Abstract
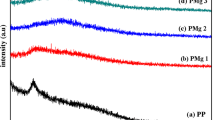
In this paper, the solution casting technique was used to develop the magnesium-conducting solid polymer electrolyte (PE) using cellulose acetate (CA) with the magnesium nitrate (Mg(NO3)2·6H2O) salt. The crystalline/amorphous nature of CA with different concentrations of Mg(NO3)2·6H2O polymer electrolytes was studied by X-ray diffraction (XRD) analysis. The highest ionic conductivity of 9.19 × 10−4 S/cm was found for 60 wt.%CA/40 wt.% Mg(NO3)2·6H2O PE by Ac impedance spectroscopy at room temperature. The polymer membranes are subjected to FTIR analysis. The glass transition temperatures (Tg) of the PEs were determined using differential scanning calorimeter (DSC). Then, the transference number of Mg2+ ion for 60 wt.%CA/40 wt.% Mg(NO3)2·6H2O PE was measured as 0.35 using Evan’s method. The electrochemical stability of 3.65 V was observed for the highest ionic conducting PE by linear sweep voltammetry. The cyclic voltammetry (CV) study was done for highest ionic conducting polymer electrolyte. Finally, the primary magnesium battery was constructed with the highest ionic conducting PE, and its performance was studied.











Similar content being viewed by others
References
Ye L, Feng Z (2010) Polymer electrolytes fundamentals and applications 551 © Wood head publishing Limited
Mohiuddin M, Kumar B, Haque S (2017) Biopolymer composites in photovoltaics and photodetectors. Biopolymer Composites in Electronics:459–486. https://doi.org/10.1016/b978-0-12-809261-3.00017-6
Mohanty AK et al. (2005) Natural fibers. Biopolymers and Biocomposites © CRC press
McCray B, Vilker VL, Nobe K (1991) Reverse osmosis cellulose acetate membranes II dependence of transport properties on acetyl content. J Membr Sci 59(3):317–330. https://doi.org/10.1016/s0376-7388(00)80820-0
Abidin SZZ, Ali AMM, Hassan OH, Yahya MZA (2013) Electrochemical studies on cellulose acetate-LiBOB polymer gel electrolytes. Int J Electrochem Sci 8:7320–7326
Monisha S, Selvasekarapandian S, Mathavan T, Milton Franklin Benial A, Manoharan S, Karthikeyan S (2016) Preparation and characterization of biopolymer electrolyte based on cellulose acetate for potential applications in energy storage devices. J Mater Sci Mater Electron 27(9):9314–9324
Monisha S, Mathavan T, Selvasekarapandian S, Milton Franklin Benial A, Aristatil G, Premalatha M, Vinoth Pandi D (2017) Investigation of biopolymer electrolyte based on cellulose acetate-ammonium nitrate for potential use in electrochemical devices. Carbohydr Polym 157:38–47
Monisha S, Mathavan T, Selvasekarapandian S, Benial AMF, Latha MP (2016) Preparation and characterization of cellulose acetate and lithium nitrate for advanced electrochemical devices. Ionics 23(10):2697–2706
Ramesh S, Shanti R, Morris E (2012) Plasticizing effect of 1-allyl-3-methylimidazolium chloride in cellulose acetate based polymer electrolytes. Carbohydr Polym 87(4):2624–2629. https://doi.org/10.1016/j.carbpol.2011.11.037
Selvakumar M, Bhat DK (2008) LiClO4 doped cellulose acetate as a biodegradable polymer electrolyte for supercapacitors. J Appl Polym Sci 110(1):594–602
Sarangika HNM, Dissanayake MAKL, Senadeera GKR, Rathnayake RRDV, Pitawala HMJC (2017) Polyethylene oxide and ionic liquid-based solid polymer electrolyte for rechargeable magnesium batteries doi: https://doi.org/10.1007/s11581-016-1870-3
Kim HS, Arthur TS, Allred GD, Zajicek J, Newman JG, Rodnyansky AE, Oliver AG, Boggess WC, Muldoon J (2011) Structure and compatibility of a magnesium electrolyte with a sulphur cathode. Nat Commun 2:427
Pandey GP, Agrawal RC, Hashmi SA (2009) Magnesium ion-conducting gel polymer electrolytes dispersed with nanosized magnesium oxide. J Power Sources 190(2):563–572. https://doi.org/10.1016/j.jpowsour.2009.01.057
Manjuladevi R, Selvasekarapandian S, Thamilselvan M, Mangalam R, Monisha S, Selvin PC (2018) A study on blend polymer electrolyte based on poly(vinyl alcohol)-poly (acrylonitrile) with magnesium nitrate for magnesium battery. Ionics. 24:3493–3506. https://doi.org/10.1007/s11581-018-2500-z
Shanmuga Priya S, Karthika M, Selvasekarapandian S, Manjuladevi R, Monisha S (2018) Study of biopolymer I-carrageenan with magnesium perchlorate. Ionics 24:3861–3875. https://doi.org/10.1007/s11581-018-2535-1
Kiruthika S, Malathi M, Selvasekarapandian S, Tamilarasan K, Moniha V, Manjuladevi R (2019) Eco-friendly biopolymer electrolyte, pectin with magnesium nitrate salt, for application in electrochemical devices. J Solid State Electrochem 23:2181–2193. https://doi.org/10.1007/s10008-019-04313-6
Sangeetha P, Selvakumari TM, Selvasekarapandian S, Srikumar SR, Manjuladevi R, Mahalakshmi M (2019) Preparation and characterization of biopolymer K-carrageenan with MgCl2 and its application to electrochemical devices. Ionics. 26:233–244. https://doi.org/10.1007/s11581-019-03193-0
Mahalakshmi M, Selvanayagam S, Selvasekarapandian S, Moniha V, Manjuladevi R, Sangeetha P (2019) Characterization of biopolymer electrolytes based on cellulose acetates with magnesium perchlorate (Mg(ClO4)2) for energy storage devices. J Sci Adv Mater Devices 4:276–284. https://doi.org/10.1016/j.jsamd.2019.04.006
Shuhaimi NEA, Alias NA, Kufian MZ, Majid SR, Arof AK (2010) Characteristics of methyl cellulose-NH4NO3-PEG electrolyte and application in fuel cells. J Solid State Electrochem 14(12):2153–2159. https://doi.org/10.1007/s10008-010-1099-4
Hodge RM, Edward GH, Simon GP (1996) Water absorption and states of water in semi crystalline poly (vinyl alcohol) films. Polymer 37(8):1371–1376
Samsudin AS, Khairul WM, Isa MIN (2012) Characterization on the potential of carboxy methylcellulose for application as proton conducting biopolymer electrolytes. J Non-Cryst Solids 358(8):1104–1112. https://doi.org/10.1016/j.jnoncrysol.2012.02.004
Koenig JL (1999) Spectroscopy of polymers © Amsterdam:Elsevier 2nd ed
Sulaiman M , Rahman AA, Mohamed NS (2013) Structural, thermal and conductivity studies of magnesium nitrate – alumina composite solid electrolytes prepared via sol-gel method. Int J Electrochem Sci, 6647–6655
Prajapati GK, Gupta PN (2011) Comparative study of the electrical and dielectric properties of PVA–PEG–Al2O3–MI (M=Na, K, Ag) complex polymer electrolytes. Phys B Condens Matter 406(15–16):3108–3113. https://doi.org/10.1016/j.physb.2011.05.019
Vargas MA, Vargas RA, Mellander BE (2000) More studies on the PVAl+H3PO2+H2O proton conductor gels. Electrochim Acta 45(8–9):1399–1403. https://doi.org/10.1016/s0013-4686(99)00350-3
Boukamp BA (1986a) A nonlinear least squares fit procedure for analysis of immittance data of electrochemical systems. Solid State Ionics 20:31–44
Ramya CS, Selvasekarapandian S, Savitha T, Hirankumar G, Baskaran R, Bhuvaneswari MS, Angelo PS (2006) Conductivity and thermal behavior of proton conducting polymer electrolyte based on poly (N-vinyl pyrrolidone). Eur Polym J 42(10):2672–2677
Ramesh S, Shanti R, Morris E (2011) Discussion on the influence of DES content in CA-based polymer electrolytes. J Mater Sci 47(4):1787–1793. https://doi.org/10.1007/s10853-011-5964-z
Wagner JB, Wagner CJ (1957) Chem Rev 26-597
Evans J, Vincent CA, Bruce PG (1987) Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 28(13):2324–2328
Tiankhoon L, Ataollahi N, Hassan N H, Ahamad A (2015) J Solid State Electrochem 015–3017-2
Kumar Y, Hashmi SA, Pandey GP (2011) Ionic liquid mediated magnesium ion conduction in poly(ethylene oxide) based polymer electrolyte. Electrochim Acta 56:3864–3873. https://doi.org/10.1016/j.electacta.2011.02.035
Hambali D, Zainol NH, Othman L, Md Isa KB, Osman Z (2018) Magnesium ion conducting gel polymer electrolytes based on poly(vinylidene chloride-co-acrylonitrile) (PVdC-co-AN): a comparative study between magnesium trifluoromethane sulfonate (MgTf2) and magnesium bis(trifluoromethanesulfonimide) (Mg(TFSI)2). Ionics 15–18
Acknowledgments
Authors are thankful to Dr. RAJKUMR, Department of Chemistry, Vivekananda College, Thiruvedagam, Madurai and to Dr. K. VIGNESHWARI, Department of Chemistry, Sri Meenakshi Govt. Arts College for women, Madurai for their useful discussion with regard to FTIR spectral analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mahalakshmi, M., Selvanayagam, S., Selvasekarapandian, S. et al. Magnesium ion-conducting solid polymer electrolyte based on cellulose acetate with magnesium nitrate (Mg(NO3)2·6H2O) for electrochemical studies. Ionics 26, 4553–4565 (2020). https://doi.org/10.1007/s11581-020-03615-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-020-03615-4