Abstract
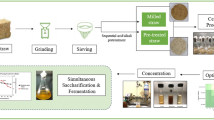
The present study reports a simple all microbial-based consolidated bioprocess for the conversion of poplar parings into ethanol. The bacterium Bacillus cereus G9241 CP 026,376 and the yeast Candida tropicalis MF 289,181 were employed for this purpose. Potential of the cellulolytic B. cereus for saccharification of the substrate and bioethanol fermentation was unveiled by employing the separate hydrolysis and fermentation (SHF) as well as simultaneous saccharification and fermentation (SSF) processes. The bacterium as well as yeast grew well in the 2% substrate hydrolyzate and the cell counts reached up to 19,701 × 107 and 852 × 106/ml at 96 h, respectively. For the bacterially fermented saccharified substrate, the HMF content reduced from 489 ± 38.1 µg/L at 24 h to 232 µg/L (52.56%) at 96 h. In case of the yeast fermented poplar hydrolyzate, the HMF content dropped down to 351.3 ± 48.6 µg/L right at first sampling point and thereafter became un-detectable. In case of SSF, HMF could appear only at 72 and 96 h of fermentation with respective values of 260.3 ± 25.8 and 243 ± 8.66 µg/L. Acetic acid content among the differently fermented substrates ranged from 460 ± 230 to 5360 ± 503 mg/L. The bacterial cellulases yielded up to 6.742 and 8.561 mg/ml of glucose and xylose of 2% substrate, respectively. In case of yeast monoculture, the glucose and xylose contents reduced down to 34.17% and 85.28%, respectively, at 24 h post-inoculation with concomitant ethanol production of 634 ± 159 mg/L. Following 24 h of co-culturing of the microbes in the substrate hydrolyzate, the glucose and xylose reduced down to 39.69% and 82%, respectively, with accompanying ethanol level of 501.38 ± 46.7 mg/L. Glucose content of 24 h incubated SSF fluids was 1568 ± 226 mg/L, whereas the xylose remained non-detectable throughout the study period. Ethanol productions at 24, 48, 72 and 96 h of incubations for the SSF experiment were 140.43 ± 44.8, 60.18 ± 13.5, 177.78 ± 23.9 and 83.48 ± 10.3 mg/L, respectively. The simple experiments reported here provide a workable model to assess the potential of suitable microbes for bioethanol production from plants’ biowastes by a consolidated bioprocess without any drastic pretreatment.





Similar content being viewed by others
References
Antczak, A., Szadkowski, J., Szadkowska, D., & Zawadzki, J. (2021). Assessment of the effectiveness of liquid hot water and steam explosion pretreatments of fast-growing poplar (Populus trichocarpa) wood. Wood Science and Technology, 1–23.
Arantes, V., & Saddler, J. N. (2011). Cellulose accessibility limits the effectiveness of minimum cellulase loading on the efficient hydrolysis of pretreated lignocellulosic substrates. Biotechnology for Biofuels, 4, 3.
Bolger, A. M., Loshe, M., & Usadel, B. (2014). Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics, 30(15), 2114–2120.
Brethauer, S., Antczak, A., Balan, R., Zielenkiewicz, T., & Studer, M. H. (2020). Steam explosion pretreatment of beechwood. Part 2: Quantification of Cellulase inhibitors and their effect on avicel hydrolysis. Energies, 13(14), 3638.
Brownlie, D. (1940). Wood as a chemical raw product: Latest developments in sugar and alcohol production. Chemistry and Industry, 59, 671–675.
Chang, T., & Yao, S. (2011). Thermophilic, lignocellulolytic bacteria for ethanol production: Current state and perspectives. Applied Microbiology and Biotechnology, 92, 13–27.
Claassen, P. A. M., van Lier, J. B., Lopez Contreras, A. M., van Niel, E. W. J., Sijtsma, L., Stams, A. J. M., de Vries, S. S., & Weusthuis, R. A. (1999). Utilisation of biomass for the supply of energy carriers. Applied Microbiology and BiotechnolOgy, 52, 741–755.
Crawford, J. T., Shan, C. W., Budsberg, E., Morgan, H., Bura, R., & Gustafson, R. (2016). Hydrocarbon bio-jet fuel from bioconversion of poplar biomass: Techno-economic assessment. Biotechnology for Biofuels, 9(1), 1–16.
Dahnum, D., Tasum, S. O., Triwahyuni, E., Nurdin, M., & Abimanyu, H. (2015). Comparison of SHF and SSF processes using enzyme and dry yeast for optimization of bioethanol production from empty fruit bunch. Energy Procedia, 68, 107–116.
Esteghlalian, A., Hashimoto, A. G., Fenske, J. J., & Penner, M. H. (1997). Modeling and optimization of the dilute sulphuric acid pretreatment of corn stover, poplar and switchgrass. Bioresource Technology, 59, 129–136.
Gille, S., & Pauly, M. (2012). O-acetylation of plant cell wall polysaccharides. Frontiers of Plant Science, 3, 12.
Harris, E. E., Beglinger, E., Hajny, G. J., & Sherrard, E. C. (1945). Hydrolysis of wood-treatment with sulfuric acid in a stationary digester. Industrial & Engineering Chemistry, 37(1), 12–23.
Hasunuma, T., & Kondo, A. (2012). Consolidated bioprocessing and simultaneous saccharification and fermentation of lignocelluloses to ethanol with thermotolerant yeast strains. Process Biochemistry, 47, 1287–1294.
Hgren, K. O., Bura, R., Lesnicki, G., Saddler, J., & Zacch, G. (2007). A comparison between simultaneous saccharification and fermentation and separate hydrolysis and fermentation using steam pretreated corn stover. Process Biochemistry, 42, 834–839.
Hussain, A., Iqbal, M. A., Javid, A., Razaq, A., Aslam, S., Hasan, A., Akmal, M., & Qazi, J. I. (2019). Application of fruit wastes as cost-effective carbon sources for biological sulphate reduction. Iranian Journal of Science and Technology Transactions, a: Science, 43(1), 33–41.
Hussain, A., & Qazi, J. I. (2012). Biological sulphate reduction using watermelon rind as a carbon source. Biologia (pakistan), 58(1–2), 85–92.
Hussain, A., Shakir, H. A., & Qazi, J. I. (2014). Anaerobic biodegradation of sulphate employing animal manure as a cost effective growth substrate. Journal of Animal and Plant Sciences, 24(3), 913–918.
Jabeen, F., Hussain, A., Younis, T., Manzoor, M., & Samiullah, K. (2019). Isolation of thermophilic Anoxybacillus beppuensis JF84 and production of thermostable amylase utilizing agro–dairy wastes. Environmental Progress and Sustainable Energy, 38(2), 417–423.
Jones, J. L., & Semrau, K. T. (1984). Wood hydrolysis for ethanol production—previous experience and the economics of selected processes. Biomass, 5(2), 109–135.
Jouzani, G. S., & Taherzadeh, M. J. (2015). Advances in consolidated bioprocessing systems for bioethanol and butanol production from biomass: A comprehensive review. Biofuel Research Journal, 5, 152–195.
Katahira, S., Mizuike, A., Fukuda, H., & Kondo, A. (2006). Ethanol fermentation from lignocellulosic hydrolysate by a recombinant xylose- and cellooligosaccharide-assimilating yeast strain. Applied Microbiology and BiotechnolOgy, 72, 1136–1143.
Klinke, H. B., Thomsen, A. B., & Ahring, B. K. (2004). Inhibition of ethanol producing yeast and bacteria by degradation products produced during pretreatment of biomass. Applied Microbiology and BiotechnolOgy, 66, 10–26.
Krumbein, W. C. (1938). Size frequency distribution of sediments and the normal phi curve. Journal of Sedimentary Petrology, 8, 84–90.
Levine, J. S. (1996). Biomass burning and global change: Remote sensing and inventory development and biomass burning in Africa (pp. 35). The MIT Press, Cambridge, Massachusetts, USA.
Lin, Y., & Tanaka, S. (2006). Ethanol fermentation from biomass resources: Current state and prospects. Applied Microbiology and Biotechnology, 69, 627–642.
Liu, L., Sun, J., Li, M., Wang, S., Pei, H., & Zhang, J. (2009). Enhanced enzymatic hydrolysis and structural features of corn stover by FeCl3 pretreatment. Bioresource Technology, 100, 5853–5858.
Liu, Z. I., Ma, M., & Song, M. (2009). Evolutionary engineered ethanologenic yeast detoxifies lignocellulosic biomass conversion inhibitors by reprogrammed pathways. Molecular Genetics and Genomics, 282, 233–244.
Mabee, W. E., Gregg, D. J., Arato, C., Berlin, A., Bura, R., Gilkes, N., Mirochnik, O., Pan, X., Pye, E. K., & Saddler, J. N. (2006). Updates on softwood-to-ethanol process development. In Twenty-Seventh Symposium on Biotechnology for Fuels and Chemicals (pp. 55–70). Humana Press.
Menon, V., & Rao, M. (2012). Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept. Progress in Energy and Combustion Science, 38(4), 522–550.
Olofsson, K., Bertilsson, M., & Liden, G. (2008). A short review of SSF an increasing process option for ethanol production from lignocellulosic feedstocks. Biotechnology for Biofuels, 1, 1–14.
Palmqvist, E., Grage, H., Meinander, N. Q., & Hahn-Hägerdal, B. (1999). Main and interaction effects of acetic acid, furfural, and phydroxybenzoic acid on growth and ethanol productivity of yeasts. Biotechnology and Bioengineering, 63, 46–55.
Phwan, C. K., Ong, H. C., Chen, W. H., Ling, T. C., Ng, E. P., & Show, P. L. (2018). Overview: Comparison of pretreatment technologies and fermentation processes of bioethanol from microalgae. Energy Conversion and Management, 173, 81–94.
Saleem, A., Hussain, A., Chaudhary, A., Ahmad, Q. A., Iqtedar, M., Javid, A., & Akram, A. M. (2020). Acid-hydrolysis optimization of pomegranate peels waste using response surface methodology for ethanol production. Biomass Conversion and Biorefinery. https://doi.org/10.1007/s13399-020-01117-x
Saleh, F., Hussain, A., Younis, T., Ali, S., Rashid, M., Ali, A., Mustafa, G., Jabeen, F., & AL-Surhaneee, A. A., Alnoman, M. M., & Qamer, S. (2020). Comparative growth potential of thermophilic amylolytic Bacillus Sp. on unconventional media food wastes and its industrial application. Saudi Journal of Biological Sciences, 27(12), 3499–3504.
Sarkar, N., Ghosh, S. K., Bannerjee, S., & Aikat, K. (2012). Bioethanol production from agricultural wastes: An overview. Renewable Energy, 37, 19–27.
Tabssum, F., Irfan, M., Shakir, H. A., & Qazi, J. I. (2018). RSM based optimization of nutritional conditions for cellulase mediated Saccharification by Bacillus cereus. Journal of Biological Engineering, 12(1), 1–10.
Tian, S., Zhu, W., Gleisner, R., Pan, X. J., & Zhu, J. Y. (2011). Comparisons of SPORL and dilute acid pretreatments for sugar and ethanol productions from aspen. Biotechnology Progress, 27, 419–427.
Tran, D. T., Yet-Pole, I., & Lin, C. W. (2013). Developing co-culture system of dominant cellulolytic Bacillus sp. THLA0409 and dominant ethanolic Klebsiella oxytoca THLC0409 for enhancing ethanol production from lignocellulosic materials. Journal of the Taiwan Institute of Chemical Engineers, 44, 762–769.
Van der Auwera, G. A., Carneiro, M. O., Hartl, C., Poplin, R., Del Angel, G., Levy-Moonshine, A., Jordan, T., Shakir, K., Roazen, D., Thibault, J., & Banks, E. (2013). From FastQ data to high-confidence variant calls: The genome analysis toolkit best practices pipeline. Current Protocols in Bioinformatics, 43(1), 11–10.
Wyman, C. E. (1996). Handbook on bioethanol: Production and Utilization. Washington, DC, Taylor & Francis.
Yee, K. L., Jansen, L. E., Lajoie, C. A., Penner, M. H., Morse, L., & Kelly, C. J. (2018). Furfural and 5-hydroxymethyl-furfural degradation using recombinant manganese peroxidase. Enzyme and Microbial Technology, 108, 59–65.
Zhang, J., Gu, F., Zhu, J. Y., & Zalesny, R. S., Jr. (2015). Using a combined hydrolysis factor to optimize high titer ethanol production from sulfite-pretreated poplar without detoxification. Bioresource Technology, 186, 223–231.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tabssum, F., Khan, M.N. Microbial-based separate and simultaneous saccharification and ethanol fermentation of poplar (Populus euramericana) substrate. Environ Dev Sustain (2022). https://doi.org/10.1007/s10668-022-02143-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10668-022-02143-7