Abstract
Cold stress is one of the most severe abiotic adverse factors limiting the growth, productivity, and spatial distribution of rice. We identified a high threshold and low temperature-sensitive mutant (lts) in our breeding program. This mutant displays severe cold sensitive phenotypes when the temperature is maintained below 20 °C for 3–5 days. Other abiotic stress responses tend to share common signal reception and gene regulation pathways, such as ABA-dependent pathways; however, the lts mutant only appears to be sensitive to cold stress. Genetic analysis indicates that this lts mutant is controlled by two major recessive loci linked within the same chromosome. The recombination value between these two loci in an indica × japonica cross of Longjing20 × lts is 12.65 %, and in an indica × indica cross of Newbonnet × lts, the recombination value is 13.93 %. Molecular mapping revealed that one of these two loci is co-segregated with RM5344 in chromosome 7. The candidate gene for this specific locus was determined by sequencing analysis, which showed that it encodes a functionally unknown protein with a transmembrane domain. The detected mutations take place not only in its coding region, including a terminal codon, but also in its promoter region, which results in polymorphism of RM5344. Thus, the recessive allele of this locus is referred to as Oslts a, and that of the other locus is referred to as Oslts b. The precise mapping of the Oslts b-associated locus is underway. These findings provide a solid base for further investigations on the molecular mechanism underlying the responses to cold stress in rice.



Similar content being viewed by others
References
Andaya VC, Mackill DJ (2003a) Mapping of QTLs associated with cold tolerance during the vegetative stage in rice. J Exp Bot 54:2579–2585
Andaya VC, Mackill DJ (2003b) QTLs conferring cold tolerance at the booting stage of rice using recombinant inbred lines from a japonica × indica cross. Theor Appl Genet 106(6):1084–1090
Andaya VC, Tai TH (2006) Fine mapping of the qCTS12 locus, a major QTL for seedling cold tolerance in rice. Theor Appl Genet 113:467–475
Chinnusamy V, Schumaker K, Zhu JK (2004) Molecular genetic perspectives on cross-talk and specificity in abiotic stress signalling in plants. J Exp Bot 55(395):225–236
Choi HI, Hong JH, Ha JO, Kang JY, Kim SY (2000) ABFs, a family of ABA-responsive element binding factors. J Biol Chem 275:1723–1730
Fujino K, Sekiguchi H, Sato T, Kiuchi H, Nonoue Y, Takeuchi Y, Ando T, Lin SY, Yano M (2004) Mapping of quantitative trait loci controlling low-temperature germinability in rice (Oryza sativa L.). Theor Appl Genet 108:794–799
Fujino K, Sekiguchi H, Matsuda Y, Sugimoto K, Ono K, Yano M (2008) Molecular identification of a major quantitative trait locus, qLTG3-1, controlling low-temperature germinability in rice. Proc Natl Acad Sci USA 105(34):12623–12628
Kaneda C, Beachell HM (1974) Response of indica ± japonica rice hybrids to low temperatures. SABRAO J 6:17–32
Kim S, Andaya V, Tai T (2011) Cold sensitivity in rice (Oryza sativa L.) is strongly correlated with a naturally occurring I99 V mutation in the multifunctional glutathione transferase isoenzyme GSTZ2. Biochem J 435:373–380
Kim SM, Suh JP, Lee CK et al (2014) QTL mapping and development of candidate gene-derived DNA markers associated with seedling cold tolerance in rice (Oryza sativa L.). Mol Gen Genet 289(3):333–343
Kosambi DD (1944) The estimation of map distances from recombination frequencys. Ann Eugene 12:172–175
Koseki M, Noriyuki K, Yonebayashi S, Maehara Y, Wang Z, Minobe Y (2010) Identification and fine mapping of a major quantitative trait locus originating from wild rice, controlling cold tolerance at the seedling stage. Mol Genet Genomics 284:45–54
Kwak TS, Vergara BS, Nanda JS, Coffman WR (1984) Inheritance of seedling cold tolerance in rice. SABRAO J 16:83–86
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newburg L (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1:174–181
Li L, Liu X, Xie K et al (2013) qLTG-9, a stable quantitative trait locus for low-temperature germination in rice (Oryza sativa L.). Theor Appl Genet 126(9):2313–2322
Liu F, Xu W, Song Q et al (2013) Microarray-assisted fine-mapping of quantitative trait loci for cold tolerance in rice. Mol Plant 6(3):757–767
Lou QJ, Chen L, Sun ZX, Xing YZ, Li J, Xu XY, Mei HW, Luo LJ (2007) A major QTL associated with cold tolerance at seedling stage in rice (Oryza sativa L.). Euphytica 158:87–94
Lu SJ, Wei H, Wang Y, Wang HM, Yang RF, Zhang XB, Tu JM (2012) Overexpression of a transcription factor OsMADS15 modifies plant architecture and flowering time in Rice (Oryza sativa L.). Plant Mol Biol Rep 30:1461–1469
Lu G, Wu FQ, Wu W et al (2014) Rice LTG1 is involved in adaptive growth and fitness under low ambient temperature. Plant J 78(3):468–480
Michelmore R, Paran I, Kesseli RV (1991) Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci USA 88(21):9828–9832
Miki D, Itoh R, Shimamoto K (2005) RNA silencing of single and multiple members in a gene family of rice. Plant Physiol 138:1903–1913
Misawa S, Mori N, Takumi S, Yoshida S, Nakamura C (2000) Mapping of QTLs for low temperature response in seedlings of rice (Oryza sativa L.). Cereal Res Commun 28(1/2):33–40
Mori M, Onishi K, Tokizono Y, Shinada H, Yoshimura T, Numao Y, Miura H, Takashi S (2011) Detection of a novel quantitative trait locus for cold tolerance at the booting stage derived from a tropical japonicarice variety Silewah. Breed Sci 61:61–68
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325
Nagamine T (1991) Genetic control to tolerance to chilling injury in rice (Oryza sativa L.). Jpn J Breed 41:35–40
Nagasawa N, Kawamoto T, Matsunaga K, Sasaki T, Nagato Y, Hinata K (1994) Cold-temperature sensitive mutants at the booting stage of rice. Breed Sci 44:53–57
Nishimura M, Hamamura K (1993) Diallel analysis of cool tolerance at the booting stage in rice varieties from Hokkaido. Ikushugaku Zasshi 43(4):557–566
Nishiyama I (1978) Male sterility caused by cooling treatment at the young microspore stage in rice plants, 23: some enzyme activities in anthers during and after the cooling. Jpn J Crop Sci 47:551–556
Panaud O, Chen X, McCouch SR (1996) Development of microsatellite markers and characterization of simple sequence length polymorphism (SSLP) in rice (Oryza sativa L.). Mol Gen Genet 252:597–607
Ruelland E, Zachowski A (2010) How plants sense temperature. Environ Exp Bot 69(3):225–232
Saito K, Miura K, Nagano K, Hayano-Saito Y, Saito A, Araki H, Kato A (1995) Chromosomal location of quantitative trait loci for cool tolerance at the booting stage in rice variety ‘Norin-PL8’. Breed Sci 45:337–340
Saito K, Miura K, Nagano K, Hayano-Saito Y, Araki H, Kato A (2001) Identification of two closely linked quantitative trait loci for cold tolerance on chromosome 4 of rice and their association with anther length. Theor Appl Genet 103:862–868
Saito K, Hayano-Saito Y, Maruyama-Funatsuki W, Sato Y, Kato A (2004) Physical mapping and putative candidate gene identification of a quantitative trait locus Ctb1 for cold tolerance at the booting stage of rice. Theor Appl Genet 109(3):515–522
Saito K, Hayano-Saito Y, Kuroki M, Sato Y (2010) Map-based cloning of the rice cold tolerance gene Ctb1. Plant Sci 179:97–102
Shinada H, Iwata N, Sato T, Fujino K (2013) Genetical and morphological characterization of cold tolerance at the fertilization stage in rice. Breed Sci 63:197–204
Shinada H, Iwata N, Sato T et al (2014) QTL pyramiding for improving of cold tolerance at fertilization stage in rice. Breed Sci 63(5):483–488
Shirasawa S, Endo T, Nakagomi K, Yamaguchi M, Nishio T (2012) Delimitation of a QTL region controlling cold tolerance at booting stage of a cultivar, ‘Lijiangxintuanheigu’, in rice (Oryza sativa L.). Theor Appl Genet 124:937–946
Suh JP, Lee K, Kim JJ, Kim SM, Cho YC, Park SH, Shin JC, Kim YG, Jena KK (2012) Identification of quantitative trait loci for seedling cold tolerance using RILs derived from a cross between japonica and tropical japonica rice cultivars. Euphytica 184:101–108
Vaultier MN, Cantrel C, Vergnolle C, Justin AM, Demandre C, BenhassaineKesri G, Cicek D, Zachowski A, Ruelland E (2006) Desaturase mutants reveal that membrane rigidification acts as a cold perception mechanism upstream of the diacylglycerol kinase pathway in Arabidopsis cells. FEBS Lett 580:4218–4223
Vigh L, Nakamoto H, Landry J, Gomez-Munoz A, Harwood JL, Horvath I (2007) Membrane regulation of the stress response from prokaryotic models to mammalian cells. Ann N Y Acad Sci 1113:40–51
Wang GL, Guo ZF (2005) Effects of chilling stress on photosynthetic rate and chlorophyll fluorescence parameter in seedlings of two rice cultivars differing in cold tolerance. Rice Sci 12(3):187–191
Wang Z, Wang F, Zhou R et al (2011) Identification of quantitative trait loci for cold tolerance during the germination and seedling stages in rice (Oryza sativa L.). Euphytica 181(3):405–413
Xiao N, Huang W, Zhang X et al (2014) Fine mapping of qRC10-2, a quantitative trait locus for cold tolerance of rice roots at seedling and mature stages. PLoS One 9(5):e96046
Yang RF, Tang QC, Wang HM, Zhang XB, Pang G, Wang H, Tu J (2011) Analyses of two rice (Oryza sativa L.) cyclin-dependent kinase inhibitors and effects of transgenic expression of OsiICK6 on plant growth and development. Ann Bot 107:1087–1101
Yang Z, Huang D, Tang W et al (2013) Mapping of quantitative trait loci underlying cold tolerance in rice seedlings via high-throughput sequencing of pooled extremes. PLoS One 8(7):e68433
Ye C, Fukai S, Godwin ID, Koh H, Reinke R, Zhou Y, Lambrides C, Jiang W, Snell P, Redoña E (2010) A QTL controlling low temperature induced spikelet sterility at booting stage in rice. Euphytica 176:291–301
Yoshida S (1973) Effects of temperature on growth of the rice plant (Oryza sativa L.) in a controlled environment. Soi Sci Plant Nutr 19(4):299–310
Yoshida S, Forno DA, Cock JH, Gomez KA (1976) Routine procedure for growing rice plants in culture solution. Laboratory manual for physiological studies of rice. The international rice research institute, Los Baños, Laguna, Philippines, pp 61–66
Zeller G, Henz SR, Widmer CK, Sachsenberg T, Rätsch G, Weigel D, Laubinger S (2009) Stress-induced changes in the Arabidopsis thaliana transcriptome analyzed using whole-genome tiling arrays. Plant J 58(6):1068–1082
Zhang ZH, Su L, Chen W, Li W, Zhu YG (2005) A major QTL conferring cold tolerance at early seedling stage using recombinant inbred lines of rice (Oryza sativa L.). Plant Sci 168:527–553
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31171166).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10681_2014_1260_MOESM1_ESM.tif
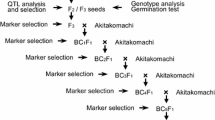
Supplementary Fig. 1 Introgression of Oslts a from lts mutant into NB via backcrossing, and maker-assisted foreground selection to obtain BC2F2 and BC2F3 lines (TIFF 52 kb)
10681_2014_1260_MOESM2_ESM.tif
Supplementary Fig. 2 Cold damage symptoms in lts mutant. Cold damage occurred at the tillering stage (a) and the maturing stage (b) when the temperature drops below 20°C for 3 to 5 days. Plants in (a) and (b) include the breeding line control (left) and the lts mutant (right) (TIFF 6025 kb)
10681_2014_1260_MOESM3_ESM.tif
Supplementary Fig. 3 Response of LJ20 (top row), NB (middle row), and lts (bottom row) seedlings to abiotic stress: (a) PEG6000; (b) NaCl; (c) ABA. The photos were taken 48 h after treatment (TIFF 11259 kb)
10681_2014_1260_MOESM4_ESM.tif
Supplementary Fig. 4 Marker analysis of mapping population derived from the cross between LJ20 (P2) and lts mutants (P1). (a) RM5344; (b) RM5752; (c) RM20916. The figure shows that the SSR marker RM5344 detected no recombinant segregants (TIFF 3134 kb)
10681_2014_1260_MOESM5_ESM.tif
Supplementary Fig. 5 (a) Indel04310 maker-assisted foreground selection of the BC2F2 segregating population derived from the backcross between lts mutants (P1) and NB (P3), with NB as recurrent parent. (b) and (c) are the phenotype identified as sensitive (S1 to S5) and insensitive (R1 to R5) in the BC2F3 line (TIFF 4543 kb)
10681_2014_1260_MOESM6_ESM.tif
Supplementary Fig. 6 RNAi plasmid construction in this study. To construct the RNAi vector, the PCR products of 5′UTR, exon3, and 3′UTR segment amplified from LJ20 genome DNA were digested with 2 pairs of restriction enzymes: KpnI/BamHI and SpeI/SacI. The differently digested fragments were then successively cloned into pTCK303 to produce the OsLTS a-5′UTR-RNAi plasmid (a), OsLTS a-exon3-RNAi plasmid (b), and OsLTS a-3′UTR-RNAi plasmid (c) (TIFF 82 kb)
Rights and permissions
About this article
Cite this article
Xu, M., Ye, X., Wang, W. et al. Genetic analysis and molecular mapping of a high threshold and low temperature-sensitive mutant in rice (Oryza sativa L.) at the seedling stage. Euphytica 203, 71–82 (2015). https://doi.org/10.1007/s10681-014-1260-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-014-1260-8