Abstract
In this study, different weight percentages of Mg(NO3)2 were added to a polymer blend composed of chitosan (CS) and methylcellulose (MC). The solution casting method was adopted to prepare the polymer electrolyte films. As the host matrix, the polymer blend consists of 70 wt.% of CS and 30 wt.% of MC. The interactions between the blend and the Mg(NO3)2 salt were revealed by FTIR analysis. The deconvolution of XRD peaks followed by the calculation of crystallinity indicates that the electrolyte samples were amorphous. The bulk resistance decreased with increasing salt content, according to the results of electrical impedance spectroscopy (EIS), and this was caused by the influence of carrier concentration. The sample containing 30 wt.% of Mg(NO3)2 was determined to have a maximum value of DC conductivity of 2.12 × 10–5 Scm−1 at room temperature by EIS analysis. The highest conducting film’s potential stability was found to be 3.65 V. It was found that the ion transference number was 0.86. The performance of the magnesium ion battery was then evaluated after it was built using the highest conducting polymer electrolyte. Considering this, the current work proposes an eco-friendly, practical, and affordable electrolyte appropriate for making electrochemical devices.
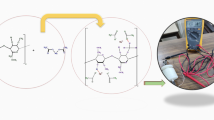
Graphical abstract (made with biorender.com)

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Electronic appliances such as smartphones, tablets, computers, and other similar devices have become ubiquitous household items, resulting in an increase in demand year after year. Almost all portable electronic devices and electric vehicles contain an energy storage system. Batteries and capacitors are the two main types of energy storage devices. The battery is made up of an anode, a cathode, and an electrolyte. The electrolyte helps transfer ions between the electrodes. A solid-state energy storage device can be constructed by inserting the solid polymer electrolyte between the cathode and anode. The solid polymer electrolyte thus serves as both an electrolyte and a separator (Wang et al. 2017). The advantages of solid polymer electrolytes include their non-volatility, leak-proof nature, wide electrochemical stability window (ESW), and ease of preparation (Khiar et al. 2018).
While lithium batteries can create short circuits due to dendrite formation, magnesium can be electrodeposited without any dendrite growth (Manjuladevi et al. 2018a, b). Compared to lithium, magnesium is a better choice for use in batteries due to its advantages, such as being less expensive, more prevalent, having a higher energy density, being considerably safer, and being easier to prepare electrodes in an atmosphere filled with oxygen (Manjuladevi et al. 2018a, b). Because of the innate benefits of magnesium metal, magnesium batteries have been proposed as a substitute for next-generation batteries. Properties such as superior ionic conductivity, high ionic transference number, a wide operating temperature range, etc., are desirable in polymer electrolytes for energy storage device applications (Mazuki et al. 2018). Unique properties of biopolymers include their low cost, renewable nature, compatibility with a wide range of solvents, abundance, excellent film formation, and biodegradability. The polymer blend technique can be used to produce a polymer blend with additional sites for ionic conduction (Aziz et al. 2017). Compared to using single polymers, blending polymers is the most effective way to increase ionic conductivity. By blending techniques we can upgrade the properties of individual polymer constituents (Aziz et al. 2020c). The primary benefit of polymer blends is easy control of physical properties through compositional changes. Blending is also one of the most efficient ways to enhance amorphous content while reducing crystalline content, which is impossible to achieve when utilizing single polymers (Aziz et al. 2020a, b, c; Kesavan et al. 2015). Salman et al. (2018) prepared a polymer electrolyte of CS:MC by incorporating lithium tetrafluoroborate and reported a room temperature conductivity value of 3.74 \(\times\) 10–6 Scm−1. Abdullah et al. (2021b) synthesized a polyblend electrolyte of CS:MC by adding lithium iodide (LiI) as salt and got an ionic conductivity value of 6.26 \(\times\) 10–6 Scm−1 for 40 wt.% of LiI salt. Even though Li-salt is thought to be the finest salt for creating polymer-salt complex electrolytes, it has a lot of disadvantages and is only found in small amounts in nature. To find an alternative salt with equivalent electrochemical characteristics, magnesium has been identified as a potential candidate. Researchers have worked on single as well as blended polymers by doping magnesium salts to get polymer electrolyte films. Polu et al. (2013) prepared a polymer electrolyte of poly(vinyl alcohol) (PVA) with Mg(NO3)2 as salt and obtained an ionic conductivity value of 7.36 \(\times\) 10–7 Scm−1. Manjuladevi et al. (2018a) have synthesized a blend polymer electrolyte by doping 0.3 m.m.% Mg(NO3)2 with PVA:poly(acrylonitrile) (PAN) which exhibited an ionic conductivity of 1.71 \(\times\) 10–3 Scm−1. Numerous blend electrolytes based on PVA:poly(vinyl pyrrolidone) (PVP), PVA:poly(ethylene glycol) (PEG), and poly(vinylidene fluoride) (PVDF):PVA have also been reported. These blends are made of synthetic polymers, which are detrimental to the environment because they cannot be decomposed easily.
This study is to develop potential magnesium ion conducting solid biodegradable blend polymer electrolytes (SBPEs) for battery applications that contain chitosan (CS) and methylcellulose (MC) as individual polymers. To solvate cations, the polymer used as the matrix needs to have polar groups in its backbone (Aziz et al. 2020b). MC has lone pair electron-containing functional groups such as OH, glycosidic bond, and methoxy groups (Nadirah et al. 2020), whereas CS has functional groups such as amine, OH, and glycosidic (C–O–C) bond groups for ion conduction.
The SBPE system CS:MC has received much attention recently due to its exceptional qualities such as abundance, non-toxicity, environmental friendliness, and strong film-forming ability (Dannoun et al. 2020; Abdullah et al. 2019; Aziz et al. 2021). Ionic conductivity of 3.74 × 10–6 Scm−1 was obtained by Salman et al. (2018) by adding lithium tetrafluoroborate to CS:MC blend polymer. Ammonium thiocyanide was used as a dopant by Aziz et al. (2019), who reported that the ionic conductivity of the CS:MC blend electrolyte was 2.81 × 10–3 Scm−1. There is very little study on CS:MC blends with magnesium salts, and there have been no studies on Mg(NO3)2 doped SBPE based on CS:MC for application in magnesium ion batteries.
The present work aims to prepare the biopolymer blend CS:MC with different amount of Mg(NO3)2 salt by the solution casting technique, and the prepared SBPE were characterized by x-ray diffraction analysis (XRD) which confirms the amorphous nature of the polymer electrolyte, Fourier transform infrared spectroscopy (FTIR) which confirms interaction between the salt and the polymer blend matrix, AC impedance analysis which gives the conductivity and the highest conducting blend polymer electrolyte has been employed in the battery studies which gives an excellent open circuit voltage.
Experimental
Materials and method
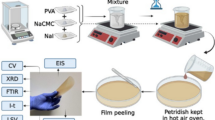
Chitosan (Viscosity 200 cps), methylcellulose (Viscosity 350–550 cps), graphite (C), and Mg metal powder were procured from Loba Chemie Pvt ltd. Mg(NO3)2 salt and acetic acid were procured from S.D. fine-chem. Pvt. limited., Mumbai, India. Manganese dioxide having a molar mass of 86.93 g/mol was procured from Labogens fine Chem, Punjab, India. Iodine (I2) was obtained from Loba Chemie Pvt. Ltd. All were used as such without any further purification to prepare the SBPE. The polymer electrolyte samples were prepared according to our previous work (Nayak et al. 2022). For a few hours, methylcellulose and chitosan are continuously stirred with a magnetic stirrer in 1% acetic acid solvent to obtain a homogenous solution. The composition of salt and polymers expressed in weight percentage is tabulated in Table 1. with reference to the article (Aziz et al. 2019). The most amorphous blend matrix is one with 70 wt.% of CS and 30 wt.% of MC (Aziz et al. 2020b). Thus, we choose to employ it in this study as the polymer matrix doped with a different weight percentage of Mg(NO3)2 salt varying from 0 to 30 wt.%. To prepare these biopolymer electrolyte complexes, homogenous solutions were carefully cast in Petri dishes and kept in a hot air oven at 60 °C for a few days. The films were then detached from the Petri dishes and kept in a desiccator at room temperature for further analysis. The prepared films had a thickness that varied from 100 to 200 µm.
An Mg (anode)/(CS–MC–Mg(NO3)2) (70–30–30)/(cathode) electrochemical cell was constructed using the highest conducting SBPE film, and the performance of the cell was studied. Anode preparation: Magnesium metal of diameter 13 mm was used as the anode. Cathode preparation: Iodine (I2), graphite (C), and the highest conducting SBPE were mixed and pressed under a 5-ton pressure to make a pellet with a diameter of 13 mm (cell 1), and MnO2 and C were combined in a 3:1 ratio to produce another pellet (cell 2), both of which were used to analyze the performance of the primary battery.
Instrumentation
Rigaku Miniflex 600 (5th gen) Cu Kα (λ = 1.54 Å) radiation was used for XRD studies (14 kV, 15 mA). With a 0.02° step size, the scanning angle range of 2θ was 3° to 80°. XRD is utilized to show the amorphous nature of the polymer electrolyte samples. The FTIR investigations were carried out using a Shimadzu IR spirit ATR-FTIR spectrometer with a resolution of 4 cm−1 in the wavenumber range of 4000–400 cm−1. The complexation between the polymer mixture and the magnesium salt was confirmed using FTIR.
The room temperature electrical conductivity of the SBPE samples was measured utilizing the precision impedance analyzer model number IM 3570, Hioki corporation, Japan, at a frequency range of 100 Hz to 5 MHz with ac voltage of 100 mV. The SBPE was inserted between the two stainless steel blocking electrodes having a contact area of 1.13 cm2 to measure the real (Z′), imaginary (Z″) part of complex impedance. A field emission scanning electron microscope (CARL ZEISS) at 25 kX magnification was used for microstructural studies. With the use of Keithley source meter 2636B, the SBPE’s potential stability was evaluated.
Using the Wagner polarization technique, transference number measurements (TNMs) were done at room temperature using the Keithley source meter 2636B by monitoring the polarization current as a function of time. With the cell arrangement, Mg ǀ CMN30 ǀ Mg, between −1 V and + 1 V, and a scan rate of 100 mV/s, a cyclic voltammogram was recorded using a potentiostat (CH Instruments model 600E series).
Results and discussion
XRD analysis
Figure 1 illustrates the x-ray diffraction pattern of pure blend and Mg salt-doped polymer electrolyte films. The XRD peak of CS occurs at 11° and 20° (Khiar 2018). For MC, the typical peaks will be around 8.5° and 20° (Aziz et al. 2020a; Abdullah et al. 2021a). It should be noted that two distinct concave peaks can be seen from the XRD pattern of the CS:MC system (see Fig. 1). All these peaks were superimposed with the amorphous hump background. The XRD pattern of the blend consists of the contribution from the CS and MC. Therefore, on deconvolution, the contribution from both components has to be considered. Thus, the peak assignments have been assigned by considering the crystalline peaks of the components. The blending of MC with CS reduces the crystalline nature of CS because of the strong interaction of MC with the CS and disrupting the hydrogen bonding of CS (Kumari et al. 2012; Liu et al. 2013; Kanimozhi et al. 2016). It is well known that ion migration occurs through the amorphous region, and higher amorphosity means easier ion migration (Arya and Sharma 2019). Earlier research on polymeric systems indicated that an increase in the broadness of the XRD peak suggests an increase in the amorphous fraction (Bdewi et al. 2016; Kumar et al. 2015). Intramolecular interactions between Mg2+ and functional groups in both CS and MC chains, as evident from FTIR studies, diminish the crystallinity of the PE system at low salt concentrations.
The XRD peaks were deconvoluted utilizing Fityk computer software (Wojdyr et al. 2004), and according to the equation
where Ac and Aa indicate the area under the crystalline peak and amorphous peak, respectively, as shown in Fig. 2 (a–g). The computed values of the degree of crystallinity of different poly-blend electrolyte systems are shown in Fig. 2 (h). The calculated values of the degree of crystallinity of the different polymer electrolyte systems indicate that these samples are amorphous in nature, and this facilitates ion conduction.
FTIR study
FTIR measurements were carried out to confirm the interactions between the CS: MC blend polymers and the Mg(NO3)2 salt (Sownthari and Suthanthiraraj 2013). Complexation occurs when the functional groups in the polymer matrix and metal salt ions coordinate. If complexation has occurred, this interaction will change/shift the intensity of the absorption band, which will influence the local structure of the polymer backbone and the active infrared modes of vibration (Manjuladevi et al. 2018a). The C–O bond in ether was found to have a bending peak around 1057 cm−1 for MC polymer (Aziz et al. 2020b).
The peak at 1026 cm−1 in the IR spectra corresponds to the C-O stretch in the polymer backbone. A peak around 2900 cm−1, is associated with the C–H stretching mode. This peak is then shifted towards a higher wavenumber when the salt concentration is raised, which explains the interaction between the C–H of polymer and \({NO}_{3}^{-}\) of salt. The C = O (amide I) stretching and -NH (secondary amide II) bending bands are located at 1641 cm−1 and 1550 cm−1, respectively, in the blended film’s spectra, as shown in Fig. 3. Table 2 lists these band positions, which are almost identical to those reported in other investigations (Wang et al. 2017; Buraidah and Arof 2011; Kadir et al. 2011). The absorption band caused by −OH stretching is found in a broad peak form at 3626–3132 cm−1, arising from adding salt to the system. The doping has brought about a significant change in the band position as well as in the band structure with a prominent shift in the -OH peak (Jeyabanu et al. 2018). It may be deduced from these FTIR investigations that the Mg(NO3)2 salt and the CS:MC blend have formed a complex. The formation of the ion dipole between the \({NO}_{3}^{-}\) anion with the \(C-H\) group has resulted in the shift in peak corresponding to \(C-H\) stretching. The band observed at 1411 cm−1 corresponds to \(-C{H}_{2}\) bending and the peak at 1379 cm−1 ascribed to \(C{H}_{3}\) symmetrical deformation of chitosan polymer (Lawrie et al. 2007). FTIR spectra of \(Mg ({{NO}_{3})}_{2 }.6{H}_{2}O\) salt exhibits bands at 3347 cm−1 that corresponds to \(-OH\) stretching, band at 1647 cm−1 ascribed to \(N=O\) bending, a prominent broad band at 1351 cm−1 corresponds to combined effect of \(N=O\) bending and \(N-O\) stretching and a band at 818 cm−1 corresponds to out of plane deformation mode of \({NO}_{3}^{-}\)(Graham et al. 2016).
The Mg(NO3)2 salt’s possible interaction with the poly-blend is shown in Fig. 4. Mg2+ acts as Lewis acid, and \({NO}_{3}^{-}\) act as Lewis base. The shift in the wavenumber of the -OH band indicates the formation of ion–dipole Mg2+…….OH complex (Fig. 4). A Lewis acid–base adduct, a compound that contains a coordinate covalent bond between the Lewis acid and the Lewis base is formed. The interaction between two polymers in a polymer blend is caused by hydrogen bonding (Nadirah et al. 2020).
Transport parameters
To understand the prominent parameters that have influenced the ionic conductivity in the electrolyte transport parameters: charge density \((\eta )\), Ionic mobility (µ), and diffusion coefficient (D) are evaluated from FTIR deconvolution technique using non-linear fitting software. For the FTIR deconvolution, the region between 1200 to 1500 cm−1 has been chosen since the peak at 1351 cm−1 corresponds to various modes of \({NO}_{3}^{-}\) an anion that overlaps in this region. This wavenumber region from 1200 to 1500 cm−1 that is chosen for deconvolution is reflected in the work of Shetty et al. (2021) for the solid polymer electrolyte systems.
Fall of \({NO}_{3}^{-}\) a peak in the wavenumber region between 1200 to 1500 cm−1 has brought about a change in the intensity and band structure. The change that is observed in this region is due to the interaction between \({NO}_{3}^{-}\) ion via hydrogen bond with the functional group present in the polymer blend. The deconvoluted FTIR spectra consist (Refer to Fig. 5) of 4 bands, the band observed between the wavenumber region 1323 to 1326 cm−1 corresponds to the free anions and the band observed at the higher wavenumber side from 1399 to 1406 cm−1 corresponds to contact ion pairs since the energy required to form contact ion pairs is more than that of the free anion, therefore, band at higher wave number corresponds to contact ion pairs (Saadiah et al. 2020).
The percentage of free anions available in the bulk of the poly-blend is evaluated by considering the area under the free anions \(\left({A}_{f}\right)\) and contact ion \(\left({A}_{c}\right)\) peak in the deconvoluted spectra using Eq. (2)
Transport parameters: charge density \((\eta )\), Ionic mobility (µ), and diffusion coefficient (D) are evaluated from the following Eqs. (3), (5), and (6)
where M is the number of moles of dopant (\(Mg ({{NO}_{3})}_{2 })\), \({N}_{A}\) is the Avogadro’s constant (6.02 \(\times\, {10}^{23}\)/mol), \({V}_{Total}\) is the total volume of the SPEs system, \({k}_{B}\) is the Boltzmann constant (1.38 \(\times {10}^{-23}\,{\mathrm{JK}}^{-1}\)), T is the absolute temperature in Kelvin and e is the electric charge (1.602 \(\times {10}^{-19}\) C).
Impedance analysis
The Nyquist plots of pristine and doped PEs are shown in Fig. 6. An inclined spike and a depressed semicircle can be seen in a Nyquist plot of a pure poly-blend sample. (Perumal et al. 2019). The polymer electrolyte’s bulk resistance and capacitance cause the high-frequency semicircle, while the free charge accumulation at the electrode/electrolyte interface causes the low-frequency spike’s blocking capacitance (Pritam et al. 2019). In our study, a high-frequency semicircle and a low-frequency non-vertical spike have been observed for the biopolymer electrolyte CMN0 and all other compositions of poly-blend with different concentrations of Mg(NO3)2 salt (Kiruthika et al. 2020). The high-frequency semicircle presents bulk conductance, equivalent to the parallel combination of bulk resistance (Rb) and bulk capacitance of the PE (Abdulkareem 2021). While the low-frequency region exhibits behavior similar to a constant phase element (CPE), the high-frequency region exhibits an association of bulk resistance and bulk capacitance [2]. The leaky capacitor and CPE are analogous. The depressed semicircle can be explained through the CPE rather than a capacitor, and the low-frequency tail is introduced as another CPE. A resistor is described as the migration of the Mg2+ ion, whereas a capacitor is the immovable polymer backbone chain that polarizes in the alternating field (Rani et al. 2015; Sownthari and Suthanthiraraj 2013).
Impedance analysis was carried out to arrive at the ionic conductivity \(\left(\sigma \right)\) of the poly-blend system using the relation (7)
where t is the thickness of the film, A is the contact area of the sample with the electrode and \({R}_{b}\) is the bulk resistance of the sample that is obtained by fitting the Nyquist plot using EIS spectrum analyzer software (Bondarenko 2005) with less than 10% error using an equivalent circuit as depicted in Fig. 6.
A clear picture of ionic conductivity correlation with transport parameters is understandable from Fig. 7. Charge carrier density (η) increased with increased salt concentration, indicating the dissociation of salt by the functional group of the polymer. Direct correlation of η with conductivity (\(\upsigma \propto\upeta )\) was not observed due to a fall of ionic conductivity in the case of CMN10 and CMN25 samples and is due to a decrease in ionic mobility and diffusivity as per the transport parameters calculated from FTIR deconvolution technique and justified due to increase in crystallinity that may hinder the motion of ions in the SBPE system. CMN20 sample exhibited the highest diffusion coefficient and mobility and is due to a decrease in crystallinity due to polymer-ion interaction. From impedance spectroscopy analysis, it has been found that the SBPE CMN30 has the highest ionic conductivity value of 2.12 × 10–5 Scm−1.
Frequency-dependent study of conductivity:
Figure 8 shows the frequency-dependent ac conductivity of the SBPE with various amounts of Mg(NO3)2. The below Fig. 8 shows three distinct regions, that is, low-frequency dispersion region which shows the space charge polarization at the blocking electrode, a middle-frequency independent plateau region showing the DC conductivity of the prepared SBPE due to ion migration to neighboring sites and the high-frequency region which indicates the bulk relaxation process (Rathika and Suthanthiraraj 2016).
SEM analysis
SEM images can be used as an effective tool to gain information about the morphological aspects of the solid polymer electrolyte samples. The SEM micrographs of the pure blend and blend doped with various concentration of Mg(NO3)2 salt is shown in Fig. 9. The detection of phase separations and interfaces using SEM has been widely used to investigate the compatibility between different components of blended polymer electrolytes. CMN0 has smooth surface morphology due to the absence of salt in the blend matrix. CMN5 and CMN10 are almost similar with an intergranular appearance on the surface without any phase separation. While in CMN15 and CMN20 with the increase in salt concentration there might be an increase in the interaction of salt with polymer chains, hence the occurrence of pores on the surface can be observed. These pores facilitate the movement of ions and segmental motion of the polymer matrix (Yap et al. 2013), hence the crystallinity decreased, as depicted in XRD studies. Furthermore, with the increase in salt concentration (CMN25 and 30) the surface was wrinkled and has minute pores indicating enhanced interaction between them. Moreover, in all the salt-containing blends as there is no agglomeration of salt on the surface of the blend electrolyte systems, the smooth surface aids the free transfer of ions within the polymer matrix and is similar to enhanced number density data (Abdulkareem 2021).
I-V Characterization
The ESW of the SBPE samples has been studied and the I-V plot of the same is shown in Fig. 10. The current begins to progressively increase as the potential increases. The ESW of the SBPE samples has been calculated and presented in Table 3. It was found that the ESW gradually raises and reaches a maximum value of 3.65 V for 30 wt.% of Mg(NO3)2. This implies that the magnesium-ion conducting SBPE system is electrochemically stable up to 3.65 V, which is an acceptable range for use in magnesium-ion primary batteries (Manjuladevi et al. 2018a). The maximum ESW of 2.05 V was obtained for the SBPE based on pectin with MgCl2 reported by Kiruthika et al. (2020), Manjuladevi et al. (2018a) reported a stability window of 3.4 V for the PVA:PAN blend with magnesium nitrate as a salt. The obtained ESW value is better than the reported values.
Transference number measurement (TNM)
Studying the TNM is essential to employ the SBPE in device applications. A TNM investigation is used to determine the dominant charge carrier species (Manjuladevi et al. 2017). The total ion and electron transference numbers can be calculated using the following equations:
where Ii is the starting current and Iss is the steady-state current (Polu et al. 2015).
Figure 11 shows the electrolyte polarization at 0.5 V. At first, both the ion and the electron cause a significant current value. The electrodes were made of SS, which is known to block ions, so the current value decreased noticeably before stabilizing (Aziz et al. 2019). The highest conducting SBPE’s total ion transport number was found to be 0.86. It was found that ions were the dominant charge carriers (Ponmani et al. 2018; Polu et al. 2012).
Cyclic voltammogram (CV) studies
For use in Mg batteries, the electrochemical reversibility of Mg/Mg2+ from an SBPE needs to be thoroughly studied. The following two symmetrical cells were subjected to CV measurements using SBPE with 30 wt.% of Mg(NO3)2.
First cell: SS/CMN30/SS.
Second cell: Mg/CMN30/Mg.
Figures 12 (a) and (b) show the first cell’s and second cell’s CV plots, which were obtained at a scanning rate of 100 mVs−1. The CV trace shows a distinct cathodic and anodic peak for the second cell, while the first cell does not have such peaks (Fig. 12). The first cell has a higher current magnitude than the second cell. The second cell CV also displays an anodic peak and a minor cathodic peak. This shows that cathodic deposition and anodic magnesium dissolution at the electrode/electrolyte interface occur in the second cell. This confirms the compatibility of Mg ions in the prepared SBPE (Cyriac et al. 2022).
Study of Mg ion primary battery
To study the practical applicability of these polymer electrolytes for primary batteries, a primary magnesium ion battery is built by incorporating the highest conducting sample i.e., CMN30 as the electrolyte in between the magnesium metal pellet (anode) and cathode materials using the battery holder. Choosing the anode materials in solid-state batteries depends on the mobile species in the electrolyte (Radhakrishna 1990). In this work, a magnesium metal pellet is used as an anode material. To study the influence of electrode material on the performance of the battery, we have chosen two types of cathode materials. One of the cell systems is Mg/CMN30/(I2 + C + CMN30) designated as cell 1 and the other one is Mg/CMN30/(MnO2 + C) designated as cell 2. In cell 1, iodine plays the role of “active cathode.” Having graphite increases the cathode material’s electronic conductivity (Rao et al. 2012). With the addition of polymer electrolytes, the cathode mixture has a larger surface area for interfacial contact, which lowers interfacial resistance and improves battery performance (Austin 1973). The cell parameters are listed in Table 4. In cell 1, an initial OCV of 1.66 V (Refer to Fig. 13a) was reduced to 1.50 V and maintained the same for 24 h. After connecting a load of 100 kΩ, the voltage of 1.50 V was reduced to 1.03 V during the time duration of 24 h. Initially, 1.2 V is maintained constant for 12 h, and this constant region is called the plateau region. In Cell 2 (Refer Fig. 13b), an initial OCV of 1.92 V was reduced to 1.80 V and maintained the same for 24 h. After connecting a load of 100 kΩ, the voltage of 1.80 V was reduced to 0.89 V during the time duration of 10 h. The first rapid drop in cell voltage may have been due to polarization or the formation of a thin layer of magnesium salt at the electrode/electrolyte interface (Polu and Kumar 2013). As indicated in Table 5, the findings of the current investigation are comparable to those of earlier studies. The performance of the cell 1 is better than the cell 2 because the discharge time for plateau region of cell 1 is around 12 to 13 h whereas that of cell 2 is only 8 to 9 h. This indicates that the present polymer electrolyte may be used in solid-state batteries.
Conclusions
The solution casting approach was employed to synthesize the SBPE using CS:MC with the varied quantity of Mg(NO3)2. CS and MC were used as the biopolymer blend matrix where ions provided by Mg(NO3)2 participate in the conduction process. The XRD analysis reveals that the prepared polymer electrolyte was amorphous in nature. FTIR study validates the complex formation between the biopolymer and the magnesium nitrate salt. From EIS analysis, it has been found that 30 wt.% magnesium nitrate added sample, i.e., CMN30, exhibits the highest ionic conductivity of 2.12 × 10–5 Scm−1 at room temperature. The electrochemical stability for the highest conducting sample is found to be 3.65 V which is sufficient for practical applications in electrochemical devices such as a battery. The total ionic transference number of the highest conducting electrolyte was 0.86. The magnesium ion electrochemical cells assembled using the CMN30 SBPE membrane which acts both as an electrolyte and separator show an open circuit voltage of 1.92 V at room temperature. Thus CS:MC:Mg(NO3)2 system demonstrates as an efficient SBPE for the futuristic magnesium ion batteries.
Data availability
No data or material is provided with this manuscript.
References
Abdulkareem SS (2021) Structural, morphological and electrical properties of chitosan/methylcellulose blend polymer doped with different concentrations of NH4NO3. Mater Res Express. https://doi.org/10.1088/2053-1591/ac1d69
Abdullah OGH, Hanna RR, Salman YAK (2019) Structural and electrical conductivity of CH:MC bio-poly-blend films: optimize the perfect composition of the blend system. Bull Mater Sci 42:1–7. https://doi.org/10.1007/s12034-019-1742-3
Abdullah AM, Aziz SB, Saeed SR (2021) Structural and electrical properties of polyvinyl alcohol (PVA): methyl cellulose (MC) based solid polymer blend electrolytes inserted with sodium iodide (NaI) salt. Arab J Chem 14:103388. https://doi.org/10.1016/j.arabjc.2021.103388
Abdullah OG, Hanna RR, Ahmed HT et al (2021) Conductivity and dielectric properties of lithium-ion biopolymer blend electrolyte based film. Results Phys 24:104135. https://doi.org/10.1016/j.rinp.2021.104135
Arya A, Sharma AL (2019) Electrolyte for energy storage/conversion (Li+, Na+, Mg2+) devices based on PVC and their associated polymer: a comprehensive review. J Solid State Electrochem 23:997–1059. https://doi.org/10.1007/s10008-019-04203-x
Austin LG (1973) Techn ca]l Notes. 120:4–7
Aziz SB, Abdullah OG, Hussein SA, Ahmed HM (2017) Effect of PVA blending on structural and ion transport properties of CS: AgNt-based polymer electrolyte membrane. Polymers (Basel). https://doi.org/10.3390/polym9110622
Aziz SB, Hamsan MH, Abdullah RM, Kadir MFZ (2019) A promising polymer blend electrolytes based on chitosan: Methyl cellulose for EDLC application with high specific capacitance and energy density. Molecules. https://doi.org/10.3390/molecules24132503
Aziz SB, Brevik I, Hamsan MH et al (2020a) Compatible solid polymer electrolyte based on methyl cellulose for energy storage application: Structural, electrical, and electrochemical properties. Polymers (basel) 12:1–19. https://doi.org/10.3390/polym12102257
Aziz SB, Hamsan MH, Abdullah RM, Abdulwahid RT, Brza MA, Marif AS, Kadir MFZ (2020b) Protonic EDLC cell based chitosan methylcellulose solid Polym blend electrolytes. Ionics 26(4):1829–1840. https://doi.org/10.1007/s11581-020-03498-5
Aziz SB, Hamsan MH, Nofal MM et al (2020c) From cellulose, shrimp and crab shells to energy storage EDLC cells: the study of structural and electrochemical properties of proton conducting chitosan-based biopolymer blend electrolytes. Polymers (basel) 12:1–24. https://doi.org/10.3390/polym12071526
Aziz SB, Asnawi ASFM, Kadir MFZ et al (2021) Structural, electrical and electrochemical properties of glycerolized biopolymers based on chitosan (Cs): methylcellulose (mc) for energy storage application. Polymers (Basel). https://doi.org/10.3390/polym13081183
Bdewi SF, Abdullah OG, Aziz BK, Mutar AAR (2016) Synthesis, structural and optical characterization of MgO nanocrystalline embedded in PVA matrix. J Inorg Organomet Polym Mater 26:326–334. https://doi.org/10.1007/s10904-015-0321-3
Bondarenko AS RGA (2005) No Title. 89–102
Buraidah MH, Arof AK (2011) Characterization of chitosan/PVA blended electrolyte doped with NH4I. J Non Cryst Solids 357:3261–3266. https://doi.org/10.1016/j.jnoncrysol.2011.05.021
Cyriac V, Ismayil NIM et al (2022) Ionic conductivity enhancement of PVA: carboxymethyl cellulose poly-blend electrolyte films through the doping of NaI salt. Cellulose 29:3271–3291. https://doi.org/10.1007/s10570-022-04483-z
Dannoun EMA, Aziz SB, Brza MA et al (2020) The study of plasticized solid polymer blend electrolytes based on natural polymers and their application for energy storage EDLC devices. Polymers (basel) 12:1–19. https://doi.org/10.3390/polym12112531
Graham M, Shchukina E, De Castro PF, Shchukin D (2016) Nanocapsules containing salt hydrate phase change materials for thermal energy storage. J Mater Chem A 4:16906–16912. https://doi.org/10.1039/c6ta06189c
Jeyabanu K, Siva V, Nallamuthu N et al (2018) Investigation of electrochemical studies of magnesium ion conducting poly(vinyl alcohol)–poly(vinyl pyrrolidone) based blend polymers. J Nanosci Nanotechnol 18:1103–1109. https://doi.org/10.1166/jnn.2018.13969
Kadir MFZ, Aspanut Z, Majid SR, Arof AK (2011) FTIR studies of plasticized poly(vinyl alcohol)-chitosan blend doped with NH4NO3 polymer electrolyte membrane. Spectrochim Acta Part A Mol Biomol Spectrosc 78:1068–1074. https://doi.org/10.1016/j.saa.2010.12.051
Kanimozhi K, Khaleel Basha S, Sugantha Kumari V (2016) Processing and characterization of chitosan/PVA and methylcellulose porous scaffolds for tissue engineering. Mater Sci Eng C 61:484–491. https://doi.org/10.1016/j.msec.2015.12.084
Kesavan K, Mathew CM, Rajendran S et al (2015) Solid polymer blend electrolyte based on poly(ethylene oxide) and poly(vinyl pyrrolidone) for lithium secondary batteries. Brazilian J Phys 45:19–27. https://doi.org/10.1007/s13538-014-0279-6
Khiar ASA (2018) Electrical and structural studies of polymer electrolyte based on 2 chitosan/ methyl cellulose blend doped with BMIMTFSI. Mater Res Express. https://doi.org/10.1088/2053-1591/aac25b
Kiruthika S, Malathi M, Selvasekarapandian S et al (2020) Conducting biopolymer electrolyte based on pectin with magnesium chloride salt for magnesium battery application. Polym Bull 77:6299–6317. https://doi.org/10.1007/s00289-019-03071-9
Kumar M, Tiwari T, Chauhan JK, Srivastava N (2015) Understanding the ion dynamics and relaxation behavior from impedance spectroscopy of NaI doped zwitterionic polymer system. Mater Res Express. https://doi.org/10.1088/2053-1591/1/4/045003
Kumari VS, Basha SK, Sudha PN (2012) Physicochemical and morphological evaluation of chitosan/poly(vinyl alcohol)/methylcellulose chemically cross-linked ternary blends. Polym Bull 68:1387–1393. https://doi.org/10.1007/s00289-011-0645-2
Lawrie G, Keen I, Drew B et al (2007) Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromol 8:2533–2541. https://doi.org/10.1021/bm070014y
Liu PT, Wei XM, Liu Z (2013) Miscibility study of chitosan and methylcellulose blends. Adv Mater Res 750–752:802–805. https://doi.org/10.4028/www.scientific.net/AMR.750-752.802
Mahalakshmi M, Selvanayagam S, Selvasekarapandian S et al (2020) Magnesium ion-conducting solid polymer electrolyte based on cellulose acetate with magnesium nitrate (Mg(NO3)2·6H2O) for electrochemical studies. Ionics (kiel) 26:4553–4565. https://doi.org/10.1007/s11581-020-03615-4
Manjuladevi R, Thamilselvan M, Selvasekarapandian S et al (2017) Mg-ion conducting blend polymer electrolyte based on poly(vinyl alcohol)-poly (acrylonitrile) with magnesium perchlorate. Solid State Ionics 308:90–100. https://doi.org/10.1016/j.ssi.2017.06.002
Manjuladevi R, Selvasekarapandian S, Thamilselvan M et al (2018a) A study on blend polymer electrolyte based on poly(vinyl alcohol)-poly (acrylonitrile) with magnesium nitrate for magnesium battery. Ionics (kiel) 24:3493–3506. https://doi.org/10.1007/s11581-018-2500-z
Manjuladevi R, Thamilselvan M, Selvasekarapandian S et al (2018b) Preparation and characterization of blend polymer electrolyte film based on poly(vinyl alcohol)-poly(acrylonitrile)/MgCl2 for energy storage devices. Ionics (kiel) 24:1083–1095. https://doi.org/10.1007/s11581-017-2273-9
Mazuki NF, Rasali NMJ, Saadiah MA, Samsudin AS (2018) Irregularities trend in electrical conductivity of CMC/PVA-NH4Cl based solid biopolymer electrolytes. AIP Conf Proc 10(1063):5066862
Nadirah BN, Ong CC, Saheed MSM et al (2020) Structural and conductivity studies of polyacrylonitrile/methylcellulose blend based electrolytes embedded with lithium iodide. Int J Hydrogen Energy 45:19590–19600. https://doi.org/10.1016/j.ijhydene.2020.05.016
Nayak P, Cyriac V, Hegde S, Sanjeev G (2022) Magnesium ion conducting free-standing biopolymer blend electrolyte films for electrochemical device application. J Non Cryst Solids 592:121741. https://doi.org/10.1016/j.jnoncrysol.2022.121741
Perumal P, Abhilash KP, Sivaraj P, Selvin PC (2019) Study on Mg-ion conducting solid biopolymer electrolytes based on tamarind seed polysaccharide for magnesium ion batteries. Mater Res Bull 118:110490. https://doi.org/10.1016/j.materresbull.2019.05.015
Polu AR, Kumar R (2013) Preparation and characterization of pva based solid polymer electrolytes for electrochemical cell applications. Chin J Polym Sci Eng Ed 31:641–648. https://doi.org/10.1007/s10118-013-1246-3
Polu AR, Kumar R, Kumar KV (2012) Ionic conductivity and electrochemical cell studies of new Mg2+ ion conducting PVA/PEG based polymer blend electrolytes. Adv Mater Lett 3:406–409. https://doi.org/10.5185/amlett.2012.6375
Polu AR, Kumar R, Rhee HW (2015) Magnesium ion conducting solid polymer blend electrolyte based on biodegradable polymers and application in solid-state batteries. Ionics (kiel) 21:125–132. https://doi.org/10.1007/s11581-014-1174-4
Ponmani S, Kalaiselvimary J, Ramesh Prabhu M (2018) Structural, electrical, and electrochemical properties of poly(vinylidene fluoride-co-hexaflouropropylene)/poly(vinyl acetate)-based polymer blend electrolytes for rechargeable magnesium ion batteries. J Solid State Electrochem 22:2605–2615. https://doi.org/10.1007/s10008-018-3971-6
Pritam AA, Sharma AL (2019) Dielectric relaxations and transport properties parameter analysis of novel blended solid polymer electrolyte for sodium-ion rechargeable batteries. J Mater Sci 54:7131–7155. https://doi.org/10.1007/s10853-019-03381-3
Rani MSA, Mohamed NS, Isa MIN (2015) Investigation of the ionic conduction mechanism in carboxymethyl cellulose/chitosan biopolymer blend electrolyte impregnated with ammonium nitrate. Int J Polym Anal Charact 20:491–503. https://doi.org/10.1080/1023666X.2015.1050803
Rao CVS, Ravi M, Raja V et al (2012) Preparation and characterization of PVP-based polymer electrolytes for solid-state battery applications. Iran Polym J Engl Ed 21:531–536. https://doi.org/10.1007/s13726-012-0058-6
Rathika R, Suthanthiraraj SA (2016) Ionic interactions and dielectric relaxation of PEO/PVDF-Mg[(CF3SO2)2N2)] blend electrolytes for magnesium ion rechargeable batteries. Macromol Res 24:422–428. https://doi.org/10.1007/s13233-016-4053-1
Saadiah MA, Nagao Y, Samsudin AS (2020) Proton (H+) transport properties of CMC–PVA blended polymer solid electrolyte doped with NH4NO3. Int J Hydrogen Energy 45:14880–14896. https://doi.org/10.1016/j.ijhydene.2020.03.213
Salman YAK, Abdullah OG, Hanna RR, Aziz SB (2018) Conductivity and electrical properties of chitosan - methylcellulose blend biopolymer electrolyte incorporated with lithium tetrafluoroborate. Int J Electrochem Sci 13:3185–3199. https://doi.org/10.20964/2018.04.25
Sarma RVGK, Radhakrishna S (1990) Silver borotungstate glasses: new electrolyte for solid state electrochemical cell. Solid State Ionics 204:483–486. https://doi.org/10.1016/0167-2738(90)90385-5
Shetty SK, Ismayil HS et al (2021) Dielectric relaxations and ion transport study of NaCMC:NaNO3 solid polymer electrolyte films. Ionics (kiel) 27:2509–2525. https://doi.org/10.1007/s11581-021-04023-y
Sownthari K, Suthanthiraraj SA (2013) Synthesis and characterization of an electrolyte system based on a biodegradable polymer. Express Polym Lett 7:495–504. https://doi.org/10.3144/expresspolymlett.2013.46
Wang J, Song S, Gao S et al (2017) Mg-ion conducting gel polymer electrolyte membranes containing biodegradable chitosan: preparation, structural, electrical and electrochemical properties. Polym Test 62:278–286. https://doi.org/10.1016/j.polymertesting.2017.07.016
Wojdyr M, Gierlotka S, Palosz B (2004) FITYK – peak-fitting software with support for powder patterns analysis. Acta Crystallogr Sect A Found Crystallogr 60:s246–s246. https://doi.org/10.1107/s010876730409511x
Yap YL, You AH, Teo LL, Hanapei H (2013) Inorganic filler sizes effect on ionic conductivity in polyethylene oxide (PEO) composite polymer electrolyte. Int J Electrochem Sci 8:2154–2163
Acknowledgements
One of the authors Pradeep Nayak acknowledges the financial assistance from the “University Grants Commission, New Delhi, Government of India” in the form of “Junior Research Fellowship”, sanction order: 1237/ (CSIR-UGC NET DEC. 2017) dated 21-01-2019.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. Pradeep Nayak has received funding from University Grants Commission (UGC), New Delhi, Government of India as Junior Research Fellowship (JRF) with sanction number UGC-Ref. No. 1237/ (CSIR-UGC NET DEC. 2017) dated 21–01-2019.
Author information
Authors and Affiliations
Contributions
PN: Conceptualization, Methodology, Data curation, Investigation, Writing-Original draft preparation. YNS: Visualization, Resources, Data curation, Investigation. SD: Resources. I: Methodology, Investigation, Validation, Resources, Supervision. SKS: Formal analysis, Validation. VC: Formal analysis, Writing- Reviewing and Editing.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to infuence the work reported in this paper.
Consent for publication
The authors hereby consent to publication of the present research work in this journal, if selected for publication.
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nayak, P., Sudhakar, Y.N., De, S. et al. Modifying the microstructure of chitosan/methylcellulose polymer blend via magnesium nitrate doping to enhance its ionic conductivity for energy storage application. Cellulose 30, 4401–4419 (2023). https://doi.org/10.1007/s10570-023-05114-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05114-x