Abstract
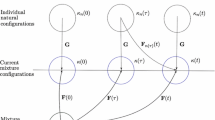
A sustained change in blood flow results in an arterial adaptation that can be thought to consist of two general steps: an immediate vasoactive response that seeks to return the wall shear stress to its homeostatic value, and a long-term growth and remodeling process that seeks to restore the intramural stresses and, if needed, the wall shear stress toward their homeostatic values. Few papers present mathematical models of arterial growth and remodeling in general, and fewer yet address flow-induced changes. Of these, most prior models build upon the concept of “kinematic growth” proposed by Skalak in the early 1980s (Skalak R (1981) In: Proceedings of the IUTAM Symposium on finite elasticity. Martinus Nijhoff, The Hague, pp 347–355). Such approaches address important consequences of growth and remodeling, but not the fundamental means by which such changes occur. In this paper, therefore, we present a new approach for mathematically modeling arterial adaptations and, in particular, flow-induced alterations. The model is motivated by observations reported in the literature and is based on a locally homogenized, constrained mixture theory. Specifically, we develop a 3-D constitutive relation for stress in terms of the responses of the three primary load-bearing constituents and their time-varying mass fractions, with the latter accounting for the kinetics of the turnover of cells and extracellular matrix in changing, stressed configurations. Of particular importance is the concept that the natural configurations of the individual constituents can evolve separately and that this leads to changes in the overall material properties and empirically inferred residual stress field of the vessel. Potential applications are discussed, but there is a pressing need for new, theoretically motivated data to allow the prescription of specific functional forms of the requisite constitutive relations and the values of the associated material parameters.




Similar content being viewed by others
Notes
Whereas homeostasis is a process that acts to maintain or restore a stable condition or state, we use the modifier homeostatic to refer to quantities in that stable condition or state.
See Humphrey and Rajagopal (2002) for detailed definitions of terms such as adaptation, growth, and remodeling. Briefly, however, growth implies a change in mass and remodeling a change in microstructure.
That is, the presence of self-equilibrating internal stresses in the absence of boundary tractions.
Clearly, s < 0 represents development, which is not considered explicitly herein.
Many consider mass production and removal via reaction (proliferation/apoptosis) and diffusion (migration) equations, but we focus on the net effect independent of the specific means.
References
Barocas VH, Tranquillo RT (1997) An anisotropic biphasic theory of tissue-equivalent mechanics: the interplay among cell traction, fibrillar network deformation, fibril alignment, and cell contact guidance. ASME J Biomech Eng 119:137–145
Busse R, Fleming I (1998) Pulsatile stretch and shear stress: physical stimuli determining the production of endothelium-derived relaxing factors. J Vasc Res 35:73–84
Cho A, Courtman DW, Langille BL (1995) Apoptosis (programmed cell death) in arteries of the neonatal lamb. Circ Res 76:168–175
Cho A, Mitchell L, Koopmans D, Langille BL (1997) Effects of changes in blood flow rate on cell death and cell proliferation in carotid arteries of immature rabbits. Circ Res 81:328–337
Chuong CJ, Fung YC (1986) On residual stress in arteries. ASME J Biomech Eng 108:189–192
Davies PF (1995) Flow-mediated endothelial mechanotransduction. Physiol Rev 75:519–560
Davis MJ, Hill MA (1999) Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79:387–423
Driss AB, Benessiano J, Poitevin P, Levy BI, Michel J-B (1997) Arterial expansive remodeling induced by high flow rates. Am J Physiol 272:H851–H858
Epstein M, Maugin GA (2000) Thermomechanics of volumetric growth in uniform bodies. Int J Plast 16:951–978
Fath SW, Burkhart HM, Miller SC, Dalsong MC, Unthank JL (1998) Wall remodeling after wall shear rate normalization in rat mesenteric arterial collaterals. J Vasc Res 35:257–264
Frangos JA, McIntire LV, Ives CL (1985) Flow effects on prostacyclin by cultured human endothelial cells. Science 227:1477–1479
Fridez P, Rachev A, Meister J-J, Hayashi K, Stergiopulos N (2001) Model of geometric and smooth muscle tone adaptation of carotid artery subject to step change in pressure. Am J Physiol 280:H2752–H2760
Fung YC (1990) Biomechanics. Motion, flow, stress, and growth. Springer Verlag, New York Berlin Heidelberg
Fung YC, Liu SQ (1989) Change of residual strains in arteries due to hypertrophy caused by aortic constriction. Circ Res 65:1340–1349
Furchgott RF, Zawadzki JV (1980) The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288:373–376
Gibbons GH, Dzau VJ (1994) The emerging concept of vascular remodeling. N Eng J Med 330:1431–1438
Girerd X, London G, Boutouyrie P, Mourad J-J, Safar M, Laurent S (1996) Remodeling of the radial artery in response to a chronic increase in shear stress. Hypertension 27:799–803
Greenwald SE, Moore JE, Jr, Rachev A, Kane TPC, Meister J-J (1997) Experimental investigation of the distribution of residual strains in the artery wall. J Biomech Eng 119:438–444
Grinnell F (1994) Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol 124:401–404
Guyton JR, Hartley CJ (1985) Flow restriction of one carotid artery in juvenile rats inhibits growth of arterial diameter. Am J Physiol 248:H540–H546
Guzman RJ, Abe K, Zarins C (1997) Flow-induced arterial enlargement is inhibited by suppression of nitric oxide synthase activity in vivo. Surgery 122:273–280
Harris AK, D Stopak, P Wild (1981) Fibroblast traction as a mechanism for collagen morphogenesis. Nature 290:249–251
Holtz J, Forstermann U, Pohl U, Giesler M, Bassenge E (1984) Flow-dependent, endothelium-mediated dilation of epicardial coronary arteries in conscious dogs: effects of cyclooxygenase inhibition. J Cardiovasc Pharm 6:1161–1169
Holzapfel GA, Gasser TC, Ogden RW (2000) A new constitutive framework for arterial wall mechanics and a comparative study of material models. J Elast 61:1–48
Humphrey JD (1999) Remodeling of a collagenous tissue at fixed lengths. ASME J Biomech Eng 121:591–597
Humphrey JD (2001) Stress, strain, and mechanotransduction in cells. ASME J Biomech Eng 123:638–641
Humphrey JD (2002) Cardiovascular solid mechanics: cells, tissues, and organs. Springer-Verlag, New York Berlin Heidelber
Humphrey JD, Na S (2002) Elastodynamics and arterial wall stress. Ann Biomed Eng 30:509–523
Humphrey JD, Rajagopal KR (2002) A constrained mixture model for growth and remodeling of soft tissues. Math Model Meth Appl Sci 12:407–430
Kamiya A, Togawa T (1980) Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am J Physiol 239:H14–H21
Klisch SM, Sah RL, Hoger A (2000) A growth mixture theory for cartilage. In: Casey J and Bao G (eds) Mechanics in biology. ASME AMD 242: 229–242
Langille BL (1993) Remodeling of developing and mature arteries: endothelium, smooth muscle, and matrix. J Cardio Pharm 21:S11–S17
Langille BL, Bendeck MP, Keeley FW (1989) Adaptations of carotid arteries of young and mature rabbits to reduced carotid blood flow. Am J Physiol 256:H931–H939
Langille BL, O’Donnell F (1986) Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium dependent. Science 231:405–407
Lanir Y (1979) Constitutive equations for fibrous connective tissues. J Biomech 16:1–12
Lehman RM, Owens GK, Kassel NF, Hongo K (1991) Mechanism of enlargement of major cerebral collateral arteries in rabbits. Stroke 22:499–504
Libby P (2000) Changing concepts of atherogenesis. J Int Med 247:349–358
Lu X, Zhao JB, Wang GR, Gregersen H, Kassab GS (2001) Remodeling of the zero-stress state of femoral arteries in response to flow overload. Am J Physiol 280:H1547–1559
Masuda H, Bassiouny H, Glagov S, Zarins CK (1989) Artery wall restructuring in response to increased flow. Surg Forum 40:285–286
McCarthy NJ, Bennett MR (2000) The regulation of vascular smooth muscle cell apoptosis. Cardiovasc Res 45:747–755
Niedermuller H, Skalicky M, Hofecker G, Kment A (1977) Investigations on the kinetics of collagen metabolism in young and old rats. Exp Gerontol 21:159–168
Nissen R, Cardinale GJ, Undenfriend S (1978) Increased turnover of arterial collagen in hypertensive rats. Proc Natl Acad Sci USA 75:451–453
Papadaki M, Eskin SG (1997) Effects of fluid shear stress on gene regulation of vascular cells. Biotechnol Prog 13:209–221
Rachev A (2000) A model of arterial adaptation to alterations in blood flow. J Elast 61:83–111
Rachev A, Hayashi K (1999) Theoretical study of the effects of vascular smooth muscle contraction on strain and stress distributions in arteries. Ann Biomed Eng 27:459–468
Rachev A, Stergiopulos N, Meister J-J (1998) A model for geometric and mechanical adaptation of arteries to sustained hypertension. ASME J Biomech Eng 120:9–17
Rajagopal KR, Tao L (1995) Mechanics of mixtures. World Scientific, Singapore
Rao IJ, Humphrey JD, Rajagopal KR (2003) Growth and remodeling in a dynamically loaded uniaxial tissue. Comput Model Eng Sci 4:439–456
Resnick N, Gimbrone MA (1995) Hemodynamic forces are complex regulators of endothelial gene expression. FASEB J 9:874–882
Rodbard S (1975) Vascular caliber. Cardiol 60:4–49
Rodriguez EK, Hoger A, McCulloch AD (1994) Stress-dependent finite growth in soft elastic tissues. J Biomech 27:455–467
Rosen LA, Hollis TH, Sharma MG (1974) Alterations in bovine endothelial histidine decarboxylase activity following exposure to shear stress. Exp Mol Pathol 20:329–343
Roy P, Petroll WM, Chuong CJ, Cavanagh HD, Jester JV (1999) Effect of cell migration on the maintenance of tension on a collagen matrix. Ann Biomed Eng 27:721–730
Schretzenmayr A (1933) Uber kreislaufregulatorische Vorgange an den grossen Arterien bei der Muskelarbeit. Pflugers Arch Ges Physiol 232:743–748
Skalak R (1981) Growth as a finite displacement shield. In: Carlson DE, Shield RT (eds) Proceedings of the IUTAM Symposium on finite elasticity. Martinus Nijhoff, The Hague, pp 347–355
Stenmark KR, Mecham RP (1997) Cellular and molecular mechanisms of pulmonary vascular remodeling. Ann Rev Physiol 59:89–144
Stergiopulos N, Vulliemoz S, Rachev A, Meister J-J, Greenwald SE (2001) Assessing the homogeneity of the elastic properties and composition of the pig aortic media. J Vasc Res 38:237–246
Taber LA (1995) Biomechanics of growth, remodeling, and morphogenesis. Appl Mech Rev 48:487–545
Taber LA (1998) A model of aortic growth based on fluid shear and fiber stresses. ASME J Biomech Eng 120:348–354
Taber LA, Humphrey JD (2001) Stress modulated growth, residual stress, and vascular heterogeneity. ASME J Biomech Eng 123:528–535
Thoma R (1893) Untersuchagen über die histogenese und histomechanik des Gefassystems. Enke, Stuttgart
Tozeren A, Skalak R (1988) Interaction of stress and growth in fibrous tissue. J Theor Biol 130:337–350
Tronc F, Wassef M, Esposito B, Henrion D, Glagov S, Tedgui A (1996) Role of NO in flow-induced remodeling of the rabbit common carotid artery. Arterioscler Thromb Vasc Biol 16:1256–1262
Truesdell C, Noll W (1965) The nonlinear field theories of mechanics. In: Flugge S (ed) Handbuch der Physik, vol III:3. Springer-Verlag, Berlin Heidelber New York
von Maltzahn WW, Warriyar RG, Keitzer WF (1984) Experimental measurements of elastic properties of media and adventitia of bovine carotid arteries. J Biomech 17:839–847
Vossoughi J, Hedjazi Z, Borris FS (1993) Intimal residual stress and strain in large arteries. In: 1993 ASME Advances in bioengineering. Amercan Society of Mechanical Engineers, New York
Wilson E, Meininger GA (2002) Extracellular matrix changes and vascular smooth muscle signaling. In: Hagen T (ed) Mechanisms of cardiovascular aging 11:183–199
Zhao S, Suciu A, Ziegler T, Moore JE, Burki E, Meister J-J, Brunner HR (1995) Synergistic effects of fluid shear stress and cyclic circumferential stretch on vascular endothelial cell morphology and cytoskeleton. Arterioscler Thromb Vasc Biol 15:1781–1786
Zeller PJ, Skalak TC (1998) Contribution of individual structural components in determining the zero-stress state in small arteries. J Vasc Res 35:8–17
Acknowledgements
This work was supported, in part, by NSF grant BES-0084644 and NIH grants HL-64372 and HL-58856 (sub-contract from Duke University, M. Friedman, PI).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Humphrey, J.D., Rajagopal, K.R. A constrained mixture model for arterial adaptations to a sustained step change in blood flow. Biomech Model Mechanobiol 2, 109–126 (2003). https://doi.org/10.1007/s10237-003-0033-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-003-0033-4