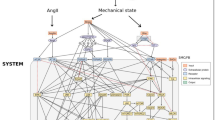
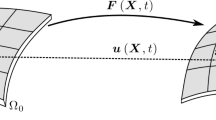Abstract
There is a need to develop multiscale models of vascular adaptations to understand tissue-level manifestations of cellular level mechanisms. Continuum-based biomechanical models are well suited for relating blood pressures and flows to stress-mediated changes in geometry and properties, but less so for describing underlying mechanobiological processes. Discrete stochastic agent-based models are well suited for representing biological processes at a cellular level, but not for describing tissue-level mechanical changes. We present here a conceptually new approach to facilitate the coupling of continuum and agent-based models. Because of ubiquitous limitations in both the tissue- and cell-level data from which one derives constitutive relations for continuum models and rule-sets for agent-based models, we suggest that model verification should enforce congruency across scales. That is, multiscale model parameters initially determined from data sets representing different scales should be refined, when possible, to ensure that common outputs are consistent. Potential advantages of this approach are illustrated by comparing simulated aortic responses to a sustained increase in blood pressure predicted by continuum and agent-based models both before and after instituting a genetic algorithm to refine 16 objectively bounded model parameters. We show that congruency-based parameter refinement not only yielded increased consistency across scales, it also yielded predictions that are closer to in vivo observations.




Similar content being viewed by others
References
Baek, S., K. R. Rajagopal, and J. D. Humphrey. A theoretical model of enlarging intracranial fusiform aneurysms. J. Biomech. Eng. 128:142–149, 2006.
Baek, S., A. Valentin, and J. D. Humphrey. Biochemomechanics of cerebral vasospasm and its resolution: II Constitutive relations and model simulations. Ann. Biomed. Eng. 35:1498–1509, 2007.
Cho, A., D. W. Courtman, and B. L. Langille. Apoptosis (programmed cell death) in arteries of the neonatal lamb. Circ. Res. 76:168–175, 1995.
Dancu, M. B., D. E. Berardi, J. P. Vanden Heuvel, and J. M. Tarbell. Asynchronous shear stress and circumferential strain reduces endothelial NO synthase and cyclooxygenase-2 but induces endothelin-1 gene expression in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 24:2088–2094, 2004.
Davis, E. C. Stability of elastin in the developing mouse aorta: a quantitative radioautographic study. Histochemistry 100:17–26, 1993.
Faury, G., M. Pezet, R. H. Knutsen, W. A. Boyle, S. P. Heximer, S. E. McLean, R. K. Minkes, K. J. Blumer, A. Kovacs, D. P. Kelly, et al. Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J. Clin. Invest. 112:1419–1428, 2003.
Fischer, G. M., and J. G. Llaurado. Collagen and elastin content in canine arteries selected from functionally different vascular beds. Circ. Res. 19:394–399, 1966.
Garcia-Lopez, G., F. Vadillo-Ortega, H. Merchant-Larios, R. Maida-Claros, M. Osorio, D. Soriano-Becerril, H. Flores-Herrera, J. Beltran-Montoya, Y. Garfias-Becerra, and V. Zaga-Clavellina. Evidence of in vitro differential secretion of 72 and 92 kDa type IV collagenases after selective exposure to lipopolysaccharide in human fetal membranes. Mol. Hum. Reprod. 13:409–418, 2007.
Gelman, R. A., D. C. Poppke, and K. A. Piez. Collagen fibril formation in vitro. The role of the nonhelical terminal regions. J. Biol. Chem. 254:11741–11745, 1979.
Gelman, R. A., B. R. Williams, and K. A. Piez. Collagen fibril formation. Evidence for a multistep process. J. Biol. Chem. 254:180–186, 1979.
Greve, J. M., A. S. Les, B. T. Tang, M. T. Draney Blomme, N. M. Wilson, R. L. Dalman, N. J. Pelc, and C. A. Taylor. Allometric scaling of wall shear stress from mice to humans: quantification using cine phase-contrast MRI and computational fluid dynamics. Am. J. Physiol. Heart Circ. Physiol. 291:H1700–H1708, 2006.
Guo, X., and G. S. Kassab. Variation of mechanical properties along the length of the aorta in C57bl/6 mice. Am. J. Physiol. Heart Circ. Physiol. 285:H2614–H2622, 2003.
Hu, J. J., S. Baek, and J. D. Humphrey. Stress-strain behavior of the passive basilar artery in normotension and hypertension. J. Biomech. 40:2559–2563, 2007.
Humphrey, J. D. Cardiovascular Solid Mechanics: Cells Tissues and Organs (1st ed.). New York: Springer, 2002.
Humphrey, J. D. Vascular adaptation and mechanical homeostasis at tissue, cellular, and sub-cellular levels. Cell Biochem. Biophys. 50:53–78, 2008.
Humphrey, J. D., and K. R. Rajagopal. A constrained mixture model for arterial adaptations to a sustained step change in blood flow. Biomech. Model Mechanobiol. 2:109–126, 2003.
Kao, W. W., R. A. Berg, and D. J. Prockop. Kinetics for the secretion of procollagen by freshly isolated tendon cells. J. Biol. Chem. 252:8391–8397, 1977.
Kim, Y. S., Z. S. Galis, A. Rachev, H. C. Han, and R. P. Vito. Matrix metalloproteinase-2 and -9 are associated with high stresses predicted using a nonlinear heterogeneous model of arteries. J. Biomech. Eng. 131:011009, 2009.
Langille, B. L. Remodeling of developing and mature arteries: endothelium, smooth muscle, and matrix. J. Cardiovasc. Pharmacol. 21(Suppl 1):S11–S17, 1993.
Langille, B. L. Arterial remodeling: relation to hemodynamics. Can. J. Physiol. Pharmacol. 74:834–841, 1996.
Lehoux, S., and B. I. Levy. Collateral artery growth: making the most of what you have. Circ. Res. 99:567–569, 2006.
Li, S., N. F. Huang, and S. Hsu. Mechanotransduction in endothelial cell migration. J. Cell Biochem. 96:1110–1126, 2005.
Li, Z., S. Moore, and M. Z. Alavi. Mitogenic factors released from smooth muscle cells are responsible for neointimal cell proliferation after balloon catheter deendothelialization. Exp. Mol. Pathol. 63:77–86, 1995.
Ma, Y. H., S. Ling, and H. E. Ives. Mechanical strain increases PDGF-B and PDGF beta receptor expression in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 265:606–610, 1999.
Mata-Greenwood, E., A. Grobe, S. Kumar, Y. Noskina, and S. M. Black. Cyclic stretch increases VEGF expression in pulmonary arterial smooth muscle cells via TGF-beta1 and reactive oxygen species: a requirement for NAD(P)H oxidase. Am. J. Physiol. Lung Cell Mol. Physiol. 289:L288–L289, 2005.
Matsumoto, T., and K. Hayashi. Mechanical and dimensional adaptation of rat aorta to hypertension. J. Biomech. Eng. 116:278–283, 1994.
Morishita, R., G. H. Gibbons, M. Horiuchi, Y. Kaneda, T. Ogihara, and V. J. Dzau. Role of AP-1 complex in angiotensin II-mediated transforming growth factor-beta expression and growth of smooth muscle cells: using decoy approach against AP-1 binding site. Biochem. Biophys. Res. Commun. 243:361–367, 1998.
Niedermuller, H., M. Skalicky, G. Hofecker, and A. Kment. Investigations on the kinetics of collagen-metabolism in young and old rats. Exp. Gerontol. 12:159–168, 1977.
Okuno, T., A. Andoh, S. Bamba, Y. Araki, Y. Fujiyama, M. Fujiyama, and T. Bamba. Interleukin-1beta and tumor necrosis factor-alpha induce chemokine and matrix metalloproteinase gene expression in human colonic subepithelial myofibroblasts. Scand. J. Gastroenterol. 37:317–324, 2002.
Rachev, A., and K. Hayashi. Theoretical study of the effects of vascular smooth muscle contraction on strain and stress distributions in arteries. Ann. Biomed. Eng. 27:459–468, 1999.
Rachev, A., N. Stergiopulos, and J. J. Meister. Theoretical study of dynamics of arterial wall remodeling in response to changes in blood pressure. J. Biomech. 29:635–642, 1996.
Robertson, S. H., C. K. Smith, A. L. Langhans, S. E. McLinden, M. A. Oberhardt, K. R. Jakab, B. Dzamba, D. W. DeSimone, J. A. Papin, and S. M. Peirce. Multiscale computational analysis of Xenopus laevis morphogenesis reveals key insights of systems-level behavior. BMC Syst. Biol. 1:46, 2007.
Shapiro, S. D., S. K. Endicott, M. A. Province, J. A. Pierce, and E. J. Campbell. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J. Clin. Invest. 87:1828–1834, 1991.
Stenmark, K. R., and R. P. Mecham. Cellular and molecular mechanisms of pulmonary vascular remodeling. Annu. Rev. Physiol. 59:89–144, 1997.
Taber, L. A. A model for aortic growth based on fluid shear and fiber stresses. J. Biomech. Eng. 120:348–354, 1998.
Thorne, B. C., A. M. Bailey, D. W. DeSimone, and S. M. Peirce. Agent-based modeling of multicell morphogenic processes during development. Birth Defects Res. C Embryo Today 81:344–353, 2007.
Thorne, B. C., H. N. Hayenga, J. D. Humphrey, and S. M. Peirce. Towards a multi-scale computational model of arterial adaptation in hypertension: verification of a multi-cell agent-based model. Front. Comput. Physiol. Med. 2:1–12, 2011.
Valentin, A., L. Cardamone, S. Baek, and J. D. Humphrey. Complementary vasoactivity and matrix remodelling in arterial adaptations to altered flow and pressure. J. R. Soc. Interface 6:293–306, 2009.
Valentin, A., and J. D. Humphrey. Evaluation of fundamental hypotheses underlying constrained mixture models of arterial growth and remodelling. Philos. Transact. A Math. Phys. Sci. 367:3585–3606, 2009.
Valentin, A., and J. D. Humphrey. Parameter sensitivity study of a constrained mixture model of arterial growth and remodeling. J. Biomech. Eng. 131:101006, 2009.
Wolinsky, H. Effects of hypertension and its reversal on the thoracic aorta of male and female rats. Morphological and chemical studies. Circ. Res. 28:622–637, 1971.
Wolinsky, H. Long-term effects of hypertension on the rat aortic wall and their relation to concurrent aging changes. Morphological and chemical studies. Circ. Res. 30:301–309, 1972.
Wolinsky, H., and S. Glagov. A lamellar unit of aortic medial structure and function in mammals. Circ. Res. 20:99–111, 1967.
Ziegler, T., K. Bouzourene, V. J. Harrison, H. R. Brunner, and D. Hayoz. Influence of oscillatory and unidirectional flow environments on the expression of endothelin and nitric oxide synthase in cultured endothelial cells. Arterioscler. Thromb. Vasc. Biol. 18:686–692, 1998.
Acknowledgments
This work was supported, in part, via NIH grants HL-082838 (to SMP) and HL-086418 (to JDH).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Cheng Dong oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
Symbol (units) | Classification | Definition |
|---|---|---|
\( K_{{\sigma_{\theta } }}^{\text{c}} \) | CMM: Bounded | Gain parameter governing circumferential-stress mediated rate of production of collagen. See Eq. (1) |
\( K_{{\sigma_{\theta } }}^{\text{m}} \) | CMM: Bounded | Gain parameter governing circumferential-stress mediated rate of production of smooth muscle. See Eq. (1) |
\( K_{{\tau_{w} }}^{\text{c}} \) | CMM: Bounded | Gain parameter governing shear-stress mediated rate of production of collagen. See Eq. (1) |
\( K_{{\tau_{w} }}^{\text{m}} \) | CMM: Bounded | Gain parameter governing shear-stress mediated rate of production of smooth muscle. See Eq. (1) |
MMP-10 (pg) | ABM: Bounded | Baseline mass of MMP-1 |
MMP-1%A (%) | ABM: Bounded | Percent of MMP-1 that is active |
C0 (pg) | ABM: Bounded | Baseline mass of collagen |
C TGF | ABM: Bounded | Rate parameter governing TBFb mediated rate of production of collagen (pg of collagen per pg of TGFb) |
M p | ABM: Bounded | Rate parameter governing PDGF mediated SMC proliferation rate (pg of SMC per pg of PDGF) |
M 0 (pg) | ABM: Bounded | Baseline SMC proliferation rate (pg) |
M a1 | ABM: Bounded | Apoptosis chance for SMC (1) |
M a2 | ABM: Bounded | Baseline apoptosis chance for SMC (2) |
\( {\text{PDGF}}_{{\sigma_{\theta } }} \) (pg/kPa) | ABM: Bounded | Rate parameter governing hoop stress mediated rate of production of PDGF (pg of PDGF per kPa of stress) |
PDGF0 (pg) | ABM: Bounded | Baseline mass of PDGF |
\( {\text{TGF}}\beta_{{\sigma_{\theta } }} \) (pg) | ABM: Bounded | Rate parameter governing hoop stress mediated rate of production of TGFb (pg of TGFb per kPa of stress) |
TGFβ0 (pg) | ABM: Bounded | Baseline mass of TGFb |
\( G_{h}^{\text{e}} \) | CMM: Observed | Homeostatic stretch when elastin is deposited |
\( G_{h}^{\text{c}} \) | CMM: Observed | Homeostatic stretch when collagen is deposited |
\( G_{h}^{\text{m}} \) | CMM: Observed | Homeostatic stretch when SMC is deposited |
\( c^{\text{e}} \) (kPa) | CMM: Calculated | Neo-Hookean material parameter for the stored energy of elastin |
\( c_{1}^{\text{c}} \) (kPa) | CMM: Calculated | Fung-type exponential material parameter for the stored energy of collagen (1) |
\( c_{1}^{\text{m}} \) (kPa) | CMM: Calculated | Fung-type exponential material parameter for the stored energy of SMC (1) |
\( c_{2}^{\text{c}} \) | CMM: Observed | Fung-type exponential material parameter for the stored energy of collagen (2) |
\( c_{2}^{\text{m}} \) | CMM: Observed | Fung-type exponential material parameter for the stored energy of SMC (2) |
\( T_{\text{M}} \) (kPa) | CMM: Observed | Maximum stress generated by SMC |
\( \lambda_{\text{M}} \) | CMM: Observed | Circumferential stretch where active stress is maximum |
\( \lambda_{0} \) | CMM: Observed | Circumferential stretch where active stress is zero |
\( C_{\text{B}} \) | CMM: Observed | Material parameter for the ratio of constrictor concentration to dilator concentration |
\( C_{\text{S}} \) | CMM: Observed | Scaling parameter for shear stress induced change in constrictor concentration scaling factor |
\( \phi^{\text{c}} \left( 0 \right) \) | CMM: Observed | Initial mass fraction of collagen in the arterial wall |
\( \phi^{\text{e}} \left( 0 \right) \) | CMM: Observed | Initial mass fraction of elastin in the arterial wall |
\( \phi^{\text{m}} \left( 0 \right) \) | CMM: Observed | Initial mass fraction of SMC in the arterial wall |
\( K_{h}^{\text{m}} \) (days−1) | CMM: Observed | Half-life of SMC |
\( K_{h}^{\text{c}} \) (days−1) | CMM: Observed | Half-life of collagen |
NO0 (pg) | ABM: Observed | Baseline mass of NO |
NO τw | ABM: Observed | Rate parameter governing shear stress mediated rate of production of NO (pg of NO per kPa of stress) |
\( \delta^{\text{PDGF}} \) (pg) | ABM: Observed | Parameters of the sigmoid function: |
\( \alpha^{\text{PDGF}} \) | \( {\text{PDGF}} = M\left( {\delta + \alpha \left( {1 - e^{{ - kx^{n} }} } \right)} \right) \) | |
\( k^{\text{PDGF}} \) (kPa−1) | ||
\( n^{\text{PDGF}} \) | ||
\( M^{\text{PDGF}} \) | Maximum rate of PDGF production | |
\( \delta^{{{\text{ET{-}}}1}} \) (pg) | ABM: Observed | Parameters of the sigmoid function: |
\( \alpha^{{{\text{ET{-}}}1}} \) | \( {\text{ET}}1 = M\left( {\delta + \alpha \left( {1 - e^{{ - kx^{n} }} } \right)} \right) \) | |
\( k^{{{\text{ET{-}}}1}} \) (kPa−1) | ||
\( n^{{{\text{ET{-}}}1}} \) | ||
\( M^{{{\text{ET{-}}}1}} \) | Maximum rate of ET-1 production | |
\( \delta^{{{\text{MMP{-}}}2}} \) (pg) | ABM: Observed | Parameters of the sigmoid function: |
\( \alpha^{{{\text{MMP{-}}}2}} \) | \( {\text{MMP}}2 = A\left( {M\left( {\delta + \alpha \left( {1 - e^{{ - kx^{n} }} } \right)} \right)} \right) \) | |
\( k^{{{\text{MMP{-}}}2}} \) (kPa−1) | ||
\( n^{{{\text{MMP{-}}}2}} \) | ||
\( M^{{{\text{MMP{-}}}2}} \) | Maximum rate of MMP-2 production | |
\( A^{{{\text{MMP{-}}}2}} \) (%) | Percent of MMP-2 Active | |
\( D^{{{\text{MMP{-}}}2}} \) | ABM: Observed | Degradation rate of MMP-2 |
\( \delta^{{{\text{MMP{-}}}9}} \) (pg) | ABM: Observed | Parameters of the sigmoid function: |
\( \delta^{\text{MMP{-}9}} \) | \( {\text{MMP}}9 = A\left( {M\left( {\delta + \alpha \left( {1 - e^{{ - kx^{n} }} } \right)} \right)} \right) \) | |
\( k^{{{\text{MMP{-}}}2}} \) (kPa−1) | ||
\( n^{{{\text{MMP{-}}}9}} \) | ||
\( M^{{{\text{MMP{-}}}9}} \) | Maximum rate of MMP-9 production | |
\( A^{{{\text{MMP{-}}}9}} \) (%) | Percent of MMP-9 active | |
\( D^{{{\text{MMP{-}}}9}} \) | ABM: Observed | Degradation rate of MMP-9 |
\( D^{{{\text{MMP{-}}}1}} \) | ABM: Observed | Degradation rate of MMP-1 |
Rights and permissions
About this article
Cite this article
Hayenga, H.N., Thorne, B.C., Peirce, S.M. et al. Ensuring Congruency in Multiscale Modeling: Towards Linking Agent Based and Continuum Biomechanical Models of Arterial Adaptation. Ann Biomed Eng 39, 2669–2682 (2011). https://doi.org/10.1007/s10439-011-0363-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-011-0363-9