Abstract
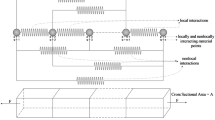
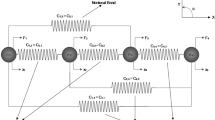
The past two decades reveal a growing role of continuum biomechanics in understanding homeostasis, adaptation, and disease progression in soft tissues. In this paper, we briefly review the two primary theoretical approaches for describing mechano-regulated soft tissue growth and remodeling on the continuum level as well as hybrid approaches that attempt to combine the advantages of these two approaches while avoiding their disadvantages. We also discuss emerging concepts, including that of mechanobiological stability. Moreover, to motivate and put into context the different theoretical approaches, we briefly review findings from mechanobiology that show the importance of mass turnover and the prestressing of both extant and new extracellular matrix in most cases of growth and remodeling. For illustrative purposes, these concepts and findings are discussed, in large part, within the context of two load-bearing, collagen dominated soft tissues—tendons/ligaments and blood vessels. We conclude by emphasizing further examples, needs, and opportunities in this exciting field of modeling soft tissues.









Similar content being viewed by others
References
Ascenzi A (1993) Biomechanics and Galileo Galilei. J Biomech 26(2):95–100
Galilei G (1638) Discorsi e dimostrazioni matematiche intorno a due nuove scienze. Lodewijk Elzevir, Leiden
Bauer L (1868) Lectures on orthopaedic surgery: delivered at the Brooklyn Medical and Surgical Institute. Wood, New York
Davis HG (1867) Conservative surgery. Appleton, New York
Nutt JJ (1913) Diseases and deformities of the foot. E.B. Treat, New York
Wolff J (1892) Das Gesetz der Transformation der Knochen. August Hirschwald, Berlin
Humphrey JD, Holzapfel GA (2012) Mechanics, mechanobiology, and modeling of human abdominal aorta and aneurysms. J Biomech 45(5):805–814
Engler AJ et al (2006) Matrix elasticity directs stem cell lineage specification. Cell 126(4):677–689
Nakagawa Y et al (1989) Effect of disuse on the ultrastructure of the achilles tendon in rats. Eur J Appl Physiol Occup Physiol 59(3):239–242
Uhthoff HK, Jaworski ZF (1978) Bone loss in response to long-term immobilisation. J Bone Joint Surg Br 60-B(3):420–429
Jaworski ZF, Liskova-Kiar M, Uhthoff HK (1980) Effect of long-term immobilisation on the pattern of bone loss in older dogs. J Bone Joint Surg Br 62(1):104–110
Humphrey JD (2008) Vascular adaptation and mechanical homeostasis at tissue, cellular, and sub-cellular levels. Cell Biochem Biophys 50(2):53–78
Makale M (2007) Cellular mechanobiology and cancer metastasis. Birth Defects Res C Embryo Today 81(4):329–343
Sumner DR et al (1998) Functional adaptation and ingrowth of bone vary as a function of hip implant stiffness. J Biomech 31(10):909–917
Taber LA (1995) Biomechanics of growth, remodeling, and morphogenesis. Appl Mech Rev 48(8):487–545
Menzel A, Kuhl E (2012) Frontiers in growth and remodeling. Mech Res Commun 42:1–14
Ambrosi D et al (2011) Perspectives on biological growth and remodeling. J Mech Phys Solids 59(4):863–883
Wolinsky H, Glagov S (1967) A lamellar unit of aortic medial structure and function in mammals. Circ Res 20(1):99–111
Wolinsky H (1972) Long-term effects of hypertension on the rat aortic wall and their relation to concurrent aging changes: morphological and chemical studies. Circ Res 30(3):301–309
Matsumoto T, Hayashi K (1996) Response of arterial wall to hypertension and residual stress. In: Hayashi K, Kamiya A, Ono K (eds) Biomechanics: functional adaptation and remodeling. Springer, Tokyo, pp 93–119. doi:10.1007/978-4-431-68317-9
Shadwick RE (1999) Mechanical design in arteries. J Exp Biol 202(23):3305–3313
Fung YC (1993) Biomechanics: mechanical properties of living tissues. Springer, Berlin
Leung DY, Glagov S, Mathews MB (1976) Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cells in vitro. Science 191(4226):475–477
Li Q et al (1998) Stretch-induced collagen synthesis in cultured smooth muscle cells from rabbit aortic media and a possible involvement of angiotensin II and transforming growth factor-beta. J Vasc Res 35(2):93–103
Simon DD, Humphrey JD (2014) Learning from tissue equivalents: biomechanics and mechanobiology. In: Brennan AB, Kirschner CM (eds) Bio-inspired materials for biomedical engineering. Wiley, Hoboken, pp 281–308. doi:10.1002/9781118843499.ch15
Brown RA et al (1998) Tensional homeostasis in dermal fibroblasts: mechanical responses to mechanical loading in three-dimensional substrates. J Cell Physiol 175(3):323–332
Ezra DG et al (2010) Changes in fibroblast mechanostat set point and mechanosensitivity: an adaptive response to mechanical stress in floppy eyelid syndrome. Invest Ophthalmol Vis Sci 51(8):3853–3863
Fung YC (1990) Biomechanics: motion, flow, stress, and growth. Springer, Berlin
Li S et al (2003) Vascular smooth muscle cells orchestrate the assembly of type I collagen via α2β1 integrin, RhoA, and fibronectin polymerization. Am J Pathol 163(3):1045–1056
Humphrey JD, Dufresne ER, Schwartz MA (2014) Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 15(12):802–812
Nissen R, Cardinale GJ, Udenfriend S (1978) Increased turnover of arterial collagen in hypertensive rats. Proc Natl Acad Sci USA 75(1):451–453
Etminan N et al (2014) Age of collagen in intracranial saccular aneurysms. Stroke 45(6):1757–1763
Arribas SM, Hinek A, Gonzalez MC (2006) Elastic fibres and vascular structure in hypertension. Pharmacol Ther 111(3):771–791
Shapiro SD et al (1991) Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest 87(5):1828–1834
O’Callaghan CJ, Williams B (2000) Mechanical strain-induced extracellular matrix production by human vascular smooth muscle cells: role of TGF-beta(1). Hypertension 36(3):319–324
Wyatt KEK, Bourne JW, Torzilli PA (2009) Deformation-dependent enzyme mechanokinetic cleavage of type I collagen. J Biomech Eng 131(5):051004
Flynn BP et al (2010) Mechanical strain stabilizes reconstituted collagen fibrils against enzymatic degradation by mammalian collagenase matrix metalloproteinase 8 (MMP-8). PLoS ONE 5(8):e12337
Maegdefessel L, Dalman RL, Tsao PS (2014) Pathogenesis of abdominal aortic aneurysms: microRNAs, proteases, genetic associations. Annu Rev Med 65:49–62
Maegdefessel L et al (2014) miR-24 limits aortic vascular inflammation and murine abdominal aneurysm development. Nat Commun 5:5214
Simon DD, Niklason LE, Humphrey JD (2014) Tissue transglutaminase, not lysyl oxidase, dominates early calcium-dependent remodeling of fibroblast-populated collagen lattices. Cells Tissues Organs 200(2):104–117
Humphrey JD, Rajagopal KR (2002) A constrained mixture model for growth and remodeling of soft tissues. Math Mod Methods Appl Sci 12(03):407–430
Baek S, Rajagopal KR, Humphrey JD (2006) A theoretical model of enlarging intracranial fusiform aneurysms. J Biomech Eng 128(1):142–149
Cyron CJ, Humphrey JD (2014) Vascular homeostasis and the concept of mechanobiological stability. Int J Eng Sci 85:203–223
Canty EG et al (2006) Actin filaments are required for fibripositor-mediated collagen fibril alignment in tendon. J Biol Chem 281(50):38592–38598
Clark K et al (2007) Myosin II and mechanotransduction: a balancing act. Trends Cell Biol 17(4):178–186
Paszek MJ et al (2005) Tensional homeostasis and the malignant phenotype. Cancer Cell 8(3):241–254
Marenzana M et al (2006) The origins and regulation of tissue tension: identification of collagen tension-fixation process in vitro. Exp Cell Res 312(4):423–433
Hinz B (2013) Matrix mechanics and regulation of the fibroblast phenotype. Periodontology 63(1):14–28
Simon DD, Horgan CO, Humphrey JD (2012) Mechanical restrictions on biological responses by adherent cells within collagen gels. J Mech Behav Biomed Mater 14:216–226
Tomasek JJ et al (2002) Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3(5):349–363
Wang N et al (2002) Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am J Physiol Cell Physiol 282(3):C606–C616
Klingberg F et al (2014) Prestress in the extracellular matrix sensitizes latent TGF-beta1 for activation. J Cell Biol 207(2):283–297
Cyron CJ, Wilson JS, Humphrey JD (2014) Mechanobiological stability: a new paradigm to understand the enlargement of aneurysms? J R Soc Interface 11(100):20140680
Jackson ZS et al (2005) Partial off-loading of longitudinal tension induces arterial tortuosity. Arterioscler Thromb Vasc Biol 25(5):957–962
Humphrey JD (2003) Review paper: continuum biomechanics of soft biological tissues. Proc R Soc Lond A Math Phys Eng Sci 459(2029):3–46
Rodriguez EK, Hoger A, McCulloch AD (1994) Stress-dependent finite growth in soft elastic tissues. J Biomech 27(4):455–467
Reese S, Govindjee S (1998) A theory of finite viscoelasticity and numerical aspects. Int J Solids Struct 35(26):3455–3482
Simo JC, Hughes TJR (2000) Computational inelasticity. Springer, New York
Schmid H et al (2012) Consistent formulation of the growth process at the kinematic and constitutive level for soft tissues composed of multiple constituents. Comput Methods Biomech Biomed Eng 15(5):547–561
Ambrosi D, Guana F (2007) Stress-modulated growth. Math Mech Solids 12(3):319–342
Himpel G et al (2005) Computational modelling of isotropic multiplicative growth. Comp Mod Eng Sci 8:119–134
Kroon M (2010) Modeling of fibroblast-controlled strengthening and remodeling of uniaxially constrained collagen gels. J Biomech Eng 132(11):111008
Driessen NJ et al (2008) Remodelling of the angular collagen fiber distribution in cardiovascular tissues. Biomech Model Mechanobiol 7(2):93–103
Pluijmert M et al (2012) Adaptive reorientation of cardiac myofibers: the long-term effect of initial and boundary conditions. Mech Res Commun 42:60–67
Eriksson TSE et al (2014) Modelling volumetric growth in a thick walled fibre reinforced artery. J Mech Phys Solids 73:134–150
Watton PN, Hill NA (2009) Evolving mechanical properties of a model of abdominal aortic aneurysm. Biomech Model Mechanobiol 8(1):25–42
Watton PN et al (2011) Modelling evolution and the evolving mechanical environment of saccular cerebral aneurysms. Biomech Model Mechanobiol 10(1):109–132
Watton PN, Ventikos Y, Holzapfel GA (2009) Modelling the mechanical response of elastin for arterial tissue. J Biomech 42(9):1320–1325
Selimovic A, Ventikos Y, Watton PN (2014) Modelling the Evolution of Cerebral Aneurysms: biomechanics, Mechanobiology and Multiscale Modelling. Procedia IUTAM 10:396–409
Watton P, Hill N, Heil M (2004) A mathematical model for the growth of the abdominal aortic aneurysm. Biomech Model Mechanobiol 3(2):98–113
Cyron CJ, Aydin RC, Humphrey JD (2016) A homogenized constrained mixture (and mechanical analog) model for growth and remodeling of soft tissue. Biomech Model Mechanobiol. doi:10.1007/s10237-016-0770-9
Rajagopal K, Srinivasa A (1998) Mechanics of the inelastic behavior of materials—Part 1, theoretical underpinnings. Int J Plast 14(10):945–967
Cyron CJ, Wilson JS, Humphrey JD (2015) Mechanobiological stability: a new paradigm to understand growth and remodeling in soft tissue. In: 9th European solid mechanics conference (ESMC 2015). Leganés-Madrid, Spain
Wilson JS, Baek S, Humphrey JD (2013) Parametric study of effects of collagen turnover on the natural history of abdominal aortic aneurysms. Proc R Soc A 469(2150):20120556
Geest JPV, Sacks MS, Vorp DA (2004) Age dependency of the biaxial biomechanical behavior of human abdominal aorta. J Biomech Eng 126(6):815–822
Haskett D et al (2010) Microstructural and biomechanical alterations of the human aorta as a function of age and location. Biomech Model Mechanobiol 9(6):725–736
Teng Z et al (2009) An experimental study on the ultimate strength of the adventitia and media of human atherosclerotic carotid arteries in circumferential and axial directions. J Biomech 42(15):2535–2539
Sommer G et al (2008) Dissection properties of the human aortic media: an experimental study. J Biomech Eng 130(2):021007
Fratzl P (2008) Collagen: structure and mechanics, an introduction. In: Fratzl P (ed) Collagen. Springer, Berlin, pp 1–13
Satta J et al (1995) Increased turnover of collagen in abdominal aortic aneurysms, demonstrated by measuring the concentration of the aminoterminal propeptide of type III procollagen in peripheral and aortal blood samples. J Vasc Surg 22(2):155–160
Shantikumar S et al (2010) Diabetes and the abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 39(2):200–207
Agnoletti D et al (2013) Central hemodynamic modifications in diabetes mellitus. Atherosclerosis 230(2):315–321
Lanne T et al (1992) Diameter and compliance in the male human abdominal aorta: influence of age and aortic aneurysm. Eur J Vasc Surg 6(2):178–184
Pearson AC et al (1994) Transesophageal echocardiographic assessment of the effects of age, gender, and hypertension on thoracic aortic wall size, thickness, and stiffness. Am Heart J 128(2):344–351
Sonesson B et al (1993) Compliance and diameter in the human abdominal aorta–the influence of age and sex. Eur J Vasc Surg 7(6):690–697
Taber LA, Eggers DW (1996) Theoretical study of stress-modulated growth in the aorta. J Theor Biol 180(4):343–357
Sáez P et al (2014) Computational modeling of hypertensive growth in the human carotid artery. Comput Mech 53(6):1183–1196
Valentin A et al (2009) Complementary vasoactivity and matrix remodelling in arterial adaptations to altered flow and pressure. J R Soc Interface 6(32):293–306
Figueroa CA et al (2009) A computational framework for fluid-solid-growth modeling in cardiovascular simulations. Comput Methods Appl Mech Eng 198(45–46):3583–3602
Kroon M, Holzapfel GA (2009) A theoretical model for fibroblast-controlled growth of saccular cerebral aneurysms. J Theor Biol 257(1):73–83
Wilson JS, Baek S, Humphrey JD (2012) Importance of initial aortic properties on the evolving regional anisotropy, stiffness and wall thickness of human abdominal aortic aneurysms. J R Soc Interface 9(74):2047–2058
Zeinali-Davarani S, Sheidaei A, Baek S (2011) A finite element model of stress-mediated vascular adaptation: application to abdominal aortic aneurysms. Comput Methods Biomech Biomed Eng 14(9):803–817
Valentín A, Humphrey J, Holzapfel GA (2013) A finite element-based constrained mixture implementation for arterial growth, remodeling, and adaptation: theory and numerical verification. Int J Numer Methods Biomed Eng 29(8):822–849
Zeinali-Davarani S, Baek S (2012) Medical image-based simulation of abdominal aortic aneurysm growth. Mech Res Commun 42:107–117
Göktepe S et al (2010) A multiscale model for eccentric and concentric cardiac growth through sarcomerogenesis. J Theor Biol 265(3):433–442
Zöllner AM et al (2012) Stretching skeletal muscle: chronic muscle lengthening through sarcomerogenesis. PLoS ONE 7(10):e45661
Creative Commons License CC BY-NC-ND 4.0. http://creativecommons.org/licenses/by-nc-nd/4.0/
Zöllner AM et al (2013) Growth on demand: reviewing the mechanobiology of stretched skin. J Mech Behav Biomed Mater 28:495–509
Grytz R, Meschke G (2010) A computational remodeling approach to predict the physiological architecture of the collagen fibril network in corneo-scleral shells. Biomech Model Mechanobiol 9(2):225–235
Grytz R et al (2012) Lamina cribrosa thickening in early glaucoma predicted by a microstructure motivated growth and remodeling approach. Mech Mater 44:99–109
Simon DD, Humphrey JD (2012) On a class of admissible constitutive behaviors in free-floating engineered tissues. Int J Non Linear Mech 47(2):173–178
Simon DD, Murtada SI, Humphrey JD (2014) Computational model of matrix remodeling and entrenchment in the free-floating fibroblast-populated collagen lattice. Int J Numer Methods Biomed Eng 30(12):1506–1529
Miller KS et al (2013) Computational growth and remodeling model for evolving tissue engineered vascular grafts in the venous circulation. In: ASME 2013 Conference on frontiers in medical devices: applications of computer modeling and simulation. American Society of Mechanical Engineers
Miller KS et al (2014) A hypothesis-driven parametric study of effects of polymeric scaffold properties on tissue engineered neovessel formation. Acta Biomater 11:283–294
Khosravi R et al (2015) Biomechanical diversity despite mechanobiological stability in tissue engineered vascular grafts 2 years post-implantation. Tissue Eng Part A 21(9–10):1529–1538
Miller KS et al (2014) Computational model of the in vivo development of a tissue engineered vein from an implanted polymeric construct. J Biomech 47(9):2080–2087
Loerakker S, Ristori T, Baaijens FP (2015) A computational analysis of cell-mediated compaction and collagen remodeling in tissue-engineered heart valves. J Mech Behav Biomed Mater 58:173–187
Acknowledgments
This work was supported, in part, by the Emmy Noether program of the German Research Foundation DFG (CY 75/2-1), the International Graduate School for Science and Engineering (IGSSE) of the Technische Universität München, and the United States NIH (R01 HL086418, U01 HL116323, and R01 HL128602).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cyron, C.J., Humphrey, J.D. Growth and remodeling of load-bearing biological soft tissues. Meccanica 52, 645–664 (2017). https://doi.org/10.1007/s11012-016-0472-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11012-016-0472-5