Abstract
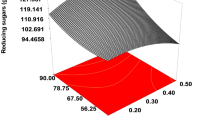
Agro-industrial wastes can be processed into valuable products. Successively, current investigation is an effort to optimize the acid hydrolysis of pomegranate peels waste (PPW) using central composite design (CCD) of response surface methodology (RSM) for ethanol production. Concentration of sulfuric acid, temperature, and time of hydrolysis were used as dependent variables, whereas reducing sugars, total carbohydrates, extractives, weight loss, hemicellulose, cellulose, and lignin contents were recorded as responses for PPW decomposition. The highest glucose level of 0.56 ± 0.04 mg mL−1 (with 5% acid concentration at 100 °C for 30 min) and carbohydrate contents of 1.53 ± 0.07 mg mL−1 (with 3% acid concentration at 75 °C for 45 min) were obtained. Subsequently, detoxification of hydrolysate was conducted employing 2.5% activated charcoal that reduced 62% of phenolic compounds. Detoxified hydrolysate was subjected to fermentation by ethanologenic yeasts: Metschnikowia sp. Y31, Metschnikowia cibodasensis Y34, and Saccharomyces cerevisiae K7 for 10 days experiment. Significant ethanol yield of 0.42 ± 0.08 g g−1 was noticed by Metschnikowia sp. Y31 on day 5 and 0.41 ± 0.07 g g−1 for Metschnikowia cibodasensis Y34 on day 2. The results demonstrated the hopeful prospect for bioethanologenesis using cellulosic wastes at marketable level.

Statement of novelty
The focus of the current study was to develop low-cost ethanologenesis by using pomegranate peels waste. Initially, the biomass hydrolysis, being extremely a critical step, was optimized through central composite design using response surface methodology by Design Expert Software. The pretreated and detoxified biomass hydrolysate was then subjected to ethanol production via fermentative yeast. The low-cost ethanol production from wastes of pomegranate can be highly valuable not only for sustainable energy production but also for effective waste management. Significant yield of ethanol was achieved while using treated pomegranate peels waste as substrate. Our findings of the present study will be helpful in developing efficient and economical strategies tending to valorize cellulosic wastes.





Similar content being viewed by others
References
Bernstad A, Jansen IC (2011) A lifecycle approach to the management of household food waste - a Swedish full-scale case study. Waste Manag 31(8):1879–1896
Cia C, Li B, Lu H, Wu W (2012) Research advances in control of N2O emission from municipal solid waste landfill sites. J Appl Ecol 23(5):1415–1422
Khan K, Shaheen S, Iqbal H, RohinaArif GMR, Khalil M, Munawaa A, Batool M, Farooq M, Khan H, Sattar AA, Ilyas K (2020) Assessment of waste management practices in hospitals of Islamabad and Abbottabad-Pakistan. Pure Appl Biol 9(1):282–289
Forastiere F, Badaloni C, Hoogh KD, Kraus MK, Martuzzi M, Mitis F, Palkovicov L, Porta D, Preiss P, Ranzi A, Perucci CA, Briggs D (2011) Health impact assessment of waste management facilities in three European countries. J Environ Health 10(53):10–53
Mahmoudkhani R, Valizadeh B, Khastoo H (2014) Greenhouse gases lifecycle assessment (GHGLCA) as a decision support tool for municipal solid waste management in Iran. J Environ Health Sci Eng 12:17
Paul S, Choudhury M, Deb U, Pegu R, Das S, Bhattacharya SS (2019) Assessing the ecological impacts of ageing on hazard potential of solid waste landfills: a green approach through vermitechnology. J Clean Prod 236:117643
Valerio F (2010) Environmental impacts of post-consumer materials managements: recycling, biological treatments, incineration. Waste Manag 30(11):2354–2361
Buratti C, Barbanera M, Testarmata F, Fantozzi F (2015) Life cycle assessment of organic waste management strategies: an Italian case study. J Clean Prod 89:125–136
Correa DF, Beyer HL, Fargione JE, Hill JD, Possingham HP, Thomas-Hall SR, Schenk PM (2019) Towards the implementation of sustainable biofuel production systems. Renew Sust Energ Rev 107:250–263
Colazo A, Sanchez A, Font X, Colon J (2015) Environmental impact of rejected materials generated in organic fraction of municipal solid waste anaerobic digestion plants: comparison of wet and dry process layout. Waste Manag 43:84–97
Bowen K, Kennedy SC, Miranda K (2010) Ethanol from sugar beets: a process and economic analysis. A Project Report of Worcester Polytechnic Institute
Kim JH, Kim HJ, Yoo SH (2020) External benefits of increasing bioethanol consumption: a choice experiment study. Appl Econ Lett 27(6):447–450
Sarkar N, Kumar GS, Bannerjee S, Aikat K (2012) Bioethanol production from agricultural wastes: an overview. Renew Energy 37:19–27
Tahany GM, Amal A (2015) Effect of agriculture waste: pomegranate fruits peel on some important phytopathogenic fungi and control of tomato damping off. J Appl Life Sci 3(3):103–113
FAO (2012) Statistical database. Food and Agriculture Organization of the United Nations. Codex Alimentarius Commission, Tunis http://www.fao.org. (Accessed May 23, 2012)
Farag RS, Abdel-Latif MS, Emam SS, Tawfeek LS (2014) Phytochemical screening and polyphenol constituents of pomegranate peels and leave juices. Agric Soil Sci 1:86–93
Malviya S, Arvind JA, Hettiarachchy N (2014) Antioxidant and antibacterial potential of pomegranate peel extracts. J Food Sci Technol 51(12):4132–4137
Aviram M, Dornfeild L, Rosenblat M, Volkova N, Kaplan M, Coleman R, Hayek T, Presser D, Fuhrman B (2000) Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am J Clin Nutr 71:1062–1076
Orzuaa MC, Mussattob SI, Contreras-Esquivela JC, Rodrigueza R, Garzaa H, Teixeirab JA, Aguilara CN (2009) Exploitation of agro industrial wastes as immobilization carrier for solid-state fermentation. Ind Crop Prod 30(1):24–27
Christaki EV, Bonos EM, Florou-Paneri PC (2011) Dietary benefits of pomegranates in humans and animals. J Food Agric Environ 9:142–144
Viuda-Martos M, Fernández-López J, Pérez-Álvarez J (2010) Pomegranate and its many functional components as related to human health: a review. Com Reviews Food Sci Food Safe 9:635–654
Mirdehghan SH, Rahemi M (2007) Seasonal changes of mineral nutrients and phenolics in pomegranate (Punica granatum L.) fruit. Sci Hortic 111:120–127
Miguel MG, Neves MA, Antunes MD (2010) Pomegranate (Punica granatum L.) a medicinal plant with myriad biological properties—a short review. J Med Plants Res 4(25):2836–2847
Ismail T, Sestili P, Akhtar S (2012) Pomegranate peel and fruit extracts: a review of potential anti-inflammatory and anti-infective effects. J Ethnopharmacol 143:397–405
Yasoubi P, Barzegar M, Sahari MA, Azizi MH (2007) Total phenolic contents and antioxidant activity of pomegranate (Punica granatum L.) peel extracts. J Agric Sci Technol 9:35–42
Li Y, Guo C, Yang J, Wei J, Xu J, Cheng S (2006) Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem 96:254–260
Suryawanshi PC, Kirtane RD, Chaudhari AB, Kothari RM (2009) Conservation and recycling of pomegranate seeds and shells for value addition. J Renew Sustain Energy 1:013107
Spilmont M, Leotoing L, Davicco MJ, Lebecque P, Mercier S, Miot-Noirault E, Pilet P, Rios L, Wittrant Y, Coxam V (2014) Pomegranate and its derivatives can improve bone health through decreased inflammation and oxidative stress in an animal model of postmenopausal osteoporosis. Eur J Nutr 53:1155–1164
Pathak PD, Mandavgane SA, Kulkarni BD (2017) Valorization of pomegranate peels: a biorefinery approach. Waste Biomass Valor 8:1127–1137
Irfan M, Asghar U, Nadeem M, Nelofer R, Syed Q, Shakir HA, Qazi JI (2016) Statistical optimization of saccharification of alkali pretreated wheat straw for bioethanol production. Waste Biomass Valor 7(6):1389–1396
Imran M, Anwar Z, Irshad M, Javid A, Hussain A, Ali S (2017) Optimization of cellulase production from a novel strain of Aspergillus tubingensis IMMIS2 through response surface methodology. Biocatal Agric Biotechnol 12:191–198
Gebremedhin M, Mishra S, Mohanty K (2018) Augmentation of native microalgae based biofuel production through statistical optimization of campus sewage wastewater as low-cost growth media. J Environ Chem Eng 6(5):6623–6632
Imran M, Hussain A, Anwar Z, Irshad M, Jabeen F (2019) Beta-glucosidase production optimization from newly isolated Aspergillus tubingensis IMMIS2 using Taguchi statistical design. Iran J Sci Technol Trans Sci 43:701–707. https://doi.org/10.1007/s40995-017-0462-z
Pandey A, Shah R, Yadav P, Verma R, Srivastava S (2020) Harvesting of freshwater microalgae Scenedesmus sp. by electro–coagulation–flocculation for biofuel production: effects on spent medium recycling and lipid extraction. Environ Sci Pollut Res 27(3):3497–3507
Dyaee N, Luti KJK (2019) Classical and statistical optimization by response surface methodology for enhancing biomass and bacteriocin production by Lactobacillus plantarum. Iraqi J Sci 60(3):494–508
Taherzadeh MJ, Karimi K (2007) Acid-based hydrolysis processes for ethanol from lignocellulosic materials: a review. BioResources 2(3):472–499
Ribeiro JAB (2010) Hydrolysis of lignocellulosic residues using cellulolytic enzyme extract produced by Trichoderm reesei ATCC 2768. Dissertation(Federal University of Rio Grande do Norte), Natal/RNBrazil (iIn Portuguese)
Avci A, Saha BC, Dien BS, Kennedy GJ, Cotta MA (2013) Response surface optimization of corn stover pretreatment using dilute phosphoric acid for enzymatic hydrolysis and ethanol production. Bioresour Technol 130:603–612
Jeong SY, Lee JW (2016) Optimization of pretreatment condition for ethanol production from oxalic acid pretreated biomass by response surface methodology. Ind Crop Prod 79:1–6
Kim D (2018) Physico-chemical conversion of lignocellulose: inhibitor effects and detoxification strategies: a mini review. Molecules 23(2):309
Silva-Fernandes T, Santos JC, Hasmann F, Rodrigues RCLB, Izario Filho HJ, Felipe MGA (2017) Biodegradable alternative for removing toxic compounds from sugarcane bagasse hemicellulosic hydrolysates for valorization in biorefineries. Bioresour Technol 243:384–392
Chaudhary A, Karita S (2017) Screening of yeast isolates from flowers for effective ethanol production. Turk J Biol 41(6):890–900
Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
Zollner N, Kirsch K (1962) Uber die quantitative Bestimmung von Lipoiden (Mikromethode) mittels der vielen naturlichen Lipoiden (allen bekannten Plasmalipoiden) gemeinsamen Sulfophosphovanillin-Reaktion. Zeitschrift Für Die Gesamte Experimentelle Medizin 135:545–561
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
AOAC (2016) Official methods of analysis (Latimer GW Jr. Ed.), 20th ed, Association of Official Analytical Chemists, Washington
Lin L, Yan R, Liu Y, Jiang W (2010) In-depth investigation of enzymatic hydrolysis of biomass wastes based on three major components: cellulose, hemicellulose and lignin. Bioresour Technol 101(21):8217–8223
Myers RH, Montgomery DC, Vining GG, Borror CM, Kowalski SM (2004) Response surface methodology: a retrospective and literature survey. J Qual Technol 36(1):53–77
Amjad M (2016) Optimization of acid saccharification by RSM and fermentation of mango peels for ethanol production. Dissertation, University of Education, Lahore, Pakistan
Ijaz A (2016) Use of melon peels for ethanol production as remedy for waste management. Dissertation, University of Education, Lahore, Pakistan
Pervaiz S (2016) Potential of yeast isolates for ethanol production from mango and banana peels. Dissertation, University of Education, Lahore, Pakistan
Siddique N (2016) Watermelon peels: a promising raw material for ethanol production. Dissertation, University of Education, Lahore, Pakistan
Begum MF, Alimon AR (2011) Bioconversion and saccharification of some lignocellulosic wastes by Aspergillus oryzae ITCC-4857.01 for fermentable sugar production. Electron J Biotechnol 14(5):1–9
Mussatto SI, Roberto IC (2005) Evaluation of nutrient supplementation to charcoal-treated and untreated rice straw hydrolysate for xylitol production by Candida guilliermondii. Braz Arch Biol Technol 48(3):497–502
Gonzalez M, Guzman B, Rudyk R, Romano E, Molina MA (2003) Spectrophotometric determination of phenolic compounds in propolis. Acta Farm Bonaer 22(3):243–248
Bonciu C, Tabacaru C, Bahrim G (2010) Yeasts isolation and selection for bioethanol production from insulin hydrolysates. Innov Rom Food Biotech 6:29
Bennete C (1971) Spectrophotometric acid dichromate method for the determination of ethyl alcohol. Am J Med Tech 37(6):217–220
Yang E, Fan L, Yan J, Jiang Y, Doucette C, Fillmore S, Walker B (2018) Influence of culture media, pH and temperature on growth and bacteriocin production of bacteriocinogenic lactic acid bacteria. AMB Expr 8:10. https://doi.org/10.1186/s13568-018-0536-0
Hasnaoui N, Wathelet B, Jiménez-Araujo A (2014) Valorization of pomegranate peel from 12 cultivars: dietary fiber composition, antioxidant capacity and functional properties. Food Chem 160:196–203
Zhu CP, Zhai XC, Li LQ, Wu XX, Li B (2015) Response surface optimization of ultrasound-assisted polysaccharides extraction from pomegranate peel. Food Chem 177:139–146
Demiray E, Karatay SE, Dönmez G (2018) Evaluation of pomegranate peel in ethanol production by Saccharomyces cerevisiae and Pichia stipitis. Energy 159:988–994
Demiray E, Ertuğrul Karatay S, Dönmez G (2019) Efficient bioethanol production from pomegranate peels by newly isolated Kluyveromyces marxianus. Energy Sources Part A Recover Util Environ Eff 42:709–718. https://doi.org/10.1080/15567036.2019.1600621
Maina MB, Oluwole FA, Ngala GM, Abdulrahman SA (2017) Comparison of the properties and yield of bioethanol from mango and orange waste. Arid Zone J Eng Technol Environ 13(6):779−789
Magyar M, da Costa SL, Jin M, Sarks C, Balan V (2016) Conversion of apple pomace waste to ethanol at industrial relevant conditions. Appl Microbiol Biotechnol 100(16):7349–7358
Boulal A, Kihal M, Khelifi C, Benali B (2016) Bioethanol production from date palm fruit waste fermentation using solar energy. Afr J Biotechnol 15(30):1621–1627
Arumugam R, Manikandan M (2011) Fermentation of pretreated hydrolyzates of banana and mango fruit wastes for ethanol production. Asian J Exp Biol Sci 2:246–256
Baud ISA, Post J, Furedy C (2006) Solid waste management and recycling: actors, partnerships and policies in Hyderabad, India and Nairobi, Kenya (Vol. 76). Springer Science and Business Media
Licht FO, Agra CEAS (2007) World biodiesel markets: the outlook to 2010. Agra Informa Limited, Kent, p 200
Jennings EW, Schell DJ (2011) Conditioning of dilute-acid pretreated corn Stover hydrolysate liquors by treatment with lime or ammonium hydroxide to improve conversion of sugars to ethanol. Bioresour Technol 102(2):1240–1245
Adnan NAA, Suhaimi SN, Abd-Aziz S, Hassan MA, Phang LY (2014) Optimization of bioethanol production from glycerol by Escherichia coli SS1. Renew Energy 66:625–633
Talebnia F, Bafrani MP, Lundin M, Taherzadeh M (2008) Optimization study of citrus wastes saccharification by dilute acid hydrolysis. BioResources 3(1):108–122
Asli AE, Qatibi A-I (2009) Ethanol production from olive cake biomass substrate. Biotechnol Bioprocess Eng 14:118–122
Unhasirikul M, Naranong N, Narkrugsa W (2012) Reducing sugar production from durian peel by hydrochloric acid hydrolysis. World Acad Sci Eng Technol 6(9):394–399
Aguilar R, Ramirez JA, Garrote G, Vazquez M (2002) Kinetic study of the acid hydrolysis of sugar cane bagasse. J Food Eng 55:309–318
Gil N, Ferreira S, Amaral ME, Domingues FC, Duarte AP (2010) The influence of dilute acid pretreatment conditions on the enzymatic saccharification of Erica spp. for bioethanol production. Ind Crop Prod 32:29–35
Toquero C, Bolado S (2014) Effect of four pretreatments on enzymatic hydrolysis and ethanol fermentation of wheat straw. Influence of inhibitors and washing. Bioresour Technol 157:68–76
Loow Y, Wu TY, Md. Jahim J, Mohammad AW, Teoh WH (2016) Typical conversion of lignocellulosic biomass into reducing sugars using dilute acid hydrolysis and alkaline pretreatment. Cellulose 23:1491–1520
Chaudhary G, Singh LK, Ghosh S (2012) Alkaline pretreatment methods followed by acid hydrolysis of Saccharum spontaneum for bioethanol production. Bioresour Technol 124:111–118
Kupiainen L, Ahola J, Tanskanen J (2014) Kinetics of formic acid-catalyzed cellulose hydrolysis. BioResources 9:2645–2658
Baadhe RR, Potumarthi R, Mekala NK (2014) Influence of dilute acid and alkali pretreatment on reducing sugar production from corncobs by crude enzymatic method: a comparative study. Bioresour Technol 162:213–217
Castro E, Nieves IU, Mullinnix MT (2014) Optimization of dilute-phosphoric-acid steam pretreatment of Eucalyptus benthamii for biofuel production. Appl Energy 125:76–83
Taherzadeh MJ, Karimi K (2011) Fermentation inhibitors in ethanol processes and different strategies to reduce their effects. In: Larroche C, Ricke SC, Dussap C-G, Gnansounou E (eds) Pandey a. Academic Press, Biofuels, pp 287–311
Kim SK, Park DH, Song SH, Wee YJ, Jeong GT (2013) Effect of fermentation inhibitors in the presence and absence of activated charcoal on the growth of Saccharomyces cerevisiae. Bioprocess Biosyst Eng 36:659–666
Łukajtis R, Kucharska K, Hołowacz I, Rybarczyk P, Wychodnik K, Słupek E, Nowak P, Kamiński M (2018) Comparison and optimization of saccharification conditions of alkaline pre-treated triticale straw for acid and enzymatic hydrolysis followed by ethanol fermentation. Energies 11:639
Moawad EY (2012) Optimizing bioethanol production by regulating yeast growth energy. Syst Synth Biol 6(3–4):61–68
Acknowledgments
The authors are thankful to the University of Education, Township Campus, Lahore, for providing research facilities to accomplish the investigation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saleem, A., Hussain, A., Chaudhary, A. et al. Acid hydrolysis optimization of pomegranate peels waste using response surface methodology for ethanol production. Biomass Conv. Bioref. 12, 1513–1524 (2022). https://doi.org/10.1007/s13399-020-01117-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01117-x