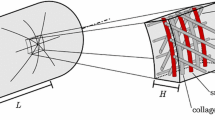
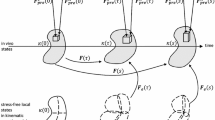
Abstract
Soft biological tissues compromise diverse cell types and extracellular matrix constituents, each of which can possess individual natural configurations, material properties, and rates of turnover. For this reason, mixture-based models of growth (changes in mass) and remodeling (change in microstructure) are well-suited for studying tissue adaptations, disease progression, and responses to injury or clinical intervention. Such approaches also can be used to design improved tissue engineered constructs to repair, replace, or regenerate tissues. Focusing on blood vessels as archetypes of soft tissues, this paper reviews a constrained mixture theory introduced twenty years ago and explores its usage since by contrasting simulations of diverse vascular conditions. The discussion is framed within the concept of mechanical homeostasis, with consideration of solid-fluid interactions, inflammation, and cell signaling highlighting both past accomplishments and future opportunities as we seek to understand better the evolving composition, geometry, and material behaviors of soft tissues under complex conditions.





Similar content being viewed by others
Data availability
Not applicable; review article.
References
Humphrey, J.D.: Continuum biomechanics of soft biological tissues. Proc. R. Soc. A 459, 3–46 (2003)
Hsu, F-H.: The influences of mechanical loads on the form of a growing elastic body. J. Biomech. 1, 303–311 (1968)
Cowin, S.C., Hegedus, D.H.: Bone remodeling I: theory of adaptive elasticity. J. Elast. 6, 313–326 (1976)
Skalak, R.: Gowth as a finite displacement field. In: Proceed IUTAM Symposium Finite Elasticity, pp. 347–355 (1981)
Rodriguez, J., Hoger, A., McCulloch, A.D.: Stress-dependent finite growth in soft elastic tissues. J. Biomech. 27, 455–467 (1994)
Humphrey, J.D., Rajagopal, K.R.: A constrained mixture model for growth and remodeling of soft tissues. Math. Models Methods Appl. Sci. 12, 407–430 (2002)
Fung, Y.C.: What are residual stresses doing in our blood vessels? Ann. Biomed. Eng. 19, 237–249 (1991)
Humphrey, J.D.: Vascular adaptation and mechanical homeostasis at tissue, cellular, and sub-cellular levels. Cell Biochem. Biophys. 50, 53–78 (2008)
Humphrey, J.D.: Remodeling of a collagenous tissue at fixed lengths. J. Biomech. Eng. 121, 591–597 (1999)
Bellini, C., Ferruzzi, J., Roccabianca, S., DiMartino, E., Humphrey, J.D.: A microstructurally-motivated model of arterial wall mechanics with mechanobiological implications. Ann. Biomed. Eng. 42, 488–502 (2014)
Cardamone, L., Valentin, A., Eberth, J.F., Humphrey, J.D.: Origin of axial prestress and residual stress in arteries. Biomech. Model. Mechanobiol. 8, 431–446 (2009)
Humphrey, J.D.: Cardiovascular Solid Mechanics: Cells, Tissues, and Organs. Springer, New York (2002)
Baek, S., Rajagopal, K.R., Humphrey, J.D.: A theoretical model of enlarging intracranial fusiform aneurysms. J. Biomech. Eng. 128, 142–149 (2006)
Valentin, A., Cardamone, L., Baek, S., Humphrey, J.D.: Complementary vasoactivity and matrix remodeling in arterial adaptations to altered flow and pressure. J. R. Soc. Interface 6, 293–306 (2009)
Latorre, M., Humphrey, J.D.: A mechanobiologically equilibrated constrained mixture model for growth and remodeling of soft tissues. Z. Angew. Math. Mech. 98, 2048–2071 (2018)
Wagneseil, J.E., Mecham, R.P.: Vascular extracellular matrix and arterial mechanics. Physiol. Rev. 89, 957–989 (2009)
Dajnowiec, D., Langille, B.L.: Arterial adaptations to chronic changes in haemodynamic function: coupling vasomotor tone to structural remodelling. Clin. Sci. (Lond.) 113, 15–23 (2007)
Hayashi, K., Naiki, T.: Adaptation and remodeling of vascular wall; biomechanical response to hypertension. J. Mech. Behav. Biomed. Mater. 2, 3–19 (2009)
Gleason, R.L., Taber, L.A., Humphrey, J.D.: A 2-D model of flow-induced alterations in the geometry, structure and properties of carotid arteries. J. Biomech. Eng. 126, 371–381 (2004)
Gleason, R.L., Humphrey, J.D.: A mixture model of arterial growth and remodeling in hypertension: altered muscle tone and tissue turnover. J. Vasc. Res. 41, 352–363 (2004)
Humphrey, J.D., Rajagopal, K.R.: A constrained mixture model for arterial adaptations to a sustained step-change in blood flow. Biomech. Model. Mechanobiol. 2, 109–126 (2003)
Rodbard, S.: Vascular caliber. Cardiology 60, 4–49 (1975)
Humphrey, J.D., Taylor, C.A.: Intracranial and abdominal aortic aneurysms: similarities, differences, and need for a new class of computational models. Annu. Rev. Biomed. Eng. 10, 221–246 (2008)
Humphrey, J.D., Holzapfel, G.A.: Mechanics, mechanobiology, and modeling of human abdominal aorta and aneurysms. J. Biomech. 45, 805–814 (2012)
Humphrey, J.D., Tellides, G.: Central artery stiffness and thoracic aortopathy. Am. J. Physiol. 316, H169–182 (2019)
Baek, S., Rajagopal, K.R., Humphrey, J.D.: Competition between radial expansion and thickening in the enlargement of an intracranial saccular aneurysm. J. Elast. 80, 13–31 (2005)
Watton, P.N., Hill, N.A., Heil, M.: A mathematical model for the growth of the abdominal aortic aneurysm. Biomech. Model. Mechanobiol. 3, 98–113 (2004)
Wilson, J.S., Baek, S., Humphrey, J.D.: Importance of initial aortic properties on the evolving regional anisotropy, stiffness, and wall thickness of human abdominal aortic aneurysms. J. R. Soc. Interface 9, 2047–2058 (2012)
Wilson, J.S., Humphrey, J.D.: Evolving anisotropy resulting from elastolytic insults in abdominal aortic aneurysms: potential clinical relevance? J. Biomech. 47, 2995–3002 (2014)
Wilson, J.S., Baek, S., Humphrey, J.D.: Parametric study of effects of collagen turnover in human abdominal aortic aneurysms. Proc. R. Soc. A 469, 20120556 (2013)
Maegdefessel, L., Azuma, J., Toh, R., et al.: Inhibition of microRNA-29b reduces murine abdominal aortic aneurysm development. J. Clin. Invest. 122, 497–506 (2012)
Cyron, C., Wilson, J.S., Humphrey, J.D.: Mechanobiological stability: a new paradigm to understand the enlargement of aneurysms. J. R. Soc. Interface 11, 20140680 (2014)
Humphrey, J.D., Tellides, G., Schwartz, M.A., Milewicz, D.M.: Role of mechanotransduction in vascular biology: focus on thoracic aortic aneurysms and dissections. Circ. Res. 116, 1448–1461 (2015)
Milewicz, D.M., Trybus, K.M., Guo, D-C., Sweeney, H.L., Regalado, E., Kamm, K., Stull, J.T.: Altered smooth muscle cell force generation as a driver of thoracic aortic aneurysms and dissections. Arterioscler. Thromb. Vasc. Biol. 37, 26–34 (2017)
Latorre, M., Humphrey, J.D.: Numerical knockouts – in silico assessment of factors predisposing to thoracic aortic aneurysm. PLoS Computational Biol. ePub ahead of print (2020)
Schriefl, A., Collins, M.J., Holzapfel, G.A., Niklason, L.E., Humphrey, J.D.: Remodeling of thrombus and collagen in an Ang-II infusion ApoE-/- model of dissecting aortic aneurysms. Thromb. Res. 130, e139–146 (2012)
Karsaj, I., Humphrey, J.D.: A mathematical model of evolving mechanical properties of intraluminal thrombus. Biorheology 46, 509–527 (2009)
Rausch, M., Humphrey, J.D.: A computational model of the biochemomechanics of an evolving occlusive thrombus. J. Elast. 129, 125–144 (2017)
Ateshian, G.A.: On the theory of reactive mixtures for modeling biological growth. Biomech. Model. Mechanobiol. 6, 423–445 (2007)
Nims, R.J., Ateshian, G.A.: Reactive constrained mixtures for modeling the solid matrix of biological tissues. J. Elast. 129, 69–105 (2017)
Humphrey, J.D., Baek, S., Niklason, L.E.: Biochemomechanics of cerebral vasospasm and its resolution: I. A new hypothesis and theoretical framework. Ann. Biomed. Eng. 35, 1485–1497 (2007)
Baek, S., Valentin, A., Humphrey, J.D.: Biochemomechanics of cerebral vasospasm and its resolution: II. Constitutive relations and model simulations. Ann. Biomed. Eng. 35, 1498–1509 (2007)
Vorp, D.A.: Biomechanics of abdominal aortic aneurysm. J. Biomech. 40, 1887–1902 (2007)
Di Achille, P., Tellides, G., Figueroa, C.A., Humphrey, J.D.: A haemodynamic predictor of intraluminal thrombus formation in abdominal aortic aneurysms. Proc. R. Soc. Lond. A 470, 20140163 (2014)
Di Achille, P., Tellides, G., Humphrey, J.D.: Hemodynamics-driven deposition of intraluminal thrombus in abdominal aortic aneurysms. Int. J. Numer. Methods Biomed. Eng. 33, e2828 (2017)
Virag, L., Wilson, J.S., Humphrey, J.D., Karsaj, I.: A computational model of biochemomechanical effects of intraluminal thrombus on the enlargement of abdominal aortic aneurysms. Ann. Biomed. Eng. 43, 2852–2867 (2015)
Virag, L., Wilson, J.S., Humphrey, J.D., Karsaj, I.: Potential biomechanical roles of risk factors in the evolution of thrombus-laden abdominal aortic aneurysms. Int. J. Numer. Methods Biomed. Eng. 33, e2893 (2017)
Ramachandra, A.B., Sankaran, S., Humphrey, J.D., Marsden, A.L.: Computational simulation of the adaptive capacity of vein grafts in response to increased pressure. J. Biomech. Eng. 137, 0310091 (2015)
Ramachandra, A.B., Humphrey, J.D., Marsden, A.L.: Gradual loading ameliorates maladaptation in computational simulations of vein growth and remodeling. J. R. Soc. Interface 14, 20160995 (2017)
Sankaran, S., Humphrey, J.D., Marsden, A.L.: Optimization and parameter sensitivity analysis for arterial growth and remodeling computations. Comput. Methods Appl. Mech. Eng. 256, 200–210 (2013)
Ramachandra, A., Latorre, M., Szafron, J., Marsden, A.L., Humphrey, J.D.: Vascular adaptation in the presence of an external support – a modeling study. J. Mech. Behav. Biomed. Mater. 110, 103943 (2020)
Valentin, A., Humphrey, J.D., Holzapfel, G.A.: A multi-layered computational model of coupled elastin degradation, vasoactive dysfunction, and collagenous stiffening in aortic aging. Ann. Biomed. Eng. 39, 2027–2045 (2011)
Humphrey, J.D., Harrison, D.G., Figueroa, C.A., Lacolley, P., Laurent, S.: Central artery stiffness in hypertension and aging: a problem with cause and consequence. Circ. Res. 118, 379–381 (2016)
Morris, S.A.: Arterial tortuosity in genetic arteriopathies. Curr. Opin. Cardiol. 30, 587–593 (2015)
Ciurică, S., Lopez-Sublet, M., Loeys, B.L., Radhouani, I., Natarajan, N., Vikkula, M., Maas, A.H., Adlam, D., Persu, A.: Arterial tortuosity: novel implications for an old phenotype. Hypertension 73, 951–960 (2019)
Han, H-C., Chesnutt, J.K., Garcia, J.R., Liu, Q., Wen, Q.: Artery buckling: new phenotypes, models, and applications. Ann. Biomed. Eng. 41, 1399–1410 (2013)
Weiss, D., Cavinato, C., Gray, A., Ramachandra, A.B., Avril, S., Humphrey, J.D., Latorre, M.: Mechanics-driven mechanobiological mechanisms of arterial tortuosity. Sci. Adv. 6, eabd3574 (2020)
Libby, P.: Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 32, 2045–2051 (2012)
Jagadesham, V.P., et al.: Abdominal aortic aneurysms: an autoimmune disease? Trends Mol. Med. 14, 522–529 (2008)
Chalouhi, N., et al.: Biology of intracranial aneurysms: role of inflammation. J. Cereb. Blood Flow Metab. 32, 1659–1676 (2012)
Dorfmuller, P., et al.: Inflammation in pulmonary arterial hypertension. Eur. Respir. J. 22, 358–363 (2003)
Mahmud, A., Freely, J.: Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension 46, 1118–1122 (2005)
Wang, M., Zhang, J., Jiang, L.Q., Spinetti, G., Pintus, G., Monticone, R., Kolodgie, F.D., Virmani, R., Lakatta, E.G.: Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension 50, 219–227 (2007)
Soares, A.G., et al.: Obesity induces artery-specific alterations: evaluation of vascular function and inflammatory and smooth muscle phenotypic markers. Biomed. Res. Int., 5038602 (2017)
Sciatti, E., Vizzardi, E., Castiello, A., Valentini, F., Bonadei, I., Gelsomino, S., Lorusso, R., Metra, M.: The role of type 2 diabetes mellitus on hypertensive-related aortic stiffness. Echocardiography 35, 798–803 (2018)
Maki-Petaja, K.M., Elkhawad, M., Cheriyan, J., Joshi, F.R., Ostor, A.J., Hall, F.C., Rudd, J.H., Wilkinson, I.B.: Anti-tumor necrosis factor-a therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation 126, 2473–2480 (2012)
Vizzardi, E., Sciatti, E., Bondadei, I., Menottil, E., Prati, F., Scodro, M., Dallapellegrina, L., Berlendis, M., Poli, P., Padoan, R., Metra, M.: Elastic aortic properties in cystic fibrosis adults without cardiovascular risk factors: a case-control study. Echocardiography 36, 1118–1122 (2019)
Medzhitov, R.: Origin and physiological roles of inflammation. Nature 454, 428–435 (2008)
Latorre, M., Humphrey, J.D.: Modeling mechano-driven and immuno-mediated aortic maladaptation in hypertension. Biomech. Model. Mechanobiol. 17, 1497–1511 (2018)
Latorre, M., Bersi, M.R., Humphrey, J.D.: Computational modeling predicts immuno-mechanical mechanisms of maladaptative aortic remodeling in hypertension. Int. J. Eng. Sci. 14, 35–46 (2019)
Kotas, M.E., Medzhitov, R.: Homeostasis, inflammation, and disease susceptibility. Cell 160, 816–827 (2015)
Niklason, L.E., Yeh, A.T., Calle, E., Bai, Y., Valentin, A., Humphrey, J.D.: Enabling tools for engineering collagenous tissues, integrating bioreactors, intravital imaging, and biomechanical modeling. Proc. Natl. Acad. Sci. 107, 3335–3339 (2010)
Miller, K.S., Lee, Y.U., Naito, Y., Breuer, C.K., Humphrey, J.D.: Computational model of the in vivo development of a tissue engineered vein from an implanted polymeric construct. J. Biomech. 47, 2080–2087 (2014)
Khosravi, R., Miller, K.S., Best, C.A., Shih, Y.C., Lee, Y-U., Yi, T., Shinoka, T., Breuer, C.K., Humphrey, J.D.: Biomechanical diversity despite mechanobiological stability in tissue engineered vascular grafts two years post-implantation. Tissue Eng., Part A 21, 1529–1538 (2015)
Szafron, J., Khosravi, R., Reinhardt, J., Best, C.A., Bersi, M.R., Yi, T., Breuer, C.K., Humphrey, J.D.: Immuno-driven and mechano-mediated neotissue formation in tissue engineered vascular grafts. Ann. Biomed. Eng. 46, 1938–1950 (2018)
Miller, K.S., Khosravi, R., Breuer, C.K., Humphrey, J.D.: A hypothesis-driven parametric study of the effects of polymeric scaffold properties on tissue engineered neovessel formation. Acta Biomater. 11, 283–294 (2015)
Szafron, J.M., Ramachandra, A.B., Breuer, C.K., Marsden, A.L., Humphrey, J.D.: Optimization of tissue engineered vascular graft design using computational modeling. Tissue Eng., Part C 25, 561–570 (2019)
Drews, J., Pepper, V.A., Best, C.A., Szafron, J.M., et al.: Spontaneous reversal of stenosis in tissue-engineered vascular grafts. Sci. Transl. Med. 12, eaax6919 (2020)
Figueroa, C.A., Baek, S., Taylor, C.A., Humphrey, J.D.: A computational framework for fluid-solid-growth modeling in cardiovascular simulations. Comput. Methods Appl. Mech. Eng. 198, 3583–3602 (2009)
Baek, S., Gleason, R.L., Rajagopal, K.R., Humphrey, J.D.: Theory of small on large: potential utility in computations of fluid-solid interactions in arteries. Comput. Methods Appl. Mech. Eng. 196, 3070–3078 (2007)
Sheidaei, A., Hunley, S.C., Zeinali-Davarani, S., Raguin, L.G., Baek, S.: Simulation of abdominal aortic aneurysm growth with updated hemodynamic loads using a realistic geometry. Med. Eng. Phys. 33, 80–88 (2011)
Wu, J., Shadden, S.C.: Coupled simulation of hemodynamics and vascular growth and remodeling in a subject-specific geometry. Ann. Biomed. Eng. 43, 1543–1554 (2015)
Hayenga, H.N., Thorne, B., Peirce, S., Humphrey, J.D.: Ensuring congruency in multiscale models: towards linking agent based and continuum biomechanical models of arterial adaptations. Ann. Biomed. Eng. 39, 2669–2682 (2011)
Irons, L., Latorre, M., Humphrey, J.D.: From transcript to tissue: multiscale modeling from cell signaling to matrix remodeling. Ann. Biomed. Eng. (2021). Accepted
Kaunas, R., Hsu, H-J.: A kinematic model of stretch-induced stress fiber turnover and reorientation. J. Theor. Biol. 257, 320–330 (2009)
Vernerey, F.J., Farsad, M.: A constrained mixture approach to mechano-sensing and force generation to contractile cells. J. Mech. Behav. Biomed. Mater. 4, 1683–1699 (2011)
Valentin, A., Humphrey, J.D., Holzapfel, G.A.: A finite element-based constrained mixture implementation for arterial growth, remodeling, and adaptation. Theory and numerical verification. Int. J. Numer. Methods Biomed. Eng. 29, 822–849 (2013)
Horat, N., Virag, L., Holzapfel, G.A., Soric, J., Karsaj, I.: A finite element implementation of a growth and remodeling model for soft biological soft tissues: verification and application to abdominal aortic aneurysms. Comput. Methods Appl. Mech. Eng. 352, 586–605 (2019)
Cyron, C., Aydin, R.C., Humphrey, J.D.: A homogenized constrained mixture (and mechanical analog model) for growth and remodeling of soft tissue. Biomech. Model. Mechanobiol. 15, 1389–1403 (2016)
Braeu, F.A., Seitz, A., Aydin, R.C., Cyron, C.J.: Homogenized constrained mixture models for anisotropic volumetric growth and remodeling. Biomech. Model. Mechanobiol. 16, 889–906 (2017)
Latorre, M., Fast, H.JD.: rate-independent, finite element implementation of a 3D constrained mixture model of soft tissue growth and remodeling. Comput. Methods Appl. Mech. Eng. 368, 113156 (2020)
Cyron, C., Humphrey, J.D.: Vascular homeostasis and the concept of mechanobiological stability. Int. J. Eng. Sci. 85, 203–223 (2014)
Wu, J., Shadden, S.C.: Stability analysis of a continuum-based constrained mixture model for vascular growth and remodeling. Biomech. Model. Mechanobiol. 15, 1669–1684 (2016)
Davies, K.J.A.: Adaptive homeostasis. Mol. Aspects Med. 49, 1–7 (2016)
Chovatiya, R., Medzhitov, R.: Stress, inflammation, and defense of homeostasis. Mol. Cell 54, 281–288 (2014)
Latorre, M., Humphrey, J.D.: Mechanobiological stability of biological soft tissues. J. Mech. Phys. Solids 125, 298–325 (2019)
Taber, L.A.: A model for aortic growth based on fluid shear and fiber stresses. J. Biomech. Eng. 120, 348–354 (1998)
Wagenseil, J.E.: A constrained mixture model for developing mouse aorta. Biomech. Model. Mechanobiol. 10, 671–687 (2011)
Humphrey, J.D., Dufrense, E., Schwartz, M.A.: Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 15, 802–812 (2014)
Emmert, M., Schmidt, B.A., Loerakker, S., et al.: Computational modeling guides tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model. Sci. Transl. Med. 10, eaan4587 (2018)
Taber, L.A., Humphrey, J.D.: Stress modulated growth, residual stress, and vascular heterogeneity. J. Biomech. Eng. 123, 528–535 (2001)
Alford, P.W., Humphrey, J.D., Taber, L.A.: Growth and remodeling in a thick-walled artery model: effects of spatial variations in wall constituents. Biomech. Model. Mechanobiol. 7, 245–262 (2008)
Valentin, A., Humphrey, J.D.: Evaluation of fundamental hypotheses underlying constrained mixture models of arterial growth and remodeling. Philos. Trans. R. Soc. Lond. A 367, 3585–3606 (2009)
Karsaj, I., Humphrey, J.D.: A 3-D framework for arterial growth and remodeling in response to altered hemodynamics. Int. J. Eng. Sci. 48, 1357–1372 (2010)
Wan, W., Hansen, L., Gleason, R.L.: A 3-D constrained mixture model for mechanically mediated vascular growth and remodeling. Biomech. Model. Mechanobiol. 9, 403–419 (2010)
Valentin, A., Holzapfel, G.A.: Constrained mixture models as tools for testing competing hypotheses in arterial biomechanics: a brief review. Mech. Res. Commun. 42, 126–133 (2012)
Satha, G., Lindstrom, S.B., Klarbring, A.: A goal function approach to remodeling of arteries uncovers mechanisms for growth instability. Biomech. Model. Mechanobiol. 13, 1243–1259 (2014)
Hald, E.S., Timm, C.D., Alford, P.W.: Amyloid beta influences vascular smooth muscle contractility and mechanoadaptation. J. Biomech. Eng. 138, 4034560 (2016)
Soares, J.S., Sacks, M.S.: A triphasic constrained mixture model of engineered tissue formation under in vitro dynamic mechanical conditioning. Biomech. Model. Mechanobiol. 15, 293–316 (2016)
Grytsan, A., Eriksson, T.S.E., Watton, P.N., Gasser, T.C.: Growth description for vessel wall adaptation: a thick-walled mixture model of abdominal aortic aneurysm evolution. Materials (Basel) 10, 994 (2017)
Mousavi, S.J., Avril, S.: Patient-specific stress analyses in the ascending thoracic aorta using a finite-element implementation of the constrained mixture theory. Biomech. Model. Mechanobiol. 16, 1765–1777 (2017)
Famaey, N., Vastmans, J., Fehervary, H., Maes, L., Vanderveken, E., Rega, F., Mousavi, S.J., Avril, S.: Numerical simulation of arterial remodeling in pulmonary autografts. Z. Angew. Math. Mech. 98, 2239–2257 (2018)
Hill, M.R., Philp, C.J., Billington, C.K., Tatler, A.L., Johnson, S.R., O’Dea, R.D., Brook, B.S.: A theoretical model of inflammation- and mechanotransduction-driven asthmatic airway remodeling. Biomech. Model. Mechanobiol. 17, 1451–1470 (2018)
Bhogal, P., Pederzani, G., Grytsan, A., Loh, Y., Brouwer, P.A., Andersson, T., Gundiah, N., Robertson, A.M., Watton, P.N., Soderman, M.: The unexplained success of stentplasty vasospasm treatment: insights using mechanistic mathematical modeling. Klin. Neuroradiol. 29, 763–774 (2019)
Lin, W.J., Iafrati, M.D., Peattie, R.A., Dorfmann, L.: Non-axisymmetric dilatation of a thick-walled aortic aneurysmal tissue. Int. J. Non-Linear Mech. 109, 172–181 (2019)
Maes, L., Fehervary, H., Vastmans, J., Mousavi, S.J., Avril, S., Famaey, N.: Constrained mixture modeling affects material parameter identification from planar biaxial tests. J. Mech. Behav. Biomed. Mater. 95, 124–135 (2019)
Mousavi, S.J., Farzaneh, S., Avril, S.: Patient-specific predictions of aneurysm growth and remodeling in the ascending thoracic aorta using the homogenized constrained mixture model. Biomech. Model. Mechanobiol. 18, 1895–1913 (2019)
Rachev, A., Shazly, T.: A structure-based constitutive model of arterial tissue considering individual natural configurations of elastin and collagen. J. Mech. Behav. Biomed. Mater. 90, 61–72 (2019)
Khosravi, R., Ramachandra, A.B., Szafron, J., Schiavazzi, D.E., Breuer, C.K., Humphrey, J.D.: A computational bio-chemo-mechanical model of in vivo tissue-engineered vascular graft development. Integr. Biol. 12, 47–63 (2020)
Wu, J., Augustin, C., Shadden, S.C.: Reconstructing vascular homeostasis by growth-based prestretch and optimal fiber deposition. J. Mech. Behav. Biomed. Mater. ePub ahead of print (2020)
Zou, D., Avril, S., Yang, H., Mousavi, S.J., Hackl, K., He, Y.: Three-dimensional numerical simulation of soft-tissue wound healing using constrained-mixture anisotropic hyperelasticity and gradient-enhanced damage mechanics. J. R. Soc. Interface 17, 20190708 (2020)
Klisch, S.M., Chen, S.S., Sah, R.L., Hoger, A.: A growth mixture theory for cartilage with application to growth-related experiments on cartilage explants. J. Biomech. Eng. 125, 169–179 (2003)
Lemon, G., King, J.R., Byrne, H.M., Jensen, O.E., Shakesheff, K.M.: Mathematical modeling of engineered tissue growth using multiphase porous flow mixture theory. J. Math. Biol. 52, 571–594 (2006)
Ambrosi, D., Preziosi, L., Vitale, G.: The insight of mixtures theory for growth and remodeling. Z. Angew. Math. Phys. 61, 177–191 (2010)
Taber, L.A.: Biomechanics of growth, remodeling, and morphogenesis. Appl. Mech. Rev. 48, 487–545 (1995)
Ambrosi, D., Ateshian, G.A., Arruda, E.M., Cowin, S.C., Dumais, J., Goriely, A., Holzapfel, G.A., Humphrey, J.D., Kemkemer, R., Kuhl, E., Olberding, J.E., Taber, L.A., Garikipati, K.: Perspectives on biological growth and remodeling. J. Mech. Phys. Solids 59, 863–883 (2011)
Ateshian, G., Humphrey, J.D.: Continuum mixture models of soft tissue growth and remodeling: past successes and future challenges. Annu. Rev. Biomed. Eng. 14, 97–111 (2012)
Menzel, A., Kuhl, E.: Frontiers in growth and remodeling. Mech. Res. Commun. 42, 1–14 (2012)
Cyron, C., Humphrey, J.D.: Growth and remodeling of load-bearing biological soft tissues. Meccanica 52, 645–664 (2017)
Gasser, T.C., Grytsan, A.: Biomechanical modeling the adaptation of soft biological tissue. Curr. Opin. Biomed. Eng. 1, 71–77 (2017)
Ambrosi, D., Ben Amar, M., Cyron, C.J., DeSimona, A., Goriely, A., Humphrey, J.D., Kuhl, E.: Growth and remodeling of living systems: perspectives, challenges, and opportunities. J. R. Soc. Interface 16, 20190233 (2019)
Goriely, A.: The Mathematics and Mechanics of Biological Growth. Springer, New York (2017)
Taber, L.A.: Continuum Modeling in Mechanobiology. Springer, New York (2020)
Acknowledgements
I thank Professor Dr. Ray Ogden for inviting this paper. I acknowledge the critical early contributions of Professor Dr. K.R. Rajagopal with whom I first sought to establish foundations for a new approach to model soft tissues. I also thank former and current students, post-doctoral fellows, and research associates who contributed so much to the conceptual advances and/or particular implementations of this theory, including Drs. R. Gleason, S. Baek, I. Karsaj, A. Valentin, H. Hayenga, J. Wilson, C. Cyron, K. Miller, R. Khosravi, M. Rausch, A. Ramachandra, J. Szafron, M. Latorre, and L. Irons. Finally, the title stems, in part, from the great 19th century French author, Alexandre Dumas (1802–1870), who penned Twenty Years After, among other novels.
Funding
This work was supported by past and current grants from the US National Science Foundation (BES-0084644) and US National Institutes of Health (R01 HL054957, R01 HL064372, R01 HL080415, R01 HL086418, R01 HL105297, U01 HL116323, R01 HL128602, P01 HL134605, R01 HL139796, U01 HL142518, R01 HL146723).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
The author declares no conflicts of interest, financial or otherwise.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Humphrey, J.D. Constrained Mixture Models of Soft Tissue Growth and Remodeling – Twenty Years After. J Elast 145, 49–75 (2021). https://doi.org/10.1007/s10659-020-09809-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10659-020-09809-1