 93:1335–1340. https://doi.org/10.1094/PDIS-93-12-1335
Steel R, Torrie J (1980) Principles and procedures of statistics. A biometrical Approach. McGraw Hill Editions, Singapore
Urashima AS, Mistura TDF, Porto LNR, Austin PD, Arias RS (2020) Genetic diversity of Puccinia kuehnii, the causal agent of orange rust of sugarcane, from Brazil. J Phytopathol 168:581–590. https://doi.org/10.1111/jph.12937
Valeriano TTB, de Souza Rolim G, Manici LM, Giustarini L, Bregaglio S (2021) A process-based model to simulate sugarcane orange rust severity from weather data in Southern Brazil. Int J Biometeorol 65:2037–2051. https://doi.org/10.1007/s00484-021-02162-5
Van Dillewijn C (1952) Botany of sugarcane. Chronica Botanica Co, Waltham
Virtudazo EV, Nojima H, Kakishima M (2001) Taxonomy of Puccinia species causing rust diseases on sugarcane. Mycoscience 42:167–175. https://doi.org/10.1007/BF02464133
Yan W (2002) Singular-value partitioning in biplot analysis of multi-environment trial data. Agron J 94:990–996. https://doi.org/10.2134/agronj2002.9900
Yan W, Tinker NA (2005) An integrated biplot analysis system for displaying, interpreting, and exploring genotype x environment interaction. Crop Sci 45:1004–1106. https://doi.org/10.2135/cropsci2004.0076
Yan W, Tinker NA (2006) Biplot analysis of multi-environment trial data: principles and applications. Can J Plant Sci 6:623–645. https://doi.org/10.4141/P05-169
Yang X, Islam MS, Sood S, Maya S, Hanson EA, Comstock J, Wang J (2018) Identifying quantitative trait loci (QTLs) and developing diagnostic markers linked to orange rust resistance in sugarcane (Saccharum spp.). Front Plant Sci 9:350. https://doi.org/10.3389/fpls.2018.00350
Yang X, Sood S, Luo Z, Todd J, Wang J (2019) Genome-wide association studies identified resistance loci to orange rust and yellow leaf virus diseases in sugarcane (Saccharum spp.). Phytopathology 109:623–631. https://doi.org/10.1094/PHYTO-08-18-0282-R
Zhao D, Glynn NC, Glaz B, Comstock JC, Sood S (2011) Orange rust effects on leaf photosynthesis and related characters of sugarcane. Plant Dis 95:640–647. https://doi.org/10.1094/PDIS-10-10-0762
Acknowledgements
The authors wish to thank the technical staff members of eRcane (F. Poiny, D. Vaitilingom, A. Corre, G. Morel, C. Sautron, and E. Sautron) for the cultural management of the field experiments and the laboratory analyses of sugarcane quality. We are very grateful to an anonymous reviewer who raised questions which helped to improve the manuscript.
Funding
This work was funded by eRcane company through the project AGIV and by ANRT (Association Nationale de la Recherche et de la Technologie) through the CIFRE PhD grant N° 2021/0634 of J. Dijoux.
Author information
Authors and Affiliations
Contributions
JYH, TD and PR conceived and designed the study and the methodology. JD, MP, CL, CH, and JYH acquired experimental data. JD, JYH and TD performed data analyses. JYH supervised the project. LB supervised the technical management of the experiments. JD, TD and JYH wrote the original draft. PR, JD, TD, LB, and JYH reviewed and edited the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dijoux, J., Dumont, T., Paysan, M. et al. Effects of orange rust on sugarcane yield traits in a multi-environment breeding program. Euphytica 219, 49 (2023). https://doi.org/10.1007/s10681-023-03177-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-023-03177-2