Abstract
Human-induced shifts in species’ ranges can increase contact between closely related species and lead to reproductive interference. In Australia, climate change and trade in stingless bee colonies is increasing the range overlap of two cryptic species: Tetragonula carbonaria and T. hockingsi. To investigate reproductive interactions between these species, we validated a diagnostic-PCR test based on the mitochondrial gene COI to ID field specimens to species. We then assessed the likelihood of reproductive interference in four ways. First, we imaged the male genitalia of each species and found no evidence of reproductive character displacement. Second, we assessed species composition of mating aggregations in an area of sympatry (Southeast Queensland) and confirmed that some males join the mating aggregations of interspecific colonies. Third, we translocated T. hockingsi colonies into the southern range of T. carbonaria (Sydney) and tracked their ability to requeen. These translocated colonies attracted mating aggregations comprised almost entirely of interspecific males, but never formed hybrid colonies; instead, queens either mated with their brothers, or the colony failed to requeen at all. Finally, we presented T. carbonaria males with either conspecific or interspecific virgin queens and found that males attempted to mate only with their own species’ queens. In all, we conclude that reproductive barriers between these species are complete with respect to “short-range” mating cues, but not for “long-range” mate attraction cues. Our study highlights that hive movements can increase some forms of pre-mating reproductive interference between managed bee species, even where the species do not actually mate or hybridize.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Closely related species that previously diverged in allopatry will sometimes be brought back into contact due to changes in climate or habitat, or due to anthropogenic dispersal (Mallet et al. 2011; Sanchez-Guillen et al. 2013). Reproductive interference may then occur, with individuals engaging in interspecific reproductive activities that produce no offspring, sterile hybrids, or otherwise incur some fitness cost (Kyogoku 2020). Such interactions occur where reproductive barriers between species are incomplete and can be especially costly for species that mate only once (Gröning and Hochkirch 2008; Shuker and Burdfield-Steel 2017). With time, natural selection is expected to reinforce reproductive isolation between incompatible species, however reproductive interference can nevertheless cause population declines in the short-term and may even lead to local extinctions (Kishi et al. 2009; Kyogoku 2015; Ting and Cutter 2018).
Bees (Anthophila) play a crucial role as primary pollinators of plants in both natural and agricultural ecosystems (Ollerton et al. 2011). Managed social bees (i.e. those kept in hives) have a special role in agriculture, as they can be readily moved into and out of flowering crops in large numbers. This includes the stingless bees (Apidae; Meliponini), a tribe of highly eusocial bees naive to the subtropical and tropical regions of the world, including Asia, Africa, Australia and Central and South America (Gruter 2020). Stingless bees are not only important wild pollinators of native plants but have also emerged in recent decades as effective managed pollinators of a range of tropical crops (Meléndez Ramírez et al. 2018). They are further managed for hive products, including honey and propolis, and in some parts of the world are popular as pets (Gruter 2020; Heard 2016). As stingless bees across the world are increasingly utilized for crop pollination and other purposes, so too is the chance that their ranges are changed by anthropomorphic dispersal (Byatt et al. 2015).
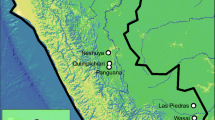
In Australia, two closely-related endemic species of stingless bees are widely propagated for both crop pollination and the pet trade: Tetragonula hockingsi and T. carbonaria (Heard 2016). Individual bees of these species are morphologically cryptic and visual species identification relies upon the architecture of their brood comb (Dollin et al. 1997; Franck et al. 2004). They are distributed along the Australian East Coast; with T. hockingsi occurring from Cape York to Southeast Queensland, and T. carbonaria occurring from Southeast Queensland to Sydney (Fig. 1). There are also some, presumably remnant, isolated populations of T. carbonaria confined to high altitude regions of North Queensland (Dollin et al. 1997). The precise natural distributions of both species are poorly resolved due to their cryptic morphology. It appears, however, that the trade of stingless bee colonies in recent decades is making T. hockingsi increasing prevalent in areas of Southeast Queensland once occupied either mostly or only by T. carbonaria (Cunningham et al. 2014). Ongoing modifications to habitat and climate in this part of Queensland might also be favouring the south-bound migration of T. hockingsi, leading to increased range overlap between the species (Fig. 1).
The approximate distributions of two morphologically cryptic stingless bees on Australia’s East Coast. The species can be identified via molecular methods or brood morphology: T. hockingsi (top, blue; clustered brood) and T. carbonaria (bottom, orange; spiral brood). The southward movement of T. hockingsi has created an area of overlap in Southeast Queensland (black box). We simulated further southward movement of T. hockingsi by translocating some colonies to Sydney (star)
Current evidence for reproductive interference between T. carbonaria and T. hockingsi is ambiguous. Some studies have suggested occasional hybridisation between T. carbonaria and T. hockingsi in an area of current sympatry in Southeast Queensland (Brito et al. 2014; Franck et al. 2004). More recently, a study of hived colonies from this region using a variety of genetic markers found no ongoing gene flow between the species, suggesting that any hybrid colonies may be F1 only, and fail to proceed to further generations (Hereward et al. 2020). Even if hybrids are never formed (that is, postzygotic barriers are complete), reproductive interference might still occur via interspecific mate attraction and/or mating (Fig. 2). In stingless bees, colonies with virgin queens that are ready to mate (requeening colonies) attract aggregations of dozens to hundreds of males that gather close to the entrance. The virgin queen then flies into the aggregation, mates with a single male and returns to the colony to begin egg-laying (Vollet-Neto et al. 2018). Thus, mate attraction in stingless bees occurs in two stages: “long-range” (males are attracted to requeening colonies and join mating aggregations) and “short-range” (males locate and attempt to mate with the queen once she enters the aggregation; Fig. 2). T. carbonaria males were reported to join the mating aggregation of an interspecific colony during a nest usurpation event (when one colony invades the nest of another; (Cunningham et al. 2014) but whether such mixed-species swarms occur regularly is unknown. Tetragonoula mate only once and workers never reproduce, even when queenless (Bueno et al. 2020; Gloag et al. 2007; Nunes et al. 2014). If interspecific matings occur but fail to produce viable offspring, then they may be costly not only to the individual male and queen, which have wasted their only mating opportunity, but also lead to colony death if subsequent virgin queens are not available.
Possible outcomes of reproductive interactions between stingless bee species or populations (depicted by different colours) that were previously isolated but are now sympatric. If mating barriers are complete, there will be no reproductive interference. Likewise, if there are neither mating barriers nor costs to hybridization then there will be no reproductive interference and then populations will re-establish gene flow. However, if there is hybrid incompatibility but pre-mating or mating barriers are incomplete, then reproductive interference will occur
In this study, we assessed whether reproductive interference occurs between T. carbonaria and T. hockingsi, with a focus on the consequences of T. hockingsi colonies continuing to move south into regions where only T. carbonaria currently occur naturally. Because the males of both species are morphologically identical, our first step was to establish a lab protocol for assigning males to species based on the mitochondrial gene cytochrome c oxidase subunit I (COI). We then assessed the likelihood of reproductive interference between the species in four ways. First, we compared the male genitalia of both species. This is because otherwise cryptic insect species may have diverged genital morphology, and such differences would suggest prezygotic barriers to mating (Masly 2012). Second, we manipulated colonies of known species to induce requeening and attract male congregations. In Southeast Queensland, where T. hockingsi has recently become common, we attracted male congregations in this way to colonies of both species and sampled males from these congregations to assess their species composition. Third, we repeated this manipulation with T. hockingsi colonies translocated further south into an area of current allopatry (Sydney) to determine whether these colonies attracted interspecific males and if hybridization occurs. Finally, we performed behavioural assays to test the attractiveness of T. hockingsi virgin queens to T. carbonraia males.
Materials and methods
A PCR diagnostic test for species identity
We first assessed the reliability of a diagnostic test that used differential amplification of the mitochondrial gene COI to assign samples to species. We extracted DNA by Chelex extraction (Walsh et al. 2013) from the abdomen of 46 workers from colonies of known species. We then designed sets of primers that targeted a species-specific ~ 300 bp region of COI. Each set paired one primer reported to amplify COI in both species in (Francoso et al. 2019) (Barhock) with one additional primer (Table 1). For each sample, we then performed two PCRs, visualised PCR products via gel electrophoresis and assigned species IDs to each sample based on which primer-set produced a band of the expected size. All T. carbonaria (N = 16) and most T. hockingsi (N = 28) in our reference set amplified only with the correct species primer pair. The remaining 7% of T. hockingsi (N = 2) amplified with both primer sets, though with a much stronger band for the T. hockingsi-specific primers.
Morphology of male genitalia
To determine whether any morphological differences in male genitalia exist between T. carbonaria and T. hockingsi, we dissected and imaged mature males collected from mating aggregations that had been stored at minus 20 °C on 100% ethanol (T. hockingsi N = 40 from Brisbane; T. carbonaria, N = 49 (N = 25 from Brisbane and N = 24 from Sydney). We first confirmed the species for each male by extracting DNA from the head and thorax (leaving the abdomen intact) and using the diagnostic COI PCR described above. We then dissected out the genitalia under a dissecting microscope with the use of Jewellers forceps (Dumont No.5) and imaged them using a ZEISS Stemi 508 stereomicroscope. We measured four characteristics from images of each specimen using Image J (Schneider et al. 2012): the gonocoxite width (mm), spathe width (mm), gonostylus length (left; mm) and penis valve length (left; mm) (Fig. 3A). During eversion Tetragonula males will open their gonostylus and penis valve and evert the penis into a queen’s reproductive tract. However, unlike honeybee males, who can be readily coerced into everting their endophallus via squeezing of the abdomen, we were unable to induce live Tetragonula males to do so prior to preservation. As a result, imaged samples were found to be at differing stages of eversion, and measurements of the penis itself were not possible. The most common eversion state across each of the three groups was “fully everted” and only fully everted samples were used for comparisons between species (Fig. 3B). We tested for differences in the morphology of male genitalia between species in two ways: (i) t-tests for each of the four variables, and (ii) a Principal Components Analysis (PCA) to identify relationships between the full variable set and species. All statistical analyses were performed in R 4.2.1 (R Core Team 2022).
A The genitalia of a T. carbonaria male, imaged using the ZEISS Stemi 508 stereomicroscope: (a) gonocoxite, (b) spathe, (c) gonostylus, (d) penis valve and (e) penis B The differing stages of Tetragonula eversion (left to right): not everted, semi-everted and fully everted. Only fully everted specimens were included in the analysis. C A PCA biplot showing the overlapping distributions measures of male genitalia between T. hockingsi (Brisbane) and T. carbonaria (triangles = Sydney, circles = Brisbane)
To visualise the finer features of Tetragonula genital morphology, we also generated images of one representative specimen of T. hockingsi (Brisbane) and T. carbonaria (Sydney) using scanning electron microscopy (SEM) at the Sydney Microscopy and Microanalysis Facility, The University of Sydney. Specimens for SEM were snap frozen at -80 °C, dissected and then placed in 1 ml of 0.1 M phosphate buffer before being transferred into the primary fixative (2.5% glutaraldehyde in 0.1 M phosphate buffer) and left to incubate at room temperature for one hour. We rinsed samples for 5 min in 0.1 M phosphate buffer and repeated this rinse three times in fresh changes of buffer. Samples were then dehydrated in the following way: 30% ethanol for five minutes repeated twice, 50% ethanol for five minutes repeated twice, 70% ethanol for five minutes repeated twice, 90% ethanol for five minutes repeated three times and 100% ethanol for five minutes repeated three times. The samples were then dehydrated and mounted onto an aluminium pin stub using double sided carbon tape, before being coated in 10 nm of gold using the ccu-010 Safematic compact coating unit. The samples were then visualised using a 15 kV accelerating voltage Jeol SEM.
Male attraction to interspecific requeening colonies in an area of sympatry
To determine if males join mating aggregations at interspecific colonies in an area of current sympatry, we analysed males sampled from mating aggregations formed in front of requeening colonies of each species in Southeast Queensland (total: N = 10 T. hockingsi, N = 6 T. carbonaria). Ten of these aggregations (all six T. carbonaria and N = 4 T. hockingsi) were collected in 2019 at sites in which no colonies of the other species were known to occur within close proximity (< 10 m) to our experimental colonies. At each site, colonies of known species kept in OATH hive boxes were induced to re-queen via hive splitting (Heard 2016). In this process, the hive is split in half and each section given an empty half-box. One half of the original colony is expected to retain the original queen, whilst the other half is forced to rear a new queen and thus attracts a male aggregation. For these aggregations, species IDs were completed using the PCR test described above. From these aggregations, as with initial trials with workers, a small number of males produced a band with both our T. carbonaria and T. hockingsi primers (N = 20 of 840). These samples were sequenced at a fragment of COI to confirm their identity and were revealed to be all T. hockingsi. For this we used PCR primers and conditions that amplified both species following (Francoso et al. 2019), with Sanger sequencing performed at Macrogen Inc., South Korea. The other six T. hockingsi aggregations were collected in 2022 by beekeepers in the Brisbane area and sent to us. These aggregations were active at the front of known T. hockingsi colonies. Males from these six aggregations were ID-ed to species directly via sequencing of COI (N = 312 males).
Tetragonula hockingsi colonies translocated south: Male attraction and requeening success
To simulate the ongoing southward movement of T. hockingsi into the range of T. carbonaria, we translocated eight T. hockingsi colonies to Sydney (33.8688° S, 151.2093° E, a region where currently only T. carbonaria occur; Fig. 1) and then monitored whether these colonies: (i) attracted local T. carbonaria males during requeening, and (ii) successfully requeened. Where our translocated T. hockingsi colonies did succeed in requeening, we then assessed whether queens had mated with T. carbonaria males to form F1 hybrids.
Translocations occurred in two batches: spring 2020 (N = 4) and spring–summer 2019 (N = 4). All colonies were kept inside of wooden OATH hives (Heard 2016). Colonies of the same batch were maintained at sites in Sydney at least 5 km from each other. Although Tetragonula males are capable of dispersing as much as 20 km from their natal nests to find mating aggregations, average male dispersal distances have been estimated at 2-3 km (Bueno et al. 2022a, b; Bueno, et al. 2023) making it unlikely that our test colonies would source mates from each other. We split each of the hives to stimulate requeening. Both halves of a split colony were then maintained at the same site at 2 m from each other. We then observed the colonies every second day over a period of six weeks and sampled any males that aggregated near the colonies. Males of these aggregations were predicted to comprise largely T. carbonaria (the only locally occurring species), however, male species ID was confirmed via diagnostic PCR assay as outlined above.
To determine the requeening success of T. hockingsi colonies translocated to Sydney, we inspected brood comb at 6 weeks post-split (16 colonies; 8 pairs). If the colonies contained no eggs or young larva, we recorded the colony as having failed to requeen. If eggs and young larva were present, we collected brood and mature foragers for genotyping. Since the life span of Tetragonula from egg to adult emergence is approximately 50 days (Heard 2016), any brood in requeened colonies would be the offspring of the new queen, whilst the foragers would carry the old queen’s genotype. We genotyped samples at eight microsatellite loci: Tc3.155, Tc4.302, Tc4.214, Tc4.287, Tc4.63 (Green et al. 2001), Tc7.13, Tang60 and Tang70 (Brito et al. 2009)PCR products were analysed on a 3130xl Genetic Analyser (Life Technologies, USA). For each colony, we determined whether genotypes were consistent with a colony having retained its original queen (predicted for one half of each split-pair) or requeened (predicted for one half of each split-pair) and in the latter case, whether the inferred genotype of the queen’s mate carried local Sydney T. carbonaria alleles (i.e. whether the colony was a putative hybrid).
Behavioural assays of T. carbonaria males
To further determine whether T. carbonaria males attempt to mate with T. hockingsi queens (i.e. short-range mate attraction), we performed behavioural assays in which virgin queens were presented to mature T. carbonaria males. To acquire sexually mature virgin queens, we collected 18 queen cells from T. hockingsi colonies of Brisbane origin (1–2 cells per colony, N = 13 colonies) and 42 queen cells from T. carbonaria colonies of Sydney origin (1–2 cells per colony, 35 colonies; summers 2020 and 2021). We collected fewer T. hockingsi than T. carbonaria queen cells due to the greater challenge of extracting undamaged cells from the clumped brood comb of T. hockingsi (relative to the spiral brood of T. carbonaria; Fig. 1). From each colony, we also collected worker brood cells (between 30–50 cells) and 20 mature adult workers. We placed the queen cell, brood and adults into 10 × 10 cm petri dishes along with an Eppendorf-cap filled with pollen, and approximately 1 ml of Tetragonula honey. We then monitored these colonies daily for queen hatchings. In total, eight T. hockingsi queens and 14 T. carbonaria queens hatched and survived until sexual maturity (8–20 days old; Bueno et al. 2022a, b).
We first used a petri dish assay for all mature queens, whereby we placed the queen into a clean petri dish (10 × 10 cm) together with 10 mature T. carbonaria males collected from local Sydney male aggregations and recorded the number of times males attempted to mount the queen’s abdomen during a 10 min observation period. Each set of 10 males was used for one assay only. We then compared the number of attempted mounts made by T. carbonaria males during these assays for conspecific (T. carbonaria, N = 14) versus interspecific (T. hockingsi, N = 8) virgin queens. Because Tetragonula naturally mate during flight in mating aggregations, we also validated our petri dish assays for a subset of mature queens with an in-swarm assay (N = 11 T. carbonaria and N = 3 T. hockingsi; summer 2021). For this assay, we inserted queens’ head and thorax into the tips of 3 mL plastic pipettes, such that they were restrained but their abdomens were exposed, and then presented them to aerial male aggregations. We then recorded the number of males that landed on and attempted to mount the queen during a 30 s interval.
Results
Morphology of male genitalia
Neither light microscopy nor SEM imagery revealed differences in male genitalia between Tetragonula species that would be likely to prevent interspecific mating (Figs. 3, 4). We cannot rule out interspecific differences in the morphology of the penis itself, as this could not be well characterized in our preserved specimens, though it appeared to be simple in shape in both species (Figs. 3, 4). Length of the gonostylus and width of the gonocoxite (mm) were greater on average in T. hockingsi than T. carbonaria (gonostylus: 0.44 mm and 0.41 mm respectively, t = 4.3, df = 76, p < 0.05; gonocoxite: 0.45 mm and 0.41 mm respectively, t = 5.3, df = 76, p < 0.05) but the size distributions of all measured genitalia components overlapped for each species (Supplementary Figure S1). Likewise, a PCA identified three principal components accounting for 90% of the total variance between species, with a biplot of PC1 (47% variance) and PC2 (23% variance) showing that samples cluster broadly by species but that species overlap in variable space (Fig. 3C).
The common structural features of the reproductive anatomy of T. carbonaria (left: A, C, E) and T. hockingsi (right: B, D, F). Images A + B depict the overall endophallus of each species. Images C + D show the distortion of the penis as a result of the dehydration process. Images E + F show the curved penis valve and gonostylus with hairs
Male attraction to interspecific requeening colonies in Southeast Queensland
Mixed species male aggregations were identified at requeening colonies of both T. hockingsi and T. carbonaria in South-East Queensland, though the majority of males in all cases were conspecifics. Of 11 male aggregations that formed at T. hockingsi colonies, six contained a small proportion of interspecific males (2–9% of T. carbonaria males per aggregation; N = 16 of 581 males total); while one of six male aggregations that formed at T. carbonaria colonies contained interspecific males (12% T. hockingsi; N = 11 of 576 males total; Fig. 5).
The proportion of conspecific (light grey) and heterospecific (dark grey) Tetragonula males in mating aggregations at requeening colonies of T. carbonaria (n = 6) and T. hockingsi (n = 10) in Southeast Queensland, and T. hockingsi translocated south to Sydney (n = 4). Number of males identified to species per mating aggregation (N) are given beside bars
Transplanted T. hockingsi colonies: male attraction and requeening success
Of the eight T. hockingsi colonies which were translocated from Southeast Queensland to Sydney and forced to requeen, five did not attract male aggregations large enough to sample during the six-week post-hive-split observation period. The remaining three attracted male aggregations within one week of the hive split, and one of these (Colony 8a) subsequently attracted a second male aggregation four months later. All four aggregations were comprised predominantly of T. carbonaria males (85–100%), with the remaining males being T. hockingsi (0–15%; Fig. 5). In each case where T. hockingsi males were present in the aggregations, they had genotypes consistent with being from the colony hosting the aggregation; that is, they were aggregating in front of their own natal colony.
Brood inspections of translocated T. hockingsi colonies at 8-weeks post-hive-split indicated that one half of each split retained the original queen in all cases. For the other halves of each colony-pair, five had successfully requeened in Sydney, while three had failed to requeen and were hopelessly queenless (no eggs, larva or queen cells). For all five of the colonies that successfully requeened, brood were consistent with the new T. hockingsi queen having mated with a brother (Supplementary Table S1). The genotype of brood from Colony 8a, which attracted two male aggregations several months apart, did not change following the second aggregation. However, we by chance sampled a T. hockingsi virgin queen flying in the second aggregation so we conclude that the colony did requeen at this time and that the new queen and her brother-mate’s genotypes matched those of the previous queen.
Behavioural assays of T. carbonaria males
T. carbonaria males presented with T. hockingsi virgin queens in petri dishes never attempted to mount queens in the mating position during the assay period (N = 8 queens assays, 10 males per assay). In contrast, T. carbonaria males attempted to mount T. carbonaria virgin queens one or more times in all assays (N = 14 assays; average = 8.6, S.E.M. = 2.6 and range = 1–21 mating attempts per assay); thus T. carbonaria males were significantly more likely to attempt mating with conspecific than heterospecific queens (Fishers Exact Test: p < 0.001). These assay results were consistent with those in which queens were presented to natural T. carbonaria male swarms, with all T. carbonaria queens attracting > 10 males within 30 s, mostly with dozens of males piling rapidly onto the queen (N = 11 queens). In contrast, T. hockingsi queens presented to real aggregations in this way failed to attract a single T. carbonaria male (N = 3 queens). Indeed, even when T. carbonaria males from these swarms were caught in the hand and placed directly onto the abdomen of a constrained T. hockingsi queen, they flew off again, sometimes after a few seconds of antennal contact with the queen’s body.
Discussion
Human activities can lead to secondary contact zones for related species, increasing the opportunity for reproductive interference between them (Kyogoku 2020). Across the tropics and subtropics, the increasing use of stingless bees in agriculture has made them more likely to be subject to regular movements and thus artificial range shifts (Jaffe et al. 2016). Here we confirm that males of the Australian stingless bees T. carbonaria and T. hockingsi can be attracted to requeening colonies of the other species, indicating incomplete divergence in the long-range male attraction signals (or cues) of each species. We find no evidence, however, that the continued movement south of T. hockingsi into regions where currently only T. carbonaria occurs will result in interspecific hybrids. Rather, we show that colonies of T. hockingsi translocated south of their current range either requeened via inbreeding, or failed to requeen at all, despite some attracting large mating aggregations of interspecific (T. carbonaria) males. Consistent with this, T. carbonaria males presented with virgin queens of both species attempted to mate only with conspecific queens and ignored potential heterospecific mates. In these bees, therefore, reproductive interference appears to occur at pre-mating stages, via long-range attraction of males to interspecific colonies, while actual mating or hybridization between the species occurs either rarely (Brito et al. 2014) or not at all.
How might long-range male attraction cues in stingless bees differ from those used in short-range mate recognition? At short-range, males are responding solely to the odours of the queen herself (Verdugo-Dardon et al. 2011). The bouquet of chemicals which attract male stingless bees to mating aggregations, however, is unknown. It is assumed to include pheromones produced by a virgin queen, but it might also comprise odours of the nest itself (e.g. propolis and resins), or the odours and scent markings left by other males (Bueno et al. 2023; Fierro et al. 2011). Given that virgin queens produce complex mixtures of volatiles, it is also possible that some have functions for long-range male attraction and others for short range mate recognition (Engels et al. 1997). In our assays, we observed T. carbonaria males from Sydney (a population naïve to contact with T. hockingsi) aggregate at translocated colonies of T. hockingsi yet show no interest in T. hockingsi virgin queens at close range. This supports the idea that the virgin queen pheromones used by males to locate queens within a mating swarm are not the only component of long-range mate attraction cues in these bees, and that other attractants are also involved. The presence of occasional interspecific males in mating aggregations has been reported in several stingless bees, usually where species are kept together in bee yards (meliponaries) (Bänziger and Khamyotchai 2014; Dos Santos et al. 2015; Kerr et al. 1962; Santos et al. 2014). Further knowledge of the composition of long-range odour cues in stingless bees are needed to understand how and when they overlap between species.
Male Tetragonula that aggregate at interspecific colonies waste their opportunity to reproduce. This is because males typically travel many kilometres in search of a mating aggregation and are unlikely to encounter more than one in their lifetime (Bänziger and Khamyotchai 2014; Bueno, Bueno, et al. 2022a, b; dos Santos et al. 2016). We therefore expect strong selection against males that join interspecific aggregations, such that populations will have lower rates of this “aggregation error” the longer they have co-occurred with related species (Kyogoku 2020). While we observed large male aggregations form at interspecific colonies in Sydney (where T. carbonaria currently occur and T. hockingsi had been experimentally translocated) only a small minority of males made aggregation errors in Southeast Queensland where they are now sympatric (around half of all aggregations contained 2–15% heterospecific males). Thus differences in the rate of male long-range error between Sydney and Southeast Queensland are consistent with the expectations of natural selection driving increasing reproductive isolation under sympatry (Kyogoku 2020).
If T. hockingsi queens cannot produce hybrid offspring with T. carbonaria males, then the fate of lone T. hockingsi colonies brought outside of their natural range is to either fail or inbreed. In this study, all T. hockingsi colonies which successfully requeened in Sydney (N = 5), were determined to have mated with their brother. Inbreeding is particularly detrimental for Hymenoptera as a consequence of their sex determination system (van Wilgenburg et al. 2006). Hymenopteran females are diploid while males are haploid. Sex in diploid embryos is ultimately determined however by zygosity at a ‘sex locus’ (or sex loci), whereby heterozygous individuals develop as females but homozygous individuals develop as diploid males that are typically inviable or sterile (Cook and Crozier 1995). Homozygosity increases when closely related individuals mate, thus increasing the chance of diploid males. Stingless bees rely on behavioural mechanisms to avoid inbreeding. For example, males will tend to avoid joining mating aggregations adjacent to their parent colony (Bueno et al. 2022a, b; Cameron et al. 2004). This decreases the probability of virgin queens meeting brothers during mating flights. Nevertheless, the incidence of inbreeding by T. hockingsi brought to Sydney shows that inbreeding can occur when other mate options are unavailable. Interestingly, we observed no excess of males among the brood of our five inbred T. hockingsi, indicating none were producing diploid males. Assuming a single locus controlling sex, Tetragonula queens should have a 50% chance of sharing a sex allele with their brother. One explanation for the absence of diploid-male in these inbred colonies is that Tetragonula workers kill queens producing diploid males, as occurs in some other stingless bees (Vollet-Neto et al. 2017); that is, only the 50% of brother-matings that produce healthy female brood may be tolerated by workers.
Male genitalia can be among the first things to diverge in otherwise cryptic species (Masly 2012). Sympatric honey bees (Apis sp) in Asia have evolved famously divergent morphologies of the male endophallus (Koeniger and Koeniger 2000). Here however, we observed no differences in the male genitalia of T. carbonaria and T. hockingsi that would suggest reproductive character displacement, aligning with Dollin’s (1997) species description. Mean sizes of some genitalia measures were marginally greater in T. hockingsi than T. carbonaria, consistent with differences in mean male body length between the species at our sample locations (T. carbonaria: 3.8–4.2 mm, T. hockingsi: 4.0–4.4 mm; Dollin et al. 1997). Reproductive interference is proposed to drive body size divergence in some insects, because males and females that differ greatly in size can less readily mate (Okuzaki et al. 2010), but the size discrepancies in Tetragonula are presumably too marginal to affect mating. We conclude therefore that either the male genitalia played no role in the evolution of reproductive barriers in these Tetragonula, or subtle differences exist between species in the morphology of the penis soft tissue that were not detectable in our preserved specimens. SEM images did highlight aspects of Tetragonula male genitalia that likely contribute to single mating by queens in these species (Smith 2019). In particular, the penis valve and gonostylus of the male are curved in the everted state, and the gonostylus is tipped with hairs, which may delay removal by other males and/or the queen.
Range shifts have occurred in various managed social bees in the past century as a result of their use in agriculture. Most of these involve invasive bee populations established outside of their natural range (e.g. Bombus terrestris, Velthuis and van Doorn 2006; Apis mellifera, Carpenter and Harpur 2021; Apis cerana, Sun et al. 2022). Interspecific mating leading to inviable brood has been reported for A. mellifera and A. cerana in artificial sympatry in China and Australia (Remnant et al. 2014), but the risk of reproductive interference is poorly known in most cases of range shifts (Byatt et al. 2015). Our study is a reminder that reproductive interactions in social bees can occur at multiple stages, and that the absence of mating or hybrids may not equate to a lack of reproductive interaction. Misdirected long-range male attraction is the mildest form of interference with respect to population stability because it affects only males and not the fitness or survival of queens on which the colony relies. Nevertheless, even mild forms of interference highlight that human-induced range shifts can shape the evolution of reproductive characters in managed species as a result of secondary contact with congeners. We propose that knowledge of the reproductive interactions between species and populations is key to ensuring that trade practices for stingless bees can best balance economic benefit with any potential ecological costs.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Bänziger H, Khamyotchai K (2014) An unusually large and persistent male swarm of the stingless bee Tetragonula laeviceps in Thailand (Hymenoptera: Apidae: Meliponini). J Melittol. https://doi.org/10.17161/jom.v0i32.4708
Brito RM, Francisco FO, Domingues-Yamada AMT, Gonçalves PHP, Pioker FC, Soares AEE, Arias MC (2009) Characterization of microsatellite loci of Tetragonisca angustula (Hymenoptera, Apidae, Meliponini). Conserv Genet Resour 1:183–187. https://doi.org/10.1007/s12686-009-9045-4
Brito RM, Francisco FO, Ho SYW, Oldroyd BP (2014) Genetic architecture of the Tetragonula carbonaria species complex of Australian stingless bees (Hymenoptera: Apidae: Meliponini). Biol J Lin Soc 113:149–161. https://doi.org/10.1111/bij.12292
Bueno FGB, Gloag R, Latty T, Ronai I (2020) Irreversible sterility of workers and high-volume egg production by queens in the stingless bee Tetragonula carbonaria. J Exper Biol. https://doi.org/10.1242/jeb.230599
Bueno FGB, Bueno BGB, Buchmann G, Heard T, Latty T, Oldroyd BP, Hosoi AE, Gloag R (2022a). Males are capable of long-distance dispersal in a social bee. Front Ecol Evol. https://doi.org/10.3389/fevo.2022.843156
Bueno FGB, Hajjar R, Colin T, Buchmann G, Gillard TL, Latty T, Gloag R (2022b) Virgin queen behaviour and mating in the stingless bee Tetragonula carbonaria (Meliponini). Insectes Soc. https://doi.org/10.1007/s00040-022-00887-z
Bueno FGB, dos Santos CF, Otesbelgue A, Menezes C, van Veen J, Blochtein B, Gloag R, Heard T, Imperatriz-Fonseca VL, Alves DA (2023) The queens of the stingless bees: from egg to adult. Insect Soc. https://doi.org/10.1007/s00040-022-00894-0
Byatt MA, Chapman NC, Latty T, Oldroyd BP (2015) The genetic consequences of the anthropogenic movement of social bees. Insectes Soc 63:15–24. https://doi.org/10.1007/s00040-015-0441-3
Cameron EC, Franck P, Oldroyd BP (2004) Genetic structure of nest aggregations and drone congregations of the southeast Asian stingless bee Trigona collina. Mol Ecol 13:2357–2364. https://doi.org/10.1111/j.1365-294X.2004.02194.x
Carpenter MH, Harpur BA (2021) Genetic past, present, and future of the honey bee (Apis mellifera) in the United States of America. Apidologie 52:63–79. https://doi.org/10.1007/s13592-020-00836-4
Cook JM, Crozier RH (1995) Sex determination and population biology in the hymenoptera. Trends Ecol Evol 10:281–286
Cunningham JP, Hereward JP, Heard TA, De Barro PJ, West SA (2014) Bees at war: interspecific battles and nest usurpation in stingless bees. Am Nat 184:777–786. https://doi.org/10.1086/678399
Dollin AE, Dollin LJ, Sakagami tlSF. (1997) Australian stingless bees of the genus Trigona (Hymenoptera: Apidae). Invertebr Syst 11:861–896. https://doi.org/10.1071/it96020
Dos Santos CF, Ferreira-Caliman MJ, Nascimento FS (2015) An alien in the group: eusocial male bees sharing nonspecific reproductive aggregations. J Insect Sci 15:157
dos Santos CF, Imperatriz-Fonseca VL, Arias MC (2016) Relatedness and dispersal distance of eusocial bee males on mating swarms. Entomol Sci 19:245–254. https://doi.org/10.1111/ens.12195
Engels W, Engels E, Francke W (1997) Ontogeny of cephalic volatile patterns in queens and mating biology of the neotropical stingless bee, Scaptotrigona postica. Invertebr Reprod Dev 31:251–256. https://doi.org/10.1080/07924259.1997.9672583
Fierro MM, Cruz-Lopez L, Sanchez D, Villanueva-Gutierrez R, Vandame R (2011) Queen volatiles as a modulator of Tetragonisca angustula drone behavior. J Chem Ecol 37:1255–1262. https://doi.org/10.1007/s10886-011-0034-1
Franck P, Cameron E, Good G, Rasplus JY, Oldroyd BPO (2004) Nest architecture and genetic differentiation in a species complex of Australian stingless bees. Mol Ecol 13:2317–2331
Francoso E, Zuntini AR, Ricardo PC, Silva JPN, Brito R, Oldroyd BP, Arias MC (2019) Conserved numts mask a highly divergent mitochondrial-COI gene in a species complex of Australian stingless bees Tetragonula (Hymenoptera: Apidae). Mitochondrial DNA A 30:806–817. https://doi.org/10.1080/24701394.2019.1665036
Gloag RS, Beekman M, Heard TA, Oldroyd BP (2007) No worker reproduction in the Australian stingless bee Trigona carbonaria Smith (Hymenoptera, Apidae). Insectes Soc 54:412–417. https://doi.org/10.1007/s00040-007-0961-6
Green CL, Franck P, Oldroyd BP (2001) Characterization of microsatellite loci for Trigona carbonaria, a stingless bee endemic to Australia. Mol Ecol Notes 1:89–92
Gröning J, Hochkirch A (2008) Reproductive interference between animal species. Q R Biol 83:257–282
Gruter C (2020) Stingless bees: their behaviour, ecology and evolution. Spinger, New York
Heard T (2016) The Australian native bee book: keeping stingless bee hives for pets, pollination and sugarbag honey. Sugarbag Bees, Brisbane
Hereward JP, Smith TJ, Gloag R, Brookes DR, Walter GH (2020) Tests of hybridisation in Tetragonula stingless bees using multiple genetic markers. bioRxiv. https://doi.org/10.1101/2020.03.08.982546
Jaffe R, Pope N, Acosta AL, Alves DA, Arias MC, De la Rua P, Francisco FO, Giannini TC, Gonzalez-Chaves A, Imperatriz-Fonseca VL, Tavares MG, Jha S, Carvalheiro LG (2016) Beekeeping practices and geographic distance, not land use, drive gene flow across tropical bees. Mol Ecol 25:5345–5358. https://doi.org/10.1111/mec.13852
Kerr WE, Zucchi R, Nakadaira JT, Butolo JE (1962) Reproduction in the social bees (Hymenoptera: Apidae). J N York Entomol Soc 70:265–276
Kishi S, Nishida T, Tsubaki Y (2009) Reproductive interference determines persistence and exclusion in species interactions. J Anim Ecol 78:1043–1049. https://doi.org/10.1111/j.1365-2656.2009.01560.x
Koeniger N, Koeniger G (2000) Reproductive isolation among species of the genus Apis. Apidologie 31:313–339
Kyogoku D (2015) Reproductive interference: ecological and evolutionary consequences of interspecific promiscuity. Popul Ecol 57:253–260. https://doi.org/10.1007/s10144-015-0486-1
Kyogoku D (2020) When does reproductive interference occur? Predictions and data. Popul Ecol 62:196–206. https://doi.org/10.1002/1438-390x.12041
Mallet J, Wynne IR, Thomas CD (2011) Hybridisation and climate change: brown argus butterflies in Britain (Polyommatus subgenus Aricia). Insect Conserv Divers 4:192–199. https://doi.org/10.1111/j.1752-4598.2010.00122.x
Masly JP (2012) 170 years of “lock-and-key”: genital morphology and reproductive isolation. Int J Evol Biol. https://doi.org/10.1155/2012/247352
Meléndez Ramírez V, Ayala R, Delfín GH (2018) Crop pollination by stingless bees. In: Vit P, Pedro S, Roubik D (eds) Pot-pollen in stingless bee melittology. Springer, New York
Nunes TM, Heard TA, Venturieri G, Oldroyd BP (2014) Emergency queens in Tetragonula carbonaria (Smith ,1854) (Hymenoptera, Apidae, Meliponini). Austral Entomol. https://doi.org/10.1111/aen.12104.
Okuzaki Y, Takami Y, Sota T (2010) Resource partitioning or reproductive isolation: the ecological role of body size differences among closely related species in sympatry. J Anim Ecol 79:383–392. https://doi.org/10.1111/j.1365-2656.2009.01645.x
Ollerton J, Winfree R, Tarrant S (2011) How many flowering plants are pollinated by animals? Oikos 120:321–326. https://doi.org/10.1111/j.1600-0706.2010.18644.x
R Core Team (2022) R: a language and environment for statistical computing. . In R Foundation for Statistical Computing. https://www.R-project.org/.
Remnant EJ, Koetz A, Tan K, Hinson E, Beekman M, Oldroyd BP (2014) Reproductive interference between honeybee species in artificial sympatry. Mol Ecol 23:1096–1107. https://doi.org/10.1111/mec.12669
Sanchez-Guillen RA, Munoz J, Rodriguez-Tapia G, Feria Arroyo TP, Cordoba-Aguilar A (2013) Climate-induced range shifts and possible hybridisation consequences in insects. PLoS ONE 8:e80531. https://doi.org/10.1371/journal.pone.0080531
Santos CFD, Menezes C, Vollet-Neto A, Imperatriz-Fonseca VL. (2014). Congregation sites and sleeping roost of male stingless bees (Hymenoptera: Apidae: Meliponini). Sociobiology. https://doi.org/10.13102/sociobiology.v61i1.115-118
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Shuker DM, Burdfield-Steel ER (2017) Reproductive interference in insects. Ecol Entomol 42:65–75. https://doi.org/10.1111/een.12450
Smith TJ (2019) Evidence for male genitalia detachment and female mate choice in the Australian stingless bee Tetragonula carbonaria. Insectes Soc 67:189–193. https://doi.org/10.1007/s00040-019-00744-6
Sun Z, Chapman NC, Raffiudin R, Putra RE, Roberts J, Widjaya C, Buchmann G, Holmes M, Gloag R (2022) Loss of mitochondrial diversity in invasive populations of Asian honey bees, Apis cerana (Hymenoptera: Apidae), in the Austral-Pacific. Austral Entomol 61:97–103. https://doi.org/10.1111/aen.12587
Ting JJ, Cutter AD (2018) Demographic consequences of reproductive interference in multi-species communities. BMC Ecol 18:46. https://doi.org/10.1186/s12898-018-0201-0
van Wilgenburg E, Driessen G, Beukeboom LW (2006) Single locus complementary sex determination in Hymenoptera: an “unintelligent” design? Front Zool 3:1. https://doi.org/10.1186/1742-9994-3-1
Velthuis HHW, van Doorn A (2006) A century of advances in bumblebee domestication and the economic and environmental aspects of its commercialization for pollination. Apidologie 37:421–451. https://doi.org/10.1051/apido:2006019
Verdugo-Dardon M, Cruz-Lopez L, Malo EA, Rojas JC, Guzman-Diaz M (2011) Olfactory attraction of Scaptotrigona mexicana drones to their virgin queen volatiles. Apidologie 42:543–550. https://doi.org/10.1007/s13592-011-0042-8
Vollet-Neto A, Oliveira RC, Schillewaert S, Alves DA, Wenseleers T, Nascimento FS, Imperatriz-Fonseca VL, Ratnieks FLW (2017) Diploid male production results in queen death in the stingless bee Scaptotrigona depilis. J Chem Ecol 43:403–410. https://doi.org/10.1007/s10886-017-0839-7
Vollet-Neto A, Koffler S, dos Santos CF, Menezes C, Nunes FMF, Hartfelder K, Imperatriz-Fonseca VL, Alves DA (2018) Recent advances in reproductive biology of stingless bees. Insectes Soc 65:201–212. https://doi.org/10.1007/s00040-018-0607-x
Walsh PS, Metzger DA, Higuchi R (2013) Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 54:134–139. https://doi.org/10.2144/000114018
Acknolwedgements
The authors acknowledge the instruments and expertise of Microscopy Australia at the Sydney Microscopy & Microanalysis Facility, University of Sydney, enabled by NCRIS, university, and state government support. We thank Aislinn Spencer and Brian Cutting for assistance in collecting samples in Brisbane. We thank Angela Crean for feedback on an early version of the study. We thank Ku-ring-gai Council Wild Things program and Alex Austin for providing T. carbonaria queen cells to rear queens for behavioural assays.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. R.G. is supported by an Australian Research Council Award (DE220100466).
Author information
Authors and Affiliations
Contributions
GP, LB, NC and RG conceived the study design. Morphological and behavioural analyses were performed by GP and RG. Field data collection and molecular analysis were performed by GP, LB, RG, FGBB, GL, TH, GB and JL. The manuscript was written by RG and GP. All authors commented on draft versions of the manuscript and read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Paul, G., Bartels, L., Bueno, F.G.B. et al. Shifting range in a stingless bee leads to pre-mating reproductive interference between species. Conserv Genet 24, 449–459 (2023). https://doi.org/10.1007/s10592-023-01512-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-023-01512-7